Introduction
Gastric cancer (GC) is the fourth most common type
of cancer worldwide, and is particularly prevalent in developing
countries (1–3). GC is associated with a high risk of
peritoneal carcinomatosis, which occurs in 5–20% of patients with
gastric cancer, and ~50% of patients with potentially curable
advanced gastric cancer die from cancer recurrence in the
peritoneum (4–6). Furthermore, peritoneal carcinomatosis is
associated with rapid progression, and has been demonstrated to
significantly decrease overall survival (7). In previous years, multimodal treatments
have emerged for patients with peritoneal carcinomatosis.
Cytoreductive surgery (CRS) combined with hyperthermic
intraperitoneal chemoperfusion (HIPEC) and systemic chemotherapy
have been proposed as beneficial treatment methods (8). This treatment has significantly improved
the loco regional control of GC and increased patient survival
rates (9–11). Generally, hyperthermia is used to
induce temperature-dependent necrosis and protein inactivation
(e.g., repair enzymes) as opposed to DNA damage. Furthermore,
thermal treatment enhances the cytotoxicity of chemotherapeutic
drugs (12).
Transient hyperthermia treatment is able to induce
the activation of cellular stress responses, specifically the
upregulation of the expression of heat shock proteins (HSPs)
(13). HSPs constitute a group of
proteins induced by heat shock or cellular stress, which are able
to inhibit the misfolding and aggregation of proteins in the cell.
HSPs are expressed in multiple types of tumor and promote the
survival of cancer cells. They have been reported to be involved in
the inhibition of apoptosis in human pancreatic, prostate and
gastric cancer cells (14).
Furthermore, the synthesis and accumulation of HSPs in tumor cells
exposed to hyperthermia are able to protect the cells from further
heat-associated cytotoxic events (15,16). HSPs
are further responsible for the resistance of cancer cells to
radiotherapy and chemotherapy, which makes them a novel target for
cancer therapy (17,18).
The upregulation of HSPs is closely associated with
a transient resistance of cells towards a subsequent second heat
shock, which may protect cells against damage induced by a second
round of thermotherapy and chemotherapy. Therefore, elucidating the
involvement of HSPs in tumor hyperthermia may provide evidence to
improve the performance of HIPEC-based treatments. In the present
study, the expression patterns of the two most well-studied,
stress-inducible members of the HSP family, HSP70 and HSP90, were
investigated in gastric cancer cells treated with HIPEC to mimic
heating. Furthermore, serum levels of these proteins were analyzed
in patients with gastric cancer prior to and following CRS plus
HIPEC treatment. The results from the in vitro experiments
indicated that the expression of HSP90 was elevated significantly
in gastric cancer cells following hyperthermic treatment. However,
the expression of HSP70 was elevated from 4 h up to 20 h
post-exposure and decreased to normal levels at 36 h post-exposure.
Furthermore, analysis of serum samples collected from 22 patients
with gastric cancer who received CRS plus HIPEC demonstrated that
serum HSP90 and HSP70 levels increased following HIPEC therapy,
peaking at 18 h post-treatment, yet returned to normal levels
following 24 h. The present study, which investigated HSP kinetics,
aimed to provide evidence to improve the efficacy of therapies that
combine the use of hyperthermia and proteasome inhibition, and
hence improve the patient outcomes. The results of the present
study specifically suggested that conducting a second round of
HIPEC or chemotherapy at least 24 h after the first treatment is
optimal to minimize any potential resistance of the tumor cells to
the thermal or chemical treatments.
Materials and methods
Cell culture and hyperthermic
treatment
Two strains of human gastric adenocarcinoma cell
lines, SGC7901 and AGS cells, were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA), for use in the
present study. Cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; GE Healthcare,
Chicago, IL, USA) and incubated at 37°C in a humidified atmosphere
of 5% CO2 in air. At 80–90% confluency, cells were
digested with a 0.25% trypsin solution (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), and collected by centrifugation at 1,000 × g
for 3 min at 37°C. Cells were seeded at a density of
5×104 cells/well in 6-well plates in 500 µl DMEM with
10% FBS. Cells were cultured at 37°C for 4 h to allow cells to
adhere, followed by cisplatin (3.5 µg/ml; Selleck Chemicals,
Houston, TX, USA) treatments for a further 1 h at 41°C. The
cisplatin-supplemented medium was replaced with DMEM with 10% FBS
and the culture was maintained at 37°C. Cells were collected prior
to and following treatment at multiple specific time points (0, 4,
8, 12, 16, 20, 24, 28, 32, 36, 40, 44 and 48 h). For
immunocytochemical (ICC) analysis of HSPs, coverslips were placed
in 6-well plates, and cells at a density of 5×104
cells/well were seeded into each well. Following 4 h incubation,
the cells were subjected to HIPEC-mimicking hyperthermic treatment
as aforementioned. The coverslips were collected and fixed for ICC
staining as described below.
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology, Nanjing, China).
Protein concentration was determined using the bicinchoninic acid
kit (Beyotime Institute of Biotechnology). Proteins were mixed with
loading buffer and heated at 70°C for 10 min and 30 µg/lane was
separated by using 7.5% SDS-PAGE gels. Electrophoresed proteins
were transferred onto polyvinylidene fluoride (PVDF) membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked for 2 h
in 5% bovine serum albumin (BSA; Beyotime Institute of
Biotechnology) at 4°C and then incubated overnight at 4°C with the
mouse monoclonal antibodies against HSP70 (cat. no. sc-2217), HSP90
(cat. no. sc-33755) and GAPDH (cat. no. sc-69778, 1:1,000; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA). The blots were then
incubated with horseradish peroxidase-conjugated goat anti-mouse
secondary antibody (cat. no. sc-2005, 1:1,000; Santa Cruz
Biotechnology Inc.). Finally, bands were visualized by enhanced
chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.) and
LabWorks Image Acquisition and Analysis Software 2 (UVP LLC,
Upland, CA, USA).
ICC staining
Coverslips were fixed with 4% paraformaldehyde for 5
min at room temperature. Following fixation, coverslips were washed
with 0.025 mol/l PBS containing 0.3% Triton X-100 (PBST) for 10
min. Endogenous peroxidase activity was inactivated by incubating
coverslips in 3% H2O2 in methanol for 30 min
at room temperature. Following three washes in PBS, antigen
retrieval was performed by heating the coverslips in a microwave
oven at 121°C for 2 min. The coverslips were cooled at room
temperature and washed in PBS, and then incubated with 10% BSA
(cat. no. P007, Beyotime Institute of Biotechnology)/PBS Tween-20
for 1 h at room temperature. Following this, coverslips were
incubated with mouse monoclonal primary antibodies against HSP70
(cat. no. sc-2217) and HSP90 (cat. no. sc-33755, 1:100; Santa Cruz
Biotechnology, Inc.) for 30 min at room temperature. Subsequent to
washing, the slides were treated with horseradish
peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG; cat.
no. sc-12358, 1:1,000; Santa Cruz Biotechnology) for 30 min at room
temperature. Following 10 min washing with PBS, sections were
incubated with Dako Detection Reagent Envision kit (Agilent
Technologies, Inc., Santa Clara, CA, USA) for 5–15 sec at room
temperature and were counterstained with hematoxylin for 1 min,
followed by dehydration with sequential ethanol washes (75, 80 and
100%) of 1 min each at room temperature. Next, the samples were
resin-sealed. Finally, the cells were observed under a Nikon
Eclipse 50i light microscope (Nikon, Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using
TRIzol™ reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), following the manufacturer's protocol. A total of 2 µg RNA
per sample was reverse transcribed into cDNA using an
iScript™ cDNA synthesis kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) according to the manufacturer's protocol. cDNA
was used for PCR amplification of HSP70 and HSP90
using an iQ™ SYBR®-Green Supermix kit
(Bio-Rad Laboratories, Inc.). The qPCRs were repeated a minimum of
three times to ensure statistical rigor. Relative quantification of
gene expression was performed according to the 2−ΔΔCt
method and normalized to the reference gene GAPDH (19). Primers are listed in Table I. The cycling conditions for qPCR were
as follows: Initial denaturation at 94°C for 2 min, followed by 40
cycles of 30 sec each at 94°C, 30 sec at 58°C and 30 sec at 72°C,
with a final extension phase of 72°C for 7 min.
 | Table I.Primers used in the present study. |
Table I.
Primers used in the present study.
| Primer name | Sequence (5′-3′) |
|---|
| GAPDH
forward |
TGTTCGTCATGGGTGTGAAC |
| GAPDH
reverse |
ATGGCATGGACTGTGGTCAT |
| HSP90
forward |
ATGGCATGGACTGTGGTCAT |
| HSP90
reverse |
GACCCATAGGTTCACCTGTGT |
| HSP70
forward |
AGTGATGGATGCAACACAGATT |
| HSP70
reverse |
CCAATGTCGTGTCAAATGCAG |
Serum collection and analysis
Serum samples were collected from 22 patients with
gastric cancer receiving CRS plus HIPEC at the Cancer Center of
Guangzhou Medical University (Guangzhou, China) between April 2009
and December 2014. Patients recruited into the study included 12
men and 10 women aged between 22–65 years, with a median age of 49
years. All patients had been diagnosed with gastric adenocarcinoma.
A total of 13 patients were diagnosed with poorly or
undifferentiated adenocarcinoma, 7 were diagnosed with highly or
moderately differentiated adenocarcinoma, and 2 patients with both
types of cancer. All of these patients were diagnosed with stage IV
gastric cancer. The present study was approved by the Ethics
Committee of the Cancer Center of Guangzhou Medical University, and
all patients provided written informed consent prior to receiving
the treatment.
Surgical procedures
All CRS and HIPEC procedures were performed by a
designated team of surgical oncologists, an anesthesiologist and
operating room staff, led by chief surgeon Dr Shuzhong Cui at the
Cancer Center of Guangzhou Medical University (Guangzhou, China).
CRS included several visceral resections of the stomach and small
intestine. A parietal peritonectomy was also performed. The
abdominal exploration was performed under general anesthesia and
hemodynamic monitoring, through a midline xiphoid-pubic incision.
Once the abdominal wall was open, detailed evaluation of peritoneal
carcinomatosis index was conducted, taking into consideration the
size and distribution, according to Sugarbaker (20). The characteristics of ascites were
also recorded. Following evaluation, maximal CRS was performed,
including the resection of the primary tumor with acceptable
margins, any involved adjacent structures, lymphadenectomy,
peritoneotomies where peritoneal surfaces were associated with the
tumor, according to the peritonectomy procedure developed by
Sugarbaker (20). A HIPEC was
performed immediately following the CRS procedure. Two inflow
drainage tubes were placed in the upper abdomen and two out flow
tubes for perfusion were placed in the lower abdomen. A 1–3 liter
volume of the heated normal saline was circulated at a rate of 600
ml/min for 60 min using the BR-TRG-I Hyperthermic Perfusion
Intraperitoneal Treatment system (Baorui Medical Technology, Co.,
Ltd., Guangzhou, China) at 43°C with 20 mg fluorouracil (Selleck
Chemicals) and 100 mg cisplatin as the chemotherapeutic agent.
Enzyme-linked immunosorbent assay
(ELISA)
The serum samples were collected prior to and
following HIPEC. HSP70 and HSP90 levels were determined in serum
samples using human HSP70 (cat. no. Eh0364) and HSP90 ELISA kits
(cat. no. Eh0366) (both from Vipotion Biotechnology Co., Ltd.,
Guangzhou, China), according to the manufacturer's protocol.
Briefly, serum samples were diluted to 1:100 in assay diluent.
Diluted samples were added to ELISA assay wells and incubated at
37°C for 40 min. Following rinsing with PBST, anti-HSP70 or
anti-HSP90 antibodies were added to each well. The plates were
incubated for 40 min at 37°C and rinsed with PBST. Plates were
incubated with a polyclonal peroxidase-conjugated goat anti-mouse
IgG secondary antibody for 15 min at 37°C. A volume of 100 enzyme
substrate was added to each well, and the assay was incubated for
15 min at 37°C. Finally, the plates were measured with a microplate
spectrophotometer (VersaMax; Molecular Devices, LLC, Sunnyvale, CA,
USA) at an absorbance of 490 nm.
Statistical analysis
Statistical analysis was performed using SPSS
statistical software (version 15.0; SPSS, Inc., Chicago, IL, USA).
In vitro experiments were representative of three repeats
and data are presented as the mean ± standard deviation (SD).
Differences between groups were analyzed using the paired Student's
t-test. Statistical differences between groups were assessed using
a one-way analysis of variance. Multiple comparisons of the means
were performed using the least significance difference test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of HSP70 and HSP90 in tumor
cells following hyperthermic treatment
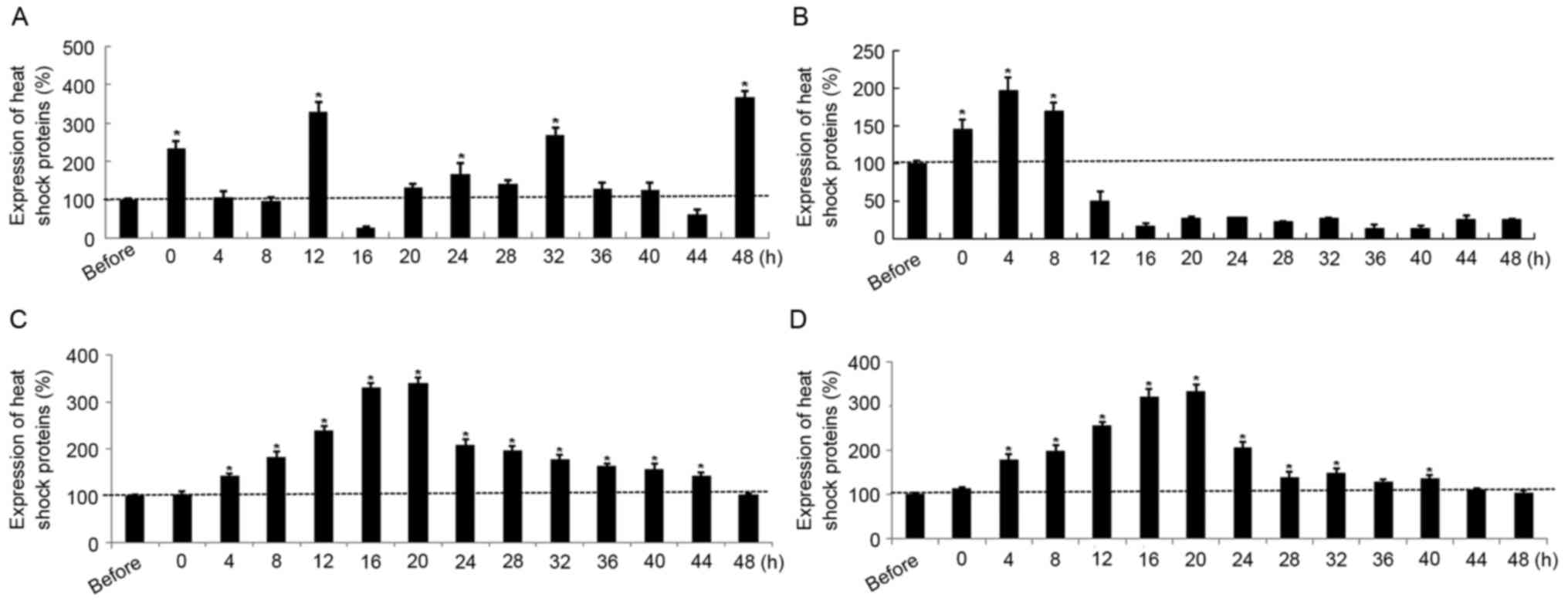
HSP90 expression was significantly elevated
in SGC7901 cells at 0, 12, 32 and 48 h post-hyperthermic treatment
compared with pre-treatment levels. However, HSP90
expression was significantly reduced at 16 h post-treatment
compared with that prior to treatment (Fig. 1A). HSP70 expression was
significantly upregulated in SGC7901 cells compared with
pre-treatment levels, up to 8 h following treatment, but
significantly decreased thereafter at 12 h post exposure until 48 h
(Fig. 1B). In contrast with the
results for SGC7901 cells, the transcription levels as a function
of time post-treatment were similar for HSP90 and
HSP70 in AGS cells; mRNA expression in each case increased
following treatment, peaked at 16–20 h (P<0.05), and decreased
gradually to pre-treatment levels during the following 24 h
(Fig. 1C and D). Furthermore, the
maximal HSP70 and HSP90 expression levels in gastric cancer cells
subjected to hyperthermic treatment were increased 2- to 4-fold
compared with the sham group. In addition, the expression of HSP70
increased at the early time points post-exposure, from 4 to 24
h.
Analysis of HSP70 and HSP90 protein
levels in tumor cells following hyperthermic treatment
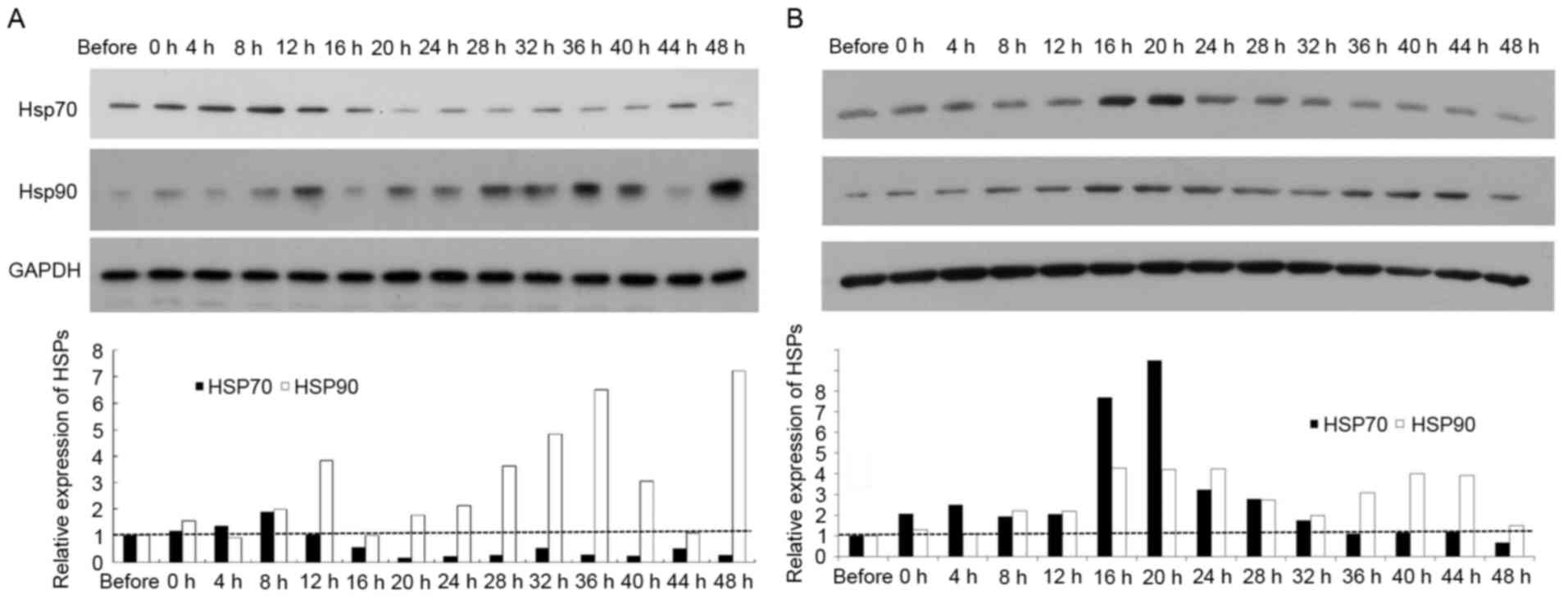
Western blotting and ICC staining were conducted to
analyze HSP70 and HSP90 protein levels in SGC7901 and AGS cells
following hyperthermic treatment. As presented in Fig. 2A, HSP90 protein levels were
significantly increased in SGC7901 cells at 12, 28, 32 and 36 h
following hyperthermic treatment, peaking at 48 h. This profile was
comparable to that for HSP90 mRNA levels in SGC7901 cells.
HSP70 protein levels in SGC7901 cells were similarly comparable to
that for HSP70 mRNA levels in SGC7901 cells. In AGS cells,
HSP70 protein levels were significantly elevated at 4 up to 20 h
post exposure and decreased to pre-treatment levels 36 h following
treatment (Fig. 2B). HSP90 protein
levels significantly increased in AGS cells following hyperthermic
treatment, with two high-expression peaks post-treatment, at 16–28
and 36–44 h. The protein levels of HSP70 and HSP90 in gastric
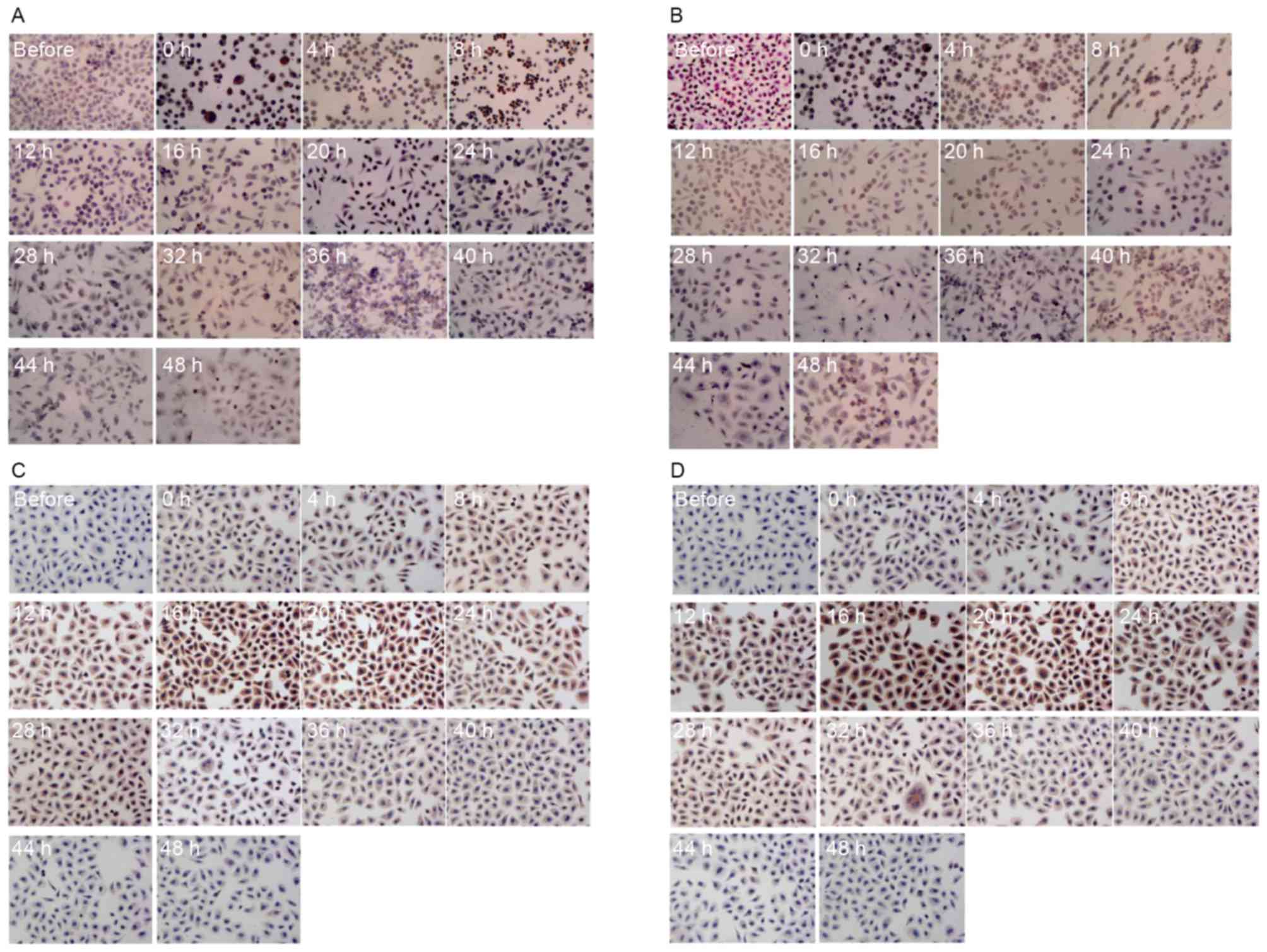
cancer cells were determined for hyperthermic stress by ICC
staining. The protein expression patterns determined using ICC
staining were revealed to be comparable to those described above
determined using qPCR and ELISA. A positive HSP70 and HSP90 signal
was indicated by a brown stain in fixed cells (Fig. 3). Consistent with the results from
western blotting and qPCR, each type of cancer cell demonstrated no
detectable staining corresponding to HSPs prior to treatment.
However, cells stained positive for HSPs following hyperthermic
treatment. Positive staining was mainly observed in the tumor cell
nucleus. The expression of HSP90 and HSP70 increased significantly
in SGC7901 and AGS cells following hyperthermic treatment. A number
of SGC7901 cells were stained positively for HSP70 and HSP90,
immediately following treatment for <8 h. Limited positive
staining was observed after 12 h (Fig. 3A
and B). Conversely, HSP70 and HSP90 levels in AGS cells were
more apparent between 12 and 24 h (Fig.
3C and D). Expression of HSP90 and HSP70 increased
significantly following hyperthermic treatment and decreased to an
almost normal level 36 h after treatment, although there were
differences between SGC7901 and AGS cells.
Serum levels of HSP70 and HSP90 in
patients with GC
To dissect the function of HSPs during HIPEC
therapy, the expression profile of HSP70 and HSP90 in patients with
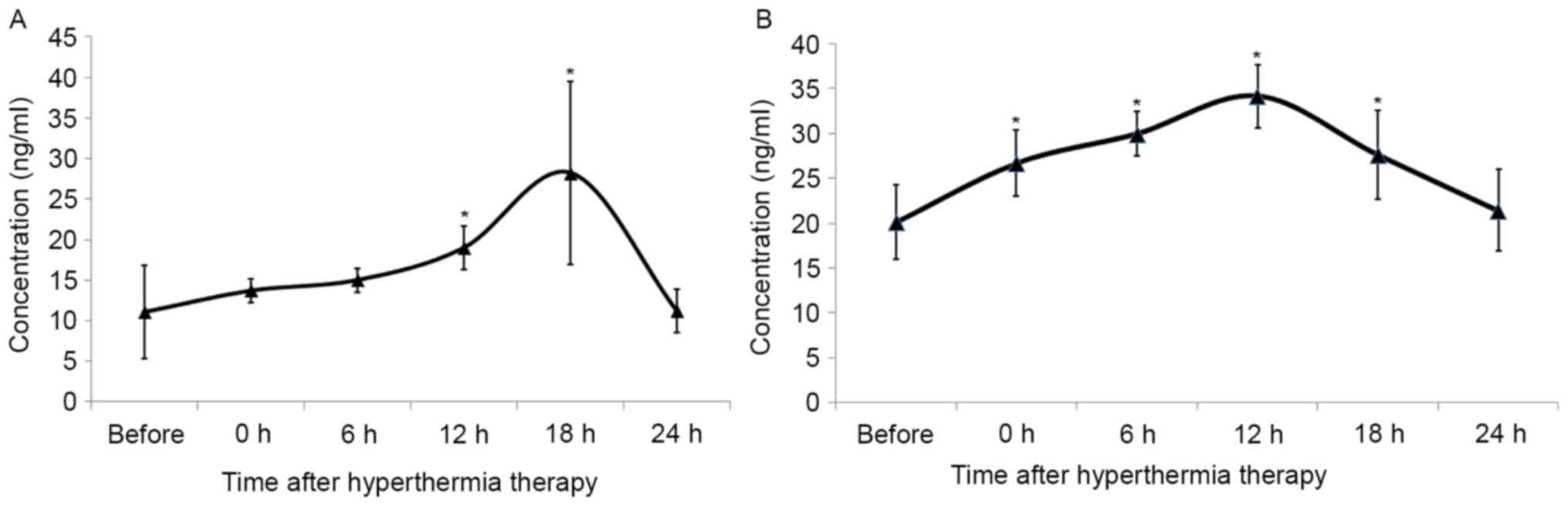
GC exposed to hyperthermia was examined. The serum levels of HSP70
and HSP90 prior to and following HIPEC therapy were analyzed. The
concentration of serum HSP90 increased following HIPEC therapy,
peaking at 18 h post-HIPEC therapy, and returned to normal levels
by 24 h post exposure (Fig. 4A).
There were no statistical differences observed between
concentrations prior to treatment and those at 0, 6 and 24 h. The
serum concentration of HSP70 increased immediately following
treatment, peaking at 12 h, and decreasing to normal levels by 24 h
post-HIPEC therapy (Fig. 4B).
Discussion
Gastric cancer is one of the most prevalent types of
cancer globally, and is particularly common in developing countries
(1–3).
Despite outstanding advances in medical technology and anticancer
therapies, the overall 5-year survival rate of patients with
resectable gastric cancer remains poor, due to a high risk of
lymphatic spread, hematogenous metastasis and peritoneal metastasis
(21). It is widely accepted that
direct mechanical contamination, spontaneous tumor rupture, local
peritoneal trauma and laparoscopic surgery are the major
predisposing factors for peritoneal metastasis (22,23). Thus,
depletion of peritoneal disseminated cancer cells may improve the
outcome for patients with gastric cancer. HIPEC is an effective
method for killing disseminated cancer cells in the peritoneal
cavity, since hyperthermia is able to enhance the efficacy and
penetration of multiple anticancer drugs. Furthermore, CRS plus
HIPEC is now considered a standard treatment for several peritoneal
carcinomas including colorectal and ovarian cancers (24,25).
Although this multimodal approach improves the
locoregional control of gastric cancer and ultimately increases the
survival of patients with this disease, HIPEC treatment results in
the activation of cellular stress responses; specifically, the
expression of HSPs, rendering tumor cells partially thermotolerant
and chemotolerant (13). Thus,
elucidating the function of HSPs in tumor hyperthermia may further
improve the performance of HIPEC-based treatments. In the present
study, HSP70 and HSP90 expression patterns were investigated in
gastric cancer cells that were subjected to hyperthermic
treatments. Furthermore, serum concentrations of HSPs were also
analyzed in patients with gastric cancer who had received
cytoreductive surgery plus HIPEC treatment. The results from the
in vitro experiments indicated that HSP90 was significantly
elevated in gastric cancer cells following hyperthermic treatment.
However, HSP70 expression increased at 4 up to 20 h post exposure
and decreased to a normal pre-treatment level by 36 h post
exposure. In addition, serum samples collected from 22 patients
with gastric cancer who had received CRS plus HIPEC, confirmed that
serum HSP70 and HSP90 levels increased and peaked at 18 h, yet
returned to normal levels 24 h post exposure. The present study
that investigated HSP kinetics may provide evidence to improve the
efficacy of therapies that combine hyperthermic treatments, and
hence improve the outcome of patients. Furthermore, these results
indicated that decreased HSP70 or HSP90 protein levels may enhance
the sensitivity of these cancer cells to CRS plus HIPEC.
HSP90 has been demonstrated to bind and stabilize
immature client proteins, a number of which are conformationally
unstable proteins involved in signal transduction pathways,
important in cell development, growth, and survival. Such proteins
include trans-membrane tyrosine kinases, signaling proteins, tumor
suppressors and cell-cycle regulators (14). Therefore, HSP90 alters protein
activity and participates in cell cycle regulation, thus altering
cellular behavior to enhance proliferation (26). HSP90 was expressed in all in
vitro experiments in the present study, which is in accordance
with its chaperone function. High levels of HSP90 protein are able
to contribute to the stabilization and refolding of proteins,
impaired by hyperthermic treatment. However, variable levels of
HSP90 expression may have a feedback effect on regulating its own
expression, in order to inhibit its further accumulation.
In contrast with HSP90, HSP70 has been revealed to
promote cell survival by interfering with apoptosis. HSP70 is
considered to be a classic apoptotic inhibitor, blocking the
intrinsic and extrinsic pathways induced by oxidative damage,
chemotherapeutics, radiation and heat-induced stress. Furthermore,
HSP70 is able to inhibit p21- and p53-dependent senescence
pathways, thereby rescuing cancer cells from apoptosis (17,27).
However, HSP70 expression is relatively low in normal cells
compared with cancer cells, which suggests that HSP70 expression is
crucial for cancer cell survival, but may not be required for
non-neoplastic cell survival under normal conditions (28). The in vitro experiments in the
present study demonstrated that HSP70 was transiently expressed in
cancer cells following hyperthermic treatment. The expression of
HSP70 prior to and 24 h following hyperthermic treatment was
relatively low. Similar results were also observed in patients
receiving HIPEC treatment, whose serum HSP70 concentration levels
peaked 12 h following treatment and decreased 24 h post-exposure.
The results from the present study suggested an anti-apoptotic
function for HSP70 in response to heat-induced stress.
Considering the multiple ways that HSPs aid cell
survival, it was anticipated that the HSPs induced by the first
round of HIPEC treatment compromised the efficacy of the following
HIPEC treatment. HSPs therefore represent promising targets for
drugs that aim to increase the effectiveness of cancer thermo- and
chemotherapy. Based on the results from the present study, delaying
chemotherapy or a second round of a HIPEC treatment for ~24 h
post-round one HIPEC treatment is highly recommended. HSPs
inhibitors, which have already received considerable attention, are
also potential targets with benefits for use in the clinic as
adjuncts to HIPEC therapy. Due to the transient high expression of
HSPs in cancer cells, HSP vaccines may also be promising adjuncts
to HIPEC therapy.
Previous studies have revealed that HSPs are induced
by hyperthermia (27). For example,
in investigations by Cui et al (29). the expression of HSP70 and HSP90 was
significantly upregulated in nasopharyngeal carcinoma cells, which
peaked at 4 h post-heat treatment, followed by a decrease to normal
levels at 24 h post-exposure. Miyagawa et al (30). further demonstrated that the
expression of HSP70 and HSP90 were upregulated in
melanoma cells, reaching a peak within 4–8 h following
hyperthermia. In addition, it was further demonstrated that
inhibition of HSP70 and HSP90 sensitizes melanoma cells to
hyperthermia. Furthermore, in patients with peritoneal
carcinomatosis from various primary tumors, HSP90 gene
expression was upregulated immediately following HIPEC therapy
(31). The aforementioned results are
consistent with the results of the present study, which
demonstrated substantial increases in the serum concentrations of
HSP70 and HSP90 from patients immediately following HIPEC
treatment. However, HSP70 and HSP90 expression levels in these
patients peaked at 18 h, and returned to initial levels 24 h
post-exposure. These inconsistencies with results from the present
study may be due to a number of factors. In the present study,
cisplatin was added as a chemotherapeutic agent to the perfusate,
whilst other previous reports investigated the effects of
hyperthermia alone. In addition, in the former two studies, HSP70
and HSP90 expression in cancer cells were measured, whilst serum
concentrations were measured in the present study. Expression
levels from in vitro experiments may therefore differ from
those in patients. Furthermore, the expression of HSP70 and HSP90
increased following HIPEC in a time-dependent manner, which was
investigated for the first time in the present study.
In conclusion, the present study is the first to
demonstrate that HSP70 and HSP90 are upregulated, then decrease to
normal, pre-treatment levels within 24 h of applying the HIPEC
procedure, in tumor cells. It is therefore advisable to apply the
second round of HIPEC or chemotherapy at least 24 h following the
first treatment to minimize any potential thermoresistance and
chemoresistance of tumor cells. Furthermore, the use of
co-inhibitors for HSP70 and HSP90 as adjuncts to HIPEC therapy
should be considered for future clinical studies. For future
studies, having analyzed the effects of the HIPEC procedure on HSPs
expression, the further aim is to elucidate the functions of HSPs
in HPIEC therapy.
Acknowledgements
The present study was supported by grants from the
PhD Start-up Funds of Guangzhou Key Medical Discipline Construction
Project, Guangzhou Medical College (grant nos. 2015A23, 2014C45 and
2012C69), Guangdong Natural Science Fund (grant no. 2013010016662)
and the National Natural Science Foundation of China (grant nos.
81201932, 81502342 and 81372493).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bieri U, Moch H, Dehler S, Korol D and
Rohrmann S: Changes in autopsy rates among cancer patients and
their impact on cancer statistics from a public health point of
view: A longitudinal study from 1980 to 2010 with data from Cancer
Registry Zurich. Virchows Arch. 466:637–643. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the Global
Burden of disease study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yonemura Y, Endou Y, Sasaki T, Hirano M,
Mizumoto A, Matsuda T, Takao N, Ichinose M, Miura M and Li Y:
Surgical treatment for peritoneal carcinomatosis from gastric
cancer. Eur J Surg Oncol. 36:1131–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coccolini F, Gheza F, Lotti M, Virzì S,
Iusco D, Ghermandi C, Melotti R, Baiocchi G, Giulini SM, Ansaloni L
and Catena F: Peritoneal carcinomatosis. World J Gastroenterol.
19:6979–6994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yonemura Y, Kawamura T, Bandou E,
Tsukiyama G, Endou Y and Miura M: The natural history of free
cancer cells in the peritoneal cavity. Recent Results Cancer Res.
169:11–23. 2007.PubMed/NCBI
|
|
7
|
Klaver YL, Lemmens VE, Creemers GJ, Rutten
HJ, Nienhuijs SW and de Hingh IH: Population-based survival of
patients with peritoneal carcinomatosis from colorectal origin in
the era of increasing use of palliative chemotherapy. Ann Oncol.
22:2250–2256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sugarbaker PH and Ryan DP: Cytoreductive
surgery plus hyperthermic perioperative chemotherapy to treat
peritoneal metastases from colorectal cancer: Standard of care or
an experimental approach? Lancet Oncol. 13:e362–e369. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blackham AU, Swett K, Eng C, Sirintrapun
J, Bergman S, Geisinger KR, Votanopoulos K, Stewart JH, Shen P and
Levine EA: Perioperative systemic chemotherapy for appendiceal
mucinous carcinoma peritonei treated with cytoreductive surgery and
hyperthermic intraperitoneal chemotherapy. J Surg Oncol.
109:740–745. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McConnell YJ, Mack LA, Gui X, Carr NJ,
Sideris L, Temple WJ, Dubé P, Chandrakumaran K, Moran BJ and Cecil
TD: Cytoreductive surgery with hyperthermic intraperitoneal
chemotherapy: An emerging treatment option for advanced goblet cell
tumors of the appendix. Ann Surg Oncol. 21:1975–1982. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Graziosi L, Cantarella F, Mingrone E,
Gunnellini M, Cavazzoni E, Liberati M and Donini A: Preliminary
results of prophylactic HIPEC in patients with locally advanced
gastric cancer. Ann Ital Chir. 84:551–556. 2013.PubMed/NCBI
|
|
12
|
Mallory M, Gogineni E, Jones GC, Greer L
and Simone CB II: Therapeutic hyperthermia: The old, the new, and
the upcoming. Crit Rev Oncol Hematol. 97:56–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kepenekian V, Aloy MT, Magné N, Passot G,
Armandy E, Decullier E, Sayag-Beaujard A, Gilly FN, Glehen O and
Rodriguez-Lafrasse C: Impact of hyperthermic intraperitoneal
chemotherapy on Hsp27 protein expression in serum of patients with
peritoneal carcinomatosis. Cell Stress Chaperones. 18:623–630.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lianos GD, Alexiou GA, Mangano A, Mangano
A, Rausei S, Boni L, Dionigi G and Roukos DH: The role of heat
shock proteins in cancer. Cancer Lett. 360:114–118. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ischia J and So AI: The role of heat shock
proteins in bladder cancer. Nat Rev Urol. 10:386–395. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zorzi E and Bonvini P: Inducible hsp70 in
the regulation of cancer cell survival: Analysis of chaperone
induction, expression and activity. Cancers (Basel). 3:3921–3956.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Chen M, Zhou J and Zhang X: HSP27,
70 and 90, anti-apoptotic proteins, in clinical cancer therapy
(Review). Int J Oncol. 45:18–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schilling D, Bayer C, Li W, Molls M,
Vaupel P and Multhoff G: Radiosensitization of normoxic and hypoxic
h1339 lung tumor cells by heat shock protein 90 inhibition is
independent of hypoxia inducible factor-1α. PLoS One. 7:e311102012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sugarbaker PH: Cytoreductive surgery and
perioperative intraperitoneal chemotherapy as a curative approach
to pseudomyxoma peritonei syndrome. Tumori. 87:S3–S5. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang C, Hua J, Li J, Zhen J, Wang F, Zhao
Q, Shuang J and Du J: Comparison of long-term results between
laparoscopy-assisted gastrectomy and open gastrectomy with D2
lymphadenectomy for advanced gastric cancer. Am J Surg.
208:391–396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kow AW, Kwon CH, Song S, Shin M, Kim JM
and Joh JW: Risk factors of peritoneal recurrence and outcome of
resected peritoneal recurrence after liver resection in
hepatocellular carcinoma: Review of 1222 cases of hepatectomy in a
tertiary institution. Ann Surg Oncol. 19:2246–2255. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rodriguez EF, Monaco SE, Khalbuss W,
Austin RM and Pantanowitz L: Abdominopelvic washings: A
comprehensive review. Cytojournal. 10:72013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Königsrainer I, Horvath P, Struller F,
Forkl V, Königsrainer A and Beckert S: Risk factors for recurrence
following complete cytoreductive surgery and HIPEC in colorectal
cancer-derived peritoneal surface malignancies. Langenbecks Arch
Surg. 398:745–749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cashin PH, Graf W, Nygren P and Mahteme H:
Cytoreductive surgery and intraperitoneal chemotherapy for
colorectal peritoneal carcinomatosis: Prognosis and treatment of
recurrences in a cohort study. Eur J Surg Oncol. 38:509–515. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sevin M, Girodon F, Garrido C and de
Thonel A: HSP90 and HSP70: Implication in inflammation processes
and therapeutic approaches for myeloproliferative neoplasms.
Mediators Inflamm. 2015:9702422015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jego G, Hazoumé A, Seigneuric R and
Garrido C: Targeting heat shock proteins in cancer. Cancer Lett.
332:275–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sherman MY and Gabai VL: Hsp70 in cancer:
Back to the future. Oncogene. 34:4153–4161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cui XB, Yu ZY, Wang W, Zheng YQ, Liu W and
Li LX: Co-inhibition of HSP70/HSP90 synergistically sensitizes
nasopharyngeal carcinoma cells to thermotherapy. Integr Cancer
Ther. 11:61–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyagawa T, Saito H, Minamiya Y, Mitobe K,
Takashima S, Takahashi N, Ito A, Imai K, Motoyama S and Ogawa J:
Inhibition of Hsp90 and 70 sensitizes melanoma cells to
hyperthermia using ferromagnetic particles with a low Curie
temperature. Int J Clin Oncol. 19:722–730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pelz JO, Vetterlein M, Grimmig T, Kerscher
AG, Moll E, Lazariotou M, Matthes N, Faber M, Germer CT,
Waaga-Gasser AM and Gasser M: Hyperthermic intraperitoneal
chemotherapy in patients with peritoneal carcinomatosis: Role of
heat shock proteins and dissecting effects of hyperthermia. Ann
Surg Oncol. 20:1105–1113. 2013. View Article : Google Scholar : PubMed/NCBI
|