Introduction
Laryngeal cancer is the most common head and neck
malignant tumor, and the second most common malignant neoplasm of
the respiratory tract. The incidence of laryngeal cancer worldwide
accounts for ~1–5% of systemic tumors, and is also the second
highest disease incidence after nasopharyngeal cancer in
otolaryngology (1,2). Laryngeal cancer seriously endangers
human life and health. Currently, chemotherapy is one of the
commonly used methods for treating laryngeal cancer, but side
effects and drug resistance significantly hinder its effectiveness
(3–5).
The development of new anticancer agents and/or novel therapeutic
strategies to enhance the chemosensitivity of laryngeal carcinoma
cells is therefore the focus of extensive research.
Cisplatin (Cis) is one of the most effective and
commonly used chemotherapeutic drugs for the treatment of locally
advanced laryngeal carcinoma (6). Cis
has been used as a single reagent or in combination with other
agents to treat this disease (7). In
addition, studies have reported that combined chemotherapy with Cis
not only improves the prevention of resistance development in
laryngeal cancer but also the survival rate of patients (8). However, Cis treatment alone may present
several issues, such as easy tolerance or the use of higher doses
after achieving appropriate efficacy and considerable toxic side
effects, which further leads to poor treatment results (9). Thus the combined use of Cis with other
cancer drugs, for appropriate compatibility or multi-target
treatment, has been applied as a crucial therapeutic strategy for
laryngeal cancer (10).
Acetazolamide (Ace) is a small heteroaromatic
sulfonamide that binds to various carbonic anhydrases with high
affinity, acting as a carbonic anhydrase (CA) inhibitor (11). Ace derivatives containing multiple
charges do not efficiently cross the cell membrane and are
restricted in binding to membrane-accessible carbonic anhydrases
(for example, CAIX and potentially CAIV and CAXII) (12). Many sulfonamides inhibit the growth of
tumor cells by inhibiting CAIX and CAXII. This finding is important
for the treatment of tumors, and these two types of CA molecules
have received much attention worldwide as novel potential
anticancer targets. Ace has an antitumor metastasis effect,
inducing the apoptosis of laryngeal cancer cells through the
inhibition of AQP1 expression (13).
Thus, the present study explored whether the Ace/Cis combination
treatment could enhance the chemosensitivity of Hep-2 laryngeal
cells.
In the current study, the drugs were used alone or
in combination, and the results demonstrated that the Ace/Cis
combination treatment was more effective in inhibiting
proliferation of laryngeal carcinoma cells, enhancing cells
apoptosis and decreasing the expression of AQP1.
Materials and methods
Cell culture and treatments
The laryngeal carcinoma cell line Hep-2 and human
umbilical vein endothelial cells (HUVECs) were obtained from the
American Type Culture Collection (Manassas, VA, USA). The Hep-2
cells were maintained in RPMI-1640 culture media (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin and streptomycin, and incubated in a 5%
CO2 humidified atmosphere at 37°C. HUVECs were cultured
in Dulbecco's modified Eagles Medium (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) with 5 mM glucose and 10% fetal bovine
serum in incubator containing 5% CO2 at 37°C.
Experiments were performed at the logarithmic phase of growth of
the cells.
For the drug treatments, Hep-2 cells were treated
with Ace (a low concentration of 1×10−8 mol/l, termed
here AceL; or a high concentration of 5×10−8 mol/l,
termed here AceH), Cis (1 µg/ml) alone, or Cis in combination with
Ace (AceL+Cis, or AceH+Cis) for 48 h. Cells that were treated with
equal volumes of vehicle were used as control. Ace was used at
1×10−8 or 5×10−8 mol/l in all experiments.
Cis was used at 1 µg/ml in all experiments. Both Cis and Ace were
dissolved in dimethyl sulfoxide (DMSO) and then added to PBS to
dilute to the final working concentrations. The final concentration
of DMSO in cultures did not exceed 0.5%. HUVECs were treated with
AceH alone, Cis alone or in combination (AceH+Cis) or control
(vehicle) for 48 h.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA from Hep-2 cells was extracted with TRIzol
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. The mRNA expression of aquaporin-1 (AQP1) was
detected by RT-PCR with GAPDH as an internal control. Reverse
transcription was performed using PrimeScript RT kit with gDNA
Eraser (Takara Biotechnology, Co., Ltd., Dalian, China). PCR
analysis was performed using SYBR Green I (Takara Biotechnology
Co., Ltd., Dalian, China). The primers used were as follows: Human
AQP1, forward 5′-ACCCGCAACTTCTCAAAC-3′ and reverse
5′-AGGCCAAGCCTCCTCTAT-3′; human GAPDH, forward
5′-ACCACAGTCCATGCCATCAC-3′ and reverse 5′-TCCACCACCCTGTTGCTGTA-3′.
The PCR reaction was performed using the following conditions: 5
min pre-denaturation at 94°C, followed by 35 cycles of 30 sec
denaturation at 94°C, 30 sec annealing at 55°C, and 30 sec
extension at 72°C; finally, 10 min extension at 72°C. The PCR
products were detected using 1.5% agarose gel electrophoresis and
the results were processed by gel imaging analysis system (SY-B175;
Guangzhou Sunnymed Electronics Ltd., Guangzhou, China).
MTT assay
The cell viability of Hep-2 cells and HUVECs was
measured by MTT assay (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Hep-2 cells and HUVECs in logarithmic growth phase were
plated in 96-well plates. Following 48 h of drug treatment as
indicated, 200 µl MTT (5 mg/ml) was added to each well. Cells were
incubated with the MTT solution at 37°C for 4 h. Then, 150 µl DMSO
was added for 5 min. The optical density (OD) values were measured
at 490 nm with a Versamax Microplate reader (Molecular Devices,
LLC, Sunnyvale, CA, USA).
Annexin V apoptosis assay
Quantification of apoptotic cells was performed by
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
double staining using a FITC-Annexin V Apoptosis Detection kit (BD
Biosciences, San Jose, CA, USA). At the logarithmic growth phase,
Hep-2 cells were placed in 6-well plates. The cells were treated
with AceL, AceH, Cis, AceL+Cis, AceH+Cis, or vehicle for 48 h.
Then, cells were washed in PBS, digested with trypsin, and
resuspended in calcium-enriched HEPES buffer. This suspension was
stained with Annexin V-FITC and PI for 15 min, as per the
manufacturer's instructions. Finally, the cells were analyzed by
FlowJo software (version 7.6.3; FlowJo, LLC, Ashland, OR, USA).
Western blot analysis
At the logarithmic growth phase, Hep-2 cells were
seeded in 6-well plates. The cells were treated with AceL, AceH,
Cis, AceL+Cis, AceH+Cis for 48 h. Cell protein was extracted by
radioimmunoprecipitation assay lysis buffer (including protease
inhibitor; Beijing ComWin Biotech Co., Ltd., Beijing, China), and
the protein concentration was measured by coomassie brilliant blue
staining. A total of 20 µg protein per lane was resolved by
SDS-PAGE (10% gel) and transferred to polyvinylidene fluoride
membranes (Merck KGaA) for 2 h. After washing, membranes were
blocked in TBS/0.1% Tween-20 (TBST) solution with 5% non-fat dry
milk for 1 h. Primary antibodies against BCL2 associated X (bax;
1:1,000 dilution; ab32503, Abcam, Cambridge, UK), BCL2 apoptosis
regulator (bcl-2; 1:1,000 dilution; ab32124, Abcam), caspase-3
(1:1,000 dilution; ab13585, Abcam), proliferating cell nuclear
antigen (PCNA; 1:5,000 dilution; sc-400037, Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), tumor protein p53 (P53;
1:5000 dilution; sc-416469, Santa Cruz Biotechnology, Inc.) and
β-actin (1:1,000 dilution; ab8226, Abcam) were diluted in TBST/3%
bovine serum albumin and incubated at room temperature for 1 h.
After washing, membranes were incubated at room temperature for 1 h
with secondary antibody (goat anti-mouse, sc-2039; Santa Cruz
Biotechnology, Inc.). Finally, membranes were developed with
enhanced chemiluminescence substrate (PerkinElmer, Inc., Waltham,
MA, USA) for 3–5 min. The results were analyzed with the Quantity
One image analysis software (version 4.62; Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Statistical analysis
The data were analyzed with SPSS version 19.0 (IBM
SPSS Inc., Chicago, IL, USA). One-way analysis of variance was used
to compare the differences between treatment groups. The
Student-Newman-Keuls post hoc test was used to compare differences
between two groups. All results were expressed as mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Combined Ace and Cis treatment
effectively reduces viability in Hep-2 cells
Compared with the control group, the high Ace
concentration (AceH, 5×10−8 mol/l), Cis (1 µg/ml) and
Cis combined with the low Ace concentration (AceL,
1×10−8 mol/) treatments significantly reduced viability
of Hep-2 cells (P<0.05, P<0.05 and P<0.05, respectively;
Fig. 1A). The effects of the AceH and
Cis combination treatment (AceH+Cis) were significantly different
from the control group (P<0.01; Fig.
1A). Notably, the AceL+Cis and AceH+Cis treatments were
statistically different compared with the Cis alone group
(P<0.05 and P<0.01, respectively; Fig. 1A). Compared with the control group,
either AceH or Cis alone or their combination treatment had no
effects on the viability of HUVECs (P>0.05; Fig. 1B). These results suggested that
combined use of Ace and Cis reduced the viability of Hep-2 cells
more effectively than AceH or Cis alone.
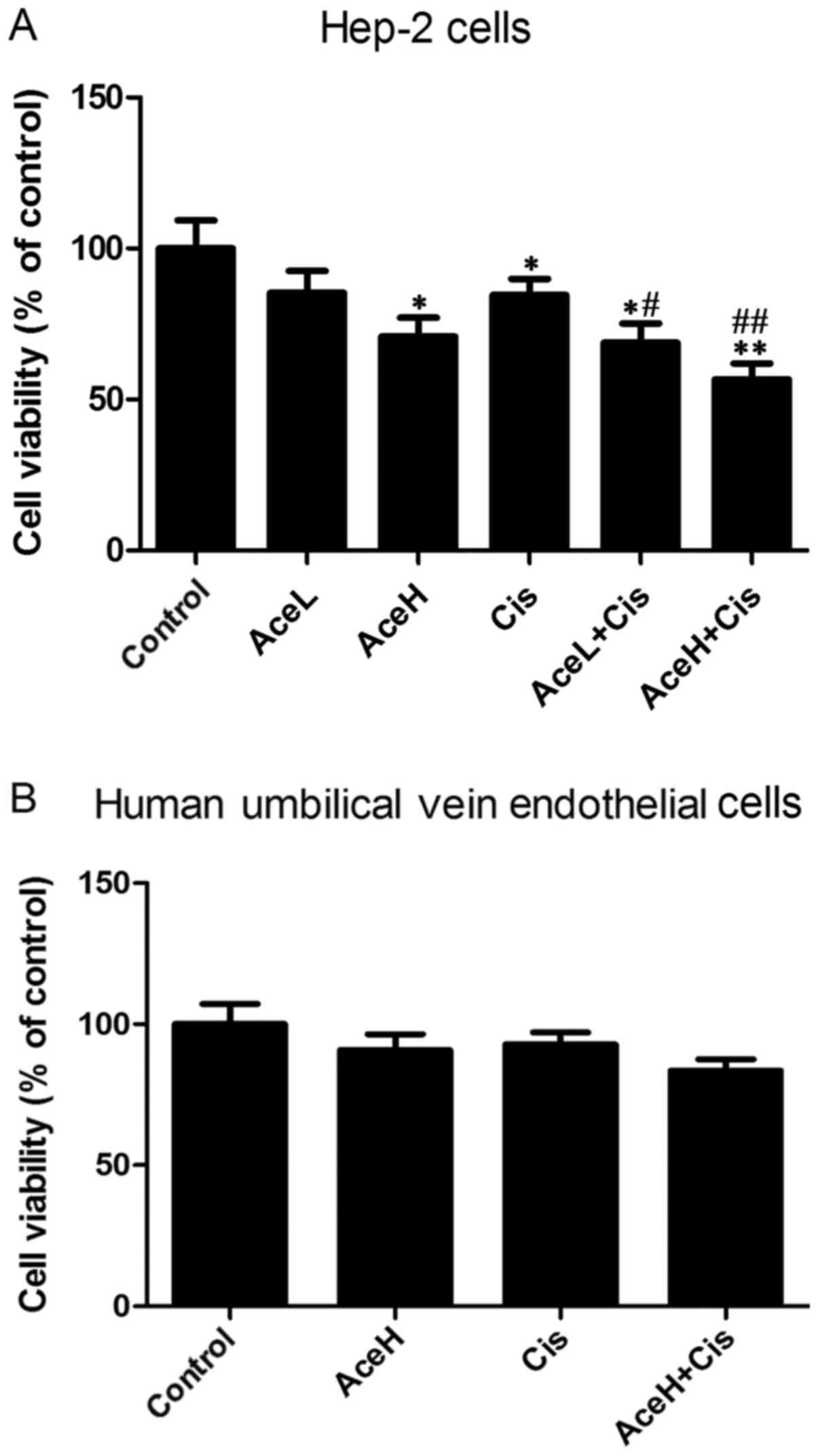 | Figure 1.Effects of Ace and/or Cis treatment on
cell viability. (A) Hep-2 cells were treated with AceL, AceH, Cis,
AceL+Cis, AceH+Cis or vehicle for 48 h. (B) Human umbilical vein
endothelial cells were treated with AceH, Cis, AceH+Cis and vehicle
for 48 h. Cells viability was determined by MTT assay. Data were
expressed as mean ± standard deviation. *P<0.05 and **P<0.01
compared with control group; #P<0.05 and
##P<0.01 compared with Cis group. Ace, acetazolamide;
Cis, cisplatin; L, low dose 1×10−8 mol/l; H, high dose
5×10−8 mol/l. |
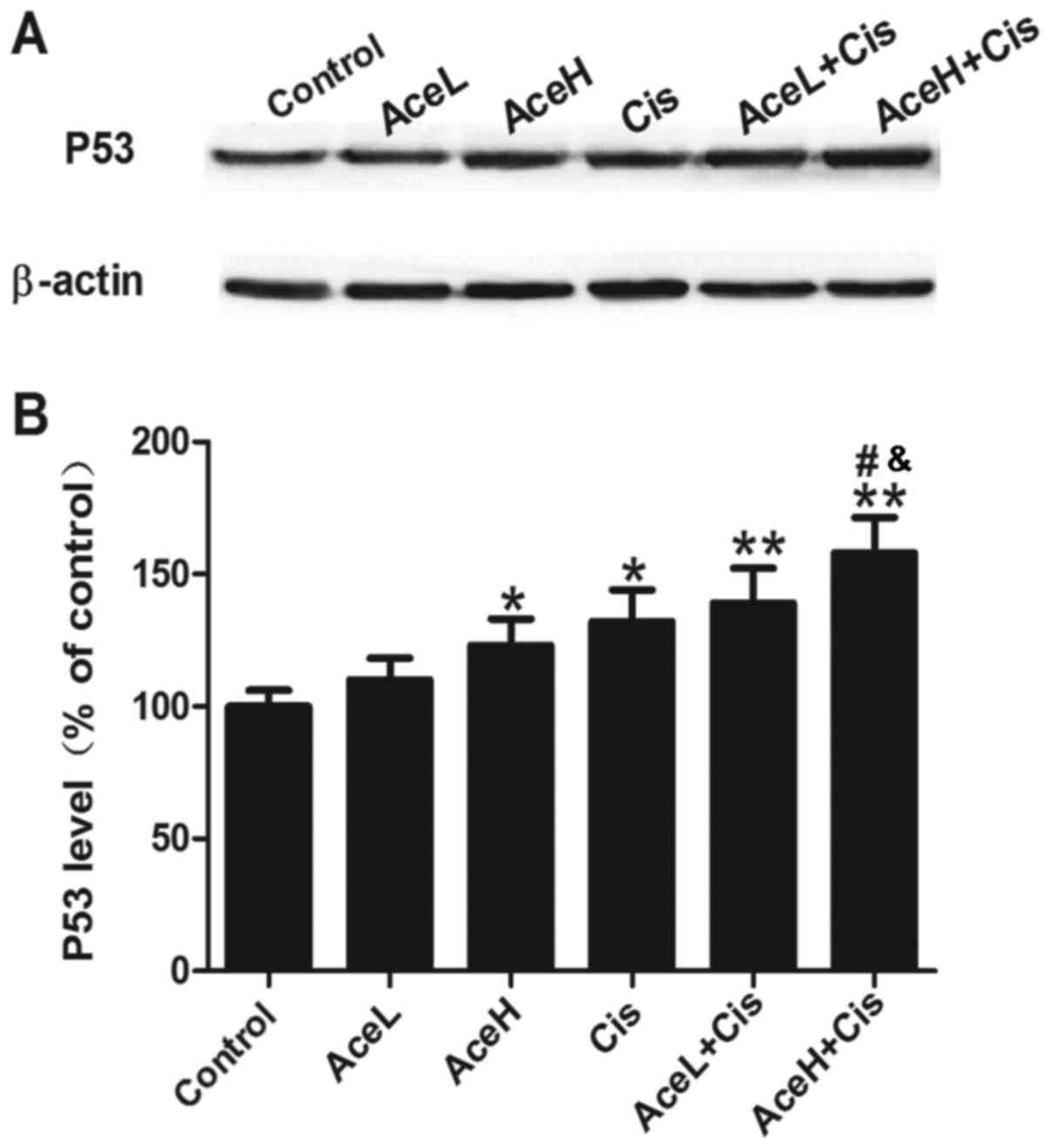
Combined Ace and Cis treatment
effectively upregulates P53 expression in Hep-2 cells
P53 is a tumor suppressor gene that has an important
role in the negative regulation of cell proliferation. Treatment
with the Ace/Cis combination significantly increased the expression
levels of P53 (Fig. 2A), as both
AceL+Cis and AceH+Cis treatments resulted in significantly
increased P53 protein expression levels compared with the control
group (P<0.01 for both; Fig. 2B).
In addition, AceH+Cis more effectively increased the expression of
P53 compared with either the AceH or Cis alone treatments
(P<0.05; Fig. 2B). These results
suggested that the effects of Ace were dose-dependent, and that the
combined use of Ace and Cis inhibited the proliferation of Hep-2
cells more effectively than AceH or Cis alone.
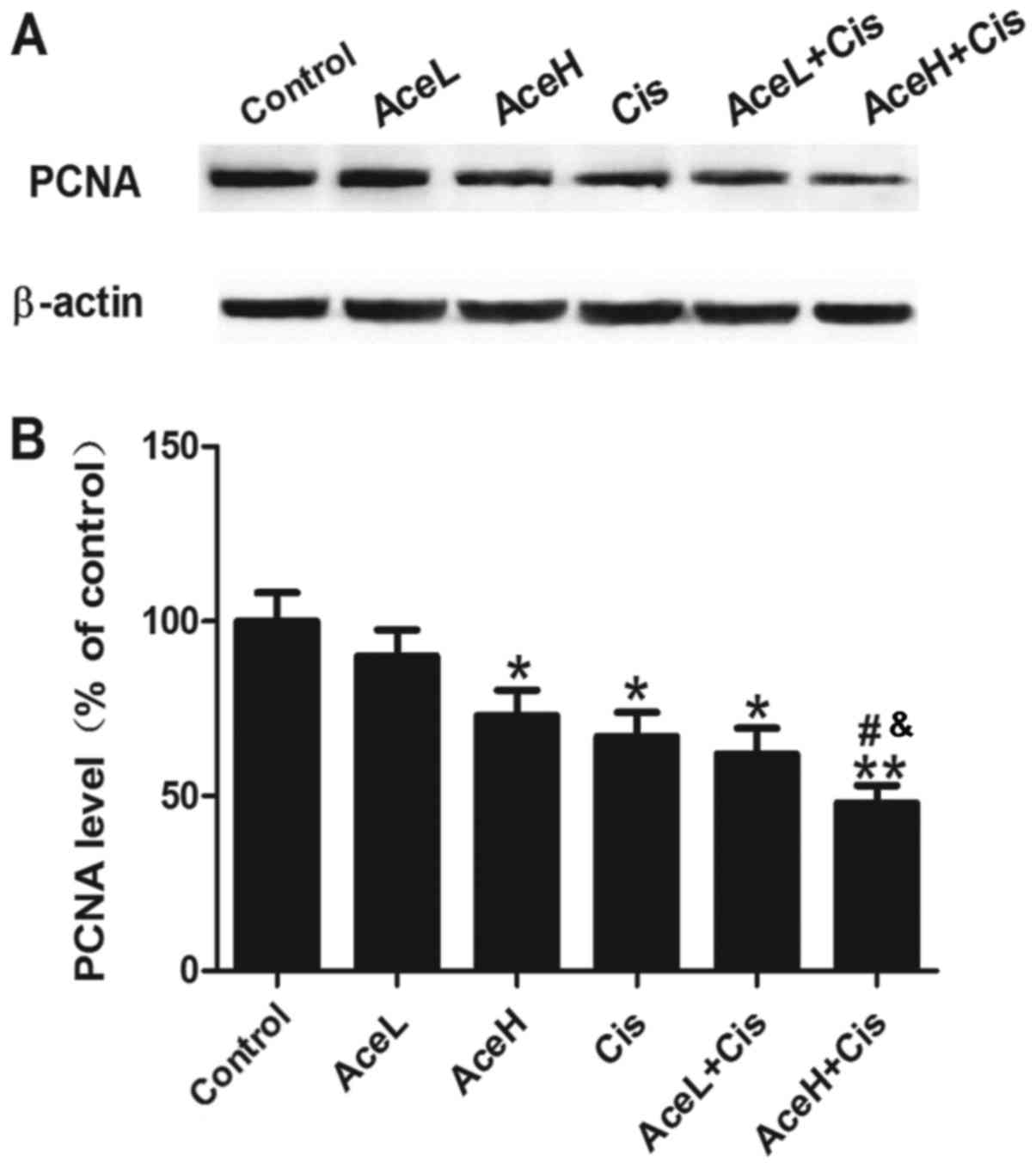
Combined Ace and Cis treatment
effectively inhibits proliferation in Hep-2 cells
Next, to observe the effects of different drug
treatments on cell proliferation, PCNA protein expression was
examined, as an indicator of tumor cell proliferation activity
(Fig. 3A). The results demonstrated
that AceH, Cis and AceL+Cis treatments significantly inhibited the
expression of PCNA compared with the control group (P<0.05 for
all; Fig. 3B), and that the effect of
AceH+Cis treatment was more significant compared with the control
group (P<0.01; Fig. 3B). In
addition, PCNA expression levels following AceH+Cis treatment were
significantly decreased compared with the AceH or Cis alone
treatments (P<0.05 for both; Fig.
3B). These results suggested that the effects of Ace on cell
proliferation were dose-dependent, and that combined treatment of
Ace and Cis decreased the proliferation of Hep-2 cells more
effectively than AceH or Cis alone.
Combined Ace and Cis treatment
effectively promotes apoptosis in Hep-2 cells
To verify whether combination therapy is superior to
the use of each drug alone, the apoptosis rate of Hep-2 cells was
further evaluated using flow cytometric analysis. After 48 h, the
combined treatment AceH+Cis significantly induced the apoptosis of
Hep-2 cells (P<0.01). In addition, the effects of AceH+Cis
combination treatment on the apoptosis of Hep-2 cells were more
pronounced compared with the AceH or AceL+Cis treatments
(P<0.05; Fig. 4).
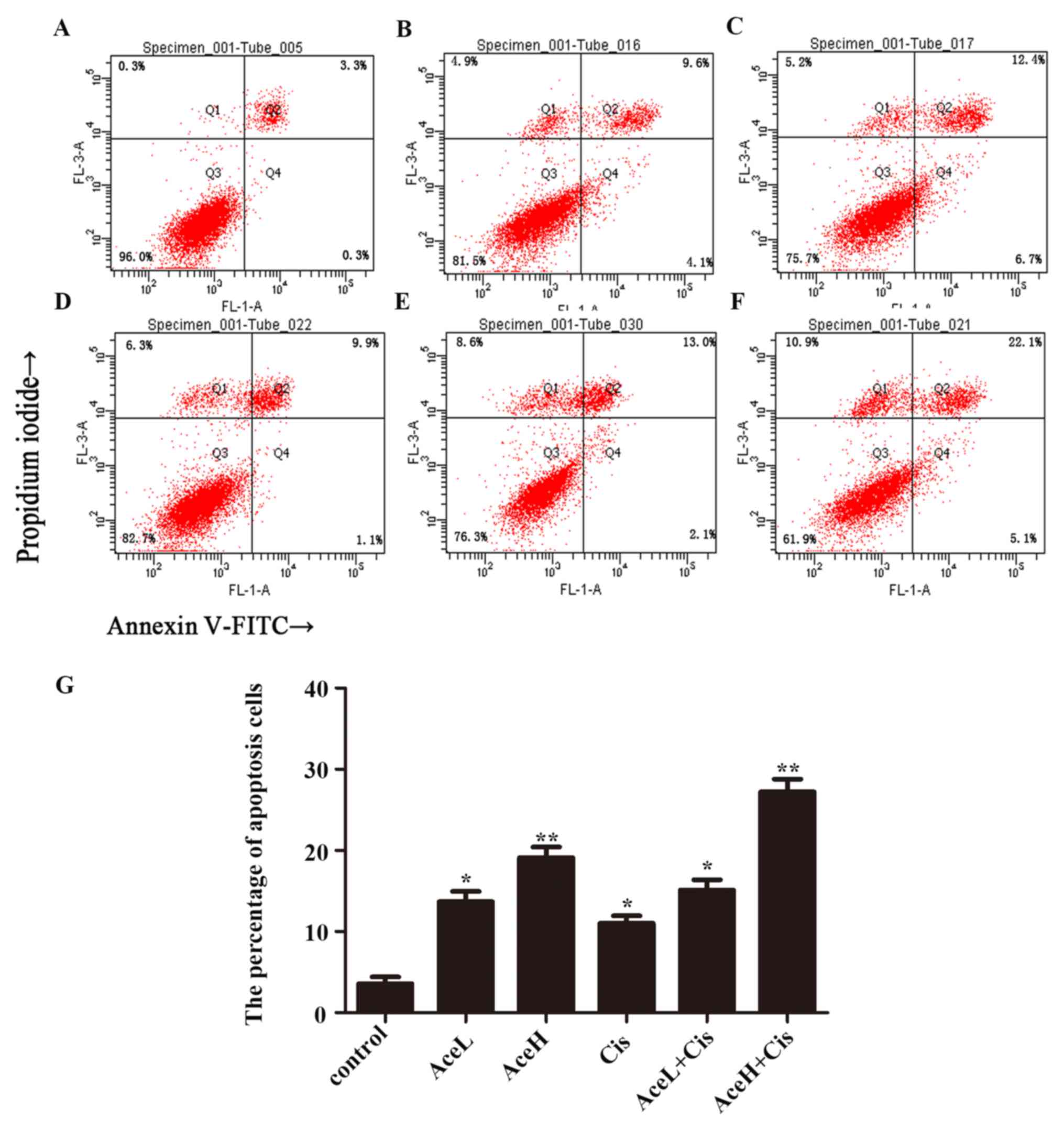 | Figure 4.Combined effect of Ace and Cis on
cell apoptosis of Hep-2 cells. Flow cytometric analysis of Annexin
V/propidium iodide double staining in Hep-2 cells following drug
treatments for 48 h. (A) Untreated (control), (B) AceL, (C) AceH,
(D) Cis, (E) AceL+Cis, (F) AceH+Cis, and (G) quantification of A-F.
*P<0.05 and **P<0.01 compared with control group. Ace,
acetazolamide; Cis, cisplatin; L, low dose 1×10−8 mol/l;
H, high dose 5×10−8 mol/l. |
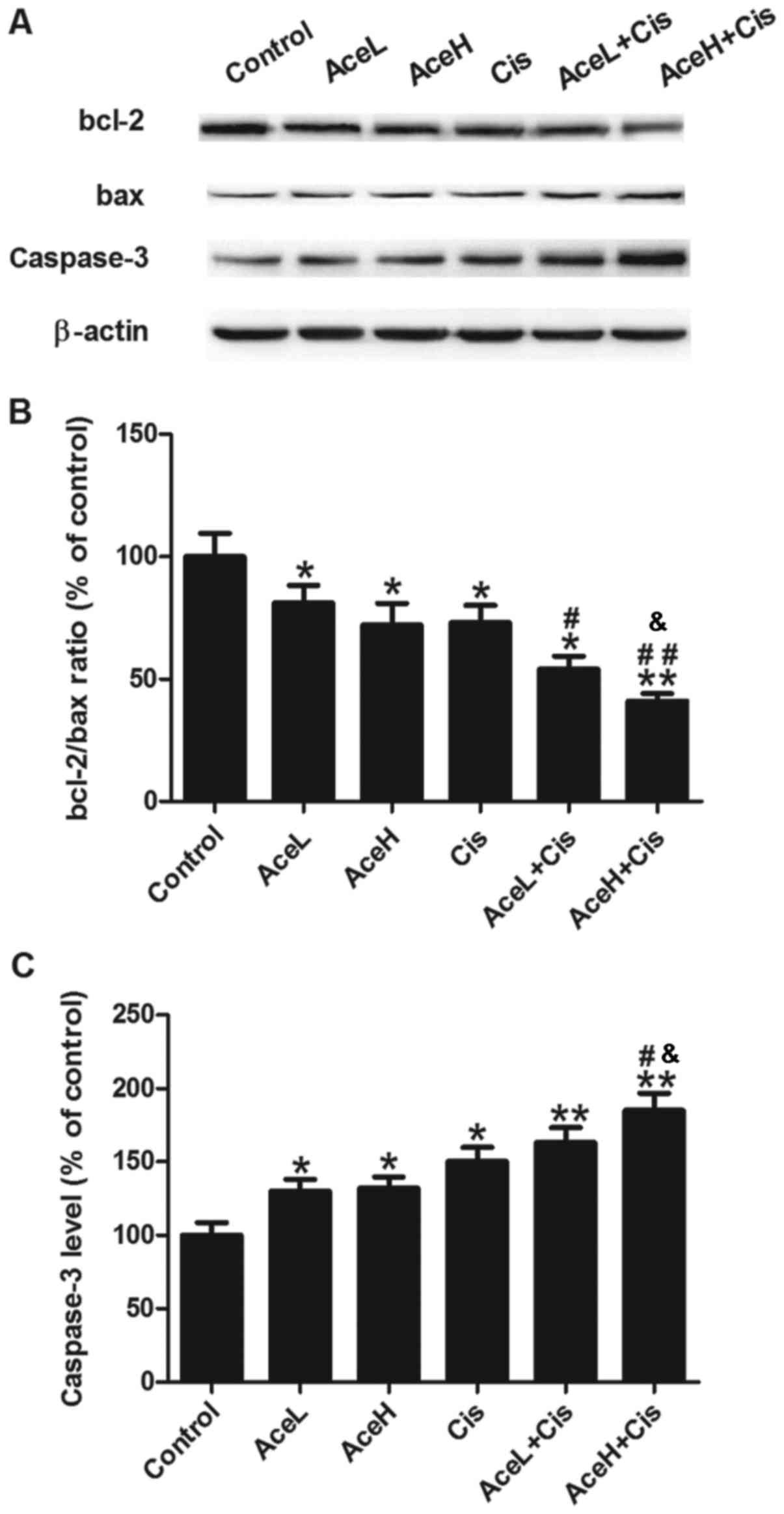
Bcl-2, bax and caspase-3 are apoptosis-related
proteins. To further assess the apoptotic response of Hep-2 cells
following the treatments, the expression levels of these proteins
were analyzed by western blotting (Fig.
5A). The Ace/Cis combination treatment significantly reduced
the bcl-2/bax expression ratio (P<0.05; Fig. 5B), and increased the expression of
caspase-3 protein (P<0.01; Fig.
5C), compared with the control group. AceL, AceH, Cis and
AceL+Cis treatments significantly reduced the bcl-2/bax ratio
compared with the control group (P<0.05 for all; Fig. 5B). However, compared with the control
group, the AceH+Cis treatment displayed a more significant
reduction of the bcl-2/bax ratio compared with the control
(P<0.01; Fig. 5B). The AceL+Cis
treatment significantly reduced the bcl-2/bax ratio compared with
the Cis group (P<0.05; Fig. 5B).
The effect of AceH+Cis was more significant compared with either
AceH or Cis alone on the inhibition of bcl-2/bax (P<0.05 and
P<0.01, respectively; Fig. 5B). As
far as the caspase-3 levels are concerned, AceL, AceH and Cis
treatments alone significantly increased caspase-3 expression
compared with the control group (P<0.05 for all; Fig. 5C). The expression of caspase-3 protein
was significantly increased in the AceL+Cis and AceH+Cis treatment
groups compared with the control group (P<0.01 for both;
Fig. 5C). Furthermore, the effects of
AceH+Cis treatment on increasing caspase-3 were more significant
compared with AceH or Cis alone (P<0.05 for both; Fig. 5C).
These results suggested that the effects of Ace on
cell apoptosis were dose-dependent, and that combined treatment of
Ace and Cis promoted apoptosis in Hep-2 cells more effectively than
AceH or Cis alone.
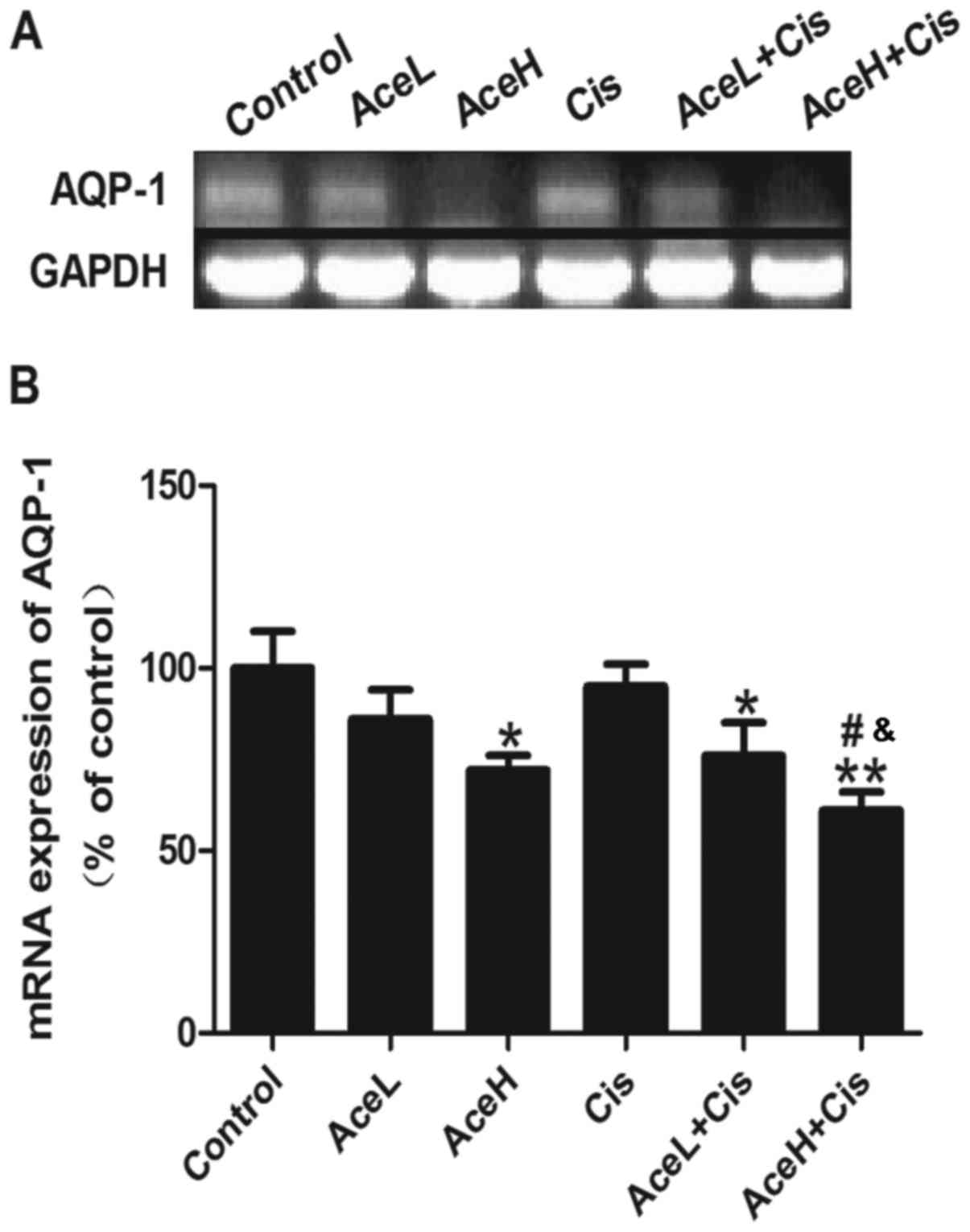
Combined Ace and Cis treatment
effectively decreased the expression of AQP1 mRNA
Combined treatment with Ace/Cis markedly decreased
the expression of AQP1 mRNA in Hep-2 cells (Fig. 6A). Both AceH and AceL+Cis treatments
decreased the expression of AQP1 mRNA in Hep-2 cells compared with
the control group (P<0.05 for both; Fig. 6B). AceH+Cis treatment also decreased
the expression of AQP1 mRNA in Hep-2 cells compared with the
control group (P<0.01; Fig. 6B).
Notably, the effects of AceH+Cis treatment on the expression of
AQP1 mRNA were more significant than those of AceH or Cis alone
(P<0.05 for both; Fig. 6B). These
results suggested that combined treatment of Ace and Cis decreased
the expression of AQP1 mRNA more effectively than AceH or Cis
alone.
Discussion
In the present study, the results demonstrated that
combined treatment with Ace and Cis inhibited the proliferation of
Hep-2 cells more effectively than each drug alone. Treatment of
laryngeal cancer cells with the Ace/Cis combination enhanced the
expression of apoptosis-related proteins and decreased the
expression of the proliferation marker PCNA. Finally, the
combination treatment increased the expression of the tumor
suppressor protein p53, thereby affecting the expression of
AQP1.
Laryngeal cancer is the most common head and neck
malignant tumor. Currently, Cis is one of the commonly used drugs
for laryngeal cancer treatment (14).
However, Cis alone has many shortcomings, such as drug resistance,
nephrotoxicity, gastrointestinal toxicity, bone marrow suppression
and ototoxicity and other considerable toxic side effects, which
limit the use of this drug (15).
Thus, currently the chemotherapy regimens for laryngeal cancer are
based on Cis combination therapies. Studies have reported that
resveratrol significantly increases the sensitivity of Hep-2
laryngeal cancer cell lines to Cis (16). Ginsenoside Rh2 or Cis alone can induce
the apoptosis of laryngeal carcinoma cells, and the combination of
the two drugs displays a synergistic effect, inducing a more
obvious anticancer effect (10). In
addition, several studies have demonstrated that other compounds
bound to Cis constitute an effective method to overcome drug
resistance and reduce adverse side effects (17,18). Ace
has received much attention in the field of cerebrovascular disease
and cancer (19). Previous studies
have indicated that a small molecule-drug conjugated product, which
was obtained using Ace and monomethylated toast oil, exerted an
effective antitumor activity in mice with renal cell carcinoma
(12). Additional studies have
demonstrated that Ace, as a treatment in mice with transplanted
human colon tumors, significantly suppressed tumor growth, with
tumor inhibition rates as high as 88.28% (20,21).
Studies have indicated that the commonly used doses of Cis in
antitumor experiments in vitro are 0.25–20 µg/ml (6–25). To
observe the effect of combination therapy and minimize the
generation of side effects, the present study selected a relatively
low effective dose of 1 µg/ml Cis. Notably, 1 µg/ml Cis effectively
inhibits the proliferation of laryngeal cancer cells, the effect of
which was less than what has been previously observed with 5 µg/ml
Cis (22,25,26). In
the present study, the anticancer effect of the combination therapy
was significantly improved compared with that observed with 1 µg/ml
Cis alone treatment, and reached or exceeded the effect of 5 µg/ml
Cis, suggesting that the combination therapy could improve the
chemical sensitivity of laryngeal cancer cells. Furthermore, the
combination of Ace and Cis displayed little cytotoxicity on normal
HUVECs. Taken together, the combined use of these drugs not only
reduced the toxicity of Cis but also promoted the chemotherapy
sensitivity of laryngeal cancer to Cis, suggesting that the Ace/Cis
combination treatment may potentially be a useful therapeutic
option for patients diagnosed with laryngeal cancer.
To further characterize the potential mechanism
underlying the synergistic effects of the combined Ace/Cis
treatment, cell apoptosis experiments were performed. The results
demonstrated that compared to treatment with Ace or Cis alone, the
cell apoptosis induced by their combination was significantly
increased, indicating that Ace enhances Cis-induced cell apoptosis
on Hep-2 cells. The main mechanism underlying the antitumor effect
of Cis involves the induction of tumor cell apoptosis. Studies have
reported that changes in tumor cell apoptosis primarily reflect
abnormal cell apoptotic signal transduction pathways and the
abnormal expression of apoptosis-related factors (22).
Ace has broad spectrum inhibition effects on
carbonic anhydrase in cells from different tissues, including human
erythrocytes, pancreas, and the central nervous system (12). Studies have previously reported that
Ace had an important role in inhibiting angiogenesis, and that this
function may be associated with the upregulation of DNA-related
proteins as observed by serum proteomics (19). To obtain a better understanding of the
mechanisms by which the Ace/Cis combination enhances the
sensitivity of laryngeal carcinoma Hep-2 cells to Cis, the present
study further investigated the expression levels of key proteins
that regulate the proliferation and apoptosis of laryngeal cancer
cells. The present results demonstrated that treatment with the
Ace/Cis combination significantly reduced the bcl-2/bax ratio and
increased the expression of caspase-3 protein. Regulation of cell
apoptosis protein is generally performed by both
apoptosis-inhibiting and apoptosis-inducing proteins. Among these
proteins, the BCL2 family is an important apoptosis regulator;
bcl-2 is an apoptosis-inhibiting protein, while bax is an
apoptosis-inducing protein. To achieve anti-apoptosis effects,
Bcl-2 and other apoptosis inhibitors block caspase-3 activity and
degrade the caspase-3 substrate poly(ADP-ribose) polymerase
(27).
Combined treatment with Ace and Cis also more
effectively inhibited the proliferation of Hep-2 cells than each
drug used alone. PCNA, a well-established proliferation marker, is
an acidic polypeptide synthesized and expressed in proliferating
laryngeal cancer cells and an essential factor in cell synthesis
(28). PCNA is expressed in the
nucleus during the late G1 phase, increases during the S phase, and
declines during the G2 and M phases. Thus, the expression levels of
PCNA have a clear correlation with cell proliferation, and are used
as an indicator of cell proliferation (29,30). The
expression levels of PCNA in cells treated with a high
concentration of Ace combined with Cis were decreased compared with
either single treatment group, suggesting that the Ace and Cis
combination inhibited the expression of PCNA, consistent with
previous studies (28–30).
The tumor suppressor gene p53 is one of the most
well-studied tumor suppressor genes in the last decade (31). P53 serves a role in cancer suppression
in a variety of mechanisms, referred to as the ‘molecular police’
to maintain the stability of the human genome. By contrast, the
mutant p53 gene has a role as a proto-oncogene, which promotes the
development and progression of tumors. P53 gene mutations are
observed in almost all human tumors (32). In tumor cells, mutant p53 loses its
ability to monitor cells, leading to continuous proliferation and
lack of cell apoptosis. The mismatched DNA cells can still enter
the S phase, eventually leading to the occurrence of cancer
(33). In the present study,
treatment of Hep-2 cells with the combination of drugs reduced the
expression of p53, which may explain the observed inhibition of
proliferation.
In the present study, the results also revealed that
the Ace/Cis combination therapy reduced the expression of AQP1 more
effectively than either agent alone. Studies have reported that Ace
is one of the most widely used aquaporin inhibitors, and its site
of action is consistent with the distribution of AQP1. The
Ace-mediated inhibition of tumor metastasis may be associated with
the downregulation of AQP1 protein expression (34). AQP1 is expressed in throat vascular
endothelial cells, and this protein can quickly transport water
into the surrounding tissue fluid through capillary endothelial
cells, which is consistent with the needs of throat cell
metabolism. Therefore, AQP1 has an important role in maintaining
the normal physiological function of the throat (35). It has been confirmed that AQP1
expression in laryngeal carcinoma vascular endothelial cells is
significantly higher than that in adjacent normal tissues (36). This finding confirms that AQP1 can
increase tumor vascular permeability and promote the rapid water
transport in tumor cells, thereby promoting tumor angiogenesis
(36). The increased expression of
AQP1 alters the osmotic pressure of tumor cell membrane, changes
the tumor cell volume and shape, and subsequently affects the
surrounding matrix infiltration, promoting tumor cell
proliferation. By contrast, if the expression of AQP1 is reduced,
then the rate of tumor migration decreases (37).
In summary, the present results have demonstrated
for the first time that Ace/Cis combination treatment inhibited
cell proliferation and promoted apoptosis in Hep-2 cells, while
decreasing the expression of AQP1. These findings suggest a
potential clinical application of the combination regimen for the
treatment of laryngeal cancer. However, a limitation of the current
report is the lack of validation using in vivo experiments.
Further studies will be required in the future to validate these
results in an in vivo setting.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HG made substantial contributions to the conception
and design of the study. HD and GL provided materials for the
study. HD, GL and HJ contributed to the collection and assembly of
data. GL and HJ analyzed and interpreted the data. All authors made
contributions in writing the manuscript. All authors approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Ace
|
acetazolamide
|
|
Cis
|
cisplatin
|
|
AQP1
|
aquaporin-1
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
AceH
|
high concentration of
acetazolamide
|
|
AceL
|
low concentration of acetazolamide
|
References
|
1
|
Markowski J, Sieroń AL, Kasperczyk K,
Ciupińskakajor M, Auguściakduma A and Likus W: Expression of the
tumor suppressor gene hypermethylated in cancer 1 in laryngeal
carcinoma. Oncol Lett. 9:2299–2302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pei SG, Wang JX, Wang XL, Zhang QJ and
Zhang H: Correlation of survivin, p53 and Ki-67 in laryngeal cancer
Hep-2 cell proliferation and invasion. Asian Pac J Trop Med.
8:636–642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang X, An L and Li X: Arsenic trioxide
induced endoplasmic reticulum stress in laryngeal squamous cell
line Hep-2 cells. Auris Nasus Larynx. 41:81–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng JZ, Yu D, Zhang H, Jin CS, Liu Y,
Zhao X, Qi XM and Liu XB: Inhibitive effect of IL-24 gene on
CD133(+) laryngeal cancer cells. Asian Pac J Trop Med. 7:867–872.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Min JW, Kim KI, Kim HA, Kim EK, Noh WC,
Jeon HB, Cho DH, Oh JS, Park IC, Hwang SG and Kim JS:
INPP4B-mediated tumor resistance is associated with modulation of
glucose metabolism via hexokinase 2 regulation in laryngeal cancer
cells. Biochem Biophys Res Commun. 440:137–142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang R, Wang ZH, Wang BQ, Zhang CM, Gao W,
Feng Y, Bai T, Zhang HL, Huang-Pu H and Wen SX: Inhibition of
autophagy-potentiated chemosensitivity to cisplatin in laryngeal
cancer Hep-2 cells. Am J Otolaryngol. 33:678–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang D and Wu X: In vitro and in vivo
targeting of bladder carcinoma with metformin in combination with
cisplatin. Oncol Lett. 10:975–981. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cimbora-Zovko T, Fritz G, Mikac N and
Osmak M: Downregulation of RhoB GTPase confers resistance to
cisplatin in human laryngeal carcinoma cells. Cancer Lett.
295:182–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu T, Peng H, Zhang M, Deng Y and Wu Z:
Cucurbitacin B, a small molecule inhibitor of the Stat3 signaling
pathway, enhances the chemosensitivity of laryngeal squamous cell
carcinoma cells to cisplatin. Eur J Pharmacol. 641:15–22. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong-Jun XU, Meng Y and Sun YX: Effects of
ginsenoside Rh_2 associated with cisplatin on human laryngeal
squamous cell carcinoma strain Hep-2. Chin J Lab Diagn. 10:506–508.
2005.
|
|
11
|
Ahlskog JK, Dumelin CE, Trüssel S, Mårlind
J and Neri D: In vivo targeting of tumor-associated carbonic
anhydrases using acetazolamide derivatives. Bioorg Med Chem Lett.
19:4851–4856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cazzamalli S, Corso AD and Neri D: Linker
stability influences the anti-tumor activity of acetazolamide-drug
conjugates for the therapy of renal cell carcinoma. J Control
Release. 246:39–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guan G and Dong Z: Effect of inhibiting
aquaporin-1 on proliferation and apoptosis of the Hep-2 cell. Lin
Chuang Er Bi Yan Hou Ke Za Zhi. 20:988–991. 2006.(In Chinese).
PubMed/NCBI
|
|
14
|
Zhang X, Zhang L and Zou Y: Research
evolution of cisplatin antitumor drugs and cisplatin-loaded. China
Mod Med. 18:25–27. 2011.
|
|
15
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhan S, Ping W, Yin W and Wei Z:
Enhancement effect of resveratrol on sensitivity of laryngeal
carcinoma Hep-2 cells to cisplatin and its mechanism. J Jilin Uni.
41:282–286. 2015.
|
|
17
|
Cui Y, Chao W, Xu D, Meng W and Quan X:
AstragalosideII inhibits autophagic flux and enhance
chemosensitivity of cisplatin in human cancer cells. Biomed
Pharmacother. 81:166–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh M, Bhui K, Singh R and Shukla Y: Tea
polyphenols enhance cisplatin chemosensitivity in cervical cancer
cells via induction of apoptosis. Life Sci. 93:7–16. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiao ZH, Lin W and Hai W: Recent progress
in clinical application of the carbonic anhydrase inhibitor,
acetazolamide. Chin J New Drugs. 17:1390–1394. 2008.
|
|
20
|
Kong B, Xiao-Hua WU and Yong LI: Effects
of aquaporin protein inhibitor acetazolamide on xenograft tumor
growth of colon cancer in nude mice. China J Modern Med.
20:1466–1465. 2010.
|
|
21
|
Bin K and Shi-Peng Z: Acetazolamide
inhibits aquaporin-1 expression and colon cancer xenograft tumor
growth. Hepatogastroenterology. 58:1502–1506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nör C, Zhang Z, Warner KA, Bernardi L,
Visioli F, Helman JI, Roesler R and Nör JE: Cisplatin induces Bmi-1
and enhances the stem cell fraction in head and neck Cancer.
Neoplasia. 16:137–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu YY, Wu TT, Zhou SH, Bao YY, Wang QY,
Fan J and Huang YP: Apigenin suppresses GLUT-1 and p-AKT expression
to enhance the chemosensitivity to cisplatin of laryngeal carcinoma
Hep-2 cells: An in vitro study. Int J Clin Exp Pathol. 7:3938–3947.
2014.PubMed/NCBI
|
|
24
|
Ju SM, Kang JG, Bae JS, Pae HO, Lyu YS and
Jeon BH: The flavonoid apigenin ameliorates cisplatin-induced
nephrotoxicity through reduction of p53 activation and promotion of
PI3K/Akt pathway in human renal proximal tubular epithelial cells.
Evid Based Complement Alternat Med. 2015:1864362015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao W, Ying L, Qin R, Liu D and Feng Q:
Silence of fibronectin 1 increases cisplatin sensitivity of
non-small cell lung cancer cell line. Biochem Biophys Res Commun.
476:35–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamauchi K, Sakurai H, Kimura T,
Wiriyasermkul P, Nagamori S, Kanai Y and Kohno N: System L amino
acid transporter inhibitor enhances anti-tumor activity of
cisplatin in a head and neck squamous cell carcinoma cell line.
Cancer Lett. 276:95–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang XK, Zheng F, Chen JH, Gao QL, Lu YP,
Wang SX, Wang CY and Ma D: Relationship between expression of
apoptosis-associated proteins and caspase-3 activity in
cisplatin-resistant human ovarian cancer cell line. Ai Zheng.
21:1288–1291. 2002.(In Chinese). PubMed/NCBI
|
|
28
|
Shi-Hong MA and Tan WH: Expression and
clinical significance of FHIT and PCAN protein in endometrial
carcinoma. J Harbin Med Uni. 43:62–65. 2009.
|
|
29
|
Sittel C, Ruiz S, Volling P, Kvasnicka HM,
Jungehülsing M and Eckel HE: Prognostic significance of Ki-67
(MIB1), PCNA and p53 in cancer of the oropharynx and oral cavity.
Oral Oncol. 35:583–589. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo GQ, Dai D and Dong-Mei WU: The
clinical implication of experession for both PCNA and p53 protein
in larynx cancer patients. Ningxia Med J. 2001.
|
|
31
|
Wang ZM, Chang-Shao XU and Sun YM: The
clinic value of the expression of p53, p16, PCNA protein in
esophageal carcinoma. Bull Chin Cancer. 15:61–62. 2006.
|
|
32
|
Zeng R: Expression of p53, p21, PCNA and
COX-2 and its relationship with recurrence in the early-stage
laryngeal cancer with negative surgical margin. Lin Chung Er Bi Yan
Hou Tou Jing Wai Ke Za Zhi. 30:349–352. 2016.(In Chinese).
PubMed/NCBI
|
|
33
|
Pfister NT, Yoh KE and Prives C: p53, DNA
damage, and NAD+ homeostasis. Cell Cycle. 13:1661–1662. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hayashi Y, Edwards NA, Proescholdt MA,
Oldfield EH and Merrill MJ: Regulation and function of aquaporin-1
in glioma cells. Neoplasia. 9:777–787. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guan B, Sun L and Dong Z: The expression
and distribution of Aquaporin 1 and Aquaporin 4 in laryngeal
carcinoma and its significance. Chin J Clin Oncol. 21:269–272.
2005.
|
|
36
|
Musumeci G, Leonardi R, Carnazza ML,
Cardile V, Pichler K, Weinberg AM and Loreto C: Aquaporin 1 (AQP1)
expression in experimentally induced osteoarthritic knee menisci:
An in vivo and in vitro study. Tissue Cell. 45:145–152. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hoque MO, Soria JC, Woo J, Lee T, Lee J,
Jang SJ, Upadhyay S, Trink B, Monitto C, Desmaze C, et al:
Aquaporin 1 is overexpressed in lung cancer and stimulates NIH-3T3
cell proliferation and anchorage-independent growth. Am J Pathol.
168:1345–1353. 2006. View Article : Google Scholar : PubMed/NCBI
|