Introduction
Malignant melanoma is the severe and life
threatening cancer of melanocytes, which has increased by ~3%
steadily throughout the last decade (1). Malignant melanoma is considered curable
when detected at an early stage. However, once malignant melanoma
has entered the advanced stages of disease, it disseminates widely
and s becomes an incurable malignancy with a very poor prognosis
(2). Due to special characteristics,
including a large intercellular distance, poorly defined cell
borders and architectural disarray (3), and usual resistance to standard
chemotherapy, there is no systemic and effective therapy at present
that has a clear effect on the overall survival of patients with
malignant melanoma (4). Although the
understanding of the molecular biology of malignant melanoma has
increased in recent years, the molecular mechanism of
melanomagenesis is not completely understood and requires further
elucidation.
Although cytotoxic chemotherapy is widely used in
patients with metastatic melanoma, it now has a limited role with
little to no response in patients (5). Historically, patients with metastatic
melanoma that were treated with conventional chemotherapies have a
poor prognosis, with median survival duration of merely 6–9 months
(6,7).
Recombinant interferon (IFN)-α has been utilized in the treatment
of patients with malignant melanoma following surgery (8). Clinically relevant benefits from IFN-α
therapy include an increase in the duration of disease-free
survival (DFS) by 9 months and also an increase in the rate of
5-year progression-free survival by 9% (9,10).
It has been indicated that the efficacy of IFN-α
therapy requires improvement (11).
IFN-α regulates the anti-proliferative effects on melanoma cells
and modulates the immune response (12). Melanoma cells are less responsive to
IFN-α compared with immune cells (13). Specifically, IFN-α has been shown to
sensitize malignant cells to increase the expression of death
receptors and by upregulating the expression of cell cycle
regulatory proteins, including p21, which is a cyclin-dependent
kinase inhibitor (14,15). These data suggested that IFN-α therapy
potentially serves a role in increasing the pro-apoptotic effects
in melanoma.
The Fas receptors and Fas ligands (Fas/FasL) system
is a key regulator of apoptosis in T-cells (16). In early- or intermediate-phase
melanomas, the Fas apoptotic pathway is active. However, the Fas
apoptotic pathway is altered in highly metastatic melanomas
(17). Furthermore, the lack of Fas
expression in malignant melanomas is associated with a poor
prognosis (18). During the
progression of melanoma, the expression of Fas is decreased and the
expression of FasL is increased (19).
Inhibitor of growth family member 4 (ING4), which is
a member of the conserved ING family, has been identified as a
novel tumor suppressor (20). ING4,
which is located on chromosome 12p13, contains two nuclear
localization signals and a highly conserved plant homeodomain
finger motif at the C-terminal end (21). The expression of ING4 is significantly
decreased in primary and metastatic melanomas compared with
dysplastic nevi (22). Furthermore,
the overexpression of ING4 resulted in a diminished colony-forming
efficiency, a decreased S phase cell population, and apoptosis was
induced in a p53-dependent manner (21,22).
In the present study, it was hypothesized that ING4
is able to enhance the anti-tumor effects of IFN-α2b by activating
the common pro-apoptotic pathways. In the present study, it was
demonstrated that the combination of overexpression of ING4 and
IFN-α2b treatment in malignant melanoma cells was able to induce
apoptosis. Apoptosis induced by this combination treatment was
associated with a decreased expression of caspase-3, and −8 as well
as poly (ADP-ribose) polymerase (PARP). Additionally, the
combination treatment also resulted in an increased expression of
cleaved caspase-3, cleaved caspase-8 and cleaved PARP. Notably, the
combination of ING4 overexpression and IFN-α2b treatment was able
to effectively induce apoptosis, which resulted in increased
Fas/FasL death signaling. Increased Fas/FasL death signaling was
associated with cellular survival and resistance to apoptosis in
melanoma cells.
The results of the present study indicate that the
effect of overexpression of ING4 was increased in the presence of
IFN-α2b, and this may be a potential treatment strategy for
melanoma.
Materials and methods
Cell culture
The human melanoma cell lines A375 and HT-144 were
cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), containing 10% (v/v) fetal bovine serum
(FBS) (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) at 37°C in a humidified incubator with 5%
CO2.
Construction of ING4 overexpression or
knockdown vectors in human melanoma cells
The pcDNA3.1-ING4 was constructed as previously
described (23). The lentiviral
vector (pLKO.1) constructs containing ING4 cDNA or 100 µM short
hairpin RNA sequences (LV-ING4-RNAi: 5′-GCTTGCCATGCAGACCTATGA-3′)
for ING4 and empty vector were purchased from Open Biosystems
(Thermo Fisher Scientific, Inc.). Lentiviral constructs were used
to transiently transfect HEK293 packaging cells along with VSV-G
pseudoviral particles. Virus-containing medium was collected from
HEK293 cells and filtered to remove non-adherent cells 2 and 3 days
post-transfection. Subconfluent A375 or HT-144 cells were incubated
with virus-containing medium and 8 µg/ml polybrene. Infected A375
or HT-144 cells were selected starting at 24 h after initial
infection using 2 µg/ml puromycin. The titer of LV-ING4-RNAi or
LV-ING4 were ~5×107 IU/ml, and the multiplicity of
infection (MOI) was 5, where >80% of cells were identified to be
green fluorescent protein (GFP)-positive. Infected A375 or HT-144
cells were passaged at least 3 times every 3–5 days prior to
further experiments. The protein levels in cells were analyzed by
western blot analysis as described below.
Cell viability assay
The human melanoma cell lines A375 and HT-144 were
trypsinized and dispensed in 96-well plates at a density of
1×105 cells/well. After incubation at various
concentrations of IFNα2b (0, 5×103, 5×104,
5×105 and 5×106 IU/l) for 24 or 48 h at 37°C,
50 µl MTT (1 mg/ml; Merck KGaA) was added to cell media. Following
incubation for 4 h at 37°C, MTT was discarded, and 150 ml DMSO was
loaded in each well. The spectrophotometric absorbance of the
samples was measured using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at 570 nm with a reference
wavelength of 655 nm. The experiments were performed at least three
times, and three wells were measured at each concentration.
Analysis of apoptosis with Annexin
V/7-aminoactinomycin D (7-AAD) staining
Apoptosis was detected using Annexin
V-phycoerythrin/7-AAD staining (Apoptosis Detection kit; cat. no.
KGA1017; Kaiji Inc, Nanjing, China; http://www.keygentec.com.cn/prd-search-KGA1017.html).
Human melanoma cell line A375 was suspended and transferred to a
sterile culture tube, 5 µl Annexin V-phycoerythrin and 5 µl 7-AAD
were added to each tube. The tubes were gently vortexed and
incubated for 15 min at room temperature in the dark.
Fluorescence-activated cell sorting (FACS) analysis was performed
using the BD FACSort flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA). Cell populations were classified into four groups:
Viable (Annexin V−, 7-AAD−), early apoptotic
(Annexin V+, 7-AAD−), late apoptotic (Annexin
V+, 7-AAD+) or necrotic (Annexin
V−, 7-AAD+). Data were analyzed using
CellQuest software (BD Biosciences, version 5.1).
Western blot analysis
Human melanoma cell line A375 was rinsed in PBS,
then the cell pellets were re-suspended in lysis buffer [137 mM
NaCl, 1% NP-40, 50 mmol/l Tris (pH 8.0), and 20% glycerol] and
normalized to total protein concentration using Bio-Rad protein
assay. Whole cell proteins (30 µg) were separated on 10% SDS-PAGE
and transferred to polyvinylidene difluoride membranes using the
BioRad electrotransfer system (Bio-Rad Laboratories, Inc.). The
membranes were incubated with antibodies against ING4 (cat. no.
sc-135742), PARP (cat. no. sc-136208), caspase 8 (cat. no.
sc-6136), caspase 3 (cat. no. sc-271759), Fas (cat. no. sc-4856),
FasL (cat. no. sc-71096) (dilution, 1:1,000; Santa Cruz
Biotechnology, Inc.) and cleaved PARP (cat. no. 5625), anti-cleaved
caspase-8 (cat. no. 8529) and cleaved caspase-3 (cat. no. 9661)
(dilution, 1:1,000; Cell Signaling Technology, Inc., Danvers, MA,
USA) for 1 h at room temperature. GAPDH (cat. no. sc-47724,
dilution, 1:1,000; Santa Cruz Biotechnology, Inc.) was used as a
loading control. The membrane was blocked using 5% fat-free milk
(Sigma-Aldrich; Merck KGaA) for 1 h at room temperature and then
incubated with the appropriate primary antibody diluted in 3% BSA
solution (Sigma-Aldrich; Merck KGaA) at 4°C overnight. Following
incubation with goat anti-mouse secondary antibody (cat. no.,
35518; 1:10,000 dilution; Thermo Fisher Scientific, Inc.) and goat
anti-rabbit secondary antibody (cat. no., A32731; 1:10,000
dilution; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature. The blots were incubated by the Odyssey Western
Blotting kit (LI-COR Biosciences, Lincoln, NE, USA) and scanned
using the Odyssey Western Detection system (LI-COR Biosciences,
Lincoln, NE, USA), followed by quantification with ImageStudio
software (LI-COR Biosciences; version 5.2.5) as previously
described (24).
Statistical analysis
Data are presented as the mean ± standard deviation.
Prism (version 6.0; GraphPad Software, Inc., La Jolla, CA, USA) was
used to perform statistical analysis. Statistical significance
between two groups was determined using an unpaired Student's
t-test. Comparisons among multiple groups were determined by
one-way or two-way analysis of variance followed by Dunnett's or
Bonferroni's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Overexpression of ING4 increases the
anti-cancer effect of IFNα2b in human melanoma cells
To assess whether the expression of ING4 enhances
the anti-cancer effect of IFNα2b, the effects of various
concentrations of IFNα2b (0, 5×103, 5×104,
5×105 and 5×106 IU/l) on the survival of
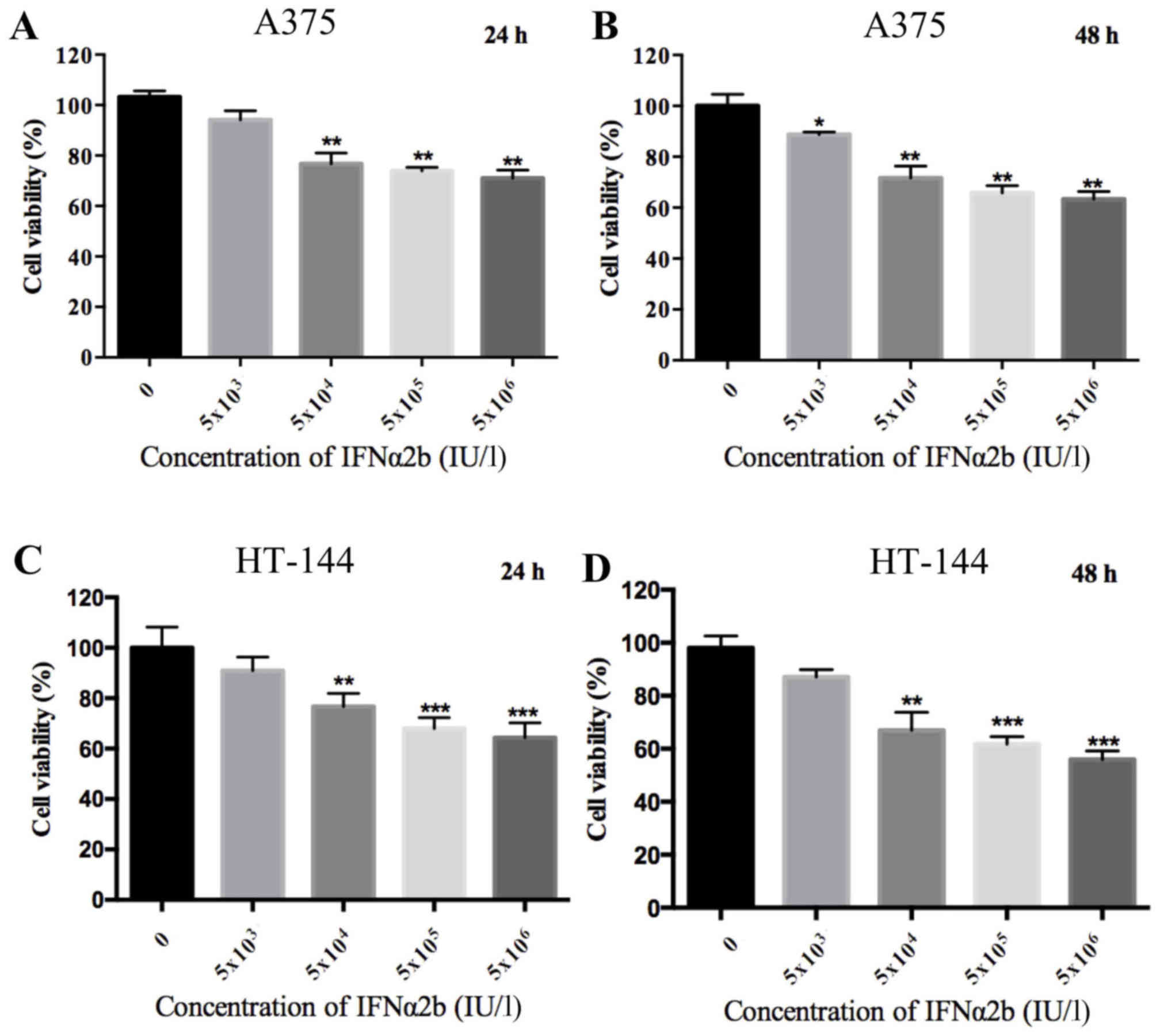
melanoma cells were examined by MTT assay. As shown in Fig. 1, a dose-dependent decrease in the
viability of melanoma cells was observed with increasing
concentrations of IFNα2b (P<0.01). However, the melanoma cell
viability in the high concentrations of IFNα2b (5×105
and 5×106 IU/l) was similar to the 5×104 IU/l
IFNα2b group.
To elucidate the function of ING4 in melanoma cells,
ING4 was either knocked down or overexpressed in A375 or HT-144
cells using LV-ING4-RNAi or LV-ING4, and the expression of ING4 was
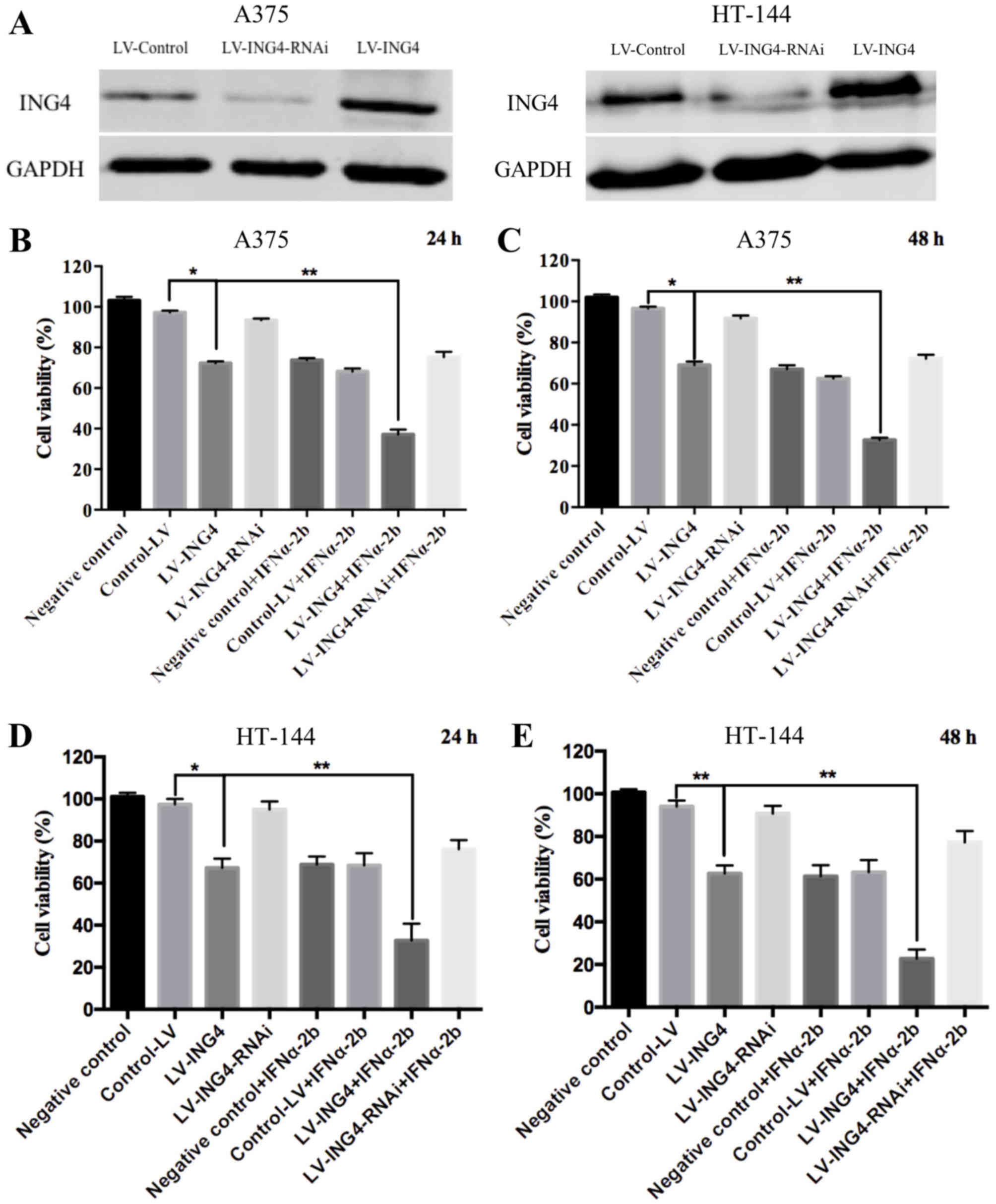
examined by western blotting. The results indicated that
downregulation of ING4 was achieved with LV-ING4-RNAi, and LV-ING4
markedly increased the expression of ING4 (Fig. 2A). The ability of ING4 to act
synergistically with IFNα2b to induce cell death of A375 or HT-144
was analyzed by MTT assay. As shown in Fig. 2B-E, the overexpression of ING4 alone
decreased the cell viability to ~70% when compared with control-LV
(~97%) for 24 or 48 h. However, IFNα2b treatment increased the
cytotoxic effect. The overexpression of ING4 reduced the viability
of IFNα2b-treated A375 cells (concentration of IFNα2b
5×104 IU/l) from 70±1.75 to 32±1.05%. These results
showed that the overexpression of ING4 in melanoma cells led to
significantly increased inhibition on cell growth compared with
IFNα2b treatment alone. However, the downregulation of ING4 alone
had no effect on the viability of melanoma cells (Fig. 2). The results illustrated that the
overexpression of ING4 is able to increase the anti-cancer effects
of IFNα2b in different types of melanoma cells (Figs. 1 and 2).
Combination treatment of A375 cell
lines with overexpression of ING4 and IFN-α2b leads to increased
apoptosis of melanoma cells
A combination of ING4 overexpression and IFN-α2b
treatment was indicated to induce apoptosis in A375 cells compared
with single treatments (Fig. 3).
Consequently, it was hypothesized that the pro-apoptotic effects of
a combination of ING4 overexpression and IFN-α2b treatment would be
greater compared with single treatments in melanoma cell lines.
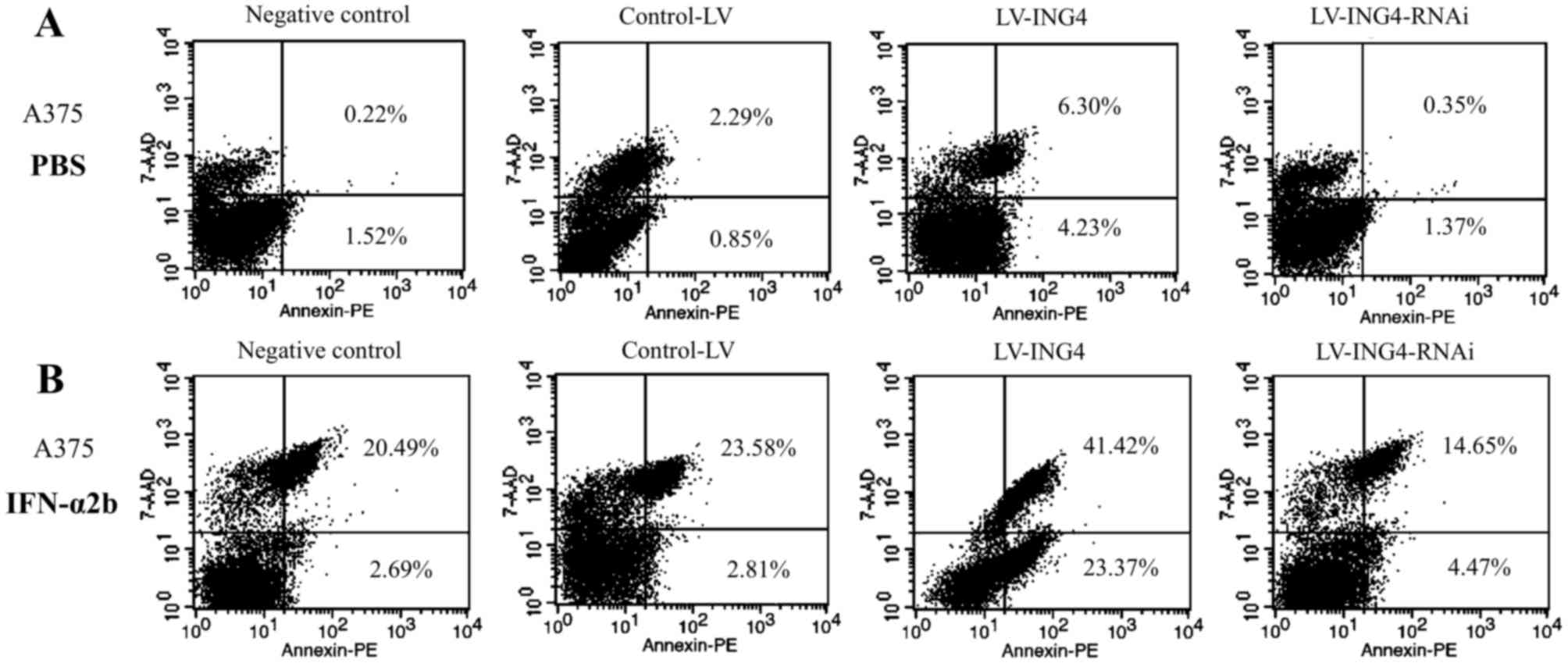
The transfection of LV-ING4 or LV-ING4-RNAi was able
to induce apoptosis in A375 cells, as assessed by FACS analysis.
The percentage of early apoptotic cells (Annexin
V+/7-AAD−) was 1.52% for control, 0.85% for
LV-control, 4.23% for LV-ING4 and 1.37% for the LV-ING4-RNAi group.
Furthermore, the percentage of late apoptotic cells (Annexin
V+/7-AAD+) was 0.22% for control, 2.29% for
LV-control, 6.30% for LV-ING4 and 0.35% for LV-ING4-RNAi (Fig. 3A).
In order to determine whether ING4 is able to
synergize with IFNα2b to induce the apoptosis of A375 cells, the
cells were treated with LV-ING4 or LV-ING4-RNAi and IFN-α2b. As
indicated in Fig. 3B, the combination
of ING4 overexpression and IFN-α2b treatment induced a greater
level of apoptosis in the A375 cells compared with single
treatments (Fig. 3B). The percentage
of early apoptotic cells of the IFN-α2b-treated A375 cells that
were transfected with LV-ING4 was 23.37%, and the percentage of
late apoptotic cells was 41.42%. By contrast, the percentage of
early apoptotic cells in the LV-control group that was treated with
IFN-α2b was 2.81%, and the percentage of late apoptotic cells was
23.58%. Therefore, there is a predominantly apoptotic effect in the
combined ING4 overexpression and IFN-α2b group in A375 cells,
compared with the LV-control combined with IFN-α2b group.
Combination of ING4 overexpression and
treatment of IFN-α2b induces the effector caspases and
Fas/FasL
The mechanisms underlying the effect of IFN-α2b
treatment and ING4 overexpression on the apoptosis of A375 cells
was investigated. The expression of a number of proteins that are
involved in cell apoptosis were detected, including PARP, cleaved
PARP, caspase-3, cleaved caspase-3, caspase-8, cleaved caspase-8,
Fas and FasL. As shown in Fig. 4, the
expression of PARP, caspase-3 and caspase-8 were markedly decreased
in A375 cells that were treated with a combination of IFN-α2b and
LV-ING4 compared with the cells that were treated with IFN-α2b
alone. Furthermore, the expression of cleaved PARP, cleaved
caspase-3 and cleaved caspase-8 were increased in A375 cells that
were treated with a combination of IFN-α2b and LV-ING4 compared
with the cells that were treated with IFN-α2b alone (Fig. 4).
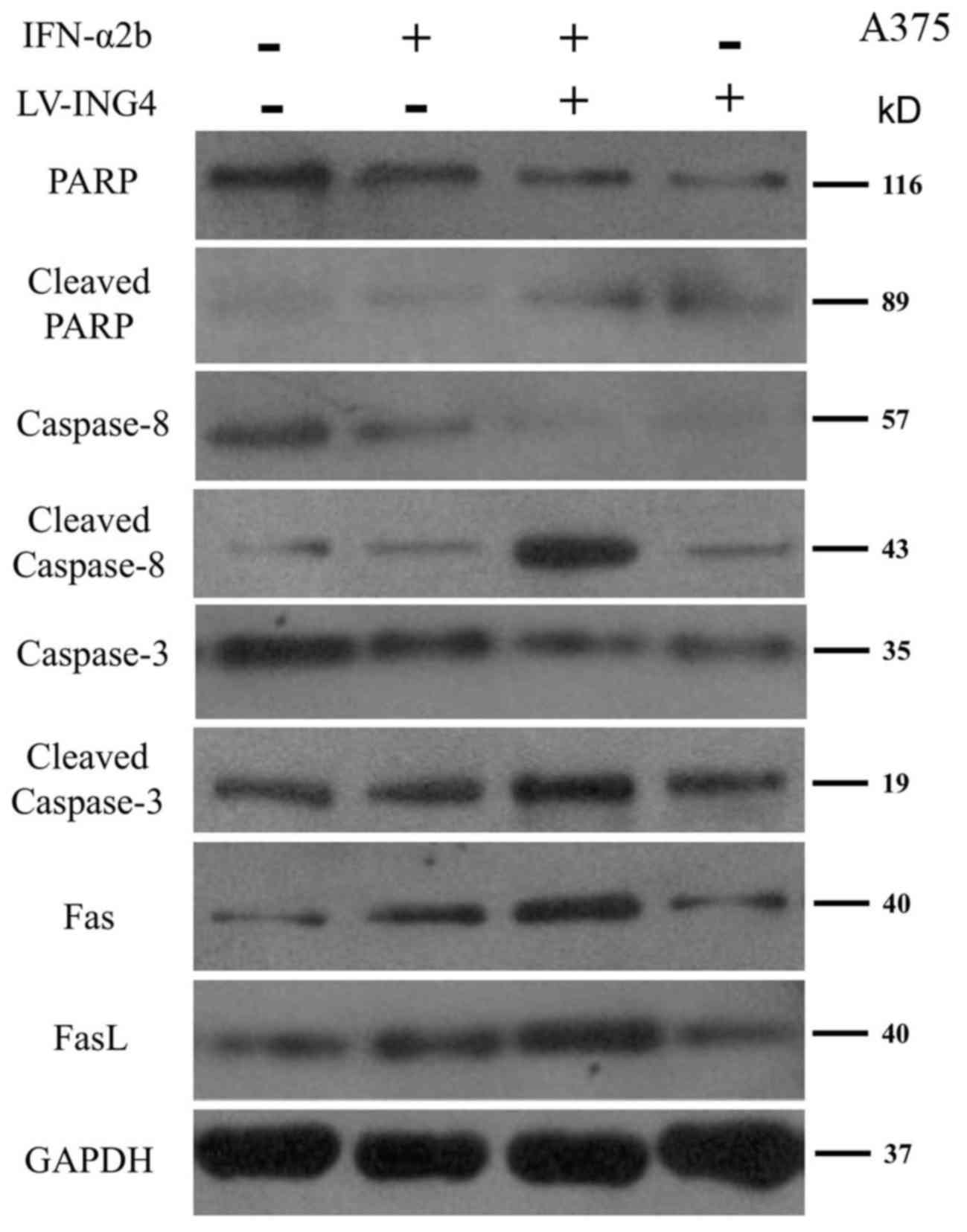 | Figure 4.Western blot analysis of PARP,
cleaved PARP, caspase 8, cleaved caspase 8, caspase 3, cleaved
caspase 3, Fas and FasL in A375 cells that were treated with PBS,
IFN-α2b, a combination of IFN-α2b and LV-ING4 or LV-ING4 alone.
ING4, inhibitor of growth family member 4; PARP, poly(ADP-ribose)
polymerase; FasL, Fas ligand; IFN, interferon. |
Discussion
The majority of patients with melanoma have a poor
response to IFN-α, which limits the clinical benefits of IFN-α2b as
a therapy (25). In the present
study, the overexpression of ING4 was indicated to enhance the
anti-tumor activity of IFN-α2b in A375 and HT-144 melanoma cells
and induce cell apoptosis, which was associated with PARP,
caspase-8 and caspase-3. In addition, the treatment of melanoma
cells with a combination of ING4 overexpression and IFN-α2b was
indicated to result in an increased level of Fas/FasL protein
(Fig. 4). Therefore, ING4 may be a
critical positive regulator of the anti-tumor effect of IFN-α2b in
melanoma, and combination treatment with ING4 overexpression and
IFN-α2b may serve a role in the treatment of melanoma.
ING4 is a strong candidate for a tumor suppressor
gene within the ING family due to its important role in a variety
of cellular processes, including oncogenesis, angiogenesis, gene
transcription, cell cycle and apoptosis (20,21,26). As a
nuclear factor, ING4 is widely expressed in normal human tissues,
but its expression is significantly decreased in various types of
cancer, including breast tumors, gliomas, and squamous cell
carcinomas (20,26). Furthermore, a previous study
identified that the expression of ING4 is significantly reduced in
human malignant melanomas (22),
suggesting that ING4 serves an important role in melanoma
tumorigenesis. Additionally, the growth of tumor cells was
significantly inhibited following the transfection of exogenous
ING4 by interfering with cell cycle progression in the human lung
adenocarcinoma cell line A549.
Apoptosis or programmed cell death consists of the
ordered disassembly of the cell from within as opposed to necrosis
or accidental cell death. Apoptosis may be induced through the
extrinsic pathway, which involves the activation of cell surface
death receptors, or the intrinsic pathway, where alterations in the
integrity of the mitochondrial membrane induce the release of
cytochrome c (27). These
pathways converge at the level of the effector caspases (caspase-3,
−6, −7 and −8) (28). Once activated,
these effector caspases cleave cytoskeletal and nuclear proteins,
such as PARP, thereby initiating cellular disassembly (28). The results of the present study
demonstrated that the expression of PARP, caspase-8 and caspase-3
is decreased in response to the treatment of melanoma cells with a
combination of ING4 and IFN-α2b, supporting the hypothesis that
cell death occurs in a manner consistent with apoptosis.
The cell death pathway mediated via Fas/FasL
interaction represents typical apoptotic signaling in various cell
types (29). In many cell types and
tissues, including HepG2, U87MG and HeLa cells, Fas and its
receptor, FasL, and caspase-8 are components of an important
cellular pathway that regulates the induction of apoptosis
(30). In the present study, the data
indicated that treatment with a combination of IFN-α2b and LV-ING4
markedly activated Fas and FasL proteins (Fig. 4). Future studies can examine the
activity of the cleaved PARP, cleaved caspase-8 and cleaved
caspase-3, which recapitulates key features of apoptosis.
In addition to its immune-enhancing effects, IFN-α
is also known to act on tumor cells to decrease cellular
proliferation and promote apoptosis (31). These processes are mediated in part
via the transcription and translation of IFN-α stimulated genes
(32). In the present study, the
pre-transfection of melanoma cells with ING4 and treatment with
IFN-α2b was demonstrated to increase the expression of Fas/FasL.
This data suggests that the overexpression of ING4 may enhance the
sensitivity of melanoma cells to the direct effects of IFN-α. IFN-α
is one of the few treatment options that are available to those
afflicted with malignant melanoma, and these suggest that there may
be methods to enhance the activity of ING4.
IFN-α2b has various effects on cancer cells.
Notably, the combination of IFN-α2b treatment and ING4
overexpression may be particularly useful as it was demonstrated in
the present study that IFN-α2b is able to induce the upregulation
of Fas/FasL. Additionally, the combination of LV-ING4 and IFN-α2b
treatment was able to significantly increase the apoptotic effect
in the examined melanoma cell lines compared with single treatments
(Fig. 2), suggesting that the
overexpression of ING4 may be effective as part of a combination
therapy regimen for advanced melanomas. It will be interesting to
test the effects of ING4 overexpression and IFN-α2b treatment in
primary cancer cells obtained from patients with metastatic
melanoma.
In summary, the present study suggests that a
combination of ING4 overexpression and IFN-α2b treatment may be a
novel treatment strategy for inducing direct, apoptotic effects on
human melanoma cells.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81072234).
Availability of data and materials
Not applicable.
Authors' contributions
LMC, JL, YCW and YW conceived and designed the
experiments. LMC, JL, HXC and YM performed the experiments and
analyzed data. LMC, JL, YW, HXC, YM, YW and YCW contributed
reagents, materials, and/or analysis tools. YCW, LMC and JL wrote
the main manuscript text. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflicts of
interest.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rubin KM and Lawrence D: Your patient with
melanoma: Staging, prognosis, and treatment. Oncology (Williston
Park). 23 8 Suppl:S13–S21. 2009.
|
|
3
|
Chan JK: Virus-associated neoplasms of the
nasopharynx and sinonasal tract: Diagnostic problems. Mod Pathol.
30(s1): S68–S83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grossman D and Altieri DC: Drug resistance
in melanoma: Mechanisms, apoptosis, and new potential therapeutic
targets. Cancer Metastasis Rev. 20:3–11. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gogas HJ, Kirkwood JM and Sondak VK:
Chemotherapy for metastatic melanoma: Time for a change. Cancer.
109:455–464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mandarà M, Nortilli R, Sava T and Cetto
GL: Chemotherapy for metastatic melanoma. Expert Rev Anticancer
Ther. 6:121–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cummins DL, Cummins JM, Pantle H,
Silverman MA, Leonard AL and Chanmugam A: Cutaneous malignant
melanoma. Mayo Clin Proc. 81:500–507. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lens MB and Dawes M: Interferon alfa
therapy for malignant melanoma: A systematic review of randomized
controlled trials. J Clin Oncol. 20:1818–1825. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sabel MS and Sondak VK: Pros and cons of
adjuvant interferon in the treatment of melanoma. Oncologist.
8:451–458. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McLoughlin JM, Zager JS, Sondak VK and
Berk LB: Treatment options for limited or symptomatic metastatic
melanoma. Cancer Control. 15:239–247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garbe C, Eigentler TK, Keilholz U,
Hauschild A and Kirkwood JM: Systematic review of medical treatment
in melanoma: current status and future prospects. Oncologist.
16:5–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lesinski GB, Anghelina M, Zimmerer J,
Bakalakos T, Badgwell B, Parihar R, Hu Y, Becknell B, Abood G,
Chaudhury AR, et al: The antitumor effects of IFN-alpha are
abrogated in a STAT1-deficient mouse. J Clin Invest. 112:170–180.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lesinski GB, Trefry J, Brasdovich M,
Kondadasula SV, Sackey K, Zimmerer JM, Chaudhury AR, Yu L, Zhang X,
Crespin TR, et al: Melanoma cells exhibit variable signal
transducer and activator of transcription 1 phosphorylation and a
reduced response to IFN-alpha compared with immune effector cells.
Clin Cancer Res. 13:5010–5019. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bearzatto A, Orlandi L, De Marco C,
Daidone MG and Zaffaroni N: Lack of p21waf1 and p27kip1 protein
induction by interferon-alpha2a in human melanoma cell lines.
Melanoma Res. 9:457–463. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Y, Wang S, Yue BG, Gobl A and Oberg
K: Effects of interferon alpha on the expression of p21cip1/waf1
and cell cycle distribution in carcinoid tumors. Cancer Invest.
20:348–356. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hahne M, Rimoldi D, Schröter M, Romero P,
Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard
D, et al: Melanoma cell expression of Fas(Apo-1/CD95) ligand:
Implications for tumor immune escape. Science. 274:1363–1366. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bullani R, Wehrli P, Viard-Leveugle I,
Rimoldi D, Cerottini JC, Saurat JH, Tschopp J and French LE:
Frequent downregulation of Fas (CD95) expression and function in
melanoma. Melanoma Res. 12:263–270. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Helmbach H, Rossmann E, Kern MA and
Schadendorf D: Drug-resistance in human melanoma. Int J Cancer.
93:617–622. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ekmekcioglu S, Okcu MF, Colome-Grimmer M,
Owen-Schaub L, Buzaid AC and Grimm EA: Differential increase of Fas
ligand expression on metastatic and thin or thick primary melanoma
cells compared with interleukin-10. Melanoma Res. 9:261–272. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim S, Chin K, Gray JW and Bishop JM: A
screen for genes that suppress loss of contact inhibition:
Identification of ING4 as a candidate tumor suppressor gene in
human cancer. Proc Natl Acad Sci USA. 101:16251–16256. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shiseki M, Nagashima M, Pedeux RM,
Kitahama-Shiseki M, Miura K, Okamura S, Onogi H, Higashimoto Y,
Appella E, Yokota J and Harris CC: p29ING4 and p28ING5 bind to p53
and p300 and enhance p53 activity. Cancer Res. 63:2373–2378.
2003.PubMed/NCBI
|
|
22
|
Li J, Martinka M and Li G: Role of ING4 in
human melanoma cell migration, invasion and patient survival.
Carcinogenesis. 29:1373–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Cai L, Liang M, Wang Y, Yang J and
Zhao Y: Ing4 induces cell growth inhibition in human lung
adenocarcinoma A549 cells by means of Wnt-1/beta-catenin signaling
pathway. Anat Rec (Hoboken). 291:593–600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bu Q, Wang A, Hamzah H, Waldman A, Jiang
K, Dong Q, Li R, Kim J, Turner D and Chang Q: CREB signaling is
involved in Rett syndrome pathogenesis. J Neurosci. 37:3735–3716.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Escudier B, Lassau N, Angevin E, Soria JC,
Chami L, Lamuraglia M, Zafarana E, Landreau V, Schwartz B, Brendel
E, et al: Phase I trial of sorafenib in combination with IFN
alpha-2a in patients with unresectable and/or metastatic renal cell
carcinoma or malignant melanoma. Clin Cancer Res. 13:1801–1809.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garkavtsev I, Kozin SV, Chernova O, Xu L,
Winkler F, Brown E, Barnett GH and Jain RK: The candidate tumour
suppressor protein ING4 regulates brain tumour growth and
angiogenesis. Nature. 428:328–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wlodkowic D, Telford W, Skommer J and
Darzynkiewicz Z: Apoptosis and beyond: Cytometry in studies of
programmed cell death. Methods Cell Biol. 103:55–98. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fulda S and Debatin K: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hohlbaum AM, Moe S and Marshak-Rothstein
A: Opposing effects of transmembrane and soluble Fas ligand
expression on inflammation and tumor cell survival. J Exp Med.
191:1209–1220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagata S and Golstein P: The Fas death
factor. Science. 267:1449–1456. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thyrell L, Erickson S, Zhivotovsky B,
Pokrovskaja K, Sangfelt O, Castro J, Einhorn S and Grandér D:
Mechanisms of interferon-alpha induced apoptosis in malignant
cells. Oncogene. 21:1251–1262. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kaur S, Sassano A, Dolniak B, Joshi S,
Majchrzakkita B, Baker DP, Hay N, Fish EN and Platanias LC: Role of
the Akt pathway in mRNA translation of interferon-stimulated genes.
Proc Natl Acad Sci USA. 105:4808–4813. 2008. View Article : Google Scholar : PubMed/NCBI
|