Introduction
Primary malignant melanoma of the esophagus (PMME)
is rare, accounting for 0.1–0.2% of all primary malignancie of the
esophagus (1). Moreover, amelanotic
malignant melanoma accounts for 10–25% of all malignant melanomas
of the esophagus and is a rarer tumor with a rapid progression and
a poor prognosis, often progressing with multiple metastases even
in the early stage of the disease; only a few case reports have
been published in the literature (1).
It is difficult to diagnose PMME, especially the amelanotic type,
with the surgically resected specimen or endoscopic biopsy tissue.
Pigment cells of normal and malignant melanocytes are useful for
the analysis of observation differentiation (2). True amelanotic malignant melanomas
produce no melanin or granules, resulting in no pigmentation and
contain stage I and/or II melanosomes (3). Accordingly, primary amelanotic malignant
melanoma of the esophagus is frequently misdiagnosed at biopsy as
poorly differentiated squamous cell carcinoma, sarcoma, spindle
cell carcinoma, or undifferentiated carcinoma. We report a case of
primary amelanotic malignant melanoma of the esophagus that was
difficult to diagnose but could be radical resection, and the
review of the literature regarding the usefulness of new markers in
diagnosis.
Case report
A 68-year-old man underwent laparoscopic curative
distal gastrectomy for early gastric cancer two years ago.
Pathological diagnosis had been stage IA: T1bN0M0 according to the
TNM classification of the International Union Against Cancer. He
had smoked 20 cigarettes per day at the age of 20 to 36 years and
drunken 720 ml of Japanese rice wine every day till the age of 48
years. His physical examination showed the scars of laparoscopic
distal gastrectomy. He also underwent endoscopic examination every
year. One year after the gastrectomy, endoscopic examination
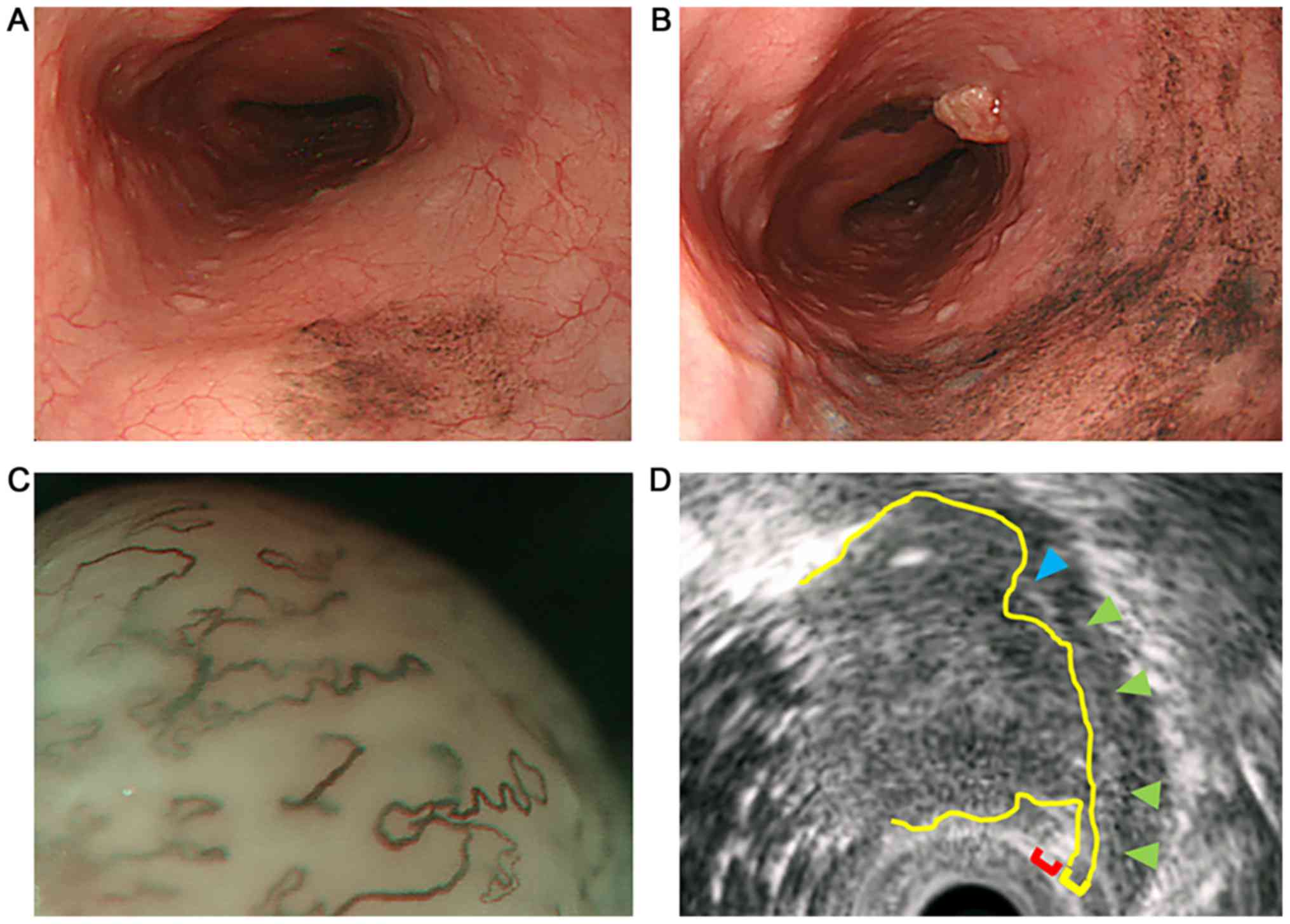
revealed the formation of melanosis in the middle thoracic
esophagus (Fig. 1A). One year later,
endoscopic examination revealed the expansion of the melanosis area
and the appearance of a protruded lesion lying adjacent to the
melanosis area (Fig. 1B). It was a
type 0-Is non-pigmented tumor with a central recess, 20 mm in
longitudinal diameter, with a clear round wall. Lugol staining
method of endoscopic examination gave a negative result. Magnifying
endoscopy demonstrated a vascular area over 3 mm and that the
vascular is extreme distention: the finding of type B3, that was
the magnifying endoscopic classification of the Japan Esophageal
Society (4) (Fig. 1C). Endoscopic ultrasonography
demonstrated that the tumor was communicated with the second layer.
The third layer disappeared by the invasion of the tumor (Fig. 1D). These findings indicated that the
depth of the tumor was beyond muscularis propria. Histology of the
biopsy specimen showed anisocytosis, nuclear enlargement, high N/C
ratio, prominent nucleoli and vacuoles. Immunohistochemical
staining was positive for S-100, but negative for cytokeratin
AE1/AE3, desmin, α-SMA, CD34, Leukocyte common antigen, HMB-45,
Melan-A, c-kit and DOG-1. We could make a diagnosis of malignant
tumor but could not reach a definite histological type. Enhanced
computed tomography from chest to pelvis did not demonstrate the
primary mass and metastases. There was accumulation in the middle
of the esophagus and no accumulations of lymph nodes and other
organs in positron-emission tomography. We did not have the
accurate diagnosis, but we confirmed the malignancy and the
necessity of the surgery. We decided to resect it. The patient
underwent trans-thoraco-abdominal curative subtotal esophagectomy.
Reconstruction was performed by pulling up the colon via the
retrosternal route; the site of anastomosis was in the neck. The
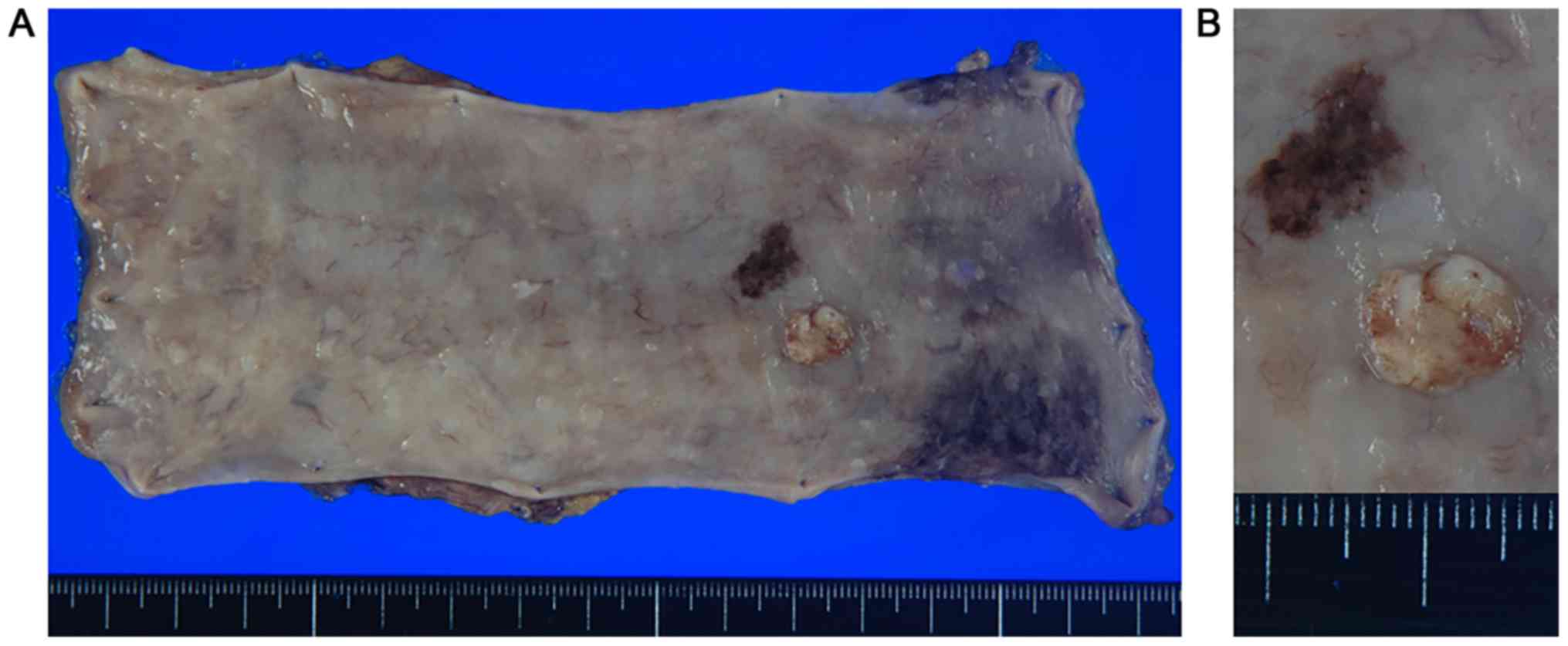
surgical specimen demonstrated a 20×15 mm non-pigmented granular
protruded lesion with a central recess next to melanosis (Fig. 2A and B). It was located in only
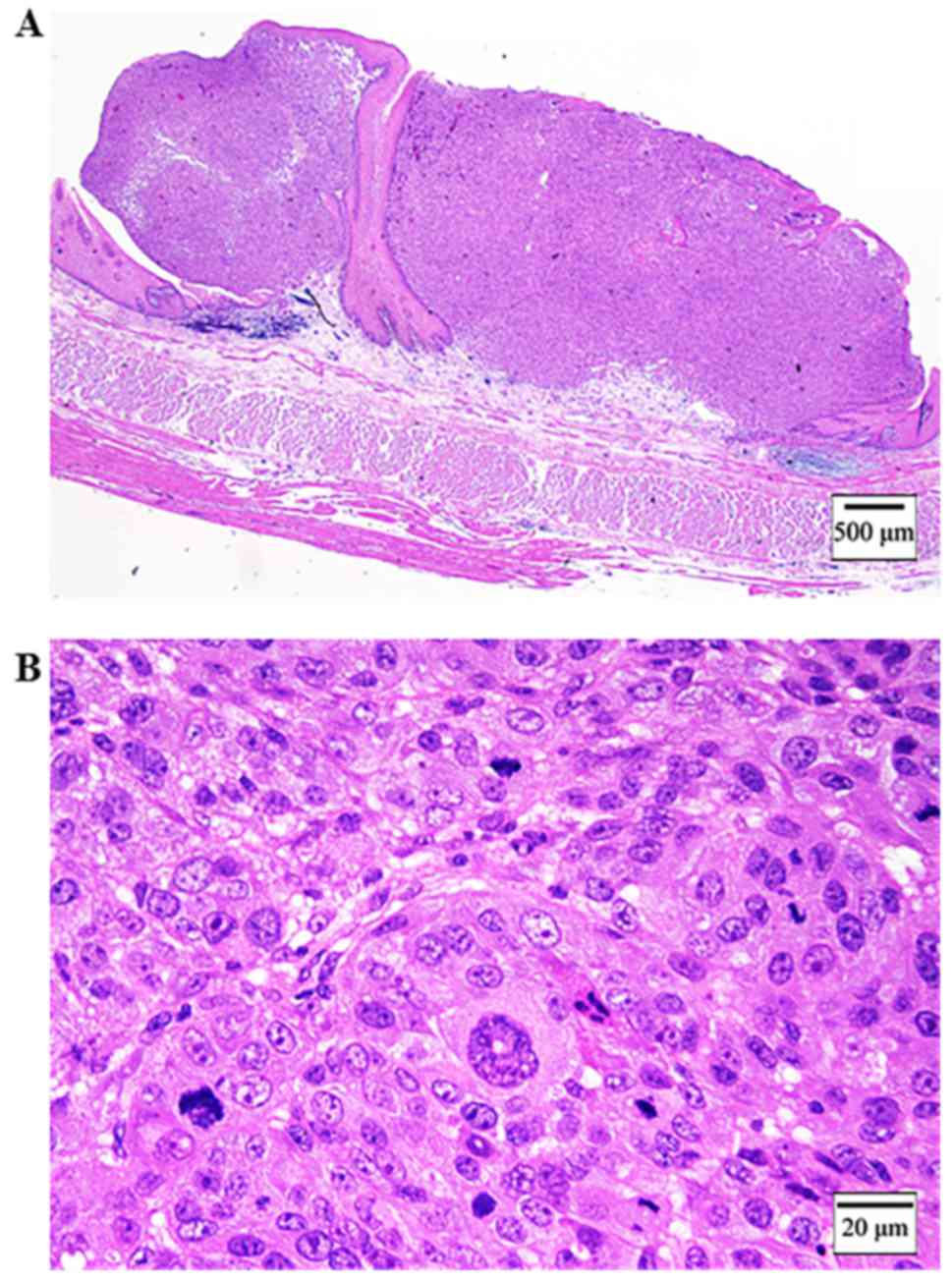
submucous coat and did not invade the muscularis mucosae (Fig. 3A). The tumor consisted of a circular
small atypical cell with anisocytosis, nuclear enlargement and
prominent nucleoli (Fig. 3B). There
was the junctional change, the identified findings of malignant
melanoma (Fig. 4A and B).
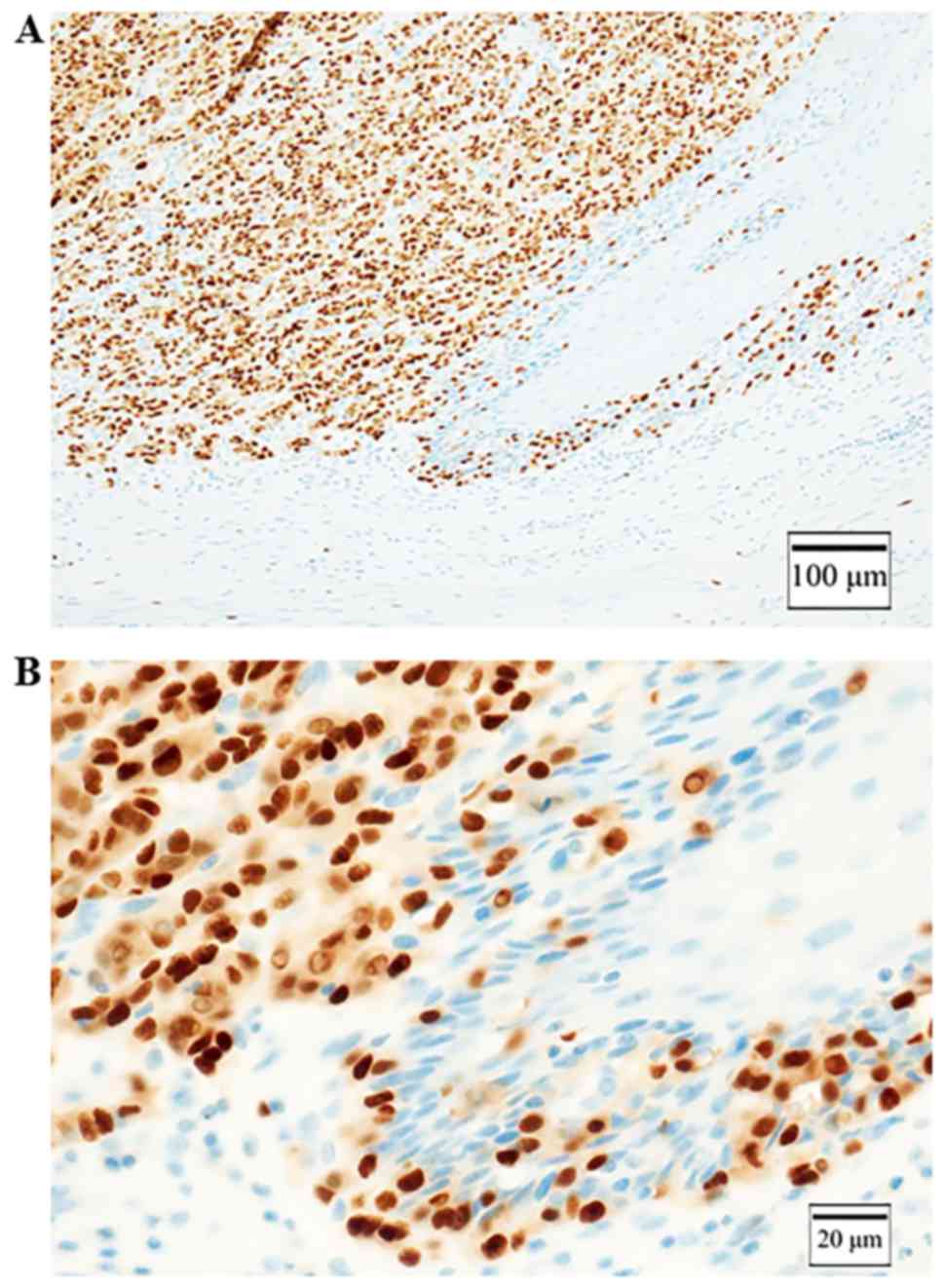
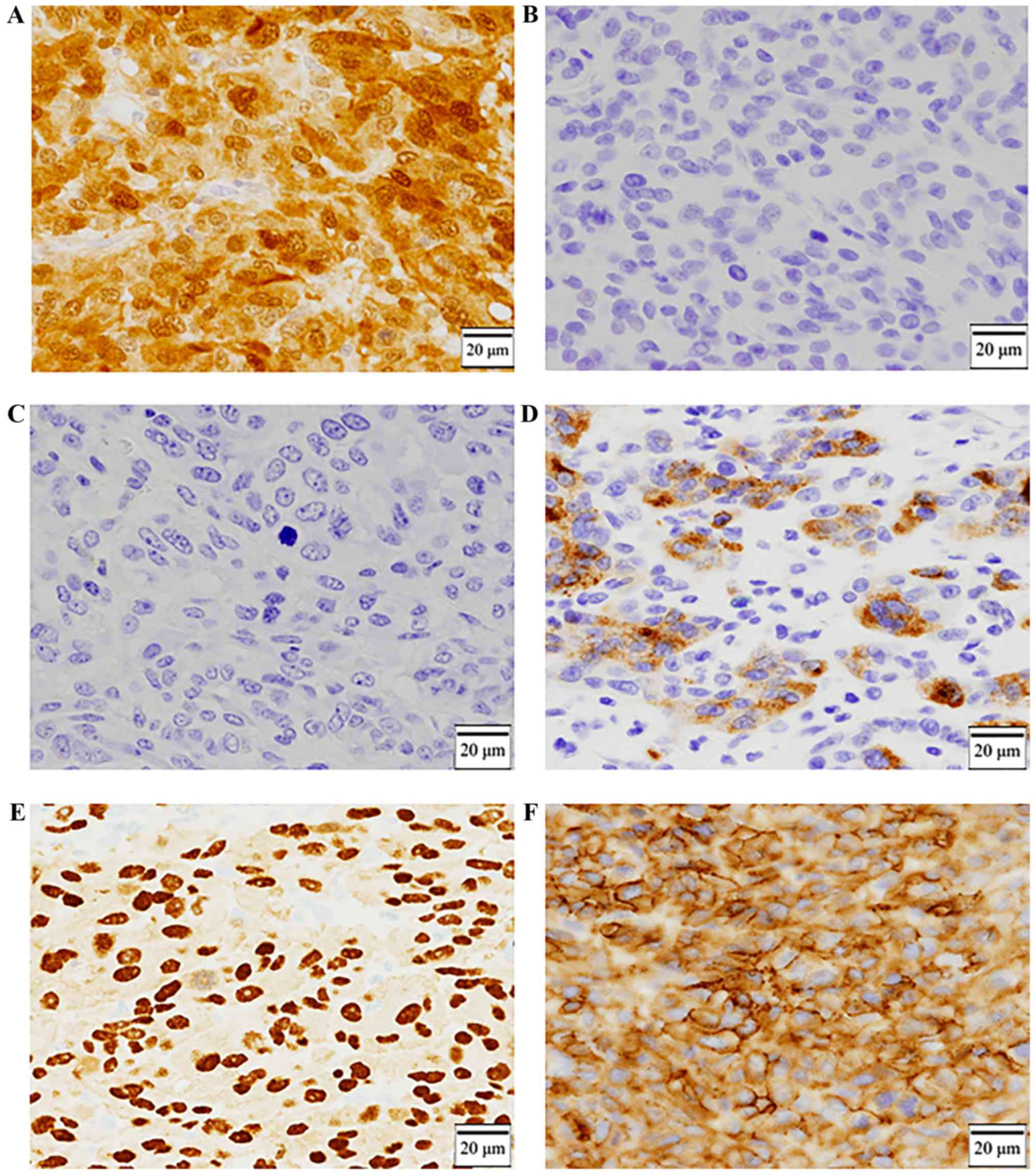
Immunohistochemical staining was positive for S-100 (Fig. 5A) and negative for HBM-45 and Melan-A
(Fig. 5B and C), and partially
positive for tyrosinase (Fig. 5D).
These results did not reveal the diagnosis. Since S-100 was
positive by immunohistochemical staining, several differential
diagnoses (such as rhabdomyo sarcoma, synovial sarcoma, malignant
peripheral nerve sheath tumor, and undifferentiated cancer) were
candidates (5). We added the
immunohistochemical examination of SOX10 (Sry-related HMg-Box gene
10) and KBA.62. It was positive for SOX10 and KBA.62 (Fig. 5E and F). We could make a diagnosis
amelanotic malignant melanoma with effort, because we confirmed the
junctional change which is a histological feature of malignant
melanoma, and furthermore, in the immunohistochemical staining
test, SOX10, KBA.62, S-100, and tyrosinase were positive in tumor
cells. There are no recurrence findings 1.5 year after the
surgery.
Discussion
Amelanotic malignant melanoma of the esophagus is a
rare disease, and only 15 such cases have been accumulated from
1996 to 2017 (6–15). Only one cases stated long survival
(6). Other case reports stated a mean
survival of approximately 9 months (7–15).
Amelanotic malignant melanoma produces no melanin pigments, and
accounts for approximately 2% of all malignant melanomas of the
esophagus (10). PMME occurs mainly
in the sixth and seventh decades of life, but may develop at any
age, with a male-to- female ratio of 2:1 (10). Volpon recommended surgical resection
as the treatment for PMME as it results in a longer mean survival
than chemo- or radiotherapy alone (14 vs. 3 months) (16). It is also difficult to diagnose
primary amelanotic malignant melanoma because melanin pigment is
absent. Diagnosis criteria of PMME are i) a typical histological
pattern of melanoma and the presence of melanin granules within the
tumor cells; ii) an origin in an area of junctional change within
the squamous epithelium; and iii) junctional change with melanotic
cells in the adjacent epithelium (1,17). The
junctional change means melanocytic proliferation in the junctional
zone between the dermis and the epidermis with its derivatives
(18). Allen and Spitz reported that
the presence of junctional activity is the most importance factor
for diagnosis (17). In this case, we
found the tumor at an early stage, and the structure of junctional
change remained. But it was very hard to reach any definite
diagnosis. This is because the markers, which were conventionally
used in the diagnosis of malignant melanoma, were not useful and
the endoscopic biopsy tissues were very small for confirming the
structure of junctional change.
S-100, HMB-45 and Melan-A are conventionally useful
marker to discriminate melanomas from other tumors. But in this
case, the conventional maker of immunohistochemical staining was
positive only S-100 and negative for the others. SOX10 and KBA.62
are relatively new markers of malignant melanoma and both were
positive in this case. As an immunochemical feature of SOX10 and
KBA.62, Sox10 was consistently expressed in benign Schwann cell
tumors of soft tissue and the GI-tract and metastatic melanoma, and
was variably present in malignant peripheral nerve sheath tumors,
in contrast, Sox10 was absent in many potential mimics of nerve
sheath tumors such as cellular neurothekeoma, meningioma,
gastrointestinal stromal tumors, PEComa, and a variety of
fibroblastic-myofibroblastic tumors (19). KBA.62 recognized an unknown
determinant expressed in melanoma cells and commonly maliganant
melanoma. This antibody was found immunoreactivity in most
metastatic melanomas, desmoplastic melanomas, and
well-differentiated squamous carcinomas (20). The sensitivity and specificity of
S-100 were reported to be 97–100 and 75–87%, respectively. The
sensitivity of HMB-45 was 69–93%. The sensitivity and specificity
were 75–92 and 95–100% for Melan-A, and 84–94 and 97–100% for
tyrosinase, respectively (3). The
sensitivity of SOX10 was reported to be 97–100%, whereas the
sensitivity of KBA62 was 93% (3).
SOX10 and KBA.62 are not relative to melanosome. SOX10 is a
transcriptional activator of microphthalmia-associated
transcription factor (MITF), MITF regulates the differentiation and
development of melanocytes and retinal pigment epithelium and is
also responsible for pigment cell-specific transcription of the
melanogenesis enzyme genes (21). The
SOX10-MITF pathway was involved in maintaining the proliferative
and tumorigenic ability, cell cycle regulation, expression of
survival factors, and metastasis formation in melanoma cells
(22). It is also considered to be
important for the specification, maturation, and maintenance of
melanocytes (23). Furthermore, it
has been shown to be a sensitive and specific marker for spindle
cell and desmoplastic melanomas (23). KBA.62 was detected in 1995 as a new
monoclonal antibody against a melanoma-associated antigen and
reacted with all histopathologic subtypes of nevi, including
junctional, intradermal, compound, Spitz, and dysplastic (24). In malignant melanoma, but it is
unknown which determinant expressed KBA62 recognizes, and the
function of KBA62 as a protein is also unknown (25). The sensitivity of anti-S-100 and
KBA.62 antibodies in detecting occult melanoma metastasis was
similar, moreover, KBA.62 identified melanoma patients who had
confirmed sentinel lymph node metastasis but were negative for
HMB-45 (25).
Melanosomes exist in four distinct stages as they
become increasingly laden with melanin pigment prior to their
transportation out of the cell into neighboring keratinocytes via
melanocyte dendrites. Stage I and II melanosomes are known as early
melanosomes because they have not initiated melanin synthesis.
Amelanotic malignant melanoma consists of melanosome stage I and/or
II, this is the reason for no pigment of amelanotic malignant
melanoma (3). Both markers, HMB-45
and Melan-A, has been associated with stage II melanosome (26). Although we did not observe it under
electron microscopy, the reason for negative HMB-45 and Melan-A may
be that, our case of amelanotic malignant melanoma consisted of
only melanosome stage I (also called premelanosomes). None of the
melanosome stage II showed that immunohistochemical staining was
negative for HMB-45 and Melan-A. Tyrosinase, partially positive in
our case, is necessary for the synthesis of melanin (27). Tyrosinase is a key enzyme in melanin
synthesis that can catalyze three different reactions: The
hydroxylation of tyrosine to 3,4-dihydroxyphenylalanine (DOPA), the
oxidation of DOPA to DOPA quinone and the oxidation of
5,6-dihydroxyindole to indole-quinone (28). In melanomas, tyrosinase can be seen as
fine granular cytoplasmic staining (29). Positive staining tends to be strong
and diffusive (30). The sensitivity
of tyrosinase for melanoma is somewhat better than HMB45, and
sensitivity decreases with increasing clinical stage and in
metastatic lesions (30). The
specificity of tyrosinase for melanoma is 97–100% (31). Tyrosinase has been found in rare
angiolipomas, a minority of angiomyolipomas and clear cell sarcomas
of the tendon sheath, and pigmented nerve sheath tumors (30). Melanin synthesis occurs within the
melanosome, a specific lysosome-related organelle that matures
through four morphologic stages (I–IV), and stage I melanosomes are
spherical vacuoles that lack tyrosinase activity and melanin
(32–34). Some amelanotic melanoma cells contain
significant levels of catalytically inactive tyrosinase molecules
and the levels of pigmentation in mammalian melanocytes are
regulated by a tyrosinase activation process (35). We think that in our case, tyrosinase
was partially positive, but not active. S-100, SOX10 and KBA.62
were not related to melanosome and the expression of melanin, such
that immunohistochemical staining was positive for them. In fact,
Cecile reported that immunohistochemical staining was negative for
HMB-45 and positive for KBA.62 in the case of amelanotic malignant
melanoma (25). Tissue staining with
conventional makers including HBM-45, Melan-A and S-100, and
histological features are useful for leading to the diagnosis of
PMME. However, when the sample size is small like an endoscopic
biopsy tissue, histological features are often not recognizable. In
our case, it may be impossible to diagnose with conventional
markers. In such cases, we believe that SOX10 and KBA.62 can be
useful new markers in the diagnosis of PMME, especially in
amelanotic malignant melanoma of the esophagus. If it is possible
to investigate the melanogenesis ability of the tumor with as
electron microscope, the diagnosis will becomes easier. In our
research, it was the limitation that we did not examine the tumor
with electron microscope.
In conclusion, HMB-45 and Melan-A, the generally
used diagnostic markers of malignant melanoma, were negative in our
case. Based on our findings, SOX10 and KBA.62 can be considered as
the new markers for the diagnosis of amelanotic malignant
melanosome.
Acknowledgements
The authors would like to thank Dr. Yoshiaki Imamura
(Pathological Department, University of Fukui, Fukui, Japan) for
his helpful comments.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
DF, JK, MM, YH and TG were part of the upper
gastrointestinal surgical treatment team that treated the present
case. DF was the attending doctor of the patient and was involved
in the followed up the patient after surgery. JK, MM and TG
contributed to the preoperative diagnosis, and JK, YH and DF
operated on the patient. The final diagnosis of the patient was
made by all authors. JK and DF provided major contributions in
writing manuscript. YH and MM provided major contributions in
immunohistochemical staining. TG revised the manuscript critically
for important intellectual content. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
This participant signed an informed consent
agreement.
Consent for publication
The patient provided consent for publication of the
data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stringa O, Valdez R, Beguerie JR,
Abbruzzese M, Lioni M, Nadales A, Iudica F, Venditti J and Roman
San A: Primary amelanotic melanoma of the esophagus. Int J
Dermatol. 45:1207–1210. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Slominski A, Tobin DJ, Shibahara S and
Wortsman J: Melanin pigmentation in mammalian skin and its hormonal
regulation. Physiol Rev. 84:1155–1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Naoki O and Akira K: The stage of
melanogenesis in amelanotic melanomaMelanoma in the
clinic-diagnosis, management and complications of malignancy. Mandi
M: InTech; London: pp. 277–286. 2011
|
|
4
|
Japan esophageal society: Japanese
classification of esophageal cancer, 11th edition: Part I.
Esopahgus. 14:1–36. 2017. View Article : Google Scholar
|
|
5
|
Ordóñez NG: Value of
melanocytic-associated immunohistochemical markers in the diagnosis
of malignant melanoma: A review and update. Hum Pathol. 45:191–205.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hirayama Y, Masahiro T, Tanaka T, Ishihara
M, Ohnishi S, Hara K, Mizuno N, Hijioka S, Okuno N, Abe T, et al:
Slow-growing amelanotic malignant melanoma of the esophagus with
long survival: A case report and review of the literature. Endosc
Int Open. 5:E1076–E1080. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramaswamy B, Bhandarkar AM, Venkitachalam
S and Trivedi S: Amelanotic malignant melanoma of the cervical
oesophagus. BMJ Case Rep 2014: pii: bcr2014204182. 2014.
|
|
8
|
Terada T: Amelanotic malignant melanoma of
the esophagus: Report of two cases with immunohistochemical and
molecular genetic study of KIT and PDGFRA. World J Gastroenterol.
15:2679–2683. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang S, Thamboo TP, Nga ME, Zin T, Cheng A
and Tan KB: C-kit positive amelanotic melanoma of the oesophagus: A
potential diagnostic pitfall. Pathology. 40:527–530. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kranzfelder M, Seidl S, Dobritz M and
Brücher BL: Amelanotic esophageal malignant melanoma: Case report
and short review of the literature. Case Rep Gastroenterol.
2:224–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stringa O, Valdez R, Beguerie JR,
Abbruzzese M, Lioni M, Nadales A, Iudica F, Venditti J and Roman
San A: Primary amelanotic melanoma of the esophagus. Int J
Dermatol. 45:1207–1210. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Simone P, Gelin M and El Nakadi I:
Amelanotic malignant melanoma of the esophagus. Report of a case.
Minerva Chir. 61:45–49. 2006.PubMed/NCBI
|
|
13
|
Suzuki Y, Aoyama N, Minamide J, Takata K
and Ogata T: Amelanotic malignant melanoma of the esophagus: Report
of a patient with recurrence successfully treated with
chemoendocrine therapy. Int J Clin Oncol. 10:204–207. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heidemann J, Lebiedz P, Herbst H, Spahn
TW, Domagk D, Domschke W and Kucharzik T: Amelanotic malignant
melanoma of the esophagus: Case report. Z Gastroenterol.
43:597–600. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee SH, Park SH, Kim HG and Kim CB:
Primary malignant melanoma of the esophagus. Yonsei Med J.
39:468–473. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Volpin E, Sauvanet A, Couvelard A and
Belghiti J: Primary malignant melanoma of the esophagus: A case
report and review of the literature. Dis Esophagus. 15:244–249.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allen AC and Spitz S: Malignant melanoma;
a clinicopathological analysis of criteria for diagnosis and
prognosis. Cancer. 6:1–45. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Levene A: On the histological diagnosis
and prognosis of malignant melanoma. J Clin Pathol. 33:101–124.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miettinen M, McCue PA, Sarlomo-Rikala M,
Biernat W, Czapiewski P, Kopczynski J, Thompson LD, Lasota J, Wang
Z and Fetsch JF: Sox10-a marker for not only schwannian and
melanocytic neoplasms but also myoepithelial cell tumors of soft
tissue: A systematic analysis of 5134 tumors. Am J Surg Pathol.
39:826–835. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaufmann O, Koch S, Burghardt J, Audring H
and Dietel M: Tyrosinase, melan-A, and KBA62 as markers for the
immunohistochemical identification of metastatic amelanotic
melanomas on paraffin sections. Mod Pathol. 11:740–746.
1998.PubMed/NCBI
|
|
21
|
Shibahara S, Takeda K, Yasumoto K, Udono
T, Watanabe K, Saito H and Takahashi K: Microphthalmia-associated
transcription factor (MITF): Multiplicity in structure, function,
and regulation. J Investig Dermatol Symp Proc. 6:99–104. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tudrej KB, Czepielewska E and
Kozłowska-Wojciechowska M: SOX10-MITF pathway activity in melanoma
cells. Arch Med Sci. 13:1493–1503. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nonaka D, Chiriboga L and Rubin BP: Sox10:
A pan-schwannian and melanocytic marker. Am J Surg Pathol.
32:1291–1298. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cohen-Kanfo E, al Saati T, Aziza J,
Ralfkiaer E, Selves J, Gorgiet B and Delsol G: Production and
characterisation of an antimelanoma monoclonal antibody KBA.62
using a new melanoma cell line reactive on paraffin wax embedded
sections. J Clin Pathol. 48:826–831. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pagès C, Rochaix P, al Saati T,
Valmary-Degano S, Boulinguez S, Launay F, Carle P, Lauwers F,
Payoux P, Le Guellec S, et al: KBA.62: A useful marker for primary
and metastatic melanomas. Hum Pathol. 39:1136–1142. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen KG, Leapman RD, Zhang G, Lai B,
Valencia JC, Cardarelli CO, Vieira WD, Hearing VJ and Gotttesman
MM: Influence of melanosome dynamics on melanoma drug sensitivity.
J Natl Cancer Inst. 101:1259–1271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tief K, Hahne M, Schmidt A and Beermann F:
Tyrosinase, the key enzyme in melanin synthesis, is expressed in
murine brain. Eur J Biochem. 241:12–16. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hearing VJ and Tsukamoto K: Enzymatic
control of pigmentation in mammals. FASEB J. 5:2902–2909. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hofbauer GF, Kamarashev J, Geertsen R,
Böni R and Dummer R: Tyrosinase immunoreactivity in formalin-fixed,
paraffin-embedded primary and metastatic melanoma: Frequency and
distribution. J Cutan Pathol. 25:204–209. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Orchard GE: Comparison of
immunohistochemical labelling of melanocyte differentiation
antibodies melan-A, tyrosinase and HMB 45 with NKIC3 and S100
protein in the evaluation of benign naevi and malignant melanoma.
Histochem J. 32:475–481. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jungbluth AA, Iversen K, Coplan K, Kolb D,
Stockert E, Chen YT, Old LJ and Busam K: T311-an anti-tyrosinase
monoclonal antibody for the detection of melanocytic lesions in
paraffin embedded tissues. Pathol Res Pract. 196:235–242. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paterson EK, Fielder TJ, MacGregor GR, Ito
S, Wakamatsu K, Gillen DL, Eby V, Boissy RE and Ganesan AK:
Tyrosinase depletion prevents the maturation of melanosomes in the
mouse hair follicle. PLoS One. 10:e01437022015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Słominski A, Moellmann G, Kuklinska E,
Bomirski A and Pawelek J: Positive regulation of melanin
pigmentation by two key substrates of the melanogenic pathway,
L-tyrosine and L-dopa. J Cell Sci. 89:287–296. 1988.PubMed/NCBI
|
|
34
|
Slominski A, Moellmann G and Kuklinska E:
L-tyrosine, L-dopa, and tyrosinase as positive regulators of the
subcellular apparatus of melanogenesis in Bomirski Ab amelanotic
melanoma cells. Pigment Cell Res. 2:109–116. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fuller BB, Iman DS and Lunsford JB:
Comparison of tyrosinase levels in amelanotic and melanotic
melanoma cell cultures by a competitive enzyme-linked
immunoadsorbent assay and by immunotitration analysis. J Cell
Physiol. 134:149–154. 1988. View Article : Google Scholar : PubMed/NCBI
|