Introduction
The treatment of multiple myeloma (MM) has been
markedly altered by the clinical use of proteasome inhibitors (PIs)
and immunomodulatory drugs (IMiDs). Two IMiDs, lenalidomide (LEN)
and pomalidomide (POM), have been characterized with potent
immunostimulatory action, such as co-stimulatory effects on T and
NK cells (1); therefore, they are
often tested in combination with monoclonal antibodies, such as
elotuzumab (2), daratumumab (3), and others (4). In addition to the stimulatory effect on
the immune system, the intracellular action of MM cells targeting
an ubiquitin E3 ligase, cereblon (1,5), followed
by the degradation of the proliferative factors, IKZF1 and IKZF3,
have been also recognized as the main effectors of anti-tumor
activity of these agents (6–8). Recently, the combination therapy of PI
and IMiDs, such as LEN plus bortezomib (BOR) and dexamethasone
(DEX), was successfully introduced to the initial treatment of MM
in both transplant eligible and non-eligible subjects (9,10), and is
considered an appropriate treatment for suitable patients, mainly
including transplant eligible or fit patients without
complications. However, the detailed mechanisms of the anti-tumor
effect of this combination therapy are poorly understood. In
addition, antagonistic activity of the combination of PI and IMiDs
was reported (11), in which the
LEN-induced degradation of IKZF1, which proceeded through the
modification of cereblon, was inhibited by proteasome inhibition.
Therefore, except for monoclonal antibodies, excellent treatment
strategies for combination with IMiDs have not been fully
developed.
The PI3K/Akt pathway is constitutively activated in
MM cells, to maintain the signals of proliferation, anti-apoptosis,
and drug resistance in MM cells (12). Therefore, several inhibitors that
target this pathway have been attempted to develop the treatment of
relapsed and refractory MM with a single treatment or in
combination with other agents.
Among these agents, afuresertib (AFU), a novel
serine/threonine kinase Akt inhibitor, has demonstrated
single-agent clinical activity and synergistic anti-myeloma effects
against MM cells in combination with BOR plus DEX therapy (13).
In the present study, we investigated a more
effective and less toxic combination of IMiDs for therapy, and
estimated the additional effect of an Akt inhibitor in the
combination treatments of IMiDs plus DEX and examined the
cytotoxicity of IMiDs plus DEX and/or AFU treatment, with both DEX
and AFU at suboptimal doses, against MM cells. We identified an
enhanced anti-myeloma effect for this combination therapy and
attempted to elucidate the mechanism underlying this enhanced
activity with a focus on the previously reported factors involved
in the functional mechanism of IMiDs or Akt inhibitors.
Materials and methods
Cell lines and primary MM cells
The human MM cell lines, JJN-3 and SK-MM-1, were
purchased from DSMZ, the German Resource Center for Biological
Material (Braunshweig, Germany). U-266 was purchased from the
American Type Culture Collection (Maanassa, VA, USA). KMS-11, NOP1,
NCI-H929, and XG-7 were kindly provided by Kawasaki Medical
University, Aichi Cancer Center, Oita University, and Dr. Bernard
Klein, respectively. All cell lines were cultured in RPMI-1640
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum, 100 U/ml penicillin and
100 U/ml streptomycin at 37°C in 5% CO2 incubator. Bone
marrow mononuclear cells (BMNCs) were isolated from three patients
with MM. MM cells were purified from BMNCs by using anti-CD138
Micro Beads (Miltenyi Biotec, Bergisch Gladbach, Germany) as
previously described (14). Two
primary MM cell sources, with abundant numbers of cells, were
selected for immunoblot analysis. All donors provided informed
written consent prior to sampling in accordance with the
Declaration of Helsinki and the present study was approved by the
institutional ethics committees of Nagoya City University Graduate
School of Medical Sciences.
Antibodies and reagents
POM and LEN were provided by Celgene Co., Ltd and
also purchased from Santa Cruz Biotechnology (Dallas, TX). AFU
(GSK2110183) was purchased from Selleck Chemicals (Houston, TX).
The antibodies to cleaved caspase-3 (no. 9664), cleaved caspase-8
(no. 9496), IKZF1 (no. 5443), p-Akt (no. 9271), Akt (no. 9272),
IRF4 (no. 4948 and no. 4964), p-FoxO1/FoxO3 (no. 9464), 4E-BP-1
(no. 9452), P-4E-BP-1 (no. 9459), and eIF4E (no. 9742) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
The antibody to actin (sc-1616) was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) and the antibody to IKZF3
(NBP-16938) was purchased from Novus Biologicals (Littleton, CO,
USA), The proteins were visualized by using goat anti-rabbit
IgG-HRP (sc-2004) and goat anti-mouse IgG-HRP (sc-2005) (Santa Cruz
Biotechnology, Inc.).
Cell proliferation assay
The proliferation of MM cell lines was assessed by
using Cell Titer 96 Aqueous One Solution cell proliferation assay
kits (Promega Corporation, Madison, WI, USA), as previously
described (15). Cells were seeded in
96-well flat bottom microplates (2×104 cells/well), and
then treated with appropriate concentration of each agent in 5%
CO2 incubator at 37°C for 72 h. Cell proliferation was
measured at the indicated concentrations relative to the control.
The absorbance at 490 nm was read using a 96-well plate reader. All
expressed values represent the average of triplicate experiments
and the IC50 values were computed by GraphPad Prism 6
(GraphPad Software, Inc., La Jolla, CA, USA) in accordance with the
manufacturer's instructions.
Apoptosis assay
The apoptosis of MM cell lines was evaluated by
using an Annexin V-FITC Apoptosis Detection Kit I (BD Biosciences,
San Jose, CA, USA) in accordance with the manufacturer's
instructions. the cells were treated with appropriate concentration
of each agents for 48 or 72 h and reacted with FITC-conjugated
Annexin V and PI for 15 min at room temperature in the dark. The
cells were analyzed on a FACS Calibur flow cytometer (BD
Biosciences) with the aid of Flow Jo software (Tree Star, Ashland,
OR, USA). In the current study, we measured the ratio of Annexin V
positive cells, generally including early and late apoptotic cells.
We defined induced apoptotic cells as Annexin V positive cells
measured at each treatment, and spontaneous apoptotic cells as
Annexin V positive cells measured under the no treatment. The
percentage of specific apoptosis (%specific apoptosis) was
calculated as follows: 100× (% induced apoptotic cells-%
spontaneous apoptotic cells)/(100-% spontaneous apoptotic cells).
All expressed values represent the mean value of triplicate
experiments.
Immunoblot analysis
The MM cell lines and primary tumor cells from
patients with MM were incubated in the presence or absence of
reagents for the indicated times. The cells were lysed in lysis
buffer [20 mM HEPES (pH 7.5), 0.1% NP40, 150 mM NaCl, 1 mM EDTA (PH
8.0), 1 tablet of phosphatase inhibitor cocktail (Roche, Basel,
Switzerland), 1 tablet of protease inhibitor cocktail (Roche)] at
4°C with sonication. The preparation and analyses of whole-cell
extracts and were performed as previously described (15). After the estimation of the total
protein content by using Bradford reagent (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany), each loaded sample was adjusted to 30 µg
per 10 µl. The lysates were separated electrophoretically by
SDS-PAGE (4–15%) (Bio-Rad Laboratories, Hercules, CA, USA) and
transferred to polyvinylidene difuroide membrane (Thermo Fisher
Scientific, Inc.) using dry blotting system (Thermo Fisher
Scientific, Inc.). The membranes were blocked with 2% skim milk for
1 h at room temperature and incubated with appropriate primary
antibodies (dilution; 1:1,000 except for actin 1:2,000) at 4°C
overnight. The membranes were incubated with secondary antibody
(anti-rabbit; 1:10,000, anti-goat; 1:5,000) for 1 h at room
temperature. The bound antibodies were detected by the enhanced
chemiluminescence reagent (GE healthcare, Chicago, IL, USA). The
results of the immunoblot analysis presented are representative of
multiple experiments.
Statistical analysis
The statistical significance of differences among
more three groups was evaluated by using ANOVA followed by post hoc
Tukey test., computed using GraphPad Prism 6 (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Inhibitory activity of pomalidomide
alone or in combination with dexamethasone against MM cell
growth
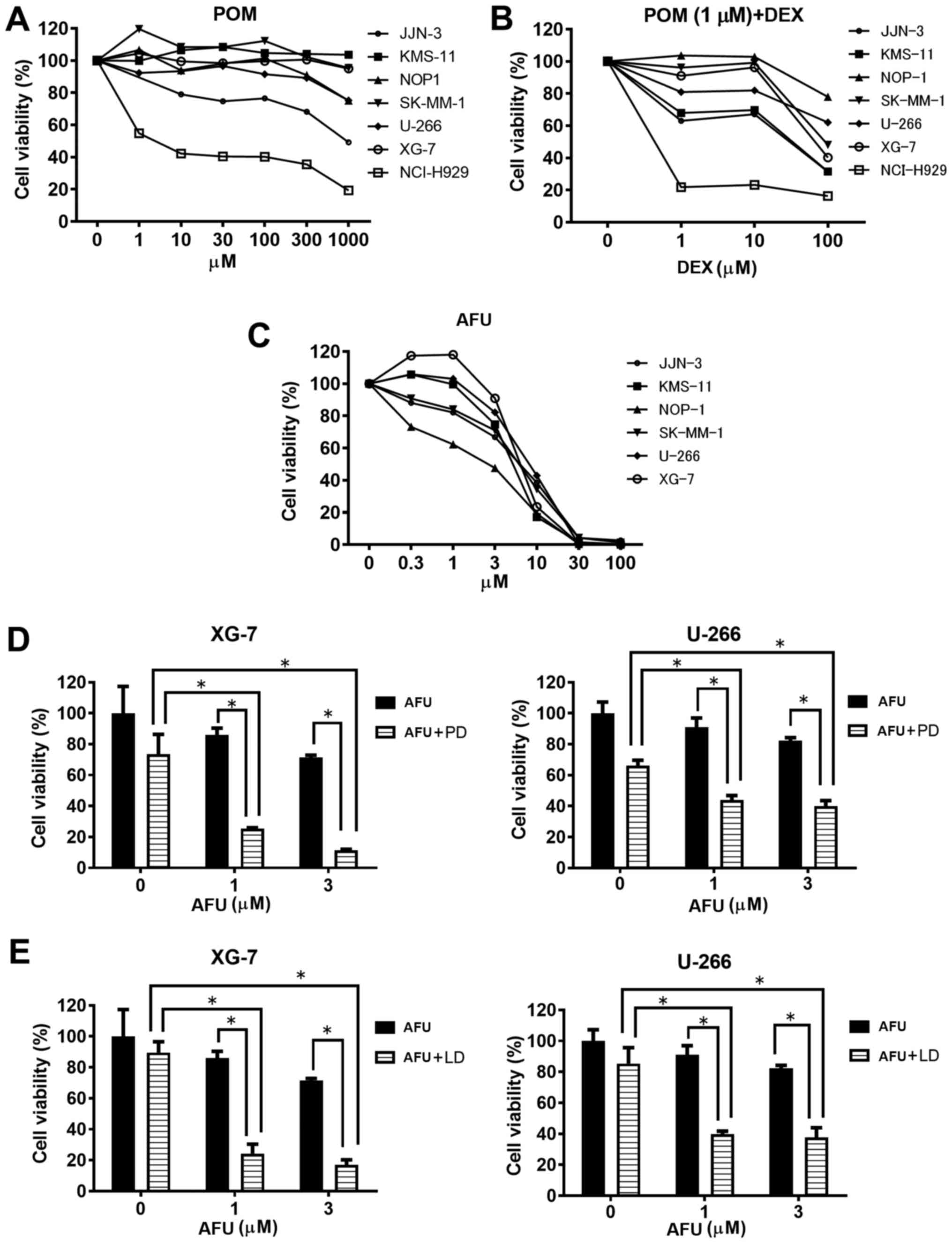
First, we determined the suboptimal doses of each
treatment, POM plus DEX (PD) and AFU, for MM cells before the
examination of the AFU-PD combination. As shown in Fig. 1A, treatment with POM alone caused only
a small inhibition of MM cell viability at all concentrations,
except in NCI-H929, which is known to have a high sensitivity to
IMiDs (6). We determined the
suboptimal dose of POM as 1 µM, and then evaluated the effect of
combination with 1 µM POM and the indicated doses of DEX in MM
cells (Fig. 1B). The PD combination
with 100 µM DEX markedly inhibited the vitality of most MM cell
lines, but only moderate inhibition occurred with 10 µM DEX. From
these results, we determined the suboptimal dose of the PD
treatment as 1 µM POM plus 10 µM DEX.
As shown in Fig. 1C,
the monotherapy of AFU caused a dose-dependent inhibition of MM
cell viability. A remarkable inhibition of viability was observed
above 10 µM AFU in most cell lines; therefore, concentrations of 1
and 3 µM were concluded as the suboptimal doses of AFU treatment.
Among the six MM cell lines tested, XG-7 and U266 showed
comparatively lower sensitivity to AFU treatment.
Inhibition activity of IMiDs plus
dexamethasone treatment in combination with AFU against MM cell
growth
Two MM cell lines, XG-7 and U266, characterized by a
comparatively lower sensitivity to AFU, were treated with the
AFU-PD combination therapy. At the suboptimal doses of PD
treatment, XG-7 showed a mild reduction (28%) in viability in
comparison with the untreated cells. Similarly, AFU treatment alone
at the suboptimal dose showed a weak reduction in cell viability at
the indicated concentrations: 10% at 1 µM and 25% at 3 µM (Fig. 1D, left). However, co-treatment with
suboptimal doses of PD and AFU resulted in an enhanced reduction of
cell viability: 75% at 1 µM AFU and 85% at 3 µM AFU. These
reductions were superior to those observed with PD or AFU alone.
The enhanced inhibition of cell viability caused by the combination
was also shown in similar tests in U266 cells (Fig. 1D, right).
Next, we examined the effect of the combination
therapy with LEN, instead of POM. Although 1 µM of LEN plus 10 µM
of DEX treatment showed a lower reduction of viability (10%) in
XG-7 cells, the combination with AFU considerably reduced the
viability at the indicated doses: 75% at 1 µM of AFU and 80% at 3
µM of AFU (Fig. 1E left), which were
very similar to the results obtained in U266 cells with identical
combination therapies (Fig. 1E
right).
Evaluation of apoptosis in MM cells
treated with the combination therapy of POM plus DEX and AFU
treatment
As shown in Fig. 2A,
PD or AFU treatment at a suboptimal dose triggered less apoptosis.
However, the combination therapy augmented the progression of
apoptosis in both XG-7 and U266 cells. The %specific apoptosis in
XG-7 cells treated with PD, AFU, or the AFU-PD combination was 0,
13.5, and 30.0, respectively (Fig.
2B, left). Similar results were also observed in U266 cells;
the %specific apoptosis induced by treatment with PD, AFU, or the
AFU-PD combination was 5.0, 0, and 22.0, respectively (Fig. 2B, right).
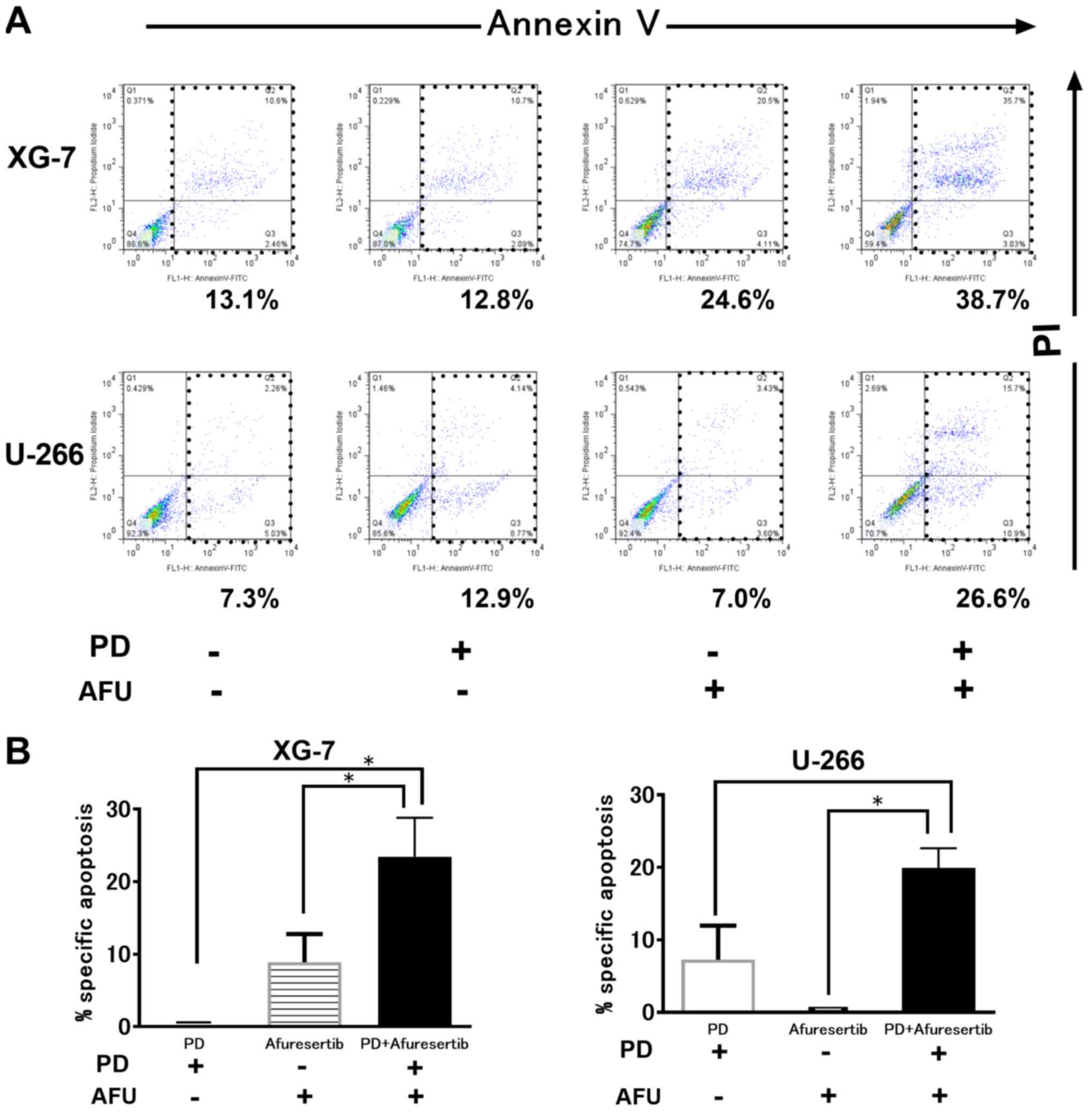 | Figure 2.Induction of cell death by suboptimal
dose of pomalidomide plus dexamethasone (PD), or afuresertib, and
the afuresertib-PD combination in MM cells. (A) The evaluation of
cell death by using Annexin V and PI staining in two MM cell lines
treated with suboptimal doses of PD, or AFU, and their combination.
AFU and PD are used as 1 µM of AFU or 1 µM of pomalidomide with 10
µM of dexamethasone, respectively. XG-7 and U266 were subjected to
the indicated treatments for 48 and 72 h, respectively. A
representative case of three independent experiments was shown. (B)
The evaluation of the percentage of specific apoptosis in two cell
lines treated with PD and or AFU. The mean value with SD bar of
three independent experiments was shown. *Represents statistically
significant (P<0.05), calculated by post hoc Tukey test. PD,
pomalidomide plus dexamethasone; AFU, Afuresertib; MM, multiple
myeloma. |
Alteration of IKZF- and Akt-regulating
substrates in MM cells after co-treatment with suboptimal doses of
POM plus DEX and/or AFU
The AFU-PD combination therapy with suboptimal doses
of PD and AFU enhanced caspase activation, as indicated by the
overexpression of cleaved caspase-3 and −8, in XG-7 cells in
comparison with the individual treatments. Similar results were
also observed in U266 cells treated in an identical manner
(Fig. 3A). PD treatment suppressed
the expression of IKZF1 and IKZF3 in both XG-7 and U266 cells, even
at suboptimal doses, which did not occur after the treatment with
AFU alone. In the AFU-PD combination therapy with suboptimal doses
of PD and AFU, the suppression of both IKZF1 and IKZF3 was further
enhanced in XG-7 cells. In U266 cells, the PD-induced suppression
of IKZF1 and IKZF3 was maintained after the addition of AFU. The
accumulation of cereblon, observed in PD treatment, was also
maintained after the co-treatment with AFU in both MM cell lines.
The expression of IRF4 was decreased by the PD treatment, and this
reduction was sustained when combined with AFU treatment in both
cell lines (Fig. 3B).
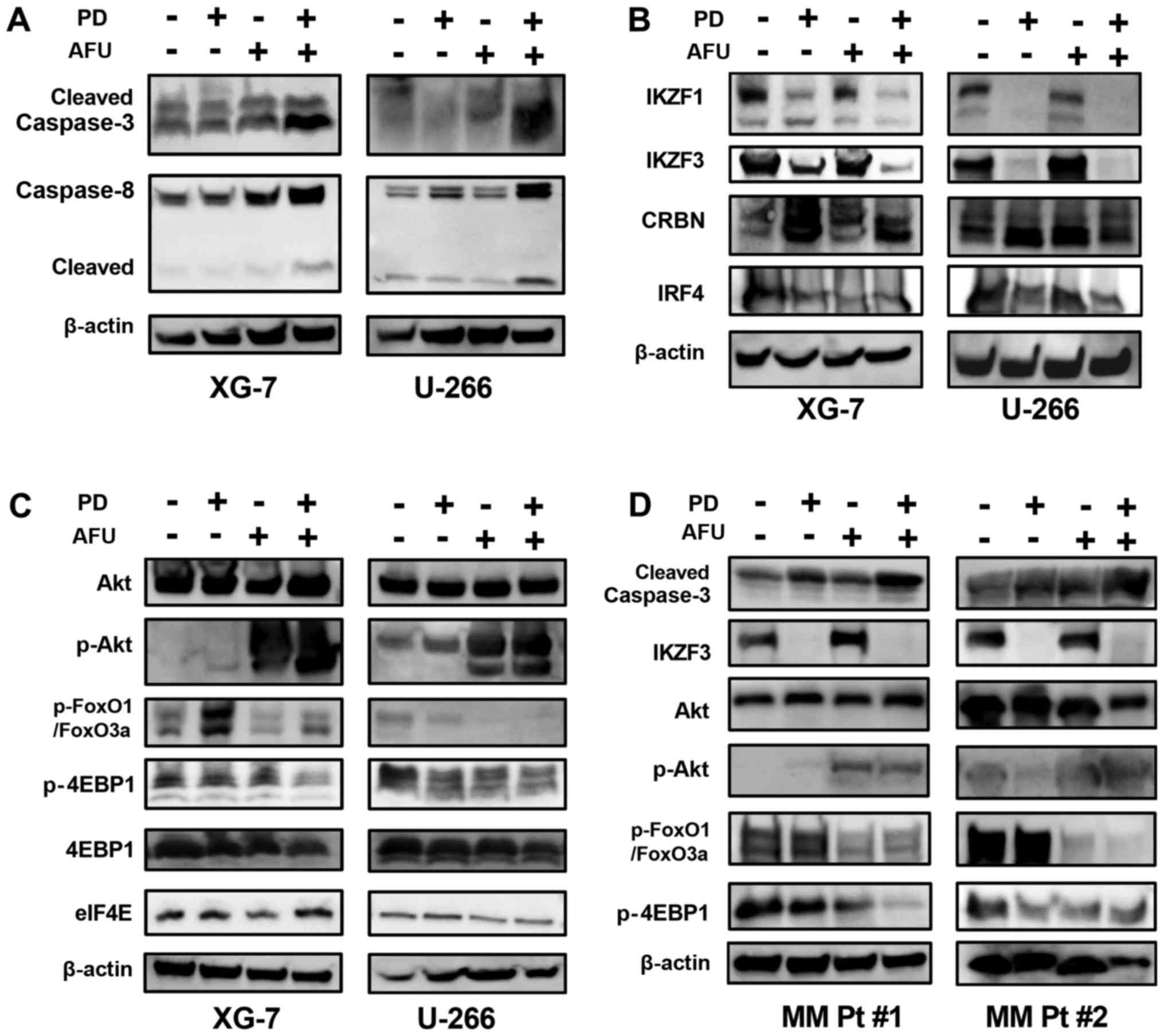 | Figure 3.Analysis of the mechanism of action of
the enhanced anti-tumor effect of the combination therapy of
suboptimal doses of pomalidomide plus dexamethasone and afuresertib
in MM cells. (A-C) The altered expression of protein substrates was
analyzed in two MM cell lines that were treated with suboptimal
doses of PD, or AFU, or the PD and AFU combination. The XG-7 and
U266 cell lines were subjected to the indicated treatments for 48
and 72 h, respectively. (A) Caspases, (B) substrates related mainly
to the working mechanism of IMiDs, (C) substrates mainly related to
the working mechanism of afuresertib, and (D) two primary MM cell
cultures, were subjected to the analysis in the same manner as the
two MM cell lines. In the expression panel of p-FoxO3a/FoxO1, the
upper band represents p-FoxO3a and the lower band represents
p-FOXO1. PD, pomalidomide plus dexamethasone; AFU, Afuresertib; MM,
multiple myeloma; IMiDs, immunomodulatory drugs. |
In agreement with other reports (16), AFU treatment triggered a feedback
increase in Akt phosphorylation in both MM cell lines, which
reflected the strong inhibition of kinase activity in Akt. Although
this feedback increase was not induced in PD treatment, it was
observed in the AFU-PD combination treatment with suboptimal doses
of AFU and PD.
FOXO1, which is considered a tumor suppressor gene
regulated by Akt signaling (17), was
highly phosphorylated in both MM cell lines. After PD treatment,
the level of phosphorylation was increased in XG-7 cells and was
slightly suppressed in U266 cells. Following the AFU-PD combination
treatment administered at suboptimal doses of each agent, the
upregulation of phosphorylated FOXO1 was inhibited in XG-7 cells,
and its expression was profoundly suppressed in U266 cells.
4EBP1, a negative regulator of eIF4e (18), was phosphorylated in both MM cell
lines (Fig. 3C). Moderate
dephosphorylation of 4EBP1 was observed in PD-treated U266 cells,
but not in XG-7 cells. However, the AFU-PD combination at
suboptimal doses of AFU and PD triggered the dephosphorylation of
4EBP1 in both MM cell lines.
Two primary MM cells derived from the patients were
treated with PD, AFU, and AFU-PD subjected to western blotting
analysis (Fig. 3D). In the PD and AFU
combination treatment, the enhanced activation of caspase-3,
dephosphorylation of FOXO1 and 4EBP1, and repression of IKZF3 were
observed in all primary MM cells.
Discussion
AFU, a member of a family of ATP-competitive
inhibitors, mainly targets the ATP binding and subsequent
phosphorylation of Akt substrates; it does not affect the
localization and self-activation of Akt. In previous reports, the
hyper-phosphorylation of Akt was commonly observed with other
ATP-competitive type inhibitors, such as A-4436548 (19) and GSK690693 (20), and a similar hyper-phosphorylation of
Akt was also observed in malignant pleural mesothelioma cells
treated with AFU (21). Although the
precise mechanism of this hyper-phosphorylation is not well
understood, it may be considered as the reflection of a homeostatic
feedback mechanism by which the cell attempts to maintain Akt
activity during the inhibition of phosphorylation of Akt substrates
caused by ATP-competitive inhibitors (19).
In general, most ATP-competitive kinase inhibitors
have been reported to inhibit additional kinases, such as PKA and
PKG1α, through off-target action (16); therefore, it cannot be completely
excluded that off-target action may be associated with the
anti-tumor effect of AFU on MM cells. In our study, Akt treatment
was tested at a sub-optimal dosage, which would serve to minimize
the effect of potential off-target action on the anti-tumor effect
of AFU on MM cells.
Several studies have reported the mechanism of
action responsible for the anti-tumor activity of IMiDs. Among
them, we have focused on two particular activities and evaluated
the alteration of factors related to the two activities that follow
the addition of a suboptimal dose of AFU to PD treatment. One of
these activities was the regulation of eIF4E, which resulted in the
inactivation of C/EBP translation and the subsequent impairment of
IRF4 transcription (18). The second
was the modulation of CRBN specificity for the ubiquitination of
proteins, which led to the downregulation of IKZF substrates
(8). Among the factors related to the
above activities, we focused mainly on two factors observed in the
combination treatment with AFU and PD: The dephosphorylation of
4EBP associated with the inactivation of eIF4E-related
transcriptional activity (22) and
the altered phosphorylation level in FOXO1, a proapoptotic factor
associated with cell cycle arrest and cell death (23–26).
A previous report demonstrated that 10 µM of IMiD
compounds downregulated eIF4E expression at the mRNA level, which
impaired C/EBP translation and inhibited MM cell growth (18). In this previous report, MM cells with
IMiD resistance did not show suppressed eIF4E expression and
sustained C/EBP translation during IMiD treatment. The suboptimal
dose, 1 µM of POM treatment could not reduce eIF4E expression in MM
cells in the present study. However, when a lower dose of an Akt
inhibitor was added, 4EBP1, a regulator of eIF4E and ordinarily
inactivated by the phosphorylation (22), was dephosphorylated and activated.
Therefore, it is speculated that this alternative activity of 4EBP1
may inhibit the activation of eIF4E through the impairment of
translation activity without the suppression of eIF4E expression at
the protein level, which leads to further suppression of IRF4. A
similar impairment of eIF4E was also observed by mTOR inhibitors in
combination with IMiDs (18,27). These results suggested that the
inactivation of the eIF4E-related translational complex by Akt or
mTOR inhibition can be an effective strategy for the treatment of
relapsed or refractory MM (RRMM) that was insensitive or resistant
to IMiDs treatment owing to the poor suppression of eIF4E
expression during IMiDs treatment.
FOXO1 is reported as a tumor suppressor gene, but
may act as a pro-apoptotic factor (23–26). Many
tumors have phosphorylated Akt, which inhibits FOXO1 activity by
direct phosphorylation and cytoplasmic sequestration, and
contributes to the maintenance of tumor cell survival. In the
present study, MM cells had highly phosphorylated FOXO1 proteins
that were regulated by Akt activation. In the current study, we
could not determine a general evaluation of the effect of PD
treatment on the phosphorylation level of FOXO1 in MM cells.
Regardless of the changes in FOXO1 phosphorylation caused by PD
treatment, the addition of AFU showed sufficient inhibition to
prevent the accelerated phosphorylation of FOXO1 in an MM cell
line, or repress the constant phosphorylation of FOXO1 in two
primary samples. These results suggest that AFU exerted a
sufficient inhibitory effect on FOXO1 phosphorylation, regardless
of the effect of PD therapy, in combination with PD. Therefore,
additional AFU treatment would be the preferred strategy to improve
the sensitivity to IMiD activity and overcome resistance to IMiD
treatment in MM cells.
IMiDs can specifically bind to cereblon, an
important modulator of an E3 ubiquitin ligase complex, and alter
the specificity of the substrates to be ubiquitinated (6). The changes in this specificity lead to
the degradation of several transcriptional factors involved in
tumor cell survival, such as IKZF1, IKZF3, and IRF4, in MM. Our
results showed that a reduced dose of PD treatment did not induce
cell death, as shown by the reduced induction of apoptosis and
lower activation of caspase, even though prominent downregulation
of IKZF1 and IKZF3 expression was observed. Hence, the
downregulation of IKZF1 and IKZF3 was considered as an early event
that is easily triggered during the action of IMiDs treatment.
Although this suppression did not occur after treatment with a
suboptimal dose of AFU, when combined with the PD treatment, the
expression of IKZF1 and IKZF3 was predominantly suppressed. The
precise mechanism of this enhanced suppression was not clear.
Recently, Liu et al (28)
proposed that kinase inhibition leads to a novel degradation
process of IKZF1, instead of through the ubiquitin-proteasome
pathway, in MM cells. In the current study, the additional
administration of AFU and the subsequent pro-apoptotic activity,
such as dephosphorylation of FOXO1, may support the PD-induced
downregulation of IKZF1 and IKZF3 expression in a manner
independent of cereblon-induced degradation. The mechanism by which
the dephosphorylation of FOXO1 is correlated with the suppression
of IKZF1 and IKZF3 expression is unclear. Recently, Alkhatib et
al demonstrated the essential role of FOXO1 in appropriate mRNA
splicing of IKZF1, which contributes to the stable expression of
IKZF1, for the somatic rearrangement of immunoglobulin genes during
B cell development (23). In MM
cells, the effect of FOXO1 on the expression of IKZF1 is unclear.
In reference to this previous report, the further study of how
FOXO1 affects the expression of IKZF1 is warranted to explain the
above concerns in MM cells.
In conclusion, the AFU-PD combination therapy with
suboptimal doses of PD and AFU exhibited remarkable anti-tumor
activity in MM cells in comparison with the individual
monotherapies. The mechanism of action of this combination therapy
was dependent on the individual activities of PD and AFU without
any interference with each other. The two subsequent actions, eIF4E
inactivation caused by 4EBP1 dephosphorylation, and the enhanced
suppression of IKZF1 and IKZF3, might induce additional effects
through the AFU-PD combination therapy. The additional treatment of
AFU with IMiD-based therapy should enhance the anti-tumor activity
of IMiDs and overcome the resistance of IMiDs treatment. This
combination therapy will exhibit more potent anti-tumor activity
and fewer side effects when used clinically. Our study has provided
the foundation for a new treatment strategy against RRMM with IMiD
insensitivity or resistance, and improved IMiD-based therapy for
patients with an intolerance to IMiD toxicity.
Acknowledgements
The authors would like to thank Ms. Chiori Fukuyama
for her technical assistance. The study received financial support
from Celgene Co., Ltd. This study was partly supported by a
Grant-in-Aids for Scientific Research from the Ministry of
Education, Culture, Sports, Science and Technology (nos. 16K07179
and 16K09855), National Cancer Center Research and Development Fund
(no. 26-A-4), and the Practical Research for Innovative Cancer
Control from Japan Agency for Medical Research and development,
AMED (no. 15ck0106077h0002).
Glossary
Abbreviations
Abbreviations:
|
AFU
|
Afuresertib
|
|
MM
|
multiple myeloma
|
|
IMiDs
|
immunomodulatory drugs
|
|
PD
|
pomalidomide plus dexamethasone
|
|
PIs
|
proteasome inhibitors
|
|
LEN
|
lenalidomide
|
|
POM
|
pomalidomide
|
|
BOR
|
bortezomib
|
|
DEX
|
dexamethasone
|
References
|
1
|
Quach H, Ritchie D, Stewart AK, Neeson P,
Harrison S, Smyth MJ and Prince HM: Mechanism of action of
immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia.
24:22–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lonial S, Dimopoulos M, Palumbo A, White
D, Grosicki S, Spicka I, Walter-Croneck A, Moreau P, Mateos MV,
Magen H, et al: Elotuzumab therapy for relapsed or refractory
multiple myeloma. N Eng J Med. 373:621–631. 2015. View Article : Google Scholar
|
|
3
|
Dimopoulos MA, Oriol A, Nahi H, San-Miguel
J, Bahlis NJ, Usmani SZ, Rabin N, Orlowski RZ, Komarnicki M, Suzuki
K, et al: Daratumumab, lenalidomide and dexamethasone for multiple
myeloma. N Eng J Med. 375:1319–1331. 2016. View Article : Google Scholar
|
|
4
|
Lonial S, Durie B, Palumbo A and
San-Miguel J: Monoclonal antibodies in the treatment of multiple
myeloma: Current status and future perspectives. Leukemia.
30:526–535. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ito T, Ando H, Suzuki T, Ogura T, Hotta K,
Imamura Y, Yamaguchi Y and Handa H: Identification of a primary
target of thalidomide teratogenicity. Science. 327:1345–1350. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lopez-Girona A, Mendy D, Ito T, Miller K,
Gandhi AK, Kang J, Karasawa S, Carmel G, Jackson P, Abbasian M, et
al: Cereblon is a direct protein target for immunomodulatory and
antiproliferative activities of lenalidomide and pomalidomide.
Leukemia. 26:2326–2335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fischer ES, Bohm K, Lydeard JR, Yang H,
Stadler MB, Cavadini S, Nagel J, Serluca F, Acker V, Lingaraju GM,
et al: Structure of the DDB1-CRBN E3 ubiquitin ligase in complex
with thalidomide. Nature. 512:49–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kronke J, Udeshi ND, Narla A, Grauman P,
Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, et al:
Lenalidomide causes selective degradation of IKZF1 and IKZF3 in
multiple myeloma cells. Science. 343:301–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chakraborty R, Muchtar E, Kumar S, Buadi
FK, Dingli D, Dispenzieri A, Hayman SR, Hogan WJ, Kapoor P, Lacy
MQ, et al: The impact of induction regimen on transplant outcome in
newly diagnosed multiple myeloma in the era of novel agents. Bone
Marrow Transplant. 52:34–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Durie BG, Hoering A, Abidi MH, Rajkumar
SV, Epstein J, Kahanic SP, Thakuri M, Reu F, Reynolds CM, Sexton R,
et al: Bortezomib with lenalidomide and dexamethasone versus
lenalidomide and dexamethasone alone in patients with newly
diagnosed myeloma without intent for immediate autologous stem-cell
transplant (SWOG S0777): A randomised, open-label, phase 3 trial.
Lancet. 389:519–527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi CX, Kortum KM, Zhu YX, Jedlowski P,
Bruins L, Braggio E and Stewart AK: Proteasome inhibitors block
Ikaros degradation by lenalidomide in multiple myeloma.
Haematologica. 100:e315–e317. 2015.PubMed/NCBI
|
|
12
|
Chapman MA, Lawrence MS, Keats JJ,
Cibulskis K, Sougnez C, Schinzel AC, Harview CL, Brunet JP, Ahmann
GJ, Adli M, et al: Initial genome sequencing and analysis of
multiple myeloma. Nature. 471:467–472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spencer A, Yoon SS, Harrison SJ, Morris
SR, Smith DA, Brigandi RA, Gauvin J, Kumar R, Opalinska JB and Chen
C: The novel AKT inhibitor afuresertib shows favorable safety,
pharmacokinetics and clinical activity in multiple myeloma. Blood.
124:2190–2195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Narita T, Ri M, Masaki A, Mori F, Ito A,
Kusumoto S, Ishida T, Komatsu H and Iida S: Lower expression of
activating transcription factors 3 and 4 correlates with shorter
progression-free survival in multiple myeloma patients receiving
bortezomib plus dexamethasone therapy. Blood Cancer J. 5:e3732015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ri M, Tashiro E, Oikawa D, Shinjo S,
Tokuda M, Yokouchi Y, Narita T, Masaki A, Ito A, Ding J, et al:
Identification of Toyocamycin, an agent cytotoxic for multiple
myeloma cells, as a potent inhibitor of ER stress-induced XBP1 mRNA
splicing. Blood Cancer J. 2:e792012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dumble M, Crouthamel MC, Zhang SY, Schaber
M, Levy D, Robell K, Liu Q, Figueroa DJ, Minthorn EA, Seefeld MA,
et al: Discovery of novel AKT inhibitors with enhanced anti-tumor
effects in combination with the MEK inhibitor. PLoS One.
9:e1008802014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Munugalavadla V, Mariathasan S, Slaga D,
Du C, Berry L, Del Rosario G, Yan Y, Boe M, Sun L, Friedman LS, et
al: The PI3K inhibitor GDC-0941 combines with existing clinical
regimens for superior activity in multiple myeloma. Oncogene.
33:316–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li S, Pal R, Monaghan SA, Schafer P,
Ouyang H, Mapara M, Galson DL and Lentzsch S: IMiD immunomodulatory
compounds block C/EBP{beta} translation through eIF4E
down-regulation resulting in inhibition of MM. Blood.
117:5157–5165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han EK, Leverson JD, McGonigal T, Shah OJ,
Woods KW, Hunter T, Giranda VL and Luo Y: Akt inhibitor A-443654
induces rapid Akt Ser-473 phosphorylation independent of mTORC1
inhibition. Oncogene. 26:5655–5661. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rhodes N, Heerding DA, Duckett DR,
Eberwein DJ, Knick VB, Lansing TJ, McConnell RT, Gilmer TM, Zhang
SY, Robell K, et al: Characterization of an Akt kinase inhibitor
with potent pharmacodynamic and antitumor activity. Cancer Res.
68:2366–2374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamaji M, Ota A, Wahiduzzaman M, Karnan S,
Hyodo T, Konishi H, Tsuzuki S, Hosokawa Y and Haniuda M: Novel
ATP-competitive Akt inhibitor afuresertib suppresses the
proliferation of malignant pleural mesothelioma cells. Cancer Med.
6:2646–2659. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Richter JD and Sonenberg N: Regulation of
cap-dependent translation by eIF4E inhibitory proteins. Nature.
433:477–480. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alkhatib A, Werner M, Hug E, Herzog S,
Eschbach C, Faraidun H, Köhler F, Wossning T and Jumaa H: FoxO1
induces Ikaros splicing to promote immunoglobulin gene
recombination. J Exp Med. 209:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Tang N, Hadden TJ and Rishi AK:
Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta.
1813:1978–1986. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu Z and Tindall DJ: FOXOs, cancer and
regulation of apoptosis. Oncogene. 27:2312–2319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Greer EL and Brunet A: FOXO transcription
factors at the interface between longevity and tumor suppression.
Oncogene. 24:7410–7425. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Raje N, Kumar S, Hideshima T, Ishitsuka K,
Chauhan D, Mitsiades C, Podar K, Le Gouill S, Richardson P, Munshi
NC, et al: Combination of the mTOR inhibitor rapamycin and CC-5013
has synergistic activity in multiple myeloma. Blood. 104:4188–4193.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Y, He X, Sui Y, Yu R and Xu G:
Transcription factor IKZF1 is degraded during the apoptosis of
multiple myeloma cells induced by kinase inhibition. FEBS Lett.
589:2233–2240. 2015. View Article : Google Scholar : PubMed/NCBI
|