Introduction
Breast cancer is the leading cause of
cancer-associated mortality among females, and is responsible for
23% of the total cases of cancer and 14% of cancer-associated
mortalities globally (1–3). Multiple chemokines are secreted by
cancer cells, and the host cannot regulate this process
autonomously (4,5). Chemokines and their receptors make up
the chemokine family, with the ligand or receptor proteins are
indicated by the letter L or R, respectively (6). C-X-C motif chemokine ligand 13 (CXCL13),
also known as B-lymphocyte chemoattractant, was originally
identified in stromal cells in B-cell follicles as regulating
subsets of T cells and the homing of B cells (7). CXCL13 serves a key role in inflammatory
diseases. In previous studies, CXCL13 was observed as being
expressed in patients with breast cancer and was associated with
cancer metastasis (8–10). Furthermore, it has been reported that
CXCL13 was particularly highly expressed in early breast cancer and
was closely associated with prognostic factors such as lymph node
positivity (11). These results
indicate that high CXCL13 expression is an adverse factor for
patients with breast cancer.
The biological effects of chemokine activity are
mediated by interactions with G protein-coupled receptors (12,13). CXCR5
is the primary receptor for CXCL13 and is expressed on a subset of
T cells, and on all B cells in the blood, cerebrospinal fluid and
lymphatic tissue (14). The
interaction between CXCR5 and CXCL13 promotes the expression of
integrin and the entrance of T cells into the lymph nodes.
Therefore, CXCR5-deficient mice have severely impaired immune
systems (15,16). It has been suggested (17) that when anti-CXCR5 or -CXCL13
antibodies are injected into the original site of breast cancer,
they can effectively block the chemotaxis of cancer cells, and that
the expression of CXCR5 is upregulated in this cancerous tissue
compared with normal breast tissue. A similar effect was observed
in the lymph nodes (the most common site of breast cancer
metastasis), where CXCL13 was upregulated (8); however, it was minimally expressed in
unassociated tissues, such as skeletal muscle or brain tissue.
Thus, cellular cluster formation and compartmentalization may be
organized through CXCR5/CXCL13 interactions in breast cancer cells.
CXCL13 and its receptor may contribute to tumor formation, with
therapeutic intervention to interrupt CXCL13/CXCR5 interactions
potentially improving the clinical course of patients with breast
cancer.
The mitogen-activated protein kinase (MAPK)
signaling pathway is downstream of estrogen receptor activation and
has been associated with breast cancer (18,19).
Extracellular-regulated protein kinases (ERK), including ERK1 and
ERK2, are essential to cell proliferation and differentiation, and
promote gene transcription and expression (20,21). It
has also been reported that ERK1/2 are activated by growth factors,
and their continuous activation promotes cellular proliferation and
malignant cell transformation (22).
Downregulation of phosphorylated (p)-ERK1/2 attenuated cell growth
and gene transcription. Recent research has associated CXCL13/CXCR5
expression with orofacial pain via ERK-mediated pro-inflammatory
cytokines (23). The MAPK/ERK1/2
pathways have been previously investigated in the context of breast
cancer (24). However, to the best of
our knowledge, whether ERK signaling can be activated by
CXCL13/CXCR5 expression in breast cancer has not been investigated.
In the present study, the aim was to explore the signal
transduction of the CXCR5/ERK pathway mediated by CXCL13 in breast
cancer mice.
Materials and methods
Cells and animal models
A total of 30 adult BALB/c mice (female; 6 weeks
old, 18–20 g) were purchased from the Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China). The animals were maintained
in a 12–12 h light-dark cycle at a temperature of 22±1°C with free
access to food and water in a specific pathogen-free environment.
All animal procedures performed in the present study were reviewed
and approved by the Animal Care and Use Committee of Yantaishan
Hospital (Yantai, China). Three experimental groups were used to
investigate the effect of CXCL13 in breast cancer: Control, Model
(inoculated with 1×105 4T1 cells) and Inhibitor
(inoculated with 4 mg/kg goat anti-mouse CXCL13 polyclonal antibody
(cat no. SAB1408778; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
prior to being inoculated with 1×105 4T1 cells). A total
of 15 BALB/c mice were randomly divided into these three groups, in
which PBS and 1×105 4T1cells were injected
subcutaneously into the right hind leg of the Control mice and
Model mice at days 6, 12 and 18 respectively (day 0 was defined as
the day they were grouped). The Inhibitor mice were perfused with 4
mg/kg goat anti-mouse CXCL13 polyclonal antibody at day −2, −1 and
0, prior to undergoing the same injection program as the Model mice
at days 6, 12 and 18.
4T1 cells were obtained from female BALB/c mice
breast cancer cell clones, which were purchased from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). Cells were cultured in Dulbecco's modified Eagle's medium
(high glucose) (Beijing Solarbio Science & Technology, Co.,
Ltd., Beijing, China) containing 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), 1,000 µg/ml
penicillin (Sigma-Aldrich; Merck KGaA) and 100 µg/ml streptomycin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C and 5%
CO2.
Measurement of tumor volume
The length (L) and width (W) of tumors were recorded
every sixth day following treatment with 4T1 cells. The tumor
volume (V) was calculated using the formula
(V=LxW2x0.52). At the age of 9 weeks, mice were
anaesthetized by an intraperitoneal injection of Pentobarbibal
(Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, China) at a dose
of 35 mg/kg under aseptic conditions, metastatic tumors were
excised from the mice and frozen in liquid nitrogen.
Hematoxylin and eosin (H&E)
staining
Tumor tissues were fixed in 4% paraformaldehyde at
4°C for 24 h. The embedded tissue was then cut into sections (5
µm). Subsequent to dewaxing with xylene, hydration was performed
using a series of graded concentrations of ethanol (100% ethanol
for 5 min, 95% ethanol for 1 min, 80% ethanol for 5 min, 75%
ethanol for 5 min and distilled water for 2 min). H&E staining
was performed using the routine method at room temperature for 12
min. Following dehydration, sections were treated with xylene at
room temperature for 10 min twice. Then tissue sections were sealed
with neutral resin and observed using a light microscope
(magnification, ×100) to check for histopathological changes.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA of the breast cancer cells from the
breast cancer tissue was extracted using TRIzol® (Takara
Bio, Inc., Otsu, Japan). The purity of RNA samples was assessed
using ultraviolet spectrophotometry and those with a 260/280 nm
ratio of 1.8–2.0 were used for reverse transcription. The volume of
RNA and buffer used were 2 and 98 µl, respectively.
Total RNA (1 µg) was reverse transcribed using a
reverse transcription kit (cat no. DRR047A; Takara Bio, Inc.)
according to the manufacturer's protocol. RT-qPCR was performed
using a Real-Time Detection system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) by SYBR Green I dye detection (Takara Bio,
Inc.). The primers used in this study are included in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Genes | Sequences |
|---|
| CXCL13 | Forward,
5′-GAGGCAGATGGAACTTGAGC-3′ |
|
| Reverse,
5′-CTGGGGATCTTCGAATGCTA-3′ |
| CXCR5 | Forward,
5′-AACTACCCGCTAACGCTGGAAATGGAC-3′ |
|
| Reverse,
5′-CACGGCAAAGGGCAAGAGAAGACC-3′ |
| ERK | Forward,
5′-TACACGCAGTTGCAGTACATCG-3′ |
|
| Reverse,
5′-CGCAGGATCTGGTAGAGGAAGT-3′ |
| β-actin | Forward,
5′-TTGTTACCAACTGGGACG-3′ |
|
| Reverse,
5′-GGCATAGAGGTCTTTACGG-3′ |
PCR amplifications were performed at 95°C for 3 min,
followed by 30 cycles at 95°C for 1 min, 56°C for 40 sec and 72°C
for 1 min. β-actin was used as an endogenous control to normalize
differences. Melting curves were created to ensure that the
production of non-specific products was avoided. Quantification was
performed by normalizing the cycle threshold values to those of
β-actin and analyzing results using the 2−ΔΔCq method
(25).
Western blot
Total protein was extracted by using the Tissue
Total Protein Lysis buffer (Sangon Biotech CO., Ltd., Shanghai,
China) according to the manufacturer's protocol. The protein
samples were incubated at 0°C for 30 min, and then centrifuged at
10,000 × g at 4°C for 8 min, and supernatants were then extracted.
Protein concentrations were quantified using a BCA Protein Assay
Reagent kit (Pierce; Thermo Fisher Scientific Inc.). Protein
samples (40 µg) were separated via 10% SDS-PAGE and transferred to
a polyvinylidene fluoride membrane. The membrane was blocked with
5% skimmed milk for 1 h at room temperature, prior to an overnight
incubation at 4°C with rabbit anti-mouse CXCL13 polyclonal antibody
(dilution, 1:500; cat no. orb101825; Biorbyt Ltd., Cambridge, UK),
rabbit anti-mouse CXCR5 monoclonal antibody (dilution, 1:500; cat
no. orb5925; Biorbyt Ltd.), rabbit anti-p-ERK polyclonal antibody
(dilution, 1:500; cat no. orb1733; Biorbyt Ltd.), rabbit anti-ERK
polyclonal antibody (dilution, 1:500; cat no. orb224458; Biorbyt
Ltd.) and rabbit anti-β-actin polyclonal antibody (dilution, 1:500;
cat no. orb 129534; Biorbyt Ltd.). The membrane was then washed 3
times for 5 min with TBST (TBS with 1 ml/l Tween-20). Finally, the
membrane was incubated with horseradish peroxidase-conjugated
secondary antibodies (dilution, 1:5,000; cat no. orb345943; Biorbyt
Ltd.) for 2 h at room temperature, and then washed 3 times for 10
min with TBST. Imaging was performed using enhanced
chemiluminescence (ECL) Prime Western Blotting Detection reagent
(GE Healthcare, Chicago, IL, USA) in a dark room. The expression of
the protein samples was standardized to β-actin, then band
densities were scanned and quantified by the Image J 2.1 software
(National Institutes of Health, Bethesda, MD, USA). The formula
used to calculate the p-ERK/ERK ratio was as follows:
P-ERK/ERK=(p-ERK/β-actin)/(ERK/β-actin).
ELISA
The normal breast tissues and breast cancer tissues
were mixed with 10X Tris-HCl buffer (cat no. ab128986; Abcam,
Cambridge, UK), vortexed and centrifuged for 20 min at 1,000 × g at
4°C. The obtained protein samples were quantified using ultraviolet
spectrophotometry and adjusted to 1 µg/µl. The concentrations of
IL-1β (cat no. EK0394), TNF-α (cat no. EK0537) and tumor growth
factor-β1 (TGF-β1) (cat no. EK0515) were detected using ELISA kits,
according to the manufacturer's protocol (Boster Biological
Technology, Pleasanton, CA, USA). The optical density (450 nm) was
read using a microplate reader and the cytokine concentrations were
calculated using a standard curve.
Statistical analysis
Results are expressed as the mean ± standard
deviation. Statistical analysis was performed using SPSS 19.0 (IBM
Corp., Armonk, NY, USA). Differences between groups were compared
by one-way analysis of variance, followed by Fisher's least
significant difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
CXCL13 inhibition reduces tumor
volume
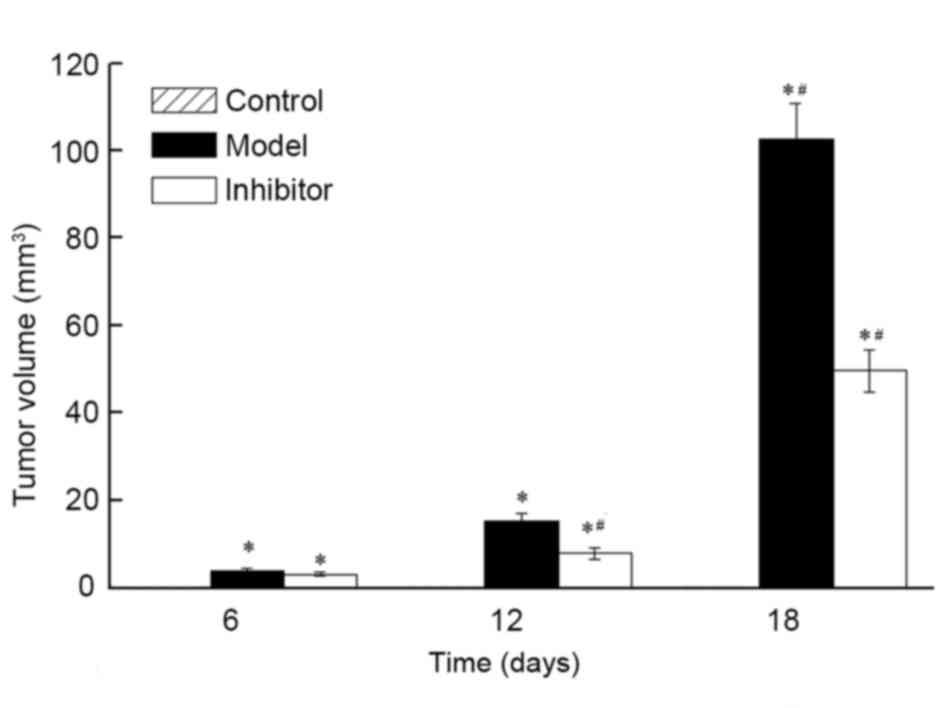
Tumor volume was examined in the Control, Model and
Inhibitor groups, and no breast cancer characteristics were present
in the Control group (Fig. 1). On
days 6, 12 and 18, the tumor volume of the Model group was
3.77±0.68, 15.31±1.62 and 102.70±8.15 mm3, respectively.
The tumor volume of the Inhibitor group on days 6, 12 and 18 was
3.12±0.49, 7.84±1.25 and 49.63±4.74 mm3, respectively.
The tumor volumes of the Model and Inhibitor groups were
significantly larger than those of the Control group (P<0.05)
The tumor volume of the Inhibitor group was significantly smaller
compared with that of the Model group (P<0.05). These results
indicate that the CXCL13 inhibitor significantly reduced the growth
of breast cancer tumors.
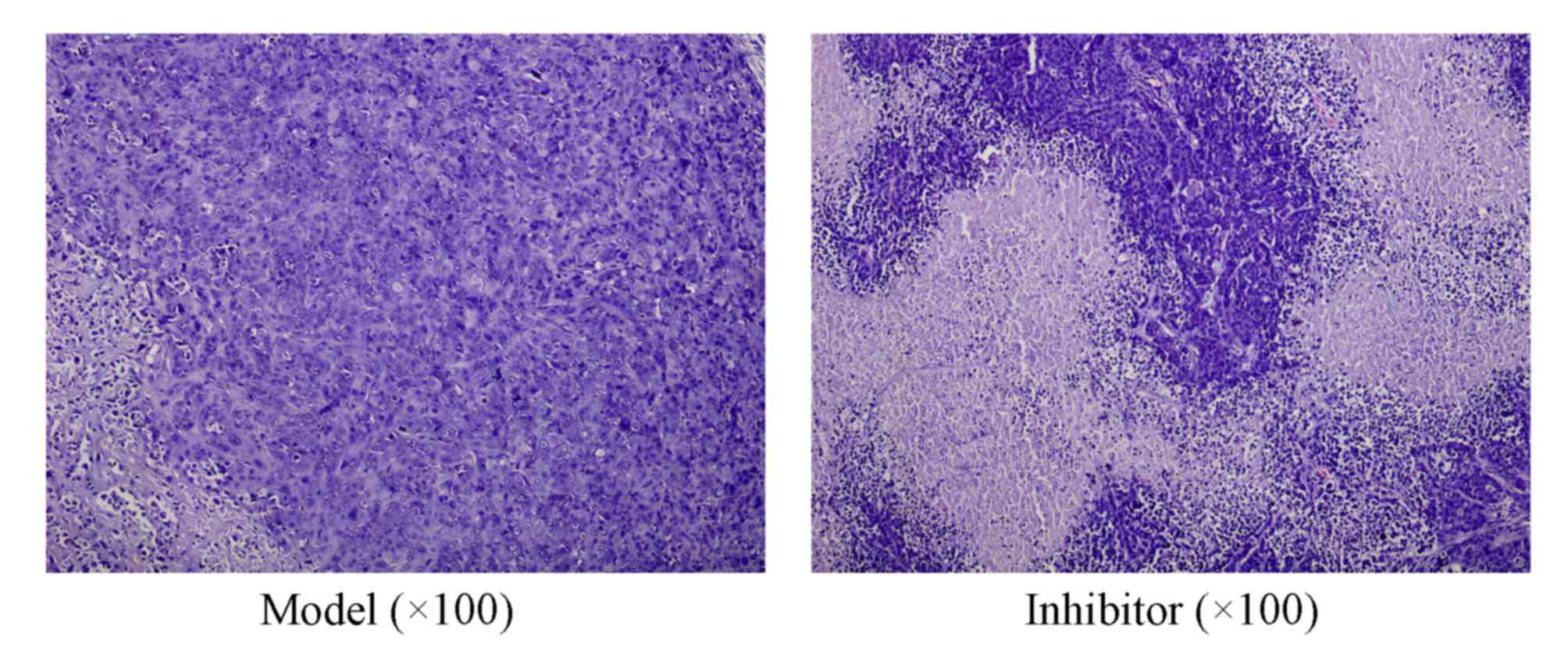
Cellular apoptosis is induced by
CXCL13 inhibition
The tumor cells in the Model group were densely
distributed in a circumambient manner and arranged in a disordered
fashion (Fig. 2). The cytoplasm was
abundant and the nucleus-cytoplasmic ratio was imbalanced, cellular
atypia was apparent and irregular mitosis was widespread. Damaged
tumor cells in the Inhibitor group were surrounded with numerous
lymphocytes and leukocytes and the growth of the tumor cells was
inhibited. These results also support the hypothesis that CXCL13
inhibition inhibits the growth of the breast cancer tumors.
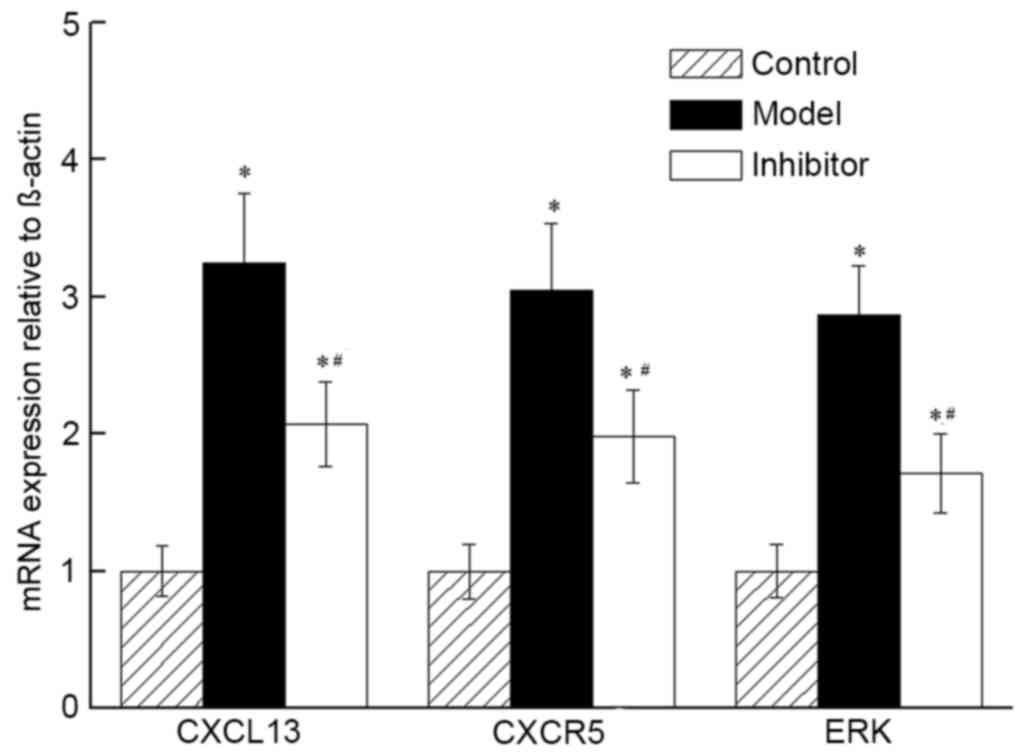
CXCL13 inhibition induces the
expression of key genes of the CXCR5/ERK signaling pathway
The relative expression of CXCL13, CXCR5 and p-ERK
mRNA in the Model group demonstrated fold changes of 3.24, 3.05 and
2.87 on days 6, 12 and 18, respectively, compared with the Control
group. The inhibitor group demonstrated fold changes of 2.07, 1.98
and 1.71 on days 6, 12 and 18, respectively, compared with the
Control group. The changes in the two groups represented a
significant increase compared with the Control group (P<0.05;
Fig. 3). The mRNA expression levels
of CXCL13, CXCR5 and p-ERK in the Inhibitor group were
significantly lower than those of the Model group (P<0.05).
Together, the results indicated that the expression levels of mRNA
in CXCL13, CXCR5 and p-ERK of the CXCR5/ERK pathway were reduced by
treatment with the CXCL13 inhibitor.
CXCR13 inhibition induces the
expression of key proteins of the CXCR5/ERK signaling pathway
There was no significant difference in total protein
expression between each group. The protein levels of CXCL13, CXCR5
and p-ERK increased as the p-ERK/ERK ratio increased in the Model
and Inhibitor groups, compared with those in the Control group
(P<0.05; Fig. 4). The level of
CXCL13, CXCR5 and p-ERK was low and the ratio of p-ERK/ERK was
decreased in the Inhibitor group compared with in the Model group
(ERK is activated by phosphorylation).
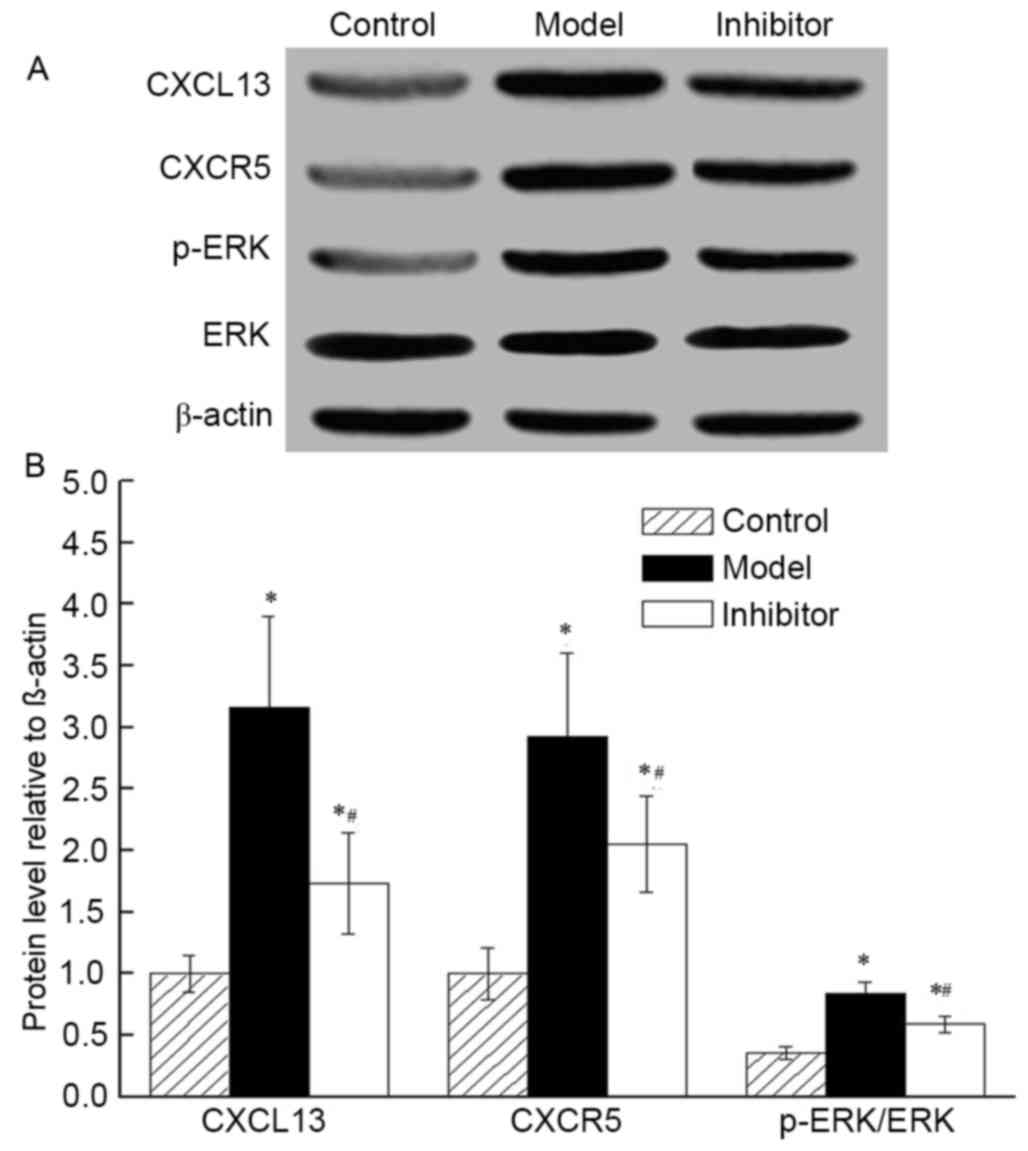 | Figure 4.Effects of CXCL13 on the expression
levels of three key proteins of the CXCR5/ERK pathway (CXCL13,
CXCR5 and p-ERK) was analyzed in the Control, Model and Inhibitor
groups. (A) Western blot analysis demonstrating that CXCL13, CXCR5
and p-ERK protein levels increased, with an increased p-ERK/ERK
ratio, in the Model and Inhibitor groups compared with those in the
Control group. The protein levels of CXCL13, CXCR5 and p-ERK
decreased and the ratio of p-ERK/ERK decreased with CXCL13
inhibition. (B) Quantification of the western blot allowed for
statistical analysis of the relative levels of proteins (normalized
to β-actin). *P<0.05 vs. Control group; #P<0.05
vs. Model group. CXCL13, C-X-C Motif Chemokine Ligand 13; CXCR5,
C-X-C motif chemokine receptor 5; ERK, extracellular-regulated
protein kinases; p-ERK, phosphorylated-ERK. |
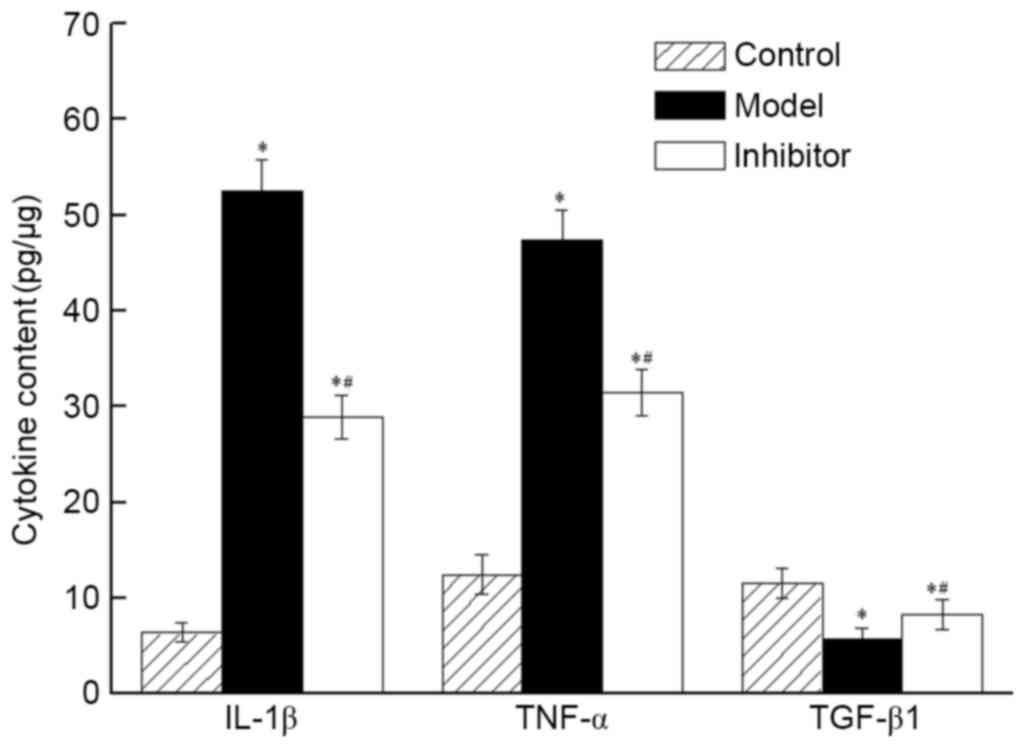
CXCL13 inhibition induces changes in
cytokine concentration in breast tumor tissues
The concentrations of IL-1β and TNF in the Model and
Inhibitor groups were significantly higher than those in the
Control group. Following CXCL13 inhibition, tumor tissues exhibited
fold-change increases in IL-1β and TNF of 8.20 and 4.51,
respectively, in the Model group, and 3.80 and 2.53, respectively,
in the Inhibitor group (P<0.05), compared with the Control group
(Fig. 5). The concentrations of IL-1β
and TNF were significantly decreased (P<0.05) and that of TGF-β1
was increased (P<0.05) in the Inhibitor group, compared with
those of the Model group. These results demonstrated that the
CXCL13 inhibitor reduced the concentration of IL-1β and TNF and
increased the concentration of TGF-β1.
Discussion
Breast cancer is a disease that affects the physical
and mental health of females worldwide, and attracts great
attention from cancer researchers. The association between
chemokines and breast cancer has been previously studied (26–28), and
research has revealed that the expression of chemokines and their
receptors serves a notable role in leukocyte maturation and
internal tumor environment stabilization. The association between
breast cancer and CXCR5/CXCL13 was first revealed in 2008; the
marked overexpression of this chemokine indicated that CXCL13/CXCR5
interactions were involved in the initiation and/or progression of
breast cancer (10). However, to the
best of our knowledge, the present study is is the first to
investigate the mediation oftheCXCR5/ERK signaling pathway by
CXCL13 in breast cancer.
In the present study, it was demonstrated that the
mean tumor volume of the Model mice was larger than that of the
Control mice, whereas the mean tumor volume of the Inhibitor mice
was smaller than that of the Model mice (P<0.05). This result
indicated that the CXCL13 inhibitor could efficiently inhibit tumor
growth. In agreement with previous research, these results
indicated that CXCL13 expression was associated with breast cancer
growth.
Cell apoptosis is an autonomic form of programmed
cell death that is required to maintain homeostasis and is
controlled by gene expression associated with the MAPK/ERK
signaling pathway (29). In contrast
to the Model group, cells of the Inhibitor group were surrounded by
numerous lymphocytes and leukocytes. This indicates that CXCL13
inhibition facilitated cell apoptosis, and led to the generation of
the hypothesis that cell apoptosis induced by CXCL13 inhibition is
associated with the expression of MAPK/ERK signaling pathway
gene/protein members.
The MAPK signaling pathway, which acts downstream of
estrogen receptor activation, has been previously associated with
the breast cancer (18,19). The MAPK/ERK signaling pathway
functions in various tissue types and is involved in the regulation
of cellular proliferation and differentiation (30). The mRNA expression of the key genes in
the CXCR5/ERK pathway, including CXCL13, CXCR5 and ERK, was
attenuated in the Inhibitor group. Simultaneously, the levels of
the CXCL13, CXCR5 and p-ERK proteins were decreased. From these
data, it can be concluded that CXCL13 serves a notable role in
mediating the CXCR5/ERK pathway. Furthermore, the ratio of
p-ERK/ERK decreased, indicating that ERK was inhibited by
phosphorylation.
IL-1β and TNF are important cytokines in regulating
different transcriptional networks in primary β-cells (31). Soria et al (1) demonstrated that the inflammatory
cytokines TNF and IL-1β were highly expressed in the tumors of
patients with relapsed disease, and that these cytokines
contributed to breast cancer development and metastasis. The
results of the present study indicate that expression of
CXCL13/CXCR5 promotes ERK activation and that CXCL13 inhibition
decreases the concentrations of IL-1β and TNF, while increasing the
level of TGF-β1. IL-1β and TNF expression is activated downstream
of the ERK signaling pathway. Together, these results suggest that
the production of IL-1β and TNF as a result of CXCL13 inhibition is
dependent on the ERK signaling pathway.
The present study demonstrated that CXCL13 was
involved in promoting tumor growth and cell apoptosis. Although
CXCL13 inhibition could not completely inhibit the growth of breast
cancer tumors, CXCL13 served a key role in breast cancer
progression via ERK-mediated production of inflammatory cytokines
IL-1β and TNF.
Overall, the results of the present study revealed
that the CXCR5/ERK pathway was mediated by CXCL13 in murine breast
cancer. CXCL13 was also involved in promoting tumor growth and cell
apoptosis, which were suppressed by the CXCL13 inhibitor. The
results of the current study demonstrated that the mRNA and protein
expression levels of CXCL13, CXCR5 and ERK were decreased in the
Inhibitor group compared with in the Model group. Simultaneously,
the ratio of p-ERK/ERK and the concentrations of IL-1β and TNF were
decreased, compared with the Model group. Therefore, the mechanism
of action of CXCL13 in breast cancer progression may be associated
with the CXCR5/ERK pathway, and the application of CXCL13
inhibitors may provide a novel therapeutic approach for the
treatment of breast cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LX, ZL, SL and JM designed the study. LX, ZL and SL
analyzed and interpreted the data. LX and JM wrote and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures performed in the present study
were reviewed and approved by the Animal Care and Use Committee of
Yantaishan Hospital (Yantai, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Soria G, Ofri-Shahak M, Haas I,
Yaal-Hahoshen N, Leider-Trejo L, Leibovich-Rivkin T, Weitzenfeld P,
Meshel T, Shabtai E, Gutman M and Ben-Baruch A: Inflammatory
mediators in breast cancer: Coordinated expression of TNF-α &
IL-1β with CCL2 & CCL5 and effects on epithelial-to-mesenchymal
transition. BMC Cancer. 11:1302011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang F, Li L, Chen Z, Zhu M and Gu Y:
MicroRNA-214 acts as a potential oncogene in breast cancer by
targeting the PTEN-PI3K/Akt signaling pathway. Int J Mol Med.
37:1421–1428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dauksa A, Jakstaitė A, Gasianec A and
Dambrauskas Z: Loss of proapoptotic gene Apaf-1 expression in
pancreatic cancer. Health Sci. 25:9–12. 2015. View Article : Google Scholar
|
|
4
|
Li Y, Zheng Y, Li T, Wang Q, Qian J, Lu Y,
Zhang M, Bi E, Yang M, Reu F, Yi Q and Cai Z: Chemokines CCL2, 3,
14 stimulate macrophage bone marrow homing, proliferation and
polarization in multiple myeloma. Oncotarget. 6:24218–24229.
2015.PubMed/NCBI
|
|
5
|
Tan KW, Evrard M, Tham M, Hong M, Huang C,
Kato M, Prevost-Blondel A, Donnadieu E, Ng LG and Abastado JP:
Tumor stroma and chemokines control T-cell migration into melanoma
following Temozolomide treatment. Oncoimmunology. 4:e9787092015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bryant VL and Slade CA: Chemokines, their
receptors and human disease: The good, the bad and the itchy.
Immunol Cell Biol. 93:364–371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Wang W, Tang L, He X, Yan X, Zhang
X, Zhu Y, Sun J, Shi Y, Ma X, et al: Chemokine (C-X-C motif) ligand
13 promotes intrahepatic chemokine (C-X-C motif) receptor 5+
lymphocyte homing and aberrant B-cell immune responses in primary
biliary cirrhosis. Hepatology. 61:1998–2007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Biswas S, Sengupta S, Chowdhury Roy S,
Jana S, Mandal G, Mandal PK, Saha N, Malhotra V, Gupta A, Kuprash
DV and Bhattacharyya A: CXCL13-CXCR5 co-expression regulates
epithelial to mesenchymal transition of breast cancer cells during
lymph node metastasis. Breast Cancer Res Treat. 143:265–276. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen L, Huang Z, Yao G, Lyu X, Li J, Hu X,
Cai Y, Li W, Li X and Ye C: The expression of CXCL13 and its
relation to unfavorable clinical characteristics in young breast
cancer. J Transl Med. 13:1682015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Panse J, Friedrichs K, Marx A, Hildebrandt
Y, Luetkens T, Barrels K, Horn C, Stahl T, Cao Y, Milde-Langosch K,
et al: Chemokine CXCL13 is overexpressed in the tumour tissue and
in the peripheral blood of breast cancer patients. Br J Cancer.
99:930–938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen L, Huang Z, Yao G, Lyu X, Li J, Hu X,
Cai Y, Li W, Ye C and Li X: Erratum to: The expression of CXCL13
and its relation to unfavorable clinical characteristics in young
breast cancer. J Transl Med. 14:3182016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dorsam RT and Gutkind JS:
G-protein-coupled receptors and cancer. Nat Rev Cancer. 7:79–94.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
del Molino del Barrio I, Kirby J and Ali
S: The role of chemokine and glycosaminoglycan interaction in
chemokine-mediated migration in vitro and in vivo. Methods Enzymol.
570:309–333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meijer J, Zeelenberg IS, Sipos B and Roos
E: The CXCR5 chemokine receptor is expressed by carcinoma cells and
promotes growth of colon carcinoma in the liver. Cancer Res.
66:9576–9582. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu Z, Zhang X, Guo H, Fu L, Pan G and Sun
Y: CXCL13-CXCR5 axis promotes the growth and invasion of colon
cancer cells via PI3K/AKT pathway. Mol Cell Biochem. 400:287–295.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu W, Qian L, Chen X and Ding B:
Prognostic significance of CXCL12, CXCR4 and CXCR7 in patients with
breast cancer. Int J Clin Exp Pathol. 8:13217–13224.
2015.PubMed/NCBI
|
|
17
|
Panse J, Friedrichs K, Marx A, Hildebrandt
Y, Luetkens T, Bartels K, Horn C, Stahl T, Cao Y, Milde-Langosch K,
et al: Chemokine CXCL13 is overexpressed in the tumour tissue and
in the peripheral blood of breast cancer patients. Br J Cancer.
99:930–938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lobenhofer EK, Huper G, Iglehart JD and
Marks JR: Inhibition of mitogen-activated protein kinase and
phosphatidylinositol 3-kinase activity in MCF-7 cells prevents
estrogen-induced mitogenesis. Cell Growth Differ. 11:99–110.
2000.PubMed/NCBI
|
|
19
|
Bosch A, Li Z, Bergamaschi A, Ellis H,
Toska E, Prat A, Tao JJ, Spratt DE, Viola-Villegas NT, Castel P, et
al: PI3K inhibition results in enhanced estrogen receptor function
and dependence in hormone receptor-positive breast cancer. Sci
Transl Med. 7:283ra512015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hindley A and Kolch W: Extracellular
signal regulated kinase (ERK)/mitogen activated protein kinase
(MAPK)-independent functions of Raf kinases. J Cell Sci.
115:1575–1581. 2002.PubMed/NCBI
|
|
21
|
Tran DD, Koch A, Saran S, Armbrecht M,
Ewald F, Koch M, Wahlicht T, Wirth D, Braun A, Nashan B, et al:
Extracellular-signal regulated kinase (Erk1/2), mitogen-activated
protein kinase-activated protein kinase 2 (MK2) and tristetraprolin
(TTP) comprehensively regulate injury-induced immediate early gene
(IEG) response in in vitro liver organ culture. Cell Signal.
28:438–447. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu S, Uppal H, Demaria M, Desprez PY,
Campisi J and Kapahi P: Simvastatin suppresses breast cancer cell
proliferation induced by senescent cells. Sci Rep. 5:178952015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Q, Cao DL, Zhang ZJ, Jiang BC and
Gao YJ: Chemokine CXCL13 mediates orofacial neuropathic pain via
CXCR5/ERK pathway in the trigeminal ganglion of mice. J
Neuroinflammation. 13:1832016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Milde-Langosch K, Bamberger AM, Rieck G,
Grund D, Hemminger G, Müller V and Löning T: Expression and
prognostic relevance of activated extracellular-regulated kinases
(ERK1/2) in breast cancer. Br J Cancer. 92:2206–2215. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Soria G and Benbaruch A: The inflammatory
chemokines CCL2 and CCL5 in breast cancer. Cancer Lett.
267:271–285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aravindan BK, Prabhakar J, Somanathan T
and Subhadra L: The role of chemokine receptor 4 and its ligand
stromal cell derived factor 1 in breast cancer. Ann Transl Med.
3:232015.PubMed/NCBI
|
|
28
|
Kitamura T and Pollard JW: Therapeutic
potential of chemokine signal inhibition for metastatic breast
cancer. Pharmacol Res. 100:266–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kudirka JC, Panupinthu N, Tesseyman MA,
Dixon SJ and Bernier SM: P2Y nucleotide receptor signaling through
MAPK/ERK is regulated by extracellular matrix: Involvement of beta3
integrins. J Cell Physiol. 213:54–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Ma L, Qi J, Shan H, Yu W and Gu
Y: MAPK/ERK signaling pathway-induced hyper-O-GlcNAcylation
enhances cancer malignancy. Mol Cell Biochem. 410:101–110. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lepen Pleić I, Secombes CJ, Bird S and
Mladineo I: Characterization of three pro-inflammatory cytokines,
TNFα1, TNFα2 and IL-1β, in cage-reared Atlantic bluefin tuna
Thunnus thynnus. Fish Shellfish Immunol. 36:98–112. 2014.
View Article : Google Scholar : PubMed/NCBI
|