Introduction
Upper-tract urothelial carcinoma (UTUC), including
ureteral and renal pelvic carcinoma, is a relatively uncommon
disease that accounts for 5–10% of cases of urothelial carcinoma
(1,2).
Radical nephroureterectomy (RNU) with excision of the bladder cuff
is the gold-standard treatment for UTUC; however, due to the
frequent multifocal nature of urothelial carcinomas, 22–47% of all
primary UTUC patients experience bladder recurrence after RNU
(3,4).
Moreover, the patients who experience bladder tumor recurrence
often require more than one transurethral resection of the bladder
tumor (TURBT), which leads to increased suffering of the patient.
Multiple bladder recurrences treated with repeated TURBT may
significantly reduce a patient's quality of life, and some patients
must undergo radical cystectomy for bladder tumor progression
during the repeated recurrences. A number of previous studies have
reported that the risk factors for bladder recurrence include tumor
multifocality, tumor site and patient gender (5–7). However,
a lack of effective markers remains a challenge with regard to the
prediction of bladder recurrence. It is necessary to increase the
amount of available data addressing second recurrence after surgery
for a first bladder recurrence, in order to establish novel
prognostic factors and predictive models.
As a major epigenetic mechanism in humans, gene
methylation plays an important role in the development, progression
and prognosis of various types of carcinoma (8–10). Our
previous study evaluated the methylation statuses of 10 selected
genes with regard to their prognostic value for bladder recurrence
of a primary UTUC treated with RNU, and found that gene methylation
was a common status and could predict bladder recurrence in UTUC
patients (11). In the present study,
to continue our previous research, we collected data from patients
who experienced bladder recurrence from a primary UTUC database,
and evaluated the predictive value of gene methylation and clinical
factors for subsequent outcomes after the surgical treatment of a
first bladder recurrence following primary UTUC treated with
RNU.
Patients and methods
Patient selection
This was a retrospective study. All patients with
primary UTUC in our database had been diagnosed with UTUC and had
undergone RNU at Peking University First Hospital (Beijing, China)
between January 2001 and December 2013. None of the patients had
received neoadjuvant chemotherapy prior to RNU. Following the
exclusion of patients with a previous history of bladder cancer,
318 patients remained in the database. Among these 318 primary UTUC
patients, 110 experienced bladder recurrence, of which 25 patients
were excluded: 9 in whom the first recurrent bladder tumor was
stage T0 or Tis, 6 who were lost to follow-up, and 10 for whom
paraffin specimens could not be obtained. A total of 85 patients
were included in the final analysis. All patients provided written
informed consent.
Diagnosis and treatment
The diagnosis, treatment and pathological
examination of primary UTUC samples were performed as described in
our previous study (11). Bladder
recurrence of UTUC was diagnosed by cystoscopy with biopsy, and
TURBT was performed according to the standard procedure. Radical
cystectomy was performed for recurrent bladder tumors where
indicated due to tumor progression; otherwise, repeated TURBT was
performed. All patients received one immediate instillation of
Mitomycin C or epirubicin within 24 h after TURBT.
All resected specimens were reviewed by two senior
pathologists who were blinded to the personal data of the patients.
Tumor stage was evaluated according to the 2002 UICC TNM
classification of malignant tumors, and tumor grade was assessed
according to the 1973 WHO classification (12). The time between primary UTUC and the
first bladder recurrence was defined as the first recurrence
interval.
Methylation analysis of gene
promoters
The methylation statuses of 10 selected genes
(ABCC6, BRCA1, CDH1, GDF15, HSPA2, RASSF1A, SALL3, THBS1,
TMEFF2, VIM) were evaluated in 117 bladder tumors (85 from a
first recurrence, and 32 from a second recurrence). DNA extraction,
bisulfite transformation and gene methylation status were evaluated
according to the procedures described in our previous study
(11). The methylation statuses of
the genes between urothelial tumors and normal tissues were not
compared as all genes investigated in this study have been
validated to have a low methylation rate in normal tissues
(13–16).
Postoperative follow-up
The patients were followed-up every 3 months for the
first 2 years after surgery, and annually thereafter at our
institution. The follow-up consisted of physical examination,
urinalysis, cytology, chest X-ray, ultrasound or CT/MRI, and
cystoscopy. Second bladder recurrence and tumor progression were
used as the endpoints in this study. Tumor progression was defined
as the presence of a pathologically confirmed, muscle-invasive
tumor (above stage T2) in the bladder during follow-up. Patients
who were still alive without a second recurrence or tumor
progression were censored at the last follow-up, and the survival
time was censored at death during follow-up.
Statistical analysis
χ2 tests were used to compare categorical
variables. Binary logistic regression was used to evaluate
methylation status with respect to tumor stage and grade. The
second bladder recurrence-free survival (BRFS) rate and
progression-free survival (PFS) rate after the surgical treatment
of the first bladder recurrence were evaluated by the Kaplan-Meier
method. Variables influencing BRFS and PFS were compared using Cox
proportional hazards regression models. Variables with P<0.05 on
univariate analysis were also assessed by multivariate analysis.
Multivariate Cox regression coefficients were then used to generate
nomograms to predict the 6-, 12-, 24- and 36-month BRFS and PFS
rates, and Harrell's concordance index (c-index) was used to
quantify the discrimination ability of these nomograms, and
calibration plots were generated to explore the performance of the
nomograms. All statistical analyses were performed using IBM SPSS
version 20.0 and R version 3.2.0. Two-sided P-values <0.05 were
considered to indicate statistical significance.
Results
Overall results of clinical
follow-up
The characteristics of the 85 patients with primary
UTUC and first bladder recurrence are presented in Table I. Of the 85 patients, 42 (49.4%) were
female and 43 (50.6%) were male, and the median age was 67 years
(range, 46–82 years). The median follow-up time was 51 months
(range, 5–161 months). During follow-up, there were 31 mortalities
(36.5%), of which 29 were due to cancer, and a total of 32 patients
(37.6%) developed second bladder recurrence. The median interval
between RNU and the first bladder recurrence was 15 months (range,
2–98 months) and the median interval between the first and second
bladder recurrences was 31 months (range, 2–126 months). A total of
16 patients experienced tumor progression during follow-up. The
median interval between the first bladder recurrence and tumor
progression was 41 months (range, 4–126 months) Of the 16 patients
with tumor progression, 8 received radical cystectomy and 8
received TURBT.
 | Table I.Characteristics of primary UTUC and
first bladder recurrence tumor of all the 85 patients. |
Table I.
Characteristics of primary UTUC and
first bladder recurrence tumor of all the 85 patients.
| Clinicopathologic
characteristics | Median (range) or
no. (%) |
|---|
| Age | 67 (46–82) |
| Gender |
|
|
Female | 42 (49.4) |
|
Male | 43 (50.6) |
| No. of subsequent
recurrence |
|
| 1 | 85 (100) |
| 2 | 15 (17.6) |
| 3 | 10 (11.8) |
| ≥4 | 7 (8.2) |
| Bladder tumor
progression |
|
|
Absent | 69 (81.2) |
|
Present | 16 (18.8) |
| Death |
|
|
Cancer-specific death | 29 (34.1) |
| Other
death | 2 (2.4) |
| Characteristics of
primary UTUC Tumor stage |
|
| Ta,
T1 | 31 (36.5) |
| T2 | 37 (43.5) |
| T3 | 17 (20.0) |
| Tumor grade |
|
| G1 | 4 (4.7) |
| G2 | 55 (64.7) |
| G3 | 26 (30.6) |
| Tumor size |
|
| Small
(<5 cm) | 76 (89.4) |
| Large
(≥5 cm) | 9 (10.6) |
| Tumor
architecture |
|
|
Papillary tumor | 72 (84.7) |
| Sessile
tumor | 13 (15.3) |
| Tumor location |
|
| Renal
pelvis | 50 (58.8) |
|
Ureter | 35 (41.2) |
| Tumor
multifocality |
|
|
Absent | 60 (70.6) |
|
Present | 25 (29.4) |
| Characteristics of
first bladder recurrence tumor |
|
| Tumor stage |
|
| Ta | 45 (53.6) |
| T1 | 35 (41.7) |
| T2 | 5 (4.7) |
| Tumor grade |
|
| G1 | 9 (10.7) |
| G2 | 59 (70.2) |
| G3 | 16 (19.0) |
| First recurrence
interval |
|
| Long
(≥8 months) | 59 (69.4) |
| Short
(<8 months) | 26 (30.6) |
| Renal function |
|
| eGFR
≥30 ml/min | 76 (89.4) |
| eGFR
<30 ml/min | 9 (10.6) |
Gene methylation status and
oncological outcomes
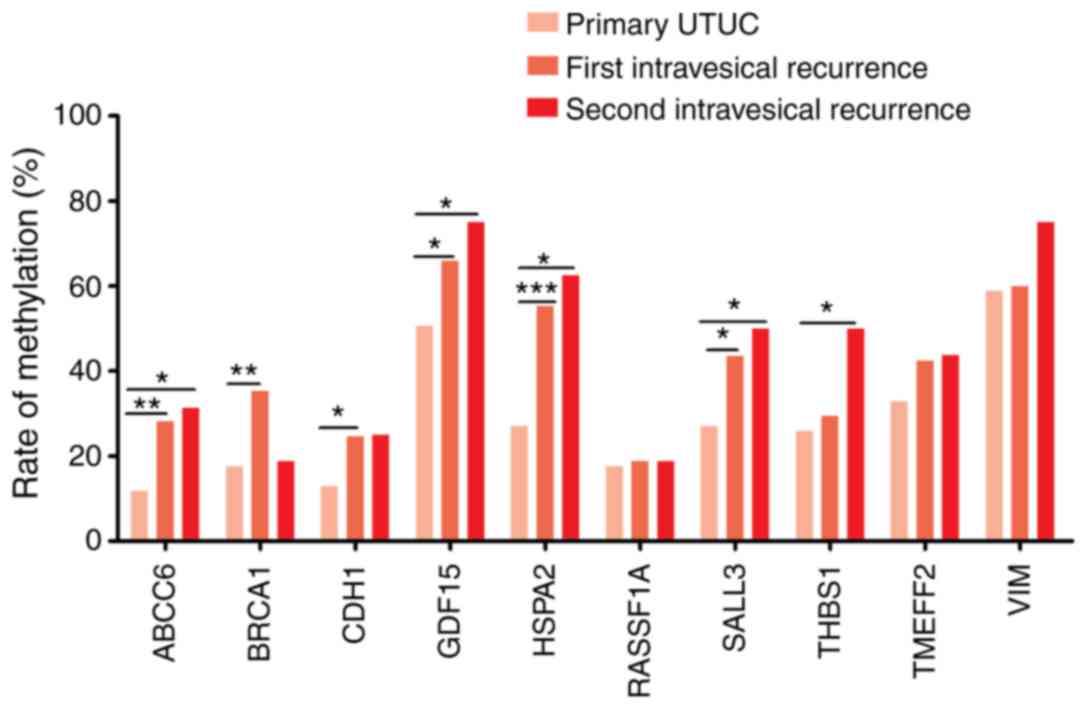
The methylation rates of the 10 selected genes in
primary UTUC, and first and second recurrent bladder tumors are
summarized in Table II and Fig. 1. GDF15 (primary, 50.6%; first
recurrence, 65.9%; second recurrence, 75%) and VIM (primary,
58.8%; first recurrence, 60%; second recurrence, 75%) had the
highest methylation rates. With the exception of BRCA1, all
genes showed a higher methylation rate in the second recurrent
tumors compared with the first recurrent tumors, and in the first
recurrent tumors compared with the primary UTUC. The associations
between gene promoter methylation statuses and pathological tumor
characteristics are shown in Table
III. Univariate analysis showed that methylated statuses in the
ABCC6, BRCA1, CDH1, GDF15, HSPA2 and RASSF1A
promoters were significantly associated with pT1/T2 stage in the
first bladder recurrence. However, on binary logistic regression
analysis, after adjusting for clinical and pathological factors,
promoter methylation status in any of the 10 genes in the first
recurrent bladder tumor was not associated with T1/T2 stage or
grade 3 malignancy.
 | Table II.The gene methylation rate of primary
UTUC, the first bladder recurrence tumor and second bladder
recurrence tumor. |
Table II.
The gene methylation rate of primary
UTUC, the first bladder recurrence tumor and second bladder
recurrence tumor.
| Gene promoter | Primary UTUC
(%) | First bladder
recurrence (%) | Second bladder
recurrence (%) |
|---|
| Total no. | 85 | 85 | 32 |
| ABCC6 |
|
|
|
|
Unmethylated | 75 (88.2) | 61 (71.8) | 22 (68.8) |
|
Methylated | 10 (11.8) | 24 (28.2) | 10 (31.3) |
| BRCA1 |
|
|
|
|
Unmethylated | 70 (82.4) | 55 (64.7) | 26 (81.3) |
|
Methylated | 15 (17.6) | 30 (35.3) | 6 (18.8) |
| CDH1 |
|
|
|
|
Unmethylated | 74 (87.1) | 64 (75.3) | 24 (75.0) |
|
Methylated | 11 (12.9) | 21 (24.7) | 8 (25.0) |
| GDF15 |
|
|
|
|
Unmethylated | 42 (49.4) | 29 (34.1) | 8 (25.0) |
|
Methylated | 43 (50.6) | 56 (65.9) | 24 (75.0) |
| HSPA2 |
|
|
|
|
Unmethylated | 62 (72.9) | 38 (44.7) | 12 (37.5) |
|
Methylated | 23 (27.1) | 47 (55.3) | 20 (62.5) |
| RASSF1A |
|
|
|
|
Unmethylated | 70 (82.4) | 69 (81.2) | 26 (81.2) |
|
Methylated | 15 (17.6) | 16 (18.8) | 6 (18.8) |
| SALL3 |
|
|
|
|
Unmethylated | 62 (72.9) | 48 (56.5) | 16 (50.0) |
|
Methylated | 23 (27.1) | 37 (43.5) | 16 (50.0) |
| THBS1 |
|
|
|
|
Unmethylated | 63 (74.1) | 60 (70.6) | 16 (50.0) |
|
Methylated | 22 (25.9) | 25 (29.4) | 16 (50.0) |
| TMEFF2 |
|
|
|
|
Unmethylated | 57 (67.1) | 49 (57.6) | 18 (56.3) |
|
Methylated | 28 (32.9) | 36 (42.4) | 14 (43.8) |
| VIM |
|
|
|
|
Unmethylated | 35 (41.2) | 34 (40.0) | 8 (25.0) |
|
Methylated | 50 (58.8) | 51 (60.0) | 24 (75.0) |
 | Table III.Predictive effect of epigenetic
biomarkers in first bladder recurrence of UTUC for high tumor stage
(T1 and T2) and grade 3 using univariable and multivariable
logistic regression. |
Table III.
Predictive effect of epigenetic
biomarkers in first bladder recurrence of UTUC for high tumor stage
(T1 and T2) and grade 3 using univariable and multivariable
logistic regression.
|
| T1 and T2 | Grade 3 |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Promoter
methylation status | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| ABCC6 | 4.626 | 1.657–12.912 | 0.003 | 1.909 | 0.503–7.241 | 0.342 | 1.70 | 0.54–5.347 | 0.364 | 1.853 | 0.398–8.631 | 0.432 |
| BRCA1 | 3.273 | 1.294–8.274 | 0.012 | 2.051 | 0.674–6.236 | 0.206 | 1.125 | 0.365–3.472 | 0.838 | 0.839 | 0.210–3.355 | 0.804 |
| CDH1 | 4.457 | 1.520–13.066 | 0.006 | 3.663 | 0.925–14.49 | 0.064 | 1.020 | 0.290–3.584 | 0.976 | 1.378 | 0.269–7.046 | 0.700 |
| GDF15 | 3.029 | 1.149–7.982 | 0.025 | 1.106 | 0.305–4.009 | 0.878 | 0.833 | 0.269–2.577 | 0.752 | 0.257 | 0.049–1.361 | 0.110 |
| HSPA2 | 3.314 | 1.335–8.222 | 0.01 | 2.006 | 0.534–7.531 | 0.303 | 1.441 | 0.472–4.406 | 0.521 | 2.510 | 0.504–12.506 | 0.261 |
| RASSF1A | 3.422 | 1.07–10.943 | 0.038 | 2.913 | 0.636–13.33 | 0.168 | 1.583 | 0.435–5.761 | 0.486 | 1.696 | 0.327–8.795 | 0.529 |
| SALL3 | 2.393 | 0.994–5.765 | 0.052 | 1.016 | 0.286–3.608 | 0.98 | 1.011 | 0.338–3.027 | 0.984 | 0.728 | 0.157–3.374 | 0.685 |
| THBS1 | 2.413 | 0.929–6.268 | 0.07 | 1.005 | 0.295–3.422 | 0.994 | 0.762 | 0.22–2.639 | 0.668 | 0.394 | 0.073–2.119 | 0.278 |
| TMEFF2 | 1.765 | 0.739–4.216 | 0.201 | 0.424 | 0.103–1.741 | 0.234 | 1.073 | 0.358–3.214 | 0.900 | 0.628 | 0.123–3.202 | 0.576 |
| VIM | 2.352 | 0.952–5.813 | 0.064 | 1.375 | 0.384–4.928 | 0.625 | 2.308 | 0.676–7.876 | 0.182 | 4.668 | 0.921–23.65 | 0.063 |
The univariate and multivariate analyses of
prognostic significance are shown in Table IV. The 12-, 24-, 36- and 60-month
BRFS rates were 75.8, 66.6, 63.6 and 58.2%, respectively. On
multivariate analysis, unmethylated GDF15 [hazard ratio
(HR)=0.36; 95% confidence interval (CI), 0.14–0.92] and methylated
VIM (HR=2.91; 95% CI, 1.11–7.61) in the first recurrent
bladder tumor, as well as male gender (HR=2.28; 95% CI, 1.06–4.87),
first recurrence interval <8 months (HR=2.34; 95% CI, 1.15–4.78)
and primary tumor size ≥5 cm (HR=3.48; 95% CI, 1.43–8.45) were
independently associated with second bladder recurrence. The 12-,
24-, 36- and 60-month PFS rates were 93.9, 84.6, 81.5 and 79.7%,
respectively. Methylated CDH1 (HR=2.91; 95% CI, 1.08–7.77)
and VIM (HR=4.91; 95% CI, 1.11–21.7) in the first recurrent
bladder tumor, male gender (HR=3.6; 95% CI, 1.1–11.73), and primary
tumor stage T2-T4 (HR=4.57; 95% CI, 1.22–17.13), multifocality
(HR=3.64; 95% CI, 1.19–11.16) and size ≥5 cm (HR=3.1; 95% CI,
1.91–10.54) were significantly associated with tumor progression on
multivariate analysis.
 | Table IV.Univariable and multivariable Cox
regression analyses predicting bladder recurrence-free survival for
UTUC patients and tumor progression of subsequent bladder
recurrence. |
Table IV.
Univariable and multivariable Cox
regression analyses predicting bladder recurrence-free survival for
UTUC patients and tumor progression of subsequent bladder
recurrence.
|
| Second bladder
recurrence | Tumor
progression |
|---|
|
|
|
|
|---|
|
| Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|
|---|
| Gene promoter
methylation status and clinicopathological parameter | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Parameters of first
bladder recurrence tumor |
|
ABCC6 (M vs. U) | 1.20 | 0.41–3.52 | 0.739 |
|
|
| 2.26 | 0.84–6.09 | 0.109 |
|
|
|
|
BRCA1 (M vs. U) | 0.57 | 0.22–1.46 | 0.241 |
|
|
| 1.06 | 0.39–2.92 | 0.909 |
|
|
|
|
CDH1 (M vs. U) | 2.85 | 1.11–7.36 | 0.030 |
|
|
| 3.12 | 1.17–8.33 | 0.023 | 2.91 | 1.08–7.77 | 0.033 |
|
GDF15 (M vs. U) | 0.35 | 0.13–0.96 | 0.042 | 0.36 | 0.14–0.92 | 0.033 | 1.06 | 0.37–3.09 | 0.906 |
|
|
|
|
HSPA2 (M vs. U) | 0.86 | 0.33–2.26 | 0.766 |
|
|
| 0.77 | 0.29–2.08 | 0.608 |
|
|
|
|
RASSF1A (M vs. U) | 1.70 | 0.56–5.17 | 0.354 |
|
|
| 1.50 | 0.48–4.65 | 0.486 |
|
|
|
|
SALL3 (M vs. U) | 0.80 | 0.32–2.02 | 0.638 |
|
|
| 1.04 | 0.39–2.80 | 0.935 |
|
|
|
|
THBS1 (M vs. U) | 0.57 | 0.19–1.66 | 0.304 |
|
|
| 1.71 | 0.62–4.72 | 0.301 |
|
|
|
|
TMEFF2 (M vs. U) | 0.61 | 0.22–1.69 | 0.346 |
|
|
| 1.09 | 0.41–2.93 | 0.865 |
|
|
|
|
VIM (M vs. U) | 3.61 | 1.32–9.87 | 0.012 | 2.91 | 1.11–7.61 | 0.029 | 5.22 | 1.18–23.03 | 0.029 | 4.91 | 1.11–21.70 | 0.036 |
| Gender (male vs.
female) | 2.40 | 1.15–5.01 | 0.019 | 2.28 | 1.06–4.87 | 0.034 | 2.77 | 1.94–8.17 | 0.045 | 3.60 | 1.10–11.73 | 0.034 |
| Age | 0.98 | 0.95–1.01 | 0.224 |
|
|
| 0.99 | 0.94–1.04 | 0.598 |
|
|
|
| First recurrence
interval (<8 vs. ≥8 months) | 1.90 | 1.04–3.82 | 0.044 | 2.34 | 1.15–4.78 | 0.019 | 0.90 | 0.31–2.60 | 0.848 |
|
|
|
| Tumor stage (T2-T4
vs. T0-T1) | 1.48 | 0.73–2.99 | 0.276 |
|
|
| 2.20 | 0.79–6.18 | 0.133 |
|
|
|
| Tumor grade (G3 vs.
G1-G2) | 1.57 | 0.71–3.51 | 0.268 |
|
|
| 1.48 | 0.48–4.60 | 0.497 |
|
|
|
| Renal function
(eGFR<30 vs. eGFR≥30 ml/min) | 0.52 | 0.13–2.19 | 0.377 |
|
|
| 0.04 | 0.00–25.92 | 0.329 |
|
|
|
| Parameters of
primary UTUC |
| Tumor stage (T2-T4
vs. T0-T1) | 1.57 | 0.73–3.40 | 0.250 |
|
|
| 3.33 | 1.94–11.72 | 0.041 | 4.57 | 1.22–17.13 | 0.024 |
| Tumor grade (G3 vs.
G1-G2) | 1.34 | 0.65–2.74 | 0.426 |
|
|
| 2.53 | 1.94–6.78 | 0.045 |
|
|
|
| Tumor size (≥5 cm
vs. <5 cm) | 3.94 | 1.67–9.32 | 0.002 | 3.48 | 1.43–8.45 | 0.006 | 4.22 | 1.31–13.57 | 0.016 | 3.10 | 1.91–10.54 | 0.041 |
| Tumor architecture
(sessile vs. papillary) | 1.31 | 0.57–3.03 | 0.530 |
|
|
| 1.85 | 0.59–5.74 | 0.287 |
|
|
|
| Tumor location
(ureter vs. renal pelvis) | 0.93 | 0.46–1.89 | 0.843 |
|
|
| 1.19 | 0.44–3.20 | 0.730 |
|
|
|
| Tumor multifocality
(yes vs. no) | 1.05 | 0.49–2.28 | 0.898 |
|
|
| 2.27 | 1.82–6.25 | 0.044 | 3.64 | 1.19–11.16 | 0.023 |
Predictive model for BRFS and PFS
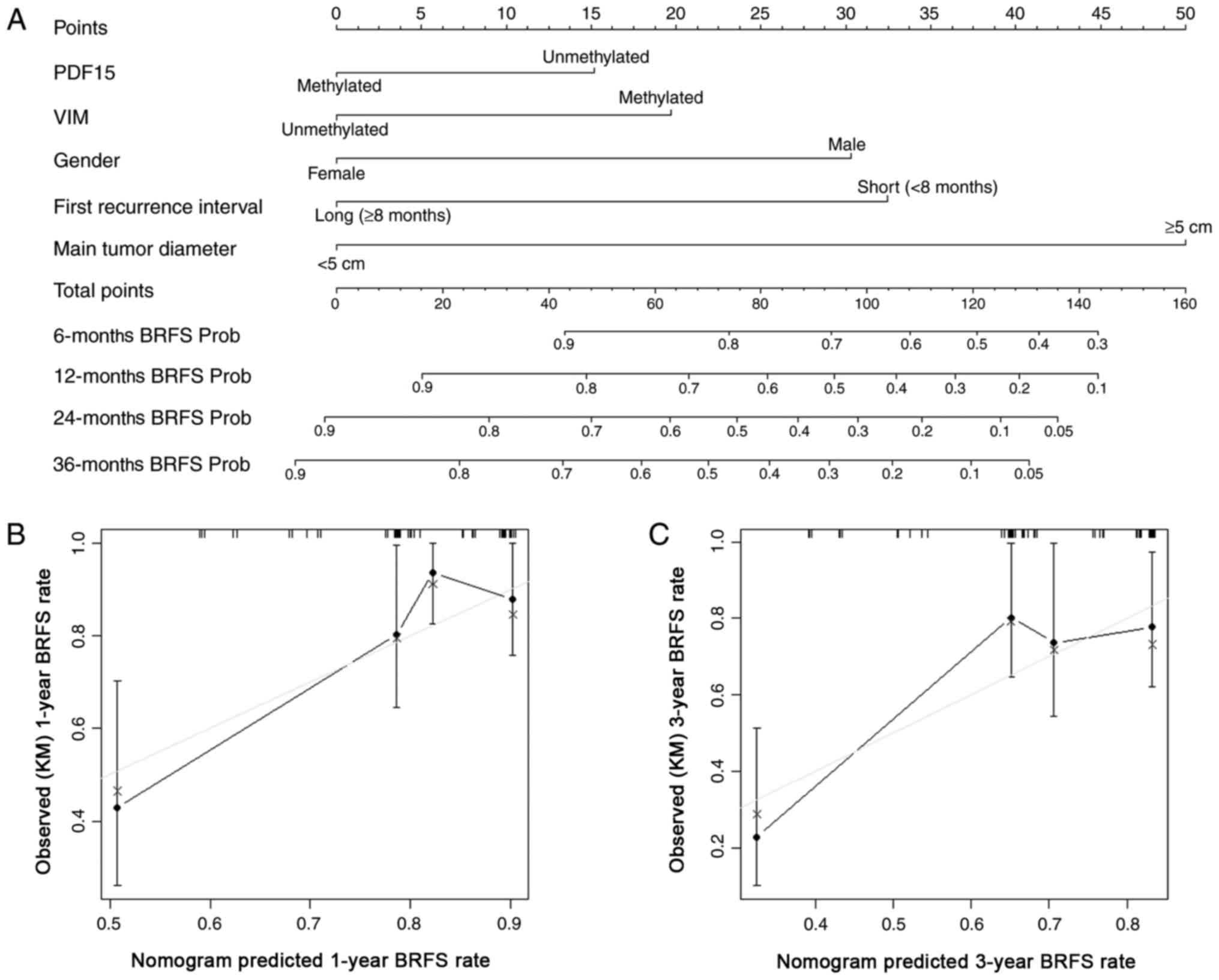
The nomogram for predicting the probability of BRFS
following surgery for first bladder recurrence is illustrated in
Fig. 2A, and the c-index of this
multivariate model was 0.71 (95% CI: 0.61–0.81). The calibration
plots at 1-year and 3-year follow-up for the nomogram are shown in
Fig. 2B and C, respectively. The
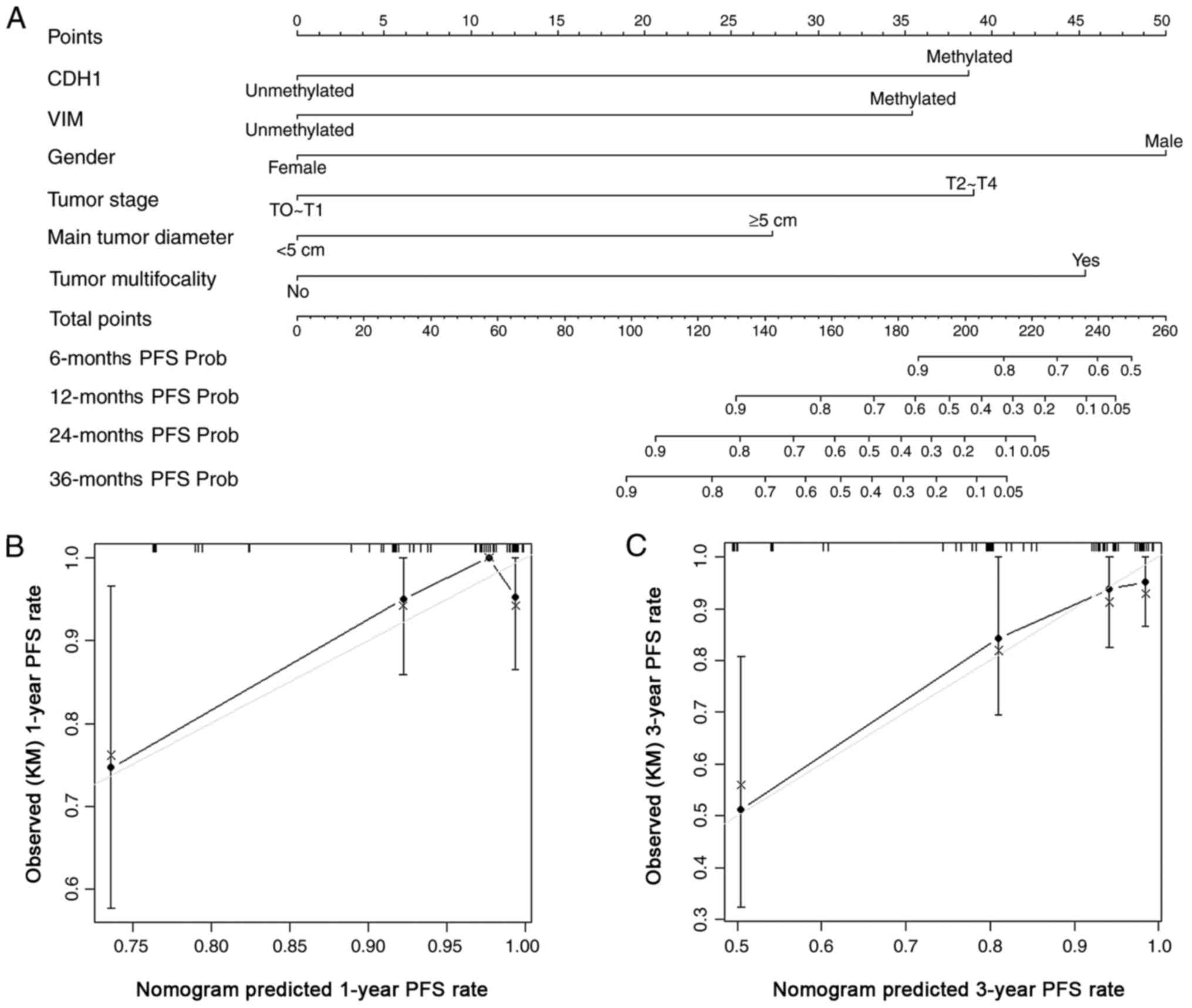
nomogram for predicting the probability of PFS after surgery for
first bladder recurrence is illustrated in Fig. 3A, and c-index of this multivariate
model was 0.88 (95% CI: 0.69–0.92). The calibration plots at 1-year
and 3-year follow-up for the nomogram are shown in Fig. 3B and C, respectively.
Discussion
UTUC is a relatively rare cancer. Approximately 60%
of UTUCs are invasive at diagnosis, compared with 15–25% of all
bladder tumors, and thus this disease has a comparatively poor
prognosis (6). The European
Association of Urology Guidelines reviewed several published
studies and reported that four different nomograms are available
for predicting survival rates postoperatively, based on standard
pathological features (17–20). Recently, several papers reported a
number of clinicopathological features and gene promoter
methylation statuses of UTUC patients that might affect the
probability of bladder recurrence, including patient gender,
smoking status, tumor multifocality, surgical management, and
GDF15 and RASSF1A promoter methylation (11,21–23).
However, although a few papers have addressed the clinical course
after the first bladder tumor relapse, none of the studies has
provided any significant recommendation concerning bladder
surveillance. In the present study, we collected data from UTUC
patients who experienced bladder recurrence following a primary
UTUC treated with RNU, and evaluated the prognostic value of gene
promoter methylation and clinical factors for the subsequent
outcomes, including repeated recurrence and progression after TURBT
for the first bladder recurrence.
DNA methylation is an important biochemical process
that is involved in the normal development of higher organisms. A
family of DNA methyltransferases transfer a methyl group from
S-adenosyl methionine to the fifth carbon of a cytosine
residue to form 5-methylcytosine, thereby catalyzing DNA
methylation. This modification can be inherited through cell
division (24). Beside its
involvement in normal development in human beings, DNA methylation
is frequently implicated in the onset or course of cancer due to
its roles in many other regulatory processes. Several studies have
reported that aberrant promoter methylation at several gene loci
was associated with bladder urothelial carcinoma (13,25–27). As
bladder urothelial carcinoma and UTUC display genomic and clinical
similarities, we selected 10 genes (ABCC6, BRCA1, CDH1, GDF15,
HSPA2, RASSF1A, SALL3, THBS1, TMEFF2 and VIM) with a
high frequency of methylation in bladder urothelial carcinoma and
evaluated their methylation statuses in UTUC and their associations
with clinical outcomes.
According to our results, gene methylation is common
in recurrent bladder tumors. Compared with our previous study that
focused on primary UTUC, we found that gene methylation rate
exhibits a significant increasing trend with the development and
recurrence of UTUC (primary UTUC to first bladder recurrence to
second recurrence); this partly confirmed the ‘intraluminal seeding
and implantation’ hypothesis, which is the classic mechanism for
bladder recurrence of primary UTUC (28,29). Our
results indicated that promoter methylation in 10 genes in the
first recurrent bladder tumor was not associated with pT1/T2 and
grade 3 malignancy. However, a former study (11) showed that certain epigenetic
biomarkers of primary UTUC were significantly associated with tumor
malignancy (pT3/T4, tumor grade 3 and positive lymph node
metastasis). Therefore, we suggested that the tumor stage and grade
of the first bladder recurrence could not be predicted by the gene
methylation status of the recurrent bladder tumor, and it was
difficult to explain this interesting phenomenon with existing
theories.
After adjusting for clinical and pathological
factors, multivariate analysis showed that male gender, a short
interval between initial RNU and first bladder recurrence,
unmethylated GDF15 and methylated VIM in the first
recurrent bladder tumor, and a primary UTUC tumor size >5 cm
were independently associated with repeated bladder relapse after
surgery for the first bladder recurrence. GDF15 encodes a
divergent member of the transforming growth factor (TGF)-β
superfamily, whose members are required for normal development,
differentiation, and tissue homeostasis. The anti-tumorigenic
activity of GDF15 has been suggested to be due to the
association between GDF15 overexpression and tumor growth
arrest and increased apoptosis (25).
Thus, we hypothesized that this anti-tumorigenic activity reduced
bladder recurrence. Male gender, methylated CDH1 and
VIM in the first recurrent bladder tumor, and high tumor
stage, large tumor size and multifocality of primary UTUC were
independently associated with bladder tumor progression. VIM,
CDH1 and GDF15 have been confirmed to be upregulated by
promoter demethylation. VIM methylation has been found to be
more frequent in bladder urothelial carcinoma and UTUC, but rare in
normal tissue, and may therefore be useful as a novel diagnosis and
detection method in urothelial cancer. Downregulation of VIM
has also been associated with increased tumor invasion,
progression, epithelial to mesenchymal transition (EMT) and poor
prognosis in various types of tumor (30–34).
Monteiro-Reis et al (31)
suggested that during early upper urinary tract carcinogenesis, the
VIM promoter is progressively methylated and the gene is
kept silenced, as in normal urothelium; by contrast, in a subset of
UTUCs, methylation is decreased, allowing for aberrant vimentin
expression, due to stimuli leading to EMT. As a consequence, these
tumors may be more prone to local invasion and systemic
dissemination, thus fostering disease progression and increasing
recurrence. In previous studies, CDH1 methylation was found
to be more frequent in colorectal cancer than in adjacent
non-neoplastic margins, and loss of CDH1 expression in
colorectal cancer was associated with an infiltrative tumor growth
pattern and lymph node metastasis (35,36). A
meta-analysis also identified CDH1 as a tumor suppressor
gene that contributes to the progression of breast cancer, and
suggested that CDH1 hypermethylation could be used as a
novel drug target for developing personalized therapy (37). Consistently, the methylation of
VIM or CDH1 predicted poor outcomes in the bladder
after surgery for a first bladder recurrence.
Previous studies (11)
have indicated that the GDF15 gene has a diverse range of
cancer-specific presentations. For bladder urothelial carcinoma,
GDF15 acts as a tumor suppressor gene, and methylation of
GDF15 is associated with tumor invasion and progression. In
the present study, we speculated that patients with GDF15
promoter methylation in the first recurrent bladder tumor may die
due to the aggressive nature of the tumor before a second
recurrence, and therefore unmethylated GDF15 in the first
recurrent bladder tumor was a risk factor for subsequent
recurrence. Compared with the predictive factors for the first
recurrent bladder tumor, the predictive factors for the primary
UTUC may be more easily applied in clinical decision-making.
Therefore, we also analyzed the gene methylation statuses in
primary UTUC; however, none showed any association with subsequent
bladder outcomes after first bladder recurrence (data not shown).
Nevertheless, certain clinicopathological parameters (T stage,
tumor size and tumor multifocality) relating to the primary UTUC
had good predictive value for subsequent bladder outcomes. Prior to
this study, two other studies had analyzed the bladder outcomes
subsequent to surgery for the first bladder recurrence following
primary UTUC. Abe et al (38)
reported that 40% of primary UTUC patients experienced bladder
recurrence, of whom 80% developed repeated bladder recurrence (in
contrast to 37.6% in the present study), and 20% eventually showed
tumor progression (compared with 18.8% in the present study).
Tanaka et al (39) identified
the independent prognostic factors for subsequent bladder outcomes,
and the c-indexes of their multivariate models to predict second
bladder recurrence and progression were 0.61 and 0.87,
respectively. By comparison, regarding the prognostic values of
gene methylation, the c-indexes of our predictive models based on
gene methylation status and clinical parameters for the prediction
of subsequent bladder recurrence and progression were 0.71 and
0.88, respectively.
There were several limitations of the present study,
partly due to the intrinsic biases of retrospective analyses, and
partly due to the small scale of our study cohort. However, while
the study cohort of 85 patients was less than that of the study by
Tanaka et al (39) (n=241),
our data were more comprehensive; in addition to routine clinical
parameters, we also evaluated the methylation statuses of 10 genes
in primary UTUC and in first recurrent bladder tumors. As a result,
we were able to construct more accurate prognostic models to
predict second bladder recurrence and tumor progression. Our
predictive models will need to be validated by further research. In
summary, to the best of our knowledge, this was the first study to
evaluate the predictive value of clinical parameters and gene
methylation status for the subsequent bladder outcomes after
surgery for a first bladder recurrence following a primary UTUC
treated by RNU. We speculate that our results may aid to achieve
more reasonable and accurate clinical decision-making, and improve
the comprehension of bladder recurrence after primary UTUC treated
by RNU.
Acknowledgements
The authors gratefully acknowledge financial support
from the Natural Science Foundation of Beijing (7122183 and
7152146), Natural Science Foundation of China (81172419 and
81372746) and the Clinical Features Research of Capital (no.
Z151100004015173). We thank G.Y. Xiong, J. Liu, and L. Zhang for
contributing their previous experience and results, and the entire
staff of the Department of Urology, Peking University First
Hospital for data collection support.
References
|
1
|
Rouprêt M, Babjuk M, Compérat E, Zigeuner
R, Sylvester R, Burger M, Cowan N, Böhle A, Van Rhijn BW, Kaasinen
E, et al: European guidelines on upper tract urothelial carcinomas:
2013 update. Eur Urol. 63:1059–1071. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kauffman EC and Raman JD: Bladder cancer
following upper tract urothelial carcinoma. Expert Rev Anticancer
Ther. 8:75–85. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ku JH, Choi WS, Kwak C and Kim HH: Bladder
cancer after nephroureterectomy in patients with urothelial
carcinoma of the upper urinary tract. Urol Oncol. 29:383–387. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu YQ, Lu J, Zhao L, Hou XF and Ma LL:
Prognostic factors for intravesical recurrence after surgery for
upper tract urothelial carcinoma in renal transplant recipients.
Beijing Da Xue Xue Bao. 47:605–610. 2015.(In Chinese). PubMed/NCBI
|
|
6
|
Rouprêt M, Babjuk M, Compêrat E, Zigeuner
R, Sylvester RJ, Burger M, Cowan NC, Böhle A, Van Rhijn BW,
Kaasinen E, et al: European association of urology guidelines on
upper urinary tract urothelial cell carcinoma: 2015 Update. Eur
Urol. 68:868–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamashita S, Ito A, Mitsuzuka K, Tochigi
T, Namima T, Soma F, Aizawa M, Ioritani N, Kaiho Y and Arai Y:
Clinical implications of intravesical recurrence after radical
nephroureterectomy for upper urinary tract urothelial carcinoma.
Int J Urol. 23:378–384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kulis M and Esteller M: DNA methylation
and cancer. Adv Genet. 70:27–56. 2010.PubMed/NCBI
|
|
9
|
Mikeska T, Bock C, Do H and Dobrovic A:
DNA methylation biomarkers in cancer: Progress towards clinical
implementation. Expert Rev Mol Diagn. 12:473–487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andres G, Ashour N, Sanchez-Chapado M,
Ropero S and Angulo JC: The study of DNA methylation in urological
cancer: Present and future. Actas Urol Esp. 37:368–375. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiong G, Liu J, Tang Q, Fan Y, Fang D,
Yang K, Xie F, Zhang M, Zhang L, Liu L, et al: Prognostic and
predictive value of epigenetic biomarkers and clinical factors in
upper tract urothelial carcinoma. Epigenomics. 7:733–744. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Epstein JI, Amin MB and Reuter VR: The
World Health Organization/International Society of Urological
Pathology consensus classification of urothelial (transitional
cell) neoplasms of the urinary bladder. Bladder Consensus
Conference Committee. Am J Surg Pathol. 22:1435–1448. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Casadio V, Molinari C, Calistri D, Tebaldi
M, Gunelli R, Serra L, Falcini F, Zingaretti C, Silvestrini R,
Amadori D and Zoli W: DNA methylation profiles as predictors of
recurrence in non muscle invasive bladder cancer: An MS-MLPA
approach. J Exp Clin Cancer Res. 32:942013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Catto JW, Azzouzi AR, Rehman I, Feeley KM,
Cross SS, Amira N, Fromont G, Sibony M, Cussenot O, Meuth M and
Hamdy FC: Promoter hypermethylation is associated with tumor
location, stage and subsequent progression in transitional cell
carcinoma. J Clin Oncol. 23:2903–2910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maruyama R, Toyooka S, Toyooka KO, Harada
K, Virmani AK, Zöchbauer-Müller S, Farinas AJ, Vakar-Lopez F, Minna
JD, Sagalowsky A, et al: Aberrant promoter methylation profile of
bladder cancer and its relationship to clinicopathological
features. Cancer Res. 61:8659–8663. 2001.PubMed/NCBI
|
|
16
|
Yu J, Zhu T, Wang Z, Zhang H, Qian Z, Xu
H, Gao B, Wang W, Gu L, Meng J, et al: A novel set of DNA
methylation markers in urine sediments for sensitive/specific
detection of bladder cancer. Clin Cancer Res. 13:7296–7304. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yates DR, Hupertan V, Colin P, Ouzzane A,
Descazeaud A, Long JA, Pignot G, Crouzet S, Rozet F, Neuzillet Y,
et al: Cancer-specific survival after radical nephroureterectomy
for upper urinary tract urothelial carcinoma: Proposal and
multi-institutional validation of a post-operative nomogram. Br J
Cancer. 106:1083–1088. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seisen T, Colin P, Hupertan V, Yates DR,
Xylinas E, Nison L, Cussenot O, Neuzillet Y, Bensalah K, Novara G,
et al: Postoperative nomogram to predict cancer-specific survival
after radical nephroureterectomy in patients with localised and/or
locally advanced upper tract urothelial carcinoma without
metastasis. BJU Int. 114:733–740. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rouprêt M, Hupertan V, Seisen T, Colin P,
Xylinas E, Yates DR, Fajkovic H, Lotan Y, Raman JD, Zigeuner R, et
al: Prediction of cancer specific survival after radical
nephroureterectomy for upper tract urothelial carcinoma:
Development of an optimized postoperative nomogram using decision
curve analysis. J Urol. 189:1662–1669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ku JH, Moon KC, Jung JH, Jeong SH, Kwak C
and Kim HH: External validation of an online nomogram in patients
undergoing radical nephroureterectomy for upper urinary tract
urothelial carcinoma. Br J Cancer. 109:1130–1136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hagiwara M, Kikuchi E, Tanaka N, Matsumoto
K, Ide H, Miyajima A, Masuda T, Nakamura S and Oya M: Impact of
smoking status on bladder tumor recurrence after radical
nephroureterectomy for upper tract urothelial carcinoma. J Urol.
189:2062–2068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kusuda Y, Miyake H, Terakawa T, Kondo Y,
Miura T and Fujisawa M: Gender as a significant predictor of
intravesical recurrence in patients with urothelial carcinoma of
the upper urinary tract following nephroureterectomy. Urol Oncol.
31:899–903. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xylinas E, Colin P, Audenet F, Phe V,
Cormier L, Cussenot O, Houlgatte A, Karsenty G, Bruyère F, Polguer
T, et al: Intravesical recurrence after radical nephroureterectomy
for upper tract urothelial carcinomas: Predictors and impact on
subsequent oncological outcomes from a national multicenter study.
World J Urol. 31:61–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hamidi T, Singh AK and Chen T: Genetic
alterations of DNA methylation machinery in human diseases.
Epigenomics. 7:247–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Costa VL, Henrique R, Danielsen SA,
Duarte-Pereira S, Eknaes M, Skotheim RI, Rodrigues A, Magalhães JS,
Oliveira J, Lothe RA, et al: Three epigenetic biomarkers, GDF15,
TMEFF2 and VIM, accurately predict bladder cancer from DNA-based
analyses of urine samples. Clin Cancer Res. 16:5842–5851. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Besaratinia A, Cockburn M and Tommasi S:
Alterations of DNA methylome in human bladder cancer. Epigenetics.
8:1013–1022. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kandimalla R, van Tilborg AA and Zwarthoff
EC: DNA methylation-based biomarkers in bladder cancer. Nat Rev
Urol. 10:327–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Habuchi T, Takahashi R, Yamada H, Kakehi
Y, Sugiyama T and Yoshida O: Metachronous multifocal development of
urothelial cancers by intraluminal seeding. Lancet. 342:1087–1088.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Habuchi T: Origin of multifocal carcinomas
of the bladder and upper urinary tract: Molecular analysis and
clinical implications. Int J Urol. 12:709–716. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huo Y, Zheng Z, Chen Y, Wang Q, Zhang Z
and Deng H: Downregulation of vimentin expression increased drug
resistance in ovarian cancer cells. Oncotarget. 7:45876–45888.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Monteiro-Reis S, Leca L, Almeida M,
Antunes L, Monteiro P, Dias PC, Morais A, Oliveira J, Henrique R
and Jerónimo C: Accurate detection of upper tract urothelial
carcinoma in tissue and urine by means of quantitative GDF15,
TMEFF2 and VIM promoter methylation. Eur J Cancer. 50:226–233.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nowakowska M, Matysiak-Burzynska Z,
Kowalska K, Pluciennik E, Dominska K and Piastowska-Ciesielska AW:
Angiotensin II promotes endometrial cancer cell survival. Oncol
Rep. 36:1101–1110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wen L, Li J, Guo H, Liu X, Zheng S, Zhang
D, Zhu W, Qu J, Guo L, Du D, et al: Genome-scale detection of
hypermethylated CpG islands in circulating cell-free DNA of
hepatocellular carcinoma patients. Cell Res. 25:1250–1264. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zuo J, Wen J, Lei M, Wen M, Li S, Lv X,
Luo Z and Wen G: Hypoxia promotes the invasion and metastasis of
laryngeal cancer cells via EMT. Med Oncol. 33:152016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miranda E, Bianchi P, Destro A, Morenghi
E, Malesci A, Santoro A, Laghi L and Roncalli M: Genetic and
epigenetic alterations in primary colorectal cancers and related
lymph node and liver metastases. Cancer. 119:266–276. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim SA, Inamura K, Yamauchi M, Nishihara
R, Mima K, Sukawa Y, Li T, Yasunari M, Morikawa T, Fitzgerald KC,
et al: Loss of CDH1 (E-cadherin) expression is associated with
infiltrative tumour growth and lymph node metastasis. Br J Cancer.
114:199–206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang R, Ding P and Yang F:
Clinicopathological significance and potential drug target of CDH1
in breast cancer: A meta-analysis and literature review. Drug Des
Devel Ther. 9:5277–5285. 2015.PubMed/NCBI
|
|
38
|
Abe T, Shinohara N, Harabayashi T, Sazawa
A, Akino T, Ishikawa S, Kubota K, Matsuno Y, Osawa T, Shibata T, et
al: Pathological characteristics and clinical course of bladder
tumour developing after nephroureterectomy. BJU Int. 105:1102–1106.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tanaka N, Kikuchi E, Kanao K, Matsumoto K,
Shirotake S, Kobayashi H, Miyazaki Y, Ide H, Obata J, Hoshino K, et
al: Independent predictors for bladder outcomes after treatment of
intravesical recurrence following radical nephroureterectomy in
patients with primary upper tract urothelial carcinoma. Ann Surg
Oncol. 21:3151–3158. 2014. View Article : Google Scholar : PubMed/NCBI
|