Introduction
Colorectal cancer ranks as the third most common
malignancy worldwide, with a global 5-year prevalence of ~3.2
million cases (1). Due to changes in
diet and lifestyle, several increasingly affluent Asian countries
(e.g., China, Japan, South Korea and Singapore) have displayed a 2-
to 4-fold increase in the incidence of colorectal carcinoma over
the past few decades (2). However,
non-polypoidal lesions and de novo colorectal neoplasms
(i.e., without preceding adenoma) are more common in Asian patient
populations. The non-polypoidal lesions are less visible, which
poses difficulties in disease screening by conventional imaging and
endoscopy (2). Therefore, the
development of alternative screening methods for colorectal
carcinoma is particularly important.
Early detection of colorectal cancer in its
localized or pre-invasive form is a more realistic approach to
screening (3). Current screening
strategies, including fecal occult blood testing and colonoscopy,
have poor levels of patient acceptability (4). The development of peripheral blood
protein biomarkers that are able to accurately and reliably detect
colorectal cancer at its earliest stages appears to be promising
alternative approach for colorectal cancer screening (5).
α-methylacyl-CoA racemase (P504S/AMACR) is
overexpressed in colorectal adenomas and cancer, and has been
demonstrated to be associated with advanced distal colorectal
adenoma (6) and tumor differentiation
(7). Furthermore, an inverse
correlation was demonstrated between p53 and B-cell lymphoma 2
(Bcl-2) expression in colorectal tumorigenesis, and abnormal
activation of Bcl-2 inhibited apoptosis in vivo (8). The Ki-67 labeling index is positively
correlated with poor survival in colorectal cancer patients
(9). Therefore, the objective of the
present study was to evaluate the protein expression of four key
genes, P504S/AMACR, p53, Bcl-2 and Ki-67/mindbomb E3 ubiquitin
protein ligase 1 (MIB-1), in a population of Chinese patients with
colorectal carcinoma. The present study aimed to shed light on the
potential applicability of these four proteins as diagnostic
biomarkers for colorectal carcinoma in Chinese patient
populations.
Materials and methods
Patients and tumor samples
Frozen tumor tissues with matched normal tissue
margins were collected from 148 Han Chinese patients (68 males and
80 females; with a mean age of 50 years old, and the ranging from
25–75 years old) that had undergone surgery at Yongchuan Hospital
(Chongqing Medical University, Chongqing, China) from January 2015
to January 2016. For each patient, the following relevant
demographic and clinical data were collected: i) Hospital/surgery
location (city, country); ii) patient age (years); iii) patient
sex; iv) risk factors [e.g., body mass index (BMI), smoking status
and excessive alcohol consumption (i.e., a male consuming >4
drinks/day or >14 drinks/week; or a female consuming >3
drinks/day or >7 drinks/week)] (10); v) tumor characteristics, including
pathological grade of tumor cells (G1-G4) (11), Clinical stage (T1-T4), samples were
staged according to the American Joint Committee on Cancer TNM
staging system (12); and vi)
survival time post-surgery (months).
Immunohistochemical staining
Immunohistochemical staining was performed using an
Ultrasensitive™ SP kit (cat. no. KIT-0105M, Maixin Biotech Co.,
Ltd., Fuzhou, China) and diaminobenzidine (DAB; Fuzhou Maixin
Biotech Co., Ltd., Fuzhou, China), according to the manufacturer's
protocols. Briefly, 4-µm thick paraffin-embedded sections, which
were fixed with 4% formaldehyde at 20°C for 24 h, were
xylene-deparaffinized at 60°C for 1 hour, rehydrated in a
descending alcohol series and rinsed in phosphate-buffered saline
(PBS). Antigen retrieval was performed by placing the slides in
boiling citric acid buffer (pH 6.0) for 5 min. The sections were
subsequently incubated in 3% hydrogen peroxide in methanol at room
temperature for 20 min to quench endogenous peroxidase activity.
Non-immune serum albumin (5%; Beyotime Institute of Biotechnology,
Haimen, China) was used to block non-specific binding at 20°C for 5
min. The tissue sections were then incubated at 37°C for 2 h with
the following monoclonal primary antibodies: sheep anti-P504S/AMACR
(cat. no. AF7508-SP; dilution, 1:500), rabbit anti-p53 (cat. no. AF
1355; dilution, 1:2,000), mouse anti-Bcl-2 (cat no. AF 810;
dilution, 1:500) and sheep anti-Ki-67/MIB-1 (cat no. AF 7617;
dilution, 1:800) (all from R&D Systems Inc., Minneapolis, MN,
USA). Next was an incubation with biotinylated goat anti-rat IgG
secondary antibodies (cat. no.; NL013, dilution, 1:1,000; R&D
Systems) at 37°C for 30 min followed by a streptavidin horseradish
peroxidase complex, according to the instructions of
Ultrasensitive™ SP kit, with intermittent PBS rinses.
Immunoreactivity was visualized with diluted DAB. Finally, sections
were rinsed with distilled water, and counterstained at 37°C for 5
min with Mayer's hematoxylin and histomounted. As a negative
control, the primary antibody was replaced with an equal amount of
normal human/rabbit/rat/mouse IgG. The positive controls were
cancer cell lines with known positive expression of P504S/AMACR,
p53, Bcl-2 or Ki-67/MIB-1.
Evaluation of
immunohistochemistry
Images of stained sections were captured in a series
of 10 randomly selected high-power fields with a laser scanning
confocal microscope (Olympus Corp., Tokyo, Japan) at magnification,
×400. The sections were then scored by the proportion and staining
intensity of positively stained tumor cells. The ‘proportion score’
of positively stained tumor cells was determined as follows: 0, no
positive tumor cells; 1, <10% positive tumor cells; 2, 10–50%
positive tumor cells; and 3, >50% positive tumor cells. The
‘staining intensity’ score was determined as follows: 0, no
staining; 1, weak staining/light yellow; 2, moderate
staining/yellow-brown; and 3, strong staining/brown.
Immunohistochemical staining was scored independently by two
investigators blinded to the clinicopathological findings. Cases
with score discrepancies were re-reviewed simultaneously by the
original two investigators and a senior pathologist until a
consensus was reached.
The ‘staining index’ was then calculated as the
staining intensity score multiplied by the proportion score. Using
this method, a staining index was obtained for P504S/AMACR, p53,
Bcl-2 and Ki-67/MIB-1 in colorectal specimens, with scores of 0, 1,
2, 3, 4, 6 or 9 for each protein biomarker. Subsequently, cut-off
values (Table I) for immunoreactivity
for each protein biomarker were based on measuring heterogeneity by
the sequential association analysis for SPA progression. This
cut-off point was used to distinguish between ‘low’ and ‘high’
expression of each protein biomarker.
 | Table I.Results of ROC curve analysis of
protein biomarkers and determination of the cut-off values. |
Table I.
Results of ROC curve analysis of
protein biomarkers and determination of the cut-off values.
| Protein | Proportion of IHC
staining | Area under ROC
curve | Cut-off value, % | Sensitivity, % | Specificity, % |
|---|
| P504S/AMACR | 6.53±4.51 | 0.62 | 8 | 60.2 | 84.2 |
| p53 | 10.39±5.28 | 0.65 | 11 | 55.1 | 78.3 |
| Bcl-2 | 18.30±13.20 | 0.72 | 21 | 66.7 | 80.3 |
| Ki-67/MIB-1 | 10.20±7.28 | 0.70 | 13 | 61.4 | 71.6 |
Western blot analysis
Total protein was extracted from the colorectal
cancer and matched normal tissue margin specimens with Cell
Extraction Buffer (cat. no. FNN0011; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Protein concentrations were determined
using a bicinchoninic acid protein assay kit, and 30 µg protein per
sample was separated by 8% SDS-PAGE and transferred onto a
methanol-activated nitrocellulose filter membrane (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Prior to immunoblotting,
membranes were blocked within 5% skimmed dry milk at 37°C for 2 h.
Expression levels were normalized to β-actin (dilution, 1:1,000;
cat. no. MAB8929; R&D Systems). The following primary
monoclonal antibodies were diluted in buffer and incubated at 4°C
overnight: sheep anti-P504S/AMACR (cat. no. AF7508-SP; R&D
Systems Inc.; dilution, 1:1,000), rabbit anti-p53 (cat. no. AF1355;
R&D Systems Inc.; dilution, 1:2,000), mouse anti-Bcl-2 (cat no.
AF810; R&D Systems Inc.; dilution, 1:1,000) and mouse
anti-Ki-67/MIB-1 (cat no. AF7617; R&D Systems Inc.; dilution,
1:1,200). Following washing in TBST, membranes were incubated with
a horseradish peroxidase-conjugated goat anti-rat secondary
antibody (cat. no. ab7097; dilution, 1:1,000; Abcam, Cambridge, UK)
for 1 h at room temperature. This experiment was repeated three
times. Bands were detected using a SuperEnhanced chemiluminescence
kit (cat. no. P1010; Applygen Technologies, Inc., Beijing,
China).
Statistical analysis
All data were analyzed using SPSS 13.0 for Windows
(SPSS, Inc., Chicago, IL, USA). χ2 and Fisher's exact
tests were used to compare P504S/AMACR, p53, Bcl-2 and Ki-67/MIB-1
protein expression (from the immunohistochemical ‘staining index’)
in the colorectal tumors and matched normal tissue margins and
various clinicopathological parameters (e.g., age, sex, BMI and
smoking status). The Student's t-test was used to analyze the
western blotting results. In order to confirm the correlations
between P504S/AMACR, p53, Bcl-2 and Ki-67/MIB-1 protein expression
and clinicopathological parameters, Spearman's rank correlation
analysis was applied. To determine whether P504S/AMACR, p53, Bcl-2
and Ki-67/MIB-1 expression levels were independent prognostic
factors in patients with colorectal carcinoma, prognostic relevance
was evaluated using multivariate Cox regression analysis. P<0.05
was considered to indicate a statistically significant difference.
Survival analysis was performed using the Kaplan-Meier method,
followed by the log-rank test (13).
Results
P504S/AMACR, p53, Bcl-2 and
Ki-67/MIB-1 protein levels are increased in colorectal tumor
tissues compared with normal tissue margins
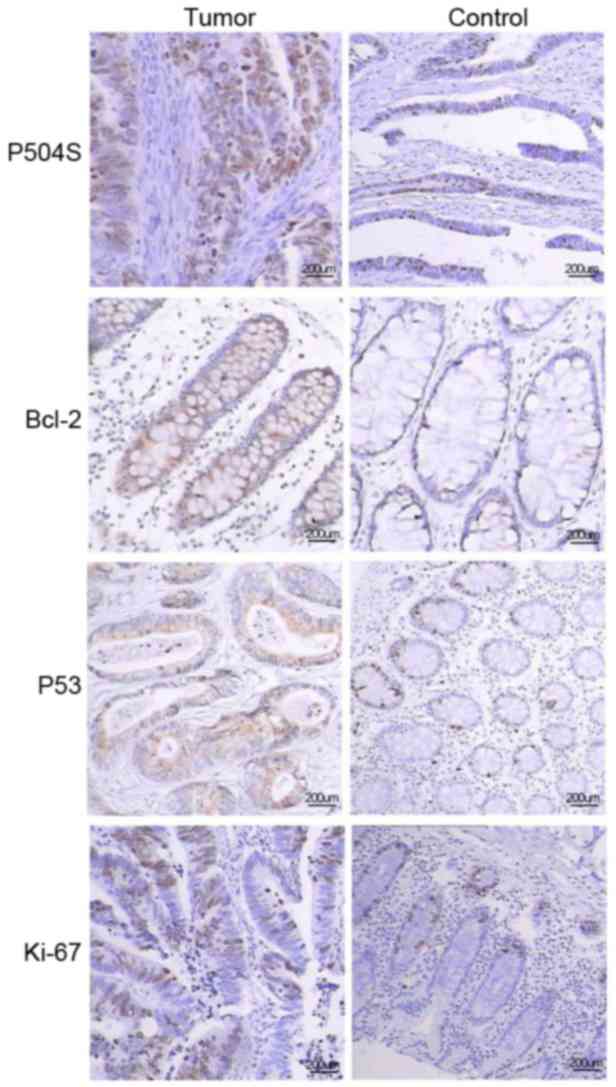
Immunohistochemical analysis revealed that a
significantly greater proportion of colorectal tumors exhibited a
high expression of all four proteins (P504S/AMACR, p53, Bcl-2 and
Ki-67/MIB-1), compared with matching normal tissue margins
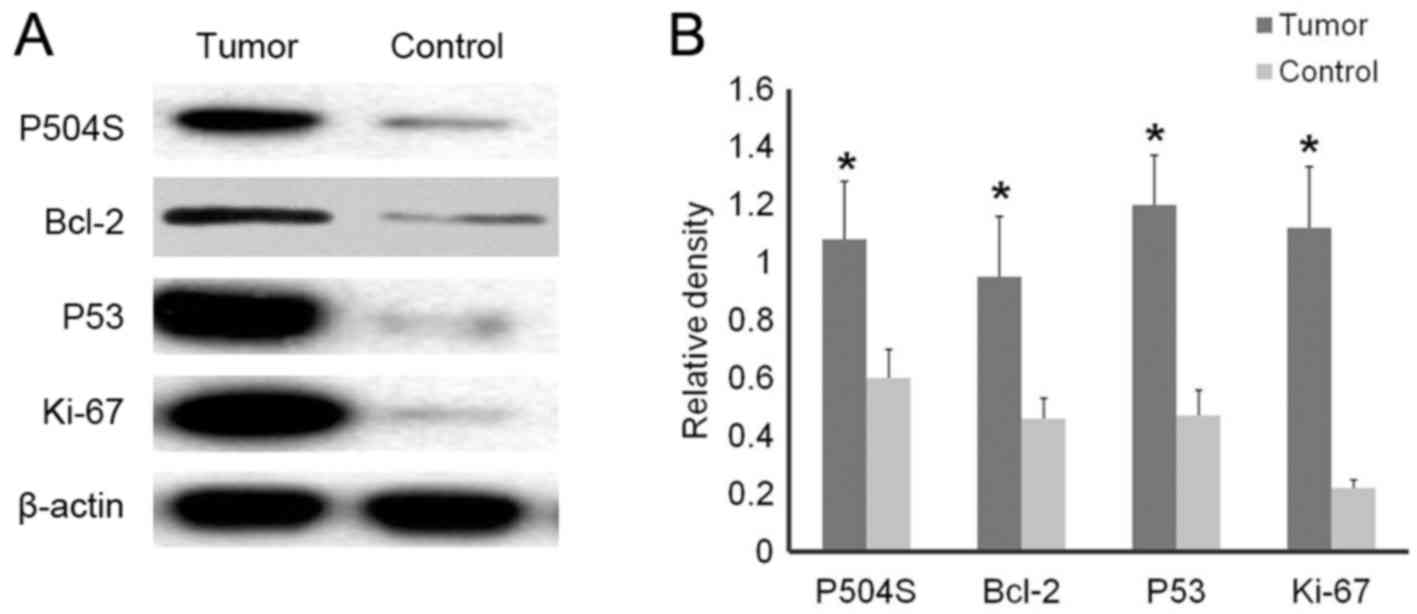
(Table II; Fig. 1). Additionally, western blot analysis
revealed significantly higher expression of the same four proteins
in colorectal tumors compared with matching normal tissue margins
(all P<0.001; Fig. 2).
 | Table II.Immunohistochemical analysis of four
proteins in colorectal carcinoma tumors and matched normal tissue
margins (control). |
Table II.
Immunohistochemical analysis of four
proteins in colorectal carcinoma tumors and matched normal tissue
margins (control).
|
| Tissue type, n |
|
|
|---|
|
|
|
|
|
|---|
| Variable | Tumor | Control | χ2
value | P-value |
|---|
| P504S/AMACR
expression |
|
| 88.80 |
<0.001a |
| High | 76 | 4 |
|
|
| Low | 72 | 144 |
|
|
| Bcl-2 expression |
|
| 97.93 |
<0.001a |
| High | 104 | 20 |
|
|
| Low | 44 | 128 |
|
|
| p53 expression |
|
| 76.85 |
<0.001a |
|
High | 88 | 16 |
|
|
|
Low | 60 | 132 |
|
|
| Ki-67/MIB-1
expression |
|
| 50.71 |
<0.001a |
|
High | 72 | 16 |
|
|
|
Low | 76 | 132 |
|
|
Higher expression of Bcl-2 was observed in patients
>55 years of age, while a lower expression was observed in
carcinomas at advanced pathological grades and TNM stages.
Statistical analysis comparing P504S/AMACR, p53, Bcl-2 and
Ki-67/MIB-1 protein expression in colorectal tumors with various
clinicopathological parameters (e.g., age, sex, BMI and smoking)
revealed that Bcl-2 expression was significantly associated with
patient age (>55 vs. <45 years), pathological grade, and TNM
stage (all P<0.05; Table III).
Additionally, the Spearman's rank correlation analysis revealed
that Bcl-2 expression, based on IHC score, was negatively
correlated with pathological grade (r=−0.827; P<0.001) and TNM
stage (r=−0.388; P=0.018).
 | Table III.Association between the expression of
four proteins and clinicopathological factors. |
Table III.
Association between the expression of
four proteins and clinicopathological factors.
|
| P504S/AMACR
expression | Bcl-2
expression | p53 expression | Ki-67/MIB-1
expression |
|---|
|
|
|
|
|
|
|---|
| Characteristic | Low | High | χ2 | P-value | Low | High | χ2 | P-value | Low | High | χ2 | P-value | Low | High | χ2 | P-value |
|---|
| Age, years |
|
| 0.10 | 0.873 |
|
| 19.75 | 0.026a |
|
| 3.02 | 0.385 |
|
| 0.13 | 0.858 |
|
<45 | 36 | 36 |
|
| 36 | 36 |
|
| 24 | 48 |
|
| 32 | 40 |
|
|
|
>55 | 36 | 40 |
|
| 12 | 64 |
|
| 36 | 40 |
|
| 36 | 40 |
|
|
| Sex |
|
| 0.06 | 0.9 |
|
| 0.22 | 0.815 |
|
| 0.43 | 0.742 |
|
| 0.08 | 0.886 |
|
Male | 36 | 32 |
|
| 36 | 44 |
|
| 48 | 40 |
|
| 44 | 32 |
|
|
|
Female | 44 | 36 |
|
| 28 | 40 |
|
| 36 | 24 |
|
| 40 | 32 |
|
|
| BMI,
kg/m2 |
|
| 1.88 | 0.791 |
|
| 0.16 | 0.98 |
|
| 0.05 | 0.994 |
|
| 0.60 | 0.928 |
|
<18.5 | 28 | 20 |
|
| 24 | 24 |
|
| 24 | 28 |
|
| 20 | 28 |
|
|
|
18.5–23.9 | 32 | 24 |
|
| 32 | 28 |
|
| 28 | 32 |
|
| 32 | 36 |
|
|
|
≥24 | 20 | 24 |
|
| 20 | 20 |
|
| 16 | 20 |
|
| 16 | 16 |
|
|
| Smoking |
|
| 0.01 | 0.97 |
|
| 1.08 | 0.603 |
|
| 0.14 | 0.85 |
|
| 0.06 | 0.9 |
|
Yes | 24 | 40 |
|
| 28 | 44 |
|
| 20 | 32 |
|
| 36 | 32 |
|
|
| No | 32 | 52 |
|
| 36 | 40 |
|
| 40 | 56 |
|
| 44 | 36 |
|
|
| Alcohol |
|
| 0.01 | 0.957 |
|
| 0.35 | 0.769 |
|
| 0.75 | 0.666 |
|
| 0.43 | 0.742 |
|
Yes | 28 | 36 |
|
| 48 | 44 |
|
| 52 | 44 |
|
| 48 | 40 |
|
|
| No | 36 | 48 |
|
| 32 | 24 |
|
| 32 | 20 |
|
| 36 | 24 |
|
|
| Pathological
grade |
|
| 2.83 | 0.622 |
|
| 91.72 |
<0.001a |
|
| 3.59 | 0.313 |
|
| 3.35 | 0.262 |
| G1 | 4 | 12 |
|
| 0 | 16 |
|
| 4 | 12 |
|
| 4 | 12 |
|
|
| G2 | 16 | 8 |
|
| 4 | 20 |
|
| 4 | 20 |
|
| 20 | 4 |
|
|
| G3 | 24 | 24 |
|
| 36 | 12 |
|
| 28 | 20 |
|
| 24 | 24 |
|
|
| G4 | 32 | 28 |
|
| 60 | 0 |
|
| 24 | 36 |
|
| 28 | 32 |
|
|
| TNM stage |
|
| 1.82 | 0.813 |
|
| 36.47 | 0.005a |
|
| 2.54 | 0.572 |
|
| 1.35 | 0.885 |
| T1 | 0 | 4 |
|
| 0 | 4 |
|
| 0 | 4 |
|
| 0 | 4 |
|
|
| T2 | 16 | 12 |
|
| 8 | 20 |
|
| 12 | 16 |
|
| 20 | 8 |
|
|
| T3 | 20 | 24 |
|
| 32 | 12 |
|
| 24 | 36 |
|
| 16 | 28 |
|
|
| T4 | 36 | 36 |
|
| 60 | 12 |
|
| 24 | 32 |
|
| 40 | 32 |
|
|
Bcl-2 may serve as a prognostic
indicator of colorectal carcinoma
To determine whether P504S/AMACR, p53, Bcl-2 and
Ki-67/MIB-1 expression levels are independent prognostic indicators
of colorectal carcinoma, The survival rate was considered to
indicate prognosis, and was evaluated by multivariate Cox
regression analysis. Only Bcl-2 expression displayed statistical
significance as a prognostic indicator of colorectal carcinoma with
a relative risk value of 0.703 (95% CI, 0.552–0.895; P<0.05;
Table IV).
 | Table IV.Multivariate Cox regression analyses
of four proteins as prognostic indicators of colorectal
carcinoma. |
Table IV.
Multivariate Cox regression analyses
of four proteins as prognostic indicators of colorectal
carcinoma.
|
| Multivariate
analysis |
|---|
|
|
|
|---|
| Variable | Relative risk (95%
confidence interval) | P-value |
|---|
| P504S/AMACR (low
vs. high) | 0.982
(0.843–1.114) | 0.816 |
| Bcl-2 (low vs.
high) | 0.703
(0.552–0.895) | 0.004a |
| p53 (low vs.
high) | 0.922
(0.731–1.162) | 0.492 |
| Ki-67/MIB-1 (low
vs. high) | 1.024
(0.763–1.375) | 0.873 |
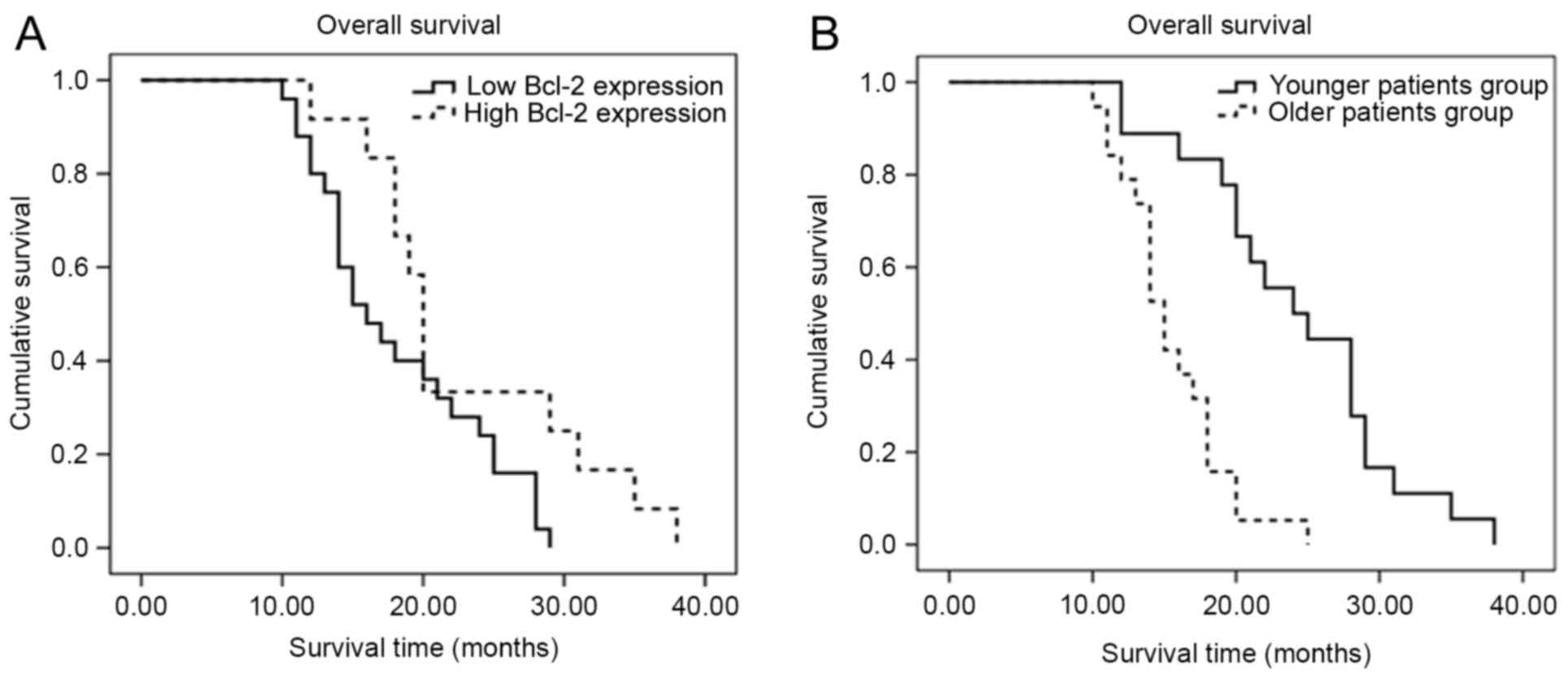
Older patients and those with a lower
expression of Bcl-2 exhibit a reduced overall survival time
Based on the aforementioned results, survival
analysis based on age and Bcl-2 expression was performed using the
Kaplan-Meier method. The Kaplan-Meier curves are plotted in
Fig. 3. Log-rank testing revealed
that low expression of Bcl-2 in patients was associated with a
reduced overall survival time when compared with patients with a
higher expression of Bcl-2 (P=0.039), and that older age (>55
years) was associated with a reduced overall survival time
(P<0.001).
Discussion
The aim of the present study was to evaluate the
protein expression of four key genes, P504S/AMACR, p53, Bcl-2 and
Ki-67/MIB-1, in order to assess their potential applicability as
diagnostic biomarkers of colorectal carcinoma in a population of
Chinese patients. Although a significantly higher expression of all
four proteins was observed in colorectal tumors compared with the
matching normal tissue margins (as determined by
immunohistochemistry and western blot analysis), only Bcl-2
downregulation was significantly correlated with advanced
pathological grade and TNM stage in these patients. Additionally,
Bcl-2 downregulation was revealed to be a prognostic indicator of a
reduced overall survival time.
Apoptosis is well-established as a highly conserved
process for eliminating damaged cells in multicellular organisms
(14,15). Therefore, defects in apoptotic
pathways may result in the survival and proliferation of
pre-malignant cells, which eventually leads to the development of
human cancer (14,16). In particular, the Bcl-2 protein family
has been identified as a key set of apoptotic regulators that
conduct the following three activities: i) Promotion of cell
survival (e.g., Bcl-2 and Bcl-xL); ii) initiation of cell death
(e.g., Bcl-2-interacting mediator of cell death and
Bcl-2-interacting domain); and iii) activation of apoptotic
effector pathways (e.g., Bcl-2-associated X protein and Bcl-2
homologous antagonist killer) (13).
With respect to Bcl-2 and tumorigenesis, elevated Bcl-2 gene
expression has been positively correlated with a poorer patient
prognosis in colorectal cancer, prostate cancer, bladder cancer,
small cell lung cancer, breast cancer, melanoma and acute myeloid
leukemia (17). Furthermore, a
previous study demonstrated that higher Bcl-2 expression
contributes to resistance to chemotherapy and radiation therapy
(17).
In contrast with these conventional findings, the
present study revealed that Bcl-2 expression was significantly
correlated with advanced pathological grade and TNM stage in
addition to reduced overall survival time in this Chinese
colorectal carcinoma patient population. This phenomenon may be
explained through colorectal tumor biology. In the colon, Bcl-2 has
been demonstrated to be more highly expressed in adenomas than in
carcinomas (18,19). As a result, colorectal tumors that
retain their Bcl-2 expression tend to display adenoma-like
characteristics and are often less aggressive, while tumors with
reduced Bcl-2 expression tend to be more aggressive (20).
In terms of tumor characteristics and clinical
outcomes, the results of the present study are in line with those
of several previous studies in colorectal cancer patients. Biden
et al (19) observed a
significant negative association between Bcl-2 expression and
microsatellite instability, as well as a significant positive
association between Bcl-2 expression and patient survival. Ofner
et al (21) associated Bcl-2
downregulation with increased tumor size, decreased lymphocytic
infiltration and an increased likelihood of a poor clinical
outcome. Baretton et al (22)
demonstrated that Bcl-2-positive carcinomas are associated with a
significantly longer disease-free survival time. Sinicrope et
al (23) demonstrated that a high
Bcl-2 expression is an independent predictor of improved
relapse-free survival, but not overall survival, following
adjustment for proliferative index, DNA ploidy and ethnicity.
Bhatavdekar et al (24)
observed a positive correlation between Bcl-2 expression and a poor
survival outcome in patients with UICC/AJCC stage I and III
colorectal carcinoma. However, in contrast to these findings, other
studies have revealed no significant association between Bcl-2
expression and prognosis in patients with colorectal cancer
(25–28). Despite numerous clinical
investigations, there remains no clear-cut association between
Bcl-2 expression and clinical outcomes in patients with colorectal
carcinoma.
As stated earlier, non-polypoidal lesions and de
novo colorectal neoplasms (i.e., without preceding adenoma)
occur more commonly in Asian patients (2). Due to the lower incidence of
adenoma-like colorectal tumors in Asian populations and the
association between Bcl-2 and an adenomatous differentiated
phenotype, it is likely that a lower Bcl-2 expression would be
associated with less differentiated, more aggressive colorectal
tumors and worse clinical outcomes in Asian patients, which is
precisely what was observed in the Chinese patient population
enrolled in the present study. Further studies in Chinese and other
East Asian (e.g., Japanese and Korean) populations that correlate
gross pathological examination (by surgery or endoscopy) with the
histopathological and molecular observations are required to
validate this hypothesis.
There are several limitations to the present study.
To begin with, the sample size used was limited to only 47
patients; a larger sample size would have provided more reliable
statistical results. Additionally, only three risk factors for
colorectal carcinoma (BMI, smoking status and excessive alcohol
consumption) were assessed in the present study. Other risk
factors, including a current diagnosis of type-2 diabetes, a
previous diagnosis of colorectal cancer, colorectal adenoma/polyps
or inflammatory bowel disease (IBD), and a family history of
colorectal cancer or colorectal adenoma/polyps, require further
investigation in future studies. Furthermore, due to resource
limitations, the expression levels of only four proteins,
P504S/AMACR, p53, Bcl-2 and Ki-67/MIB-1, were analyzed in the
present study. Other proteins that have been previously associated
with IBD and colorectal malignancy, including adenomatous polyposis
coli, retinoblastoma, deleted in colon cancer and v-Ki-ras2 Kirsten
rat sarcoma viral oncogene homolog, also require further
investigation in future studies (29). Finally, the present study did not
analyze gross tumor morphology, epigenetic factors or the mRNA
expression of the proteins examined, all of which may have provided
additional insights.
In conclusion, significantly higher expression
levels of P504S/AMACR, p53, Bcl-2 and Ki-67/MIB-1 were observed in
colorectal tumors than in matched normal tissue margins. Bcl-2
downregulation was revealed to be significantly associated with
advanced pathological grade and TNM stage in a Chinese patient
population with colorectal carcinoma. Furthermore, it was
demonstrated that Bcl-2 downregulation is a prognostic indicator of
reduced overall survival time in these patients. Bcl-2 may be a
novel target of colorectal carcinoma treatment in the Chinese
population of patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Yongchuan
Chongqing Medical University Hospital Fund (grant no.
YJYB2012009).
Availability of data and materials
All the data and materials are available upon
reasonable request.
Authors' contributions
ZL contributed to the study design. GH, QY and LB
contributed to data collection. JW contributed to the performance
of experiments. BJ and QL contributed to data analysis.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee (Institutional Review Board) of Yongchuan Hospital,
Chongqing Medical University (Chongqing, China). All subjects
recruited for the present study provided written informed consent
prior to participation.
Consent for publication
All patients signed informed consent and agreed to
the publication of the present research.
Competing interests
The authors declare that they have no competing
interests. The funding body had no role in the study design, data
collection and analysis, decision to publish or preparation of the
manuscript.
References
|
1
|
Bray F, Ren JS, Masuyer E and Ferlay J:
Global estimates of cancer prevalence for 27 sites in the adult
population in 2008. Int J Cancer. 132:1133–1145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung JJ, Lau JY, Goh KL and Leung WK: Asia
Pacific Working Group on Colorectal Cancer: Increasing incidence of
colorectal cancer in Asia: implications for screening. Lancet
Oncol. 6:871–876. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coghlin C and Murray GI: Biomarkers of
colorectal cancer: Recent advances and future challenges.
Proteomics Clin Appl. 9:64–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coghlin C and Murray GI: Progress in the
identification of plasma biomarkers of colorectal cancer.
Proteomics. 13:2227–2228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Daugherty SE, Platz EA, Shugart YY, Fallin
MD, Isaacs WB, Chatterjee N, Welch R, Huang WY and Hayes RB:
Variants in the alpha-Methylacyl-CoA racemase gene and the
association with advanced distal colorectal adenoma. Cancer
Epidemiol Biomarkers Prev. 16:1536–1542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuefer R, Varambally S, Zhou M, Lucas PC,
Loeffler M, Wolter H, Mattfeldt T, Hautmann RE, Gschwend JE,
Barrette TR, et al: alpha-Methylacyl-CoA racemase: Expression
levels of this novel cancer biomarker depend on tumor
differentiation. Am J Pathol. 161:841–848. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sinicrope FA, Ruan SB, Cleary KR, Stephens
LC, Lee JJ and Levin B: bcl-2 and p53 oncoprotein expression during
colorectal tumorigenesis. Cancer Res. 55:237–241. 1995.PubMed/NCBI
|
|
9
|
Oshima CT, Iriya K and Forones NM: Ki-67
as a prognostic marker in colorectal cancer but not in gastric
cancer. Neoplasma. 52:420–424. 2005.PubMed/NCBI
|
|
10
|
Dawson DA, Grant BF, Stinson FS and Zhou
Y: Effectiveness of the derived alcohol use disorders
identification test (AUDIT-C) in screening for alcohol use
disorders and risk drinking in the US general population. Alcohol
Clin Exp Res. 29:844–854. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haining Z, Kawai N, Miyake K, Okada M,
Okubo S, Zhang X, Fei Z and Tamiya T: Relation of LAT1/4F2hc
expression with pathological grade, proliferation and angiogenesis
in human gliomas. BMC Clin Pathol. 12:42012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goel MK, Khanna P and Kishore J:
Understanding survival analysis: Kaplan-Meier estimate. Int J
Ayurveda Res. 1:274–278. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kelly PN and Strasser A: The role of Bcl-2
and its pro-survival relatives in tumourigenesis and cancer
therapy. Cell Death Differ. 18:1414–1424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Strasser A, O'Connor L and Dixit VM:
Apoptosis signaling. Annu Rev Biochem. 69:217–245. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thomas S, Quinn BA, Das SK, Dash R, Emdad
L, Dasgupta S, Wang XY, Dent P, Reed JC, Pellecchia M, et al:
Targeting the Bcl-2 family for cancer therapy. Expert Opin Ther
Targets. 17:61–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hague A, Moorghen M, Hicks D, Chapman M
and Paraskeva C: BCL-2 expression in human colorectal adenomas and
carcinomas. Oncogene. 9:3367–3370. 1994.PubMed/NCBI
|
|
19
|
Biden KG, Simms LA, Cummings M, Buttenshaw
R, Schoch E, Searle J, Gobe G, Jass JR, Meltzer SJ, Leggett BA and
Young J: Expression of Bcl-2 protein is decreased in colorectal
adenocarcinomas with microsatellite instability. Oncogene.
18:1245–1249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shanmugam C, Katkoori VR, Jhala NC,
Grizzle WE, Siegal GP and Manne U: p53 Nuclear accumulation and
Bcl-2 expression in contiguous adenomatous components of colorectal
adenocarcinomas predict aggressive tumor behavior. J Histochem
Cytochem. 56:305–312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ofner D, Riehemann K, Maier H, Riedmann B,
Nehoda H, Tötsch M, Böcker W, Jasani B and Schmid KW:
Immunohistochemically detectable bcl-2 expression in colorectal
carcinoma: correlation with tumour stage and patient survival. Br J
Cancer. 72:981–985. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baretton GB, Diebold J, Christoforis G,
Vogt M, Müller C, Dopfer K, Schneiderbanger K, Schmidt M and Löhrs
U: Apoptosis and immunohistochemical bcl-2 expression in colorectal
adenomas and carcinomas. Aspects of carcinogenesis and prognostic
significance. Cancer. 77:255–264. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sinicrope FA, Hart J, Michelassi F and Lee
JJ: Prognostic value of bcl-2 oncoprotein expression in stage II
colon carcinoma. Clin Cancer Res. 1:1103–1110. 1995.PubMed/NCBI
|
|
24
|
Bhatavdekar JM, Patel DD, Ghosh N,
Chikhlikar PR, Trivedi TI, Suthar TP, Doctor SS, Shah NG and Balar
DB: Coexpression of Bcl-2, c-Myc, and p53 oncoproteins as
prognostic discriminants in patients with colorectal carcinoma. Dis
Colon Rectum. 40:785–790. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bukholm IK and Nesland JM: Protein
expression of p53, p21 (WAF1/CIP1), bcl-2, Bax, cyclin D1 and pRb
in human colon carcinomas. Virchows Arch. 436:224–228. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bosari S, Moneghini L, Graziani D, Lee AK,
Murray JJ, Coggi G and Viale G: bcl-2 oncoprotein in colorectal
hyperplastic polyps, adenomas, and adenocarcinomas. Hum Pathol.
26:534–540. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tollenaar RA, van Krieken JH, van Slooten
HJ, Bruinvels DJ, Nelemans KM, van den Broek LJ, Hermans J and van
Dierendonck JH: Immunohistochemical detection of p53 and Bcl-2 in
colorectal carcinoma: No evidence for prognostic significance. Br J
Cancer. 77:1842–1847. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kaklamanis L, Savage A, Whitehouse R,
Doussis-Anagnostopoulou I, Biddolph S, Tsiotos P, Mortensen N,
Gatter KC and Harris AL: Bcl-2 protein expression: Association with
p53 and prognosis in colorectal cancer. Br J Cancer. 77:1864–1869.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matkowskyj KA, Chen ZE, Rao MS and Yang
GY: Dysplastic lesions in inflammatory bowel disease: Molecular
pathogenesis to morphology. Arch Pathol Lab Med. 137:338–350. 2013.
View Article : Google Scholar : PubMed/NCBI
|