Introduction
Ovarian cancer is the leading cause of mortality in
women with gynecological malignancies (1). The standard treatment of this disease
comprises surgery followed by chemotherapy; however, the prognosis
is limited (2). Numerous molecular
targeting therapies such as poly (adenosine 5′-diphosphate-ribose)
polymerase inhibitors have been applied in the treatment of
advanced cases, but the observed effects have not been satisfactory
(3–5).
These findings suggest that there may additional molecular targets
for ovarian cancer therapy.
One of these targets may be the oncogenic protein
lysine-specific demethylase 1 (LSD1). LSD1 was initially reported
to specifically remove mono- and dimethyl groups from methylated
histone H3 at lysine 4 to suppress gene expression (6,7). LSD1 is
frequently overexpressed in numerous cancer types, including breast
(8), prostate (9), lung (10),
neuroblastoma (11) and colon cancer
(12). Importantly, the
overexpression of LSD1 promotes cell invasion and migration in
gastric cancer (13). It also
contributes to the oncogenic potential of mixed lineage
leukemia-AF9 leukemia stem cells and acute myeloid leukemia
(14,15). We and others have reported that LSD1
is upregulated in ovarian cancer tissues and cell lines (16–18);
however, the role of LSD1 in ovarian cancer requires further
investigation.
In the present study, the function of LSD1 on SKOV3
ovarian cancer cell proliferation and its role in therapeutic
response to cisplatin were investigated. The results revealed that
LSD1 promoted the proliferation and migration capacity of SKOV3
cells and enhanced their resistance to cisplatin, suggesting an
unfavorable role of LSD1 in cisplatin-based regimens.
Materials and methods
Cell lines and cell culture
Human ovarian epithelial cancer cell line SKOV3 was
a gift from Dr. Qixiang Shao (Jiangsu University, Zhenjiang,
China). The cells were cultured as described previously (18). 293T cells were cultured in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) at a temperature of 37°C under 5%
CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the LSD1-knockdown
SKOV3 cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions,
followed by treatment with DNase I (Takara Bio, Inc., Otsu, Japan).
A total of 2 µg RNA was reverse-transcribed using the PrimeScript
RT Reagent Kit (Takara Bio, Inc.), according to the manufacturer's
instructions (19). All gene
transcripts were quantified via RT-qPCR using a Bio-Rad CFX96
system (Bio-Rad, Hercules, CA, USA) with SsoFast EvaGreen Supermix
(Bio-Rad Laboratories, Inc., Hercules, CA, USA), according to the
manufacturer's instructions. The primer sequences for each gene
were as follows: Cyclin D1 forward, 5′-CAGTGCAAGGCCTGAACCTG-3′,
reverse, 5′-CTTCGATCTGCTCCTGGCAGG-3′; cyclin-dependent kinase 2
(CDK2) forward, 5′-CGAGAGATCTCTCTGCTTAAG-3′, reverse,
5′-GCATCCATGAATTTCTTGAG-3′; CDK inhibitor 1 (p21Cip1)
forward, 5′-TGATTAGCAGCGGAACAAG-3′, reverse
5′-AAACAGTCCAGGCCAGTATG-3′ and GAPDH forward,
5′-GCAAATTCCATGGCACCGTC-3′ and reverse, 5′-TCGCCCCACTTGATTTTGG-3′.
The reaction parameters were as follows: an initial step at 95°C
for 1 min, followed by 40 cycles at 94°C for 10 sec, 56~59°C for 20
sec, and 72°C for 20 sec. Following each PCR run, melting-curve
analysis was performed for each sample to verify that a single
specific product was generated. Amplicon size was confirmed by
ethidium bromide staining and 2% agarose gel electrophoresis.
Negative controls, composed of the PCR mix without nucleic acid,
were also run with each group of samples. The abundance of each
single gene was determined relative to housekeeping gene, GAPDH.
Expression levels were quantified using the comparative cycle
threshold 2−ΔΔCq method (20).
Western blot analysis
Total cellular proteins were isolated from the
LSD1-knockdown or LSD1-overexpressing SKOV3 cells in 100 mm Petri
dishes following a wash with ice-cold PBS and the addition of 200
µl Cell and Tissue Protein Extraction Reagent (Kangchen Biotech,
Shanghai, China). The protein concentration was determined using
the BCA Protein Assay (Kangchen Biotech). A total of 40 µg protein
was separated on 8~10% SDS-PAGE gels and transferred to
polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.).
The membranes were blocked with 5% milk/TBS-T (0.1% Tween-20) for 1
h and immunoprobed with an antibody (diluted in 5% BSA/TBS-T)
against LSD1 (1:1,000; cat. no. 2184S; Cell Signaling Technology,
Danvers, MA, USA), B-cell lymphoma-2 (Bcl-2)-associated X (Bax;
1:1,000; cat. no. ab32503; Abcam, Cambridge, MA, USA),
p21Cip1 (1:1,000; cat. no. ab109520; Abcam), Bcl-2
(1:500; cat. no. BS1511; Bioworld Technology, Shanghai, China),
Survivin (1:1,000; cat. no. BS8456; Bioworld Technology), snail
family transcriptional repressor 1 (Snail; 1:500; cat. no. 9782T;
Cell Signaling), Vimentin (1:500; cat. no. 9782T; Cell Signaling),
E-cadherin (1:500; cat. no. 9782T; Cell Signaling), or α-tubulin
(1:1,000; cat. no. BS1699; Bioworld Technology), overnight at 4°C.
Immunodetection was achieved following incubation with a
horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse
secondary antibody (1:10,000; BS13278 and BS12478, Bioworld
Technology) in TBS-T for 1 h at RT. ECL reagents (Millipore,
Billerica, MA, USA) were used to reveal the positive bands on the
membrane (21). Images were collected
with a ChemiDoc XRS system (Bio-Rad Laboratories, Inc.) and
densitometry analysis was performed with an image analysis program
Quantity One software v.4.6.3 (Bio-Rad Laboratories, Inc.).
Generation of stable cell lines
Lentiviruses expressing pLKO (empty vector) or
pLKO-LSD1-shRNA oligos were produced as described previously
(18). Shed virus was harvested at 48
and 72 h post-transduction. Infection of pLKO or pLKO-LSD1-shRNA
lentivirus was performed by adding 1 ml lentiviral supernatant to
SKOV3 cells at ~80% confluency in a 60 mm culture dish with 4 ml of
McCoy's 5A medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
supplemented with 8 µg/ml Polybrene (Sigma-Aldrich; Merck KGaA).
Stable knockdown clones were obtained under 1.5 µg/ml puromycin
selection for 1 week.
To generate a rTet-repressor expressing (rtTA) cell
line, 293T cells were transfected with 2 µg pLVX-Tet-On (empty
vector), 1.5 µg pHR'-CMV-8.2ΔVPR and 0.5 µg of pHR'-CMV-VSVG
(lentiviral packaging plasmids) (all kind gifts from Professor
Changdeng Hu, Purdue University, West Lafayette, IN, USA) using
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.). After 24 h
transfection, the viral supernatant was harvested and used to
infect SKOV3 cells. SKOV3 cells were then selected with 200 µg/ml
G418 (Sigma-Aldrich; Merck KGaS) for 1 week. The cells that
survived had been stably transfected with rtTA. The rtTA cells were
infected with the lentiviral particles packaged with
pLVX-Tight-Puro (control vector, obtained from Professor Changdeng
Hu; Purdue University, West Lafayette, IN, USA) or
pLVX-tight-puro-LSD1 produced as described previously (22). The rtTA cells were selected with 2.0
µg/ml puromycin for 3 days, and then maintained in the presence of
1.0 µg/ml puromycin for one week (22). The surviving cells were considered
stable clones. The stable knockdown or overexpression clones were
confirmed via western blot analysis as aforementioned.
Cell proliferation assay
Cell proliferation was assessed using Cell Counting
Kit (CCK)-8 and 5-ethynyl-2′-deoxyuridene (EdU) incorporation
assays. In the CCK-8 assay, the stable SKOV3 cell lines (5,000
cells/well) were plated in 96-well plates in 100 µl McCoy's 5A
medium per well. The cells were cultured overnight at 37°C and then
treated with 1, 10 and 100 ng/ml doxycycline (Dox; Sigma-Aldrich;
Merck KGaA) to induce LSD1 knockdown or overexpression for 24, 48
and 72 h at 37°C. A total of 1/10 volume of CCK-8 was then added to
each well and incubated for additional 2 h at 37°C. The optical
density was measured at 450 nm with a microplate reader (Bio-Rad
Model 680; Bio-Rad Laboratories, Inc.). The cells from each group
were added to 6 wells and the experiment was performed in
triplicate.
In the EdU assay, the stable SKOV3 cell lines were
plated in 24-well plates at a density of 5×104
cells/well and then treated with 100 ng/ml Dox for 48 h at 37°C.
The cells were incubated in serum-free Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) containing 50
mM EdU for 2 h at 37°C, after which the nuclei were stained with 1
µg/ml DAPI (cat. no. D9542, Sigma-Aldrich; Merck KGaA) for 10 min
at room temperature in the dark. The cells were imaged using an
Olympus IX71 fluorescence microscope with excitation wavelengths of
460 nm (green) and 420 nm (blue). The stained cells were counted in
5 randomly selected fields (×100, magnification), and the mean
value was calculated.
Cell cycle analysis
The effect of LSD1 on cell cycle phase distribution
was determined by flow cytometry. The stable LSD1-knockdown SKOV3
cells (1×106 cells/ml) were fixed in 70% ethanol for 30
min at 4°C and stained with 50 µg/ml propidium iodide
(Sigma-Aldrich; Merck KGaA) for 30 min at room temperature in the
dark. Subsequently, the cell cycle stages were measured with a flow
cytometer (FACScan®, BD Biosciences, Franklin Lakes, NJ,
USA) equipped with the CellQuest software version 3.3 (BD
Biosciences).
Cell apoptosis assay
Cell apoptosis was analyzed with the fluorescein
isothiocyanate (FITC) Annexin V apoptosis detection kit (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan). Briefly, the stable
LSD1 knockdown or overexpression SKOV3 cells (5×104
cells/well) were treated with 100 ng/ml Dox for 24 h at 37°C. After
24 h, the cells were exposed to 5 µg/ml cisplatin (Sigma-Aldrich;
Merck KGaA) in the presence of Dox for another 48 h at 37°C. The
cells were incubated in 500 µl 1X Annexin V binding buffer at a
concentration of 1×106 cells/ml. A total of 100 µl of
the solution was transferred to a 5-ml culture tube and then
stained with 5 µl each of Annexin V-FITC and propidium iodide for
15 min at room temperature in the dark. Following the addition of
400 µl the Annexin V binding buffer to each tube, the samples were
analyzed using a flow cytometer (FACScan®, BD
Biosciences) equipped with the CellQuest software version 3.3 (BD
Biosciences). All these measurements were repeated three times
independently.
Cell migration assay
The migration ability of the stable LSD1 knockdown
or overexpression SKOV3 cells was assessed as described previously
(18). Briefly, 1.5×105
cells in 300 µl of serum-free McCoy's 5A medium were placed in the
upper chamber of a Transwell system (BD Biosciences). Then, 500 µl
10% FBS-containing McCoy's 5A medium was placed in the lower
chamber to act as a chemoattractant. After incubation for 24 h at
37°C, the cells on the upper surface of the membrane (8-µm pore
size) were removed with a wet cotton swab. The cells on the lower
surface of the membrane were fixed with 4% formaldehyde for 30 min
and stained with 0.1% crystal violet (Sigma-Aldrich; Merck KGaA)
for 15 min at room temperature. The number of the stained cells
were counted under a light microscope (BX43; Olympus Corporation,
Tokyo, Japan) in 5 random fields (×100, magnification), and the
mean value was calculated. All experiments were performed with 3
replicates.
Statistical analysis
All values were presented as means ± standard error
of the mean. Differences in different groups were analyzed by
Student's t-test or one-way analysis of variance followed by
Tukey's test using SPSS 11.5 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
LSD1 promotes the proliferation of
SKOV3 cells
To investigate the effects of LSD1 on the
proliferation of SKOV3 ovarian cancer cells, stable LSD1-knockdown
(LSD1-KD) clones and LSD1-overexpressing (LSD1-OE) clones were
generated using SKOV3 cells in the present study. Total proteins
were extracted from the stable cells treated with increasing doses
of Dox for 48 h. This time point was chosen based on previous
studies (22). The results of the
present study revealed that LSD1 protein expression levels were
decreased in the LSD1-KD cells in a dose-dependent manner (Fig. 1A), and the reduced expression of LSD1
protein was observed in the pLKO-shLSD1 cells compared with the
empty vector cells (Fig. 1B). In
contrast, a dose-dependent increase in LSD1 protein expression was
observed with increasing concentrations of Dox (Fig. 1C), and the expression levels of LSD1
protein were increased in the LSD1-OE cells (Fig. 1D).
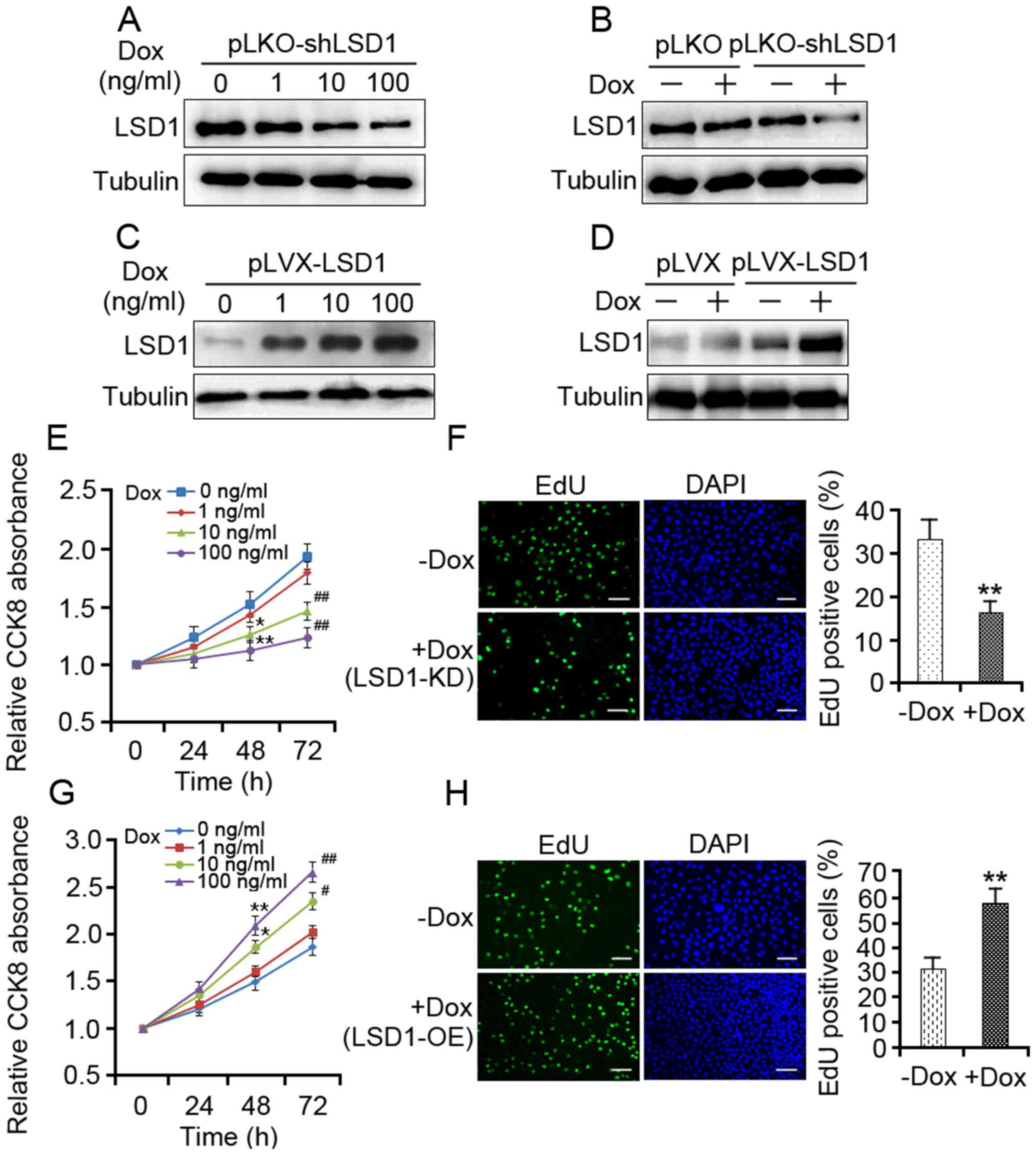 | Figure 1.LSD1 is required for the proliferation
of SKOV3 cells. (A) SKOV3 cells transduced with pLKO-LSD1-shRNA
lentivirus were treated with different dosages of Dox for 48 h.
LSD1-KD was determined by western blotting. (B) pLKO and
pLKO-LSD1-shRNA-transduced cells were treated with 100 ng/ml Dox
for 48 h followed by western blot analysis. (C) LSD1-OE SKOV3 cells
were incubated with doses of Dox for 48 h, as indicated; the
protein expression levels of LSD1 were assessed by western
blotting. (D) pLVX and pLVX-LSD1-transduced cells were treated with
100 ng/ml Dox for 48 h. Subsequently, the protein expression levels
of LSD1 were detected via western blotting. (E) LSD1-KD cells were
treated with doses of Dox as indicated, and cell viability was
assessed using the CCK-8 assay at the indicated durations. (F)
LSD1-KD cells were treated with 100 ng/ml Dox for 48 h, and cell
proliferation was assessed using the EdU incorporation assay
(green). (G) LSD1-OE cells were treated with doses of Dox as
indicated, and cell viability was assessed using the CCK-8 assay at
the indicated durations. (H) LSD1-OE cells were treated with 100
ng/ml Dox for 48 h, and cell proliferation was assessed using the
EdU incorporation assay. Cells of the new generation were detected
via EdU (green). DAPI stained nuclei (blue). Error bars represented
the data as the mean ± standard deviation (E and G, n=3; F and H,
n=4). *P<0.05 and **P<0.01, compared with the group not
treated with Dox (48 h); #P<0.05 and
##P<0.01, compared with the group not treated with
Dox (72 h). Scale bar=50 µm. CCK-8, Cell Counting Kit-8; Dox,
doxycycline; EdU, 5-ethynyl-2′-deoxyuridene; LSD1, lysine-specific
demethylase 1; shRNA, short hairpin RNA; pLKO, empty vector; pLVX,
empty vector; LSD1-KD, Dox-mediated LSD1 knockdown of cells
transduced with pLKO-LSD1-shRNA; LSD1-OE, transduced with
lentivirus expressing pLVX-LSD1. |
To understand the effect of LSD1 expression on cell
proliferation, CCK-8 and EdU assays were performed to measure the
proliferative capacity of the LSD1-KD and LSD1-OE cells. The
LSD1-KD cells demonstrated significantly reduced proliferative
ability compared with in the control (Fig. 1E and F), whereas the LSD1-OE cells
exhibited a higher proliferation rate as compared with in the
control (Fig. 1G and H).
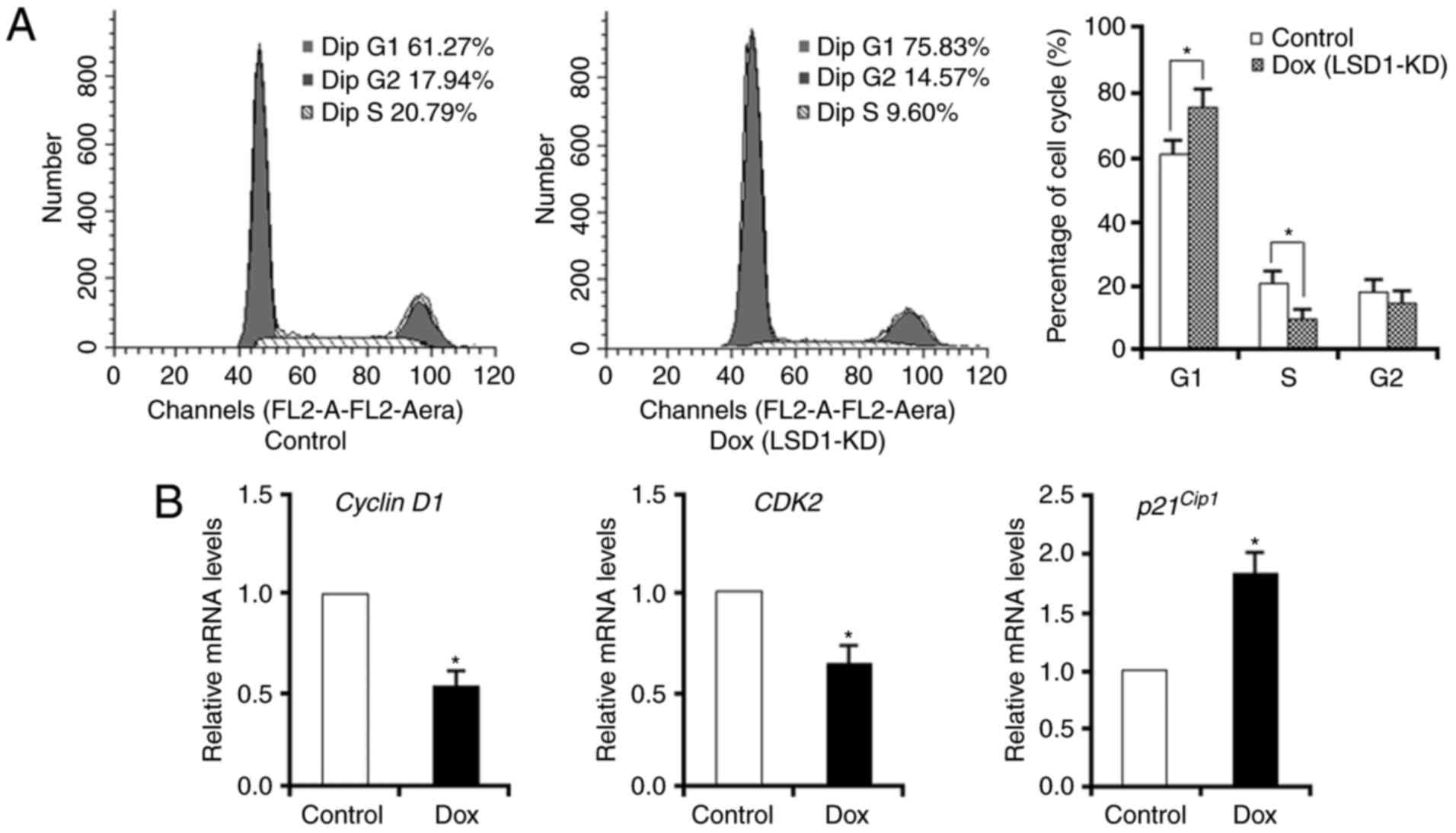
To further determine the role of LSD1 in cell
proliferation, analysis of the cell cycle was conducted via flow
cytometry. Knockdown of LSD1 led to an accumulation of cells in the
G1 phase (75.8%) compared with in the control (61.3%). In addition,
there was an notable decrease in the S-phase cell fraction of
LSD1-KD cells compared with the control (9.6 vs. 20.8%,
respectively; Fig. 2A). Furthermore,
knockdown of LSD1 was associated with the significant
downregulation of cyclin D1 and CDK2 and the upregulation of
p21Cip1 (Fig. 2B). Based
on these data, LSD1 silencing may inhibit cell-cycle progression
via the G1 phase.
LSD1 regulation of cisplatin-induced
inhibition of cell proliferation
Cisplatin resistance is a major obstacle in the
treatment of ovarian carcinoma (23).
To examine whether LSD1 serves a role in cisplatin resistance, the
proliferation of LSD1-OE and LSD1-KD cells in response to cisplatin
was analyzed. Treatment with cisplatin resulted in decreased LSD1
level in a dose-dependent manner (Fig.
3A). Cisplatin treatment also caused a dose-dependent reduction
in cell proliferation (Fig. 3B). When
the cells were cotreated with Dox to induce LSD1-OE, the
suppressive effect of cisplatin was significantly reduced (Fig. 3C). Conversely, Dox-mediated LSD1-KD
enhanced the cisplatin-induced proliferation inhibition (Fig. 3D). To further verify the involvement
of LSD1 in cisplatin-mediated proliferation inhibition, the
expression of two proliferation-associated genes were analyzed. The
present study reported that overexpression of LSD1 reversed the
cisplatin-induced downregulation of Survivin and upregulation of
p21Cip1 in the LSD1-OE cells compared with the cells
without Dox and cisplatin (Fig. 3E),
whereas knockdown of LSD1 promoted the cisplatin-induced expression
of both genes in the LSD1-KD cells compared with the cells not
treated with Dox and cisplatin (Fig.
3F). Collectively, these results demonstrated that LSD1
silencing may facilitate the cisplatin-induced proliferation
inhibition of SKOV3 cells.
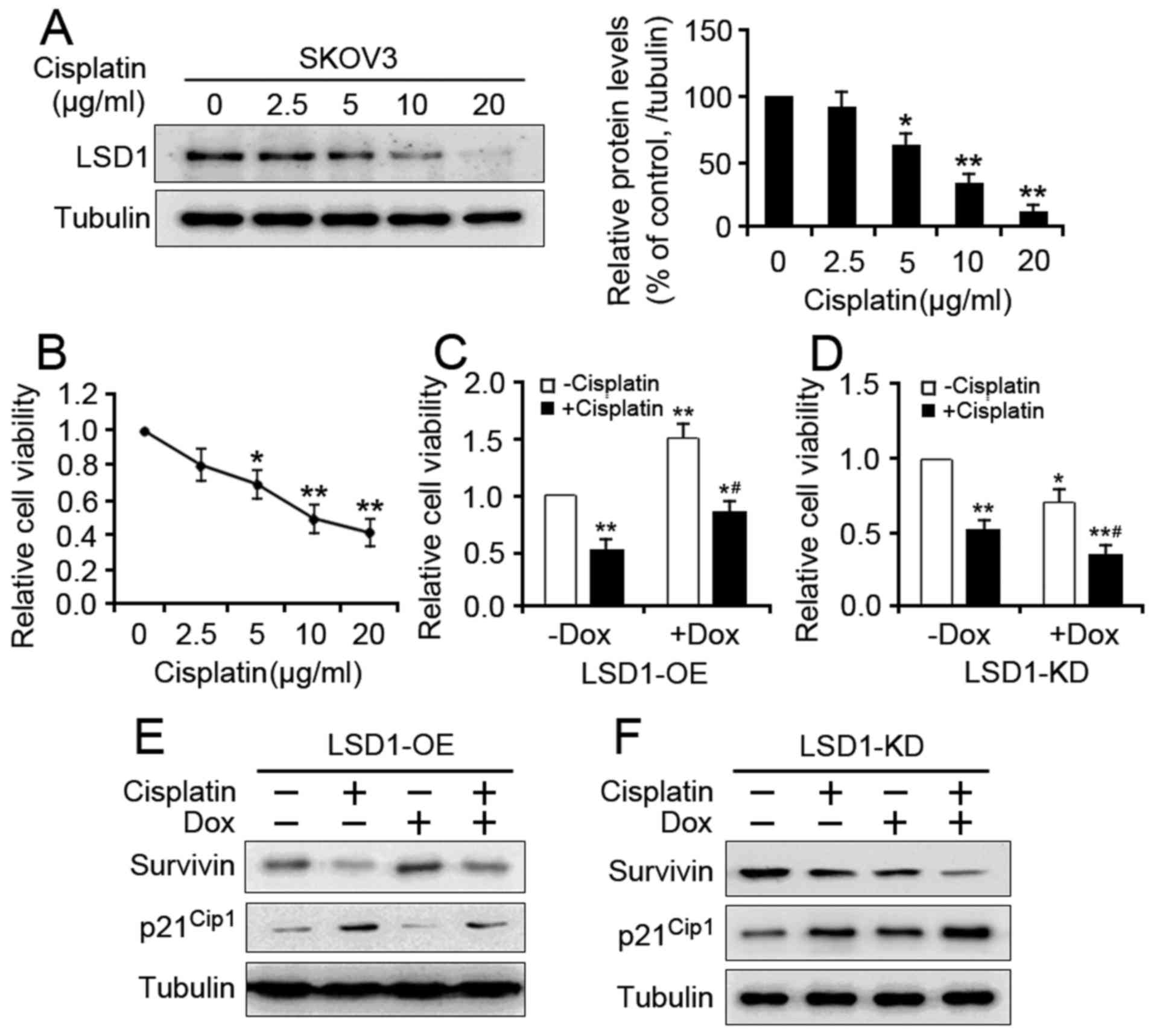 | Figure 3.Effect of LSD1 on cisplatin-induced
proliferation inhibition. (A) The untransduced SKOV3 cells were
treated with different doses of cisplatin for 24 h, after which
LSD1 protein expression levels were detected via western blotting.
(B) The unstransduced SKOV3 cells were exposed to various doses of
cisplatin, as indicated, for 48 h. Cell viability was determined
via a CCK-8 assay. Error bars represented data as the mean ±
standard error of the mean (n=3). *P<0.05 and **P<0.01,
compared with the group not treated with cisplatin. (C) LSD1-OE and
(D) LSD1-KD cells were treated with either 5 µg/ml cisplatin, 100
ng/ml Dox, or both for 48 h. After 48 h, the viability of both cell
lines was analyzed via the CCK-8 assay. Error bars represented data
as the mean ± standard error of the mean (n=3). *P<0.05 and
**P<0.01, compared with the group not treated with cisplatin and
Dox; #P<0.05, compared with the groups treated with
cisplatin or Dox alone. After 48 h of cisplatin and/or Dox
treatments, the protein expression levels of
proliferation-associated genes were detected in the (E) LSD1-OE and
(F) LSD1-KD cells via western blotting. |
Impact of LSD1 on cell apoptosis
against cisplatin
As cisplatin-induced DNA damage has been associated
with the activation of both intrinsic and extrinsic apoptotic
pathways (24,25), whether LSD1 is associated with
cisplatin-induced apoptosis was investigated in the present study.
In the presence of cisplatin, the total cell apoptosis rates in the
LSD1-KD group were significantly higher compared with in the
corresponding control group (Fig.
4A). Additionally, the expression of proapoptotic protein Bax
was notably higher in the LSD1-KD group compared with cells not
treated with cisplatin and Dox, while the level of anti-apoptotic
protein Bcl-2 was lower in the LSD1-KD group (Fig. 4B). Conversely, LSD1-OE significantly
reduced total apoptosis and partially eliminated cisplatin-induced
total apoptosis (Fig. 4C) and the
expression of Bcl-2 and Bax genes in the LSD1-OE group compared
with the group without Dox and cisplatin (Fig. 4D). These data suggested that LSD1
inhibition may promote apoptosis by enhancing cellular responses to
cisplatin.
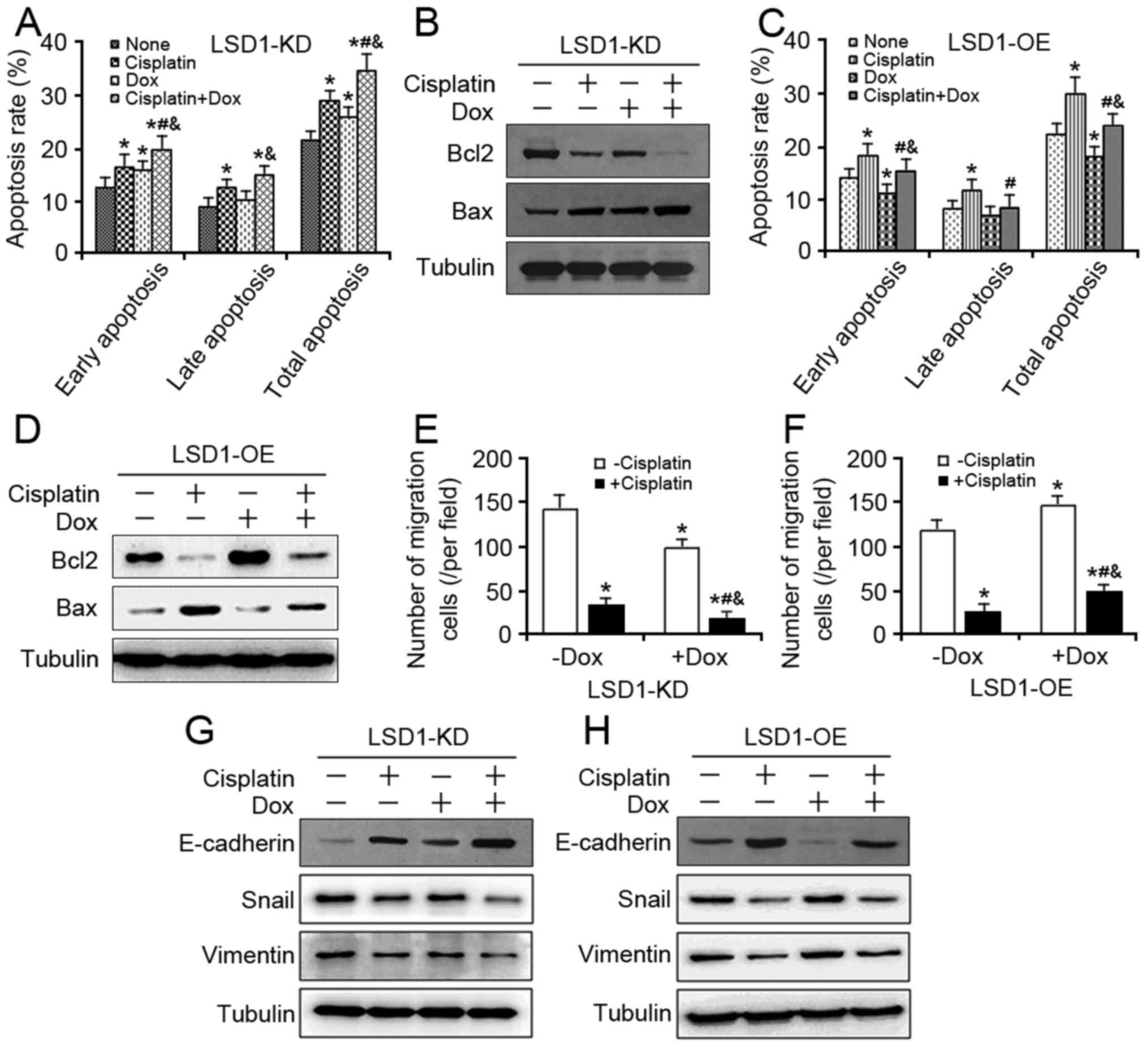 | Figure 4.Effects of LSD1 on cell apoptosis and
migration against cisplatin. (A) At 24 h after 100 ng/ml Dox
induction, the LSD1-KD cells were exposed to 5 µg/ml cisplatin for
additional 48 h, and cell apoptosis assay was performed using the
Annexin V-FITC. (B) Expression levels of Bcl2 and Bax proteins were
detected in the LSD1-KD cells via western blotting. (C) At 24 h
following induction via 100 ng/ml Dox, the LSD1-OE cells were
exposed to 5 µg/ml cisplatin for an additional 48 h, and a cell
apoptosis assay was performed using Annexin V-FITC. (D) Expression
levels of Bcl2 and Bax proteins were detected in the LSD1-OE cells
via western blotting. (E) After 24 h of induction via 100 ng/ml
Dox, the trypsinized LSD1-KD and (F) LSD1-OE cells were seeded in
Transwell inserts and cultured with 5 µg/ml cisplatin in the
presence of Dox for another 24 h, and then stained with crystal
violet. (G) Following treatment with 100 ng/ml Dox for 24 h, the
LSD1-KD and (H) LSD1-OE cells were cotreated with 5 µg/ml cisplatin
for additional 24 h, after which the protein expression levels of
epithelial-mesenchymal transition markers were detected via western
blot analysis. Error bars represented data as the means ± standard
error of the mean (n=3). *P<0.05, compared with the group not
treated with cisplatin and Dox; #P<0.05, compared
with the groups treated with cisplatin alone;
&P<0.05, compared with the groups treated with
Dox alone. Bcl2, B-cell lymphoma-2; Bax, Bcl2-associated X; FITC,
fluorescein isothiocyanate; Snail, snail family transcriptional
repressor 1. |
Effect of LSD1 on cell migration
against cisplatin
We have demonstrated previously that LSD1 promotes
ovarian cancer cell migration by regulating epithelial-mesenchymal
transition (EMT)-associated genes (22). The potential of LSD1 in cell migration
in response to cisplatin was investigated in the present study.
Cell migration following exposure to cisplatin was significantly
inhibited by LSD1-KD (Fig. 4E).
Additionally, the inhibition of cell migration was markedly
reversed by LSD1-OE (Fig. 4F). In the
presence of cisplatin, SKOV3 cells exhibited an upregulation of the
epithelial marker E-cadherin and a downregulation of the
mesenchymal markers Snail and Vimentin. When the cells were
cotreated with Dox to induce LSD1-KD, the cisplatin effects were
markedly enhanced (Fig. 4G), whereas
Dox-treated LSD1-OE reversed the effects of cisplatin (Fig. 4H). Collectively, these results
suggested that LSD1 inhibition and cisplatin may synergistically
suppress the migration of SKOV3 cells.
Discussion
LSD1 has been implicated in various types of cancers
and serves an oncogenic role in cancer cell proliferation (26,27). The
findings of the present study support that the overexpression of
LSD1 promotes cell proliferation and inhibits cell apoptosis of
SKOV3 ovarian cancer cells. Additionally, the expression levels of
LSD1 may be closely associated with the effects of cisplatin. When
LSD1 is upregulated, the inhibitory effects of cisplatin are
notably inhibited, whereas a reduction of endogenous LSD1
substantially enhances the cisplatin effects. Furthermore,
cisplatin may directly downregulate LSD1 protein expression in a
dose-response manner, suggesting that LSD1 is a downstream target
of cisplatin. Thus, cisplatin may inhibit cell proliferation by
modulating epigenetic factors, such as LSD1.
In addressing the molecular mechanisms of the LSD1
inhibitory effect on cisplatin activity, LSD1 silencing was
accompanied by a reduced expression in cyclin D1, CDK2, Survivin,
and Bcl-2 proteins as observed in the present study, which are
known regulators of cell proliferation and survival (28). Importantly, the present study
demonstrated that LSD1 knockdown plus cisplatin increased reduction
in the expression of these genes. As LSD1 activates gene
transcription via the demethylation of H3K9 (29), LSD1 may modulate these gene
expressions via epigenetic changes to mediate its cellular
function.
One of the notable findings of the present study is
that cisplatin-mediated migration inhibition may be partially
eliminated by exogenous expression of LSD1, whereas cisplatin plus
LSD1 knockdown causes significantly decreased cell migration. This
suggests that LSD1 expression is associated with the migration of
ovarian cancer cells and may serve a role in the development of
cisplatin resistance. Accumulating evidence demonstrates that EMT
serves important roles in ovarian cancer metastasis and
chemoresistance (22,30). Among the multiple factors, Snail has
been recognized as a central transcription factor that controls the
EMT program via repressing E-cadherin expression (31,32). It
has also demonstrated that mesenchymal cells, which are
characterized by the upregulation of Vimentin, may acquire
increased migratory potential during tumor progression (32). The results of the present study
revealed that LSD1 knockdown may sensitize SKOV3 cells to cisplatin
by downregulating Snail and Vimentin protein expression;
LSD1-overexpressing cells exhibit the protein expression profiles
similar to LSD1-knockdown cells. In the future, it will be
beneficial to measure whether LSD1 knockout in vivo
sensitizes ovarian tumors to cisplatin.
In conclusion, the present study revealed the role
of LSD1 in directing SKOV3 cell proliferation, as well as
resistance to cisplatin. Overexpression of LSD1 may stimulate the
expression of proliferation-associated genes, thus contributing to
the proliferation of SKOV3 cells; sustained expression of LSD1 may
overcome cisplatin-induced cell apoptosis. These data identify LSD1
as a regulator of SKOV3 cell potential and provide a possible
therapeutic strategy against ovarian cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81170573)
and Clinical Medicine Science & Technology Project of Jiangsu
Province (grant no. BL2013024).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GS, CW and QL made substantial contributions to
study conception and design, and data acquisition. XW, WL and YW
performed western blotting and cell proliferation assays. XL, JJ
and LZ participated in cell cycle analysis. XW and QS analyzed the
data and were major contributors in writing the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was endorsed by the Ethics
Committee of Jiangsu University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ali AY, Farrand L, Kim JY, Byun S, Suh JY,
Lee HJ and Tsang BK: Molecular determinants of ovarian cancer
chemoresistance: New insights into an old conundrum. Ann N Y Acad
Sci. 1271:58–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vaughan S, Coward JI, Bast RC Jr, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, et al: Rethinking ovarian cancer: Recommendations
for improving outcomes. Nat Rev Cancer. 11:719–725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Behbakht K, Sill MW, Darcy KM, Rubin SC,
Mannel RS, Waggoner S, Schilder RJ, Cai KQ, Godwin AK and Alpaugh
RK: Phase II trial of the mTOR inhibitor, temsirolimus and
evaluation of circulating tumor cells and tumor biomarkers in
persistent and recurrent epithelial ovarian and primary peritoneal
malignancies: A Gynecologic Oncology Group study. Gynecol Oncol.
123:19–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ledermann J, Harter P, Gourley C,
Friedlander M, Vergote I, Rustin G, Scott CL, Meier W,
Shapira-Frommer R, Safra T, et al: Olaparib maintenance therapy in
patients with platinum-sensitive relapsed serous ovarian cancer: A
preplanned retrospective analysis of outcomes by BRCA status in a
randomised phase 2 trial. Lancet Oncol. 15:852–861. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matulonis UA, Berlin S, Ivy P, Tyburski K,
Krasner C, Zarwan C, Berkenblit A, Campos S, Horowitz N, Cannistra
SA, et al: Cediranib, an oral inhibitor of vascular endothelial
growth factor receptor kinases, is an active drug in recurrent
epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin
Oncol. 27:5601–5606. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lan F, Nottke AC and Shi Y: Mechanisms
involved in the regulation of histone lysine demethylases. Curr
Opin Cell Biol. 20:316–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Metzger E, Wissmann M, Yin N, Müller JM,
Schneider R, Peters AH, Günther T, Buettner R and Schüle R: LSD1
demethylates repressive histone marks to promote
androgen-receptor-dependent transcription. Nature. 437:436–439.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lim S, Janzer A, Becker A, Zimmer A,
Schüle R, Buettner R and Kirfel J: Lysine-specific demethylase 1
(LSD1) is highly expressed in ER-negative breast cancers and a
biomarker predicting aggressive biology. Carcinogenesis.
31:512–520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu CY, Hsieh CY, Huang KE, Chang C and
Kang HY: Cryptotanshinone down-regulates androgen receptor
signaling by modulating lysine-specific demethylase 1 function. Int
J Cancer. 131:1423–1434. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hayami S, Kelly JD, Cho HS, Yoshimatsu M,
Unoki M, Tsunoda T, Field HI, Neal DE, Yamaue H, Ponder BA, et al:
Overexpression of LSD1 contributes to human carcinogenesis through
chromatin regulation in various cancers. Int J Cancer. 128:574–586.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schulte JH, Lim S, Schramm A, Friedrichs
N, Koster J, Versteeg R, Ora I, Pajtler K, Klein-Hitpass L,
Kuhfittig-Kulle S, et al: Lysine-specific demethylase 1 is strongly
expressed in poorly differentiated neuroblastoma: Implications for
therapy. Cancer Res. 69:2065–2071. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang Y, Greene E, Stewart Murray T,
Goodwin AC, Baylin SB, Woster PM and Casero RA Jr: Inhibition of
lysine-specific demethylase 1 by polyamine analogues results in
reexpression of aberrantly silenced genes. Proc Natl Acad Sci USA.
104:8023–8028. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng YC, Duan YC, Ma JL, Xu RM, Zi X, Lv
WL, Wang MM, Ye XW, Zhu S, Mobley D, et al:
Triazole-dithiocarbamate based selective lysine specific
demethylase 1 (LSD1) inactivators inhibit gastric cancer cell
growth, invasion, and migration. J Med Chem. 56:8543–8560. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harris WJ, Huang X, Lynch JT, Spencer GJ,
Hitchin JR, Li Y, Ciceri F, Blaser JG, Greystoke BF, Jordan AM, et
al: The histone demethylase KDM1A sustains the oncogenic potential
of MLL-AF9 leukemia stem cells. Cancer Cell. 21:473–487. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schenk T, Chen WC, Göllner S, Howell L,
Jin L, Hebestreit K, Klein HU, Popescu AC, Burnett A, Mills K, et
al: Inhibition of the LSD1 (KDM1A) demethylase reactivates the
all-trans-retinoic acid differentiation pathway in acute myeloid
leukemia. Nat Med. 18:605–611. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen C, Ge J, Lu Q, Ping G, Yang C and
Fang X: Expression of Lysine-specific demethylase 1 in human
epithelial ovarian cancer. J Ovarian Res. 8:282015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Konovalov S and Garcia-Bassets I: Analysis
of the levels of lysine-specific demethylase 1 (LSD1) mRNA in human
ovarian tumors and the effects of chemical LSD1 inhibitors in
ovarian cancer cell lines. J Ovarian Res. 6:752013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shao G, Wang J, Li Y, Liu X, Xie X, Wan X,
Yan M, Jin J, Lin Q, Zhu H, et al: Lysine-specific demethylase 1
mediates epidermal growth factor signaling to promote cell
migration in ovarian cancer cells. Sci Rep. 5:153442015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shao GB, Wang J, Zhang LP, Wu CY, Jin J,
Sang JR, Lu HY, Gong AH, Du FY and Peng WX: Aging alters histone H3
lysine 4 methylation in mouse germinal vesicle stage oocytes.
Reprod Fertil Dev. 27:419–426. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L, Wang J, Pan Y, Jin J, Sang J,
Huang P and Shao G: Expression of histone H3 lysine 4 methylation
and its demethylases in the developing mouse testis. Cell Tissue
Res. 358:875–883. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Wan X, Wei Y, Liu X, Lai W, Zhang L,
Jin J, Wu C, Shao Q, Shao G and Lin Q: LSD1-mediated epigenetic
modification contributes to ovarian cancer cell migration and
invasion. Oncol Rep. 35:3586–3592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Davis A, Tinker AV and Friedlander M:
‘Platinum resistant’ ovarian cancer: What is it, who to treat and
how to measure benefit. Gynecol Oncol. 133:624–631. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hayakawa J, Ohmichi M, Kurachi H, Ikegami
H, Kimura A, Matsuoka T, Jikihara H, Mercola D and Murata Y:
Inhibition of extracellular signal-regulated protein kinase or
c-Jun N-terminal protein kinase cascade, differentially activated
by cisplatin, sensitizes human ovarian cancer cell line. J Biol
Chem. 274:31648–31654. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Persons DL, Yazlovitskaya EM, Cui W and
Pelling JC: Cisplatin-induced activation of mitogen-activated
protein kinases in ovarian carcinoma cells: Inhibition of
extracellular signal-regulated kinase activity increases
sensitivity to cisplatin. Clin Cancer Res. 5:1007–1014.
1999.PubMed/NCBI
|
|
26
|
Ding J, Zhang ZM, Xia Y, Liao GQ, Pan Y,
Liu S, Zhang Y and Yan ZS: LSD1-mediated epigenetic modification
contributes to proliferation and metastasis of colon cancer. Br J
Cancer. 109:994–1003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang GG, Allis CD and Chi P: Chromatin
remodeling and cancer, Part I: Covalent histone modifications.
Trends Mol Med. 13:363–372. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li M, Lewis B, Capuco AV, Laucirica R and
Furth PA: WAP-TAg transgenic mice and the study of dysregulated
cell survival, proliferation, and mutation during breast
carcinogenesis. Oncogene. 19:1010–1019. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
El Mansouri FE, Nebbaki SS, Kapoor M, Afif
H, Martel-Pelletier J, Pelletier JP, Benderdour M and Fahmi H:
Lysine-specific demethylase 1-mediated demethylation of histone H3
lysine 9 contributes to interleukin 1β-induced microsomal
prostaglandin E synthase 1 expression in human osteoarthritic
chondrocytes. Arthritis Res Ther. 16:R1132014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vergara D, Merlot B, Lucot JP, Collinet P,
Vinatier D, Fournier I and Salzet M: Epithelial-mesenchymal
transition in ovarian cancer. Cancer Lett. 291:59–66. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin T, Ponn A, Hu X, Law BK and Lu J:
Requirement of the histone demethylase LSD1 in Snai1-mediated
transcriptional repression during epithelial-mesenchymal
transition. Oncogene. 29:4896–4904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin Y, Wu Y, Li J, Dong C, Ye X, Chi YI,
Evers BM and Zhou BP: The SNAG domain of Snail1 functions as a
molecular hook for recruiting lysine-specific demethylase 1. EMBO
J. 29:1803–1816. 2010. View Article : Google Scholar : PubMed/NCBI
|