Introduction
Glioma is the most common type of primary tumor of
the central nervous system (1). It
has been suggested that a small subset of glioma stem cells (GSCs)
in glioma tissues, with stem cell-like features, are the cause of
glioma initiation and recurrence (2).
Additionally, it has been also identified that GSCs have the
ability for over-proliferation, self-renewal, multi-directional
differentiation and tumor formation, which are activities closely
associated with the mechanisms of tumor angiogenesis and
chemotherapy/radiotherapy resistance (3). Therefore, they may be a crucial target
for glioma treatment and study.
The current standard treatment for patients with
glioma consists of microsurgical resection, followed by
radiotherapy and chemotherapy. Chemotherapy with concomitant and
adjuvant temozolomide (TMZ) improves the median survival and 5-year
survival rate in gliomas (4,5). TMZ is the widely recognized anti-glioma
drug and commonly used in clinics at present; however, due to its
side effects and only low concentrations delivered to the brain,
topical interstitial chemotherapy is considered as an important
means of comprehensive treatment of glioma (6). In addition, Ommaya intracapsular
injection of nimustine (ACNU) is performed to kill tumor cells, as
it reaches a sufficient concentration for activity in the brain and
has no marked systemic side effects, achieving a clinical benefit
(6). ACNU and TMZ, as alkylating
agents, modify the O6 position of DNA guanine with methyl groups,
eventually leading to cell death (7,8). The DNA
repair protein O6-methylguanine-DNA methyltransferase (MGMT) is
able to remove the methyl group with toxic and mutagenic effects
from the O6 position of guanine for O6-methylguanine repair,
thereby protecting the cells against the damage of methyl groups;
this is the primary cause of cellular resistance to alkylating
agents including TMZ and ACNU (9,10). In
tumor cells, the expression of MGMT protein is increased and
cellular resistance to alkylating agents is more enhanced. MGMT
gene promoter methylation may silence the expression of the MGMT
gene, therefore the MGMT gene promoter methylation level may be
used to assess the sensitivity of alkylating agents for the
predictive evaluation of the clinical efficacy of radiotherapy
alone compared to radiotherapy combined with chemotherapy (11,12).
Valproic acid (VPA) is a common antiepileptic drug,
and used for the treatment and prevention of brain tumor patients
with seizures. In addition to its antiepileptic properties, VPA has
been documented to inhibit cell proliferation, induce cell
differentiation and apoptosis and suppress tumor angiogenesis
(13–15).
However, a small number of studies are available on
the interaction of VPA and alkylating agents and the effects of VPA
on chemotherapy for glioma (16),
particularly for the association of VPA and initiation and
recurrence of glioma. The present study used GSCs as the target to
explore whether VPA affected the susceptibility of human glioma
cells for TMZ and ACNU in vitro, and its effects on the MGMT
promoter methylation and its expression of MGMT in glioma
cells.
Materials and methods
Specimens
The present study was approved by the Ethics
Committee of Zhengzhou No. 7 People's Hospital (Zhengzhou, China).
Written informed consent was gained from all participants. A total
of 3 glioma stem cell populations were derived from glioma tissue
obtained by surgical resection of 3 patients with glioma (2 male
and 1 female; age, 42–67; median age, 50.23±6.56) in the Department
of Neurosurgery, Henan Provincial People's Hospital (Zhengzhou,
China) from August 2012 to October 2013. Firstly, the tissue
specimens were placed in a centrifuge tube containing 10 ml DMEM
medium (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences), and cut into 0.5 mm3 blocks
following rinsing with PBS three times. They were then digested in
2 ml trypsin with PBS added to terminate the digestion, and finally
mouth pipetted repetitively to produce a monoplast suspension. The
suspension was filtered with a 100 µm sieve. Following
centrifugation at 37°C and 120 × g for 5 min, the cells were
collected and seeded into 25 cm2 flasks at a density of
5×103 cells/flask, then placed in an incubator at 37°C
and 5% CO2 and 95% humidity. The cell growth status and
morphological changes were observed under inverted phase-contrast
microscope (magnification, ×400) each day. The cells at passage 2
or 3 were collected and identified by immunofluorescence staining
of the CD133 and nestin markers of the glioma stem cells. Briefly,
cell were rinsed in PBS three times and subsequently blocked with
10% FBS (Hyclone; GE Healthcare Life Sciences) at 37°C for 15 min.
The cells were incubated with CD133 (catalog no. ab19898, 1:500;
Abcam, Cambridge, UK) and nestin (catalog no. ab11306, 1:500;
Abcam) primary antibodies overnight at 4°C. Following washing with
PBS three times, slides were incubated with tetramethylrhodamine
(TRITC)-conjugated goat anti-rabbit IgG secondary antibody (catalog
no. BA1090, 1:20,000; Wuhan Boster Biological Technology, Ltd.,
Wuhan, China) and TRITC-conjugated goat anti-mouse IgG secondary
antibody (catalog no. BA1089, 1:20,000; Wuhan Boster Biological
Technology, Ltd.) at room temperature for 1 h. Finally, cells were
identified under a fluorescence microscope (magnification, ×400;
Olympus AX80; Olympus Corporation, Tokyo, Japan).
Immunocytochemical assay
Cells were grown on glass slides as described
previously (17). Following fixation
with 4% paraformaldehyde at 37°C for 10 min and washing with PBS
three times, the slides were incubated at 37°C with 0.5% Triton
X-100, then endogenous catalase activity was blocked in 3%
H2O2 solution for 15 min at room temperature
and the slides were sealed in 10% FBS (Hyclone; GE Healthcare Life
Sciences). Subsequently, they were incubated with mouse anti-MGMT
antibody (catalog no. ZM-0461, 1:100; ZSGB-Bio; OriGene
Technologies, Inc., Beijing, China) at 25°C for 60 min and rinsed
in PBS three times. Following the addition of 50 µl amplification
reagent (reagent A; catalog no. AR1024; Wuhan Boster Biological
Technology, Ltd.) and 50 µl polymerase conjugate (reagent B;
catalog no. AR1024; Wuhan Boster Biological Technology, Ltd.) at
37°C for 10 min, 3,3′-diaminobenzidine was used as the chromogen.
The slides were counterstained with 10% hematoxylin at 37°C for 15
min, differentiated in 0.1% HCl-ethanol and re-stained blue with 1%
ammonia at 37°C for 15 min. Finally, the slides were dehydrated
using graded ethanol series (70, 80, 90, 95 and 100% ethanol). With
xylene (100%) as the clearing medium, the sections were mounted in
neutral resin, observed with a fluorescence microscope
(magnification, ×20,000) and images were captured.
Viability of glioma stem cells
As described previously (18), glioma stem cells were seeded into
96-well plates at a density of 5×103 cells/well, 20
wells of which were subjected to VPA pre-treatment in each plate: A
total of 1 µl 100 mmol/l VPA was added in each well to a final
concentration of 1 mmol/l, then the plate was incubated at 37°C for
24 h. According to MGMT protein expression, as determined by the
immunocytochemical assay, TMZ or ACNU was added to the wells of the
VPA-pretreated and VPA-untreated groups in each 96-well plate in a
gradient concentration, with each concentration used in four
repeated wells. In the MGMT-negative group, the TMZ concentration
gradient consisted of 40, 80, 120, 160 and 200 µmol/l, and the ACNU
concentration gradient was 20, 40, 60, 80 and 100 µg/ml. In the
MGMT-positive group, the TMZ gradient concentration consisted of
100, 200, 300, 400 and 500 µmol/l, and the ACNU concentration of
50, 100, 150, 200 and 250 µg/ml. The plates were incubated at 37°C
for 72 h, then 10 µl CCK-8 (catalog no. C0037; Beyotime Institute
of Biotechnology, Haimen, China) was added to each well, followed
by incubation at 37°C for 2 h. The absorbance was measured by with
a microplate reader at 450 nm, and the viability fraction of each
group was calculated using the following formula: Viability
fraction=absorbance value of experimental group-value of blank
group)/(absorbance value of control group-absorbance value of blank
group).
Apoptotic rate detection
Subsequent to culture in serum-free DMEM (Hyclone;
GE Healthcare Life Sciences), the glioma stem cells were collected
and the cell density was adjusted to 2×105 cells/ml.
Then, the cells were transferred into 6 culture flasks (25
cm2) and divided into control, VPA, TMZ, VPA + TMZ, ACNU
and VPA + ACNU groups. Cells in the VPA, VPA + TMZ and VPA + ACNU
groups were treated with 1 mmol/l VPA. After 24 h, the TMZ and VPA
+ TMZ groups were treated with TMZ, while the ACNU and VPA + ACNU
groups were treated with ACNU. MGMT-negative glioma stem cells were
subjected to 60 µg/ml ACNU or 120 mmol/l TMZ, while the
MGMT-positive glioma stem cells were treated with 150 µg/ml ACNU or
300 mmol/l TMZ. Appropriate concentrations were selected based on
the results of the CCK-8 assay. Subsequent to treatment for 72 h,
the cells were collected and the density was adjusted to
1×106 cells/ml. From this, 195 µl cell suspension was
removed and placed into a 5 ml flow tube, followed by the addition
of 5 µl Annexin V with gentle mixing; after a 3 min interval, 20
µl/ml propidium iodide solution (10 µl) was added, and the mixture
was incubated for 15 min at room temperature in a dark room. A
total of 300 µl PBS was added to the reaction tube, and the mixture
was homogenized and analyzed by flow cytometry (FACSCalibur™; BD
Biosciences, Franklin Lakes, NJ, USA). The experiment was repeated
three times.
Detection of the expression of MGMT
protein
Cells were grown on glass slides as described
previously (17) and divided into VPA
and control groups. Cells were adherent to the wall following 1 day
of culture at 37°C in the flasks, and the VPA group was treated
with 1 mmol/l VPA solution, while the same amount of sterile
deionized water was added to the control group. The slides were
incubated at 37°C for 2 days in a constant temperature incubator,
and cells were adhered firmly. The cells were rinsed twice with PBS
and fixed with 4% polylysine for 10 min at 37°C. The expression of
MGMT protein in glioma stem cells was detected by
immunocytochemistry as aforementioned.
DNA extraction and
methylation-specific polymerase chain reaction (PCR)
When 90% confluence was reached, glioma stem cells
were divided into two groups: VPA and control. Firstly, the VPA
group was treated with 1 mmol/l VPA solution, while the same amount
of sterile deionized water was added to the control group. After 3
days of culturing at 37°C, the cells were collected by
centrifugation at 37°C and 1,000 × g for 10 min. DNA extraction and
purification were performed using a Genomic DNA Purification kit
(catalog no. K0512; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Following treatment of samples with methylation-specific PCR
(MSP), as described previously (19),
MGMT gene promoter methylation amplification primers were designed
in Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
and are summarized in Table I. The
optimal PCR amplification system for MGMT gene was established: A
50 µl solution with 10 µl bead DNA, 1 µl Taq DNA polymerase (Takara
Biotechnology Co., Ltd., Dalian, China), 1 µl dNTP, 4 µl
MgCl2, 5 µl 10X Buffer, 1 µl MGMT methylation primers, 1
µl MGMT non-methylation primers and 27 µl double-distilled water.
The PCR amplification conditions were as follows: Following
pre-denaturation at 96°C for 10 min, the PCR was suspended, and the
PCR products were stored into the refrigerator at 4°C for 3 min.
Followed by the addition of the Taq DNA polymerase, the reaction
continued. The PCR amplification was then performed for 40 cycles
of 94°C for 30 sec, 59°C for 40 sec (56°C for 40 sec in the
methylation amplification cycles), 70°C for 40 sec and 70°C for 10
min. The products were stored at 4°C and separated on 1.5% agarose
gel, then observed with a GelDoc-It®2 Imager (UVP, LLC,
Phoenix, AZ, USA).
 | Table I.Methylation-specific amplification
primers for the MGMT promoter. |
Table I.
Methylation-specific amplification
primers for the MGMT promoter.
| Primers | Sequence (5′-3′) | Products, bp | Annealing
temperature, °C |
|---|
| MGMT-M | F:
TTTCGACGTTCGTAGGTTTTCGC | 83 | 59 |
|
| R:
GCACTCTTCCGAAAACGAAACG |
|
|
| MGMT-U | F:
TTTGTGTTTTGATGTTTGTAGGTTTTTGT | 91 | 59 |
|
| R:
AACTCCACACTCTTCCAAAAACAAAACA |
|
|
Determination criteria of MGMT gene
promoter methylation
If the methylated primer amplification produced
positive results and the non-methylated primer amplification
reaction was negative; this was considered to indicate complete
methylation of the gene. When the methylated and non-methylated
primer amplification samples exhibited positive bands, this was
considered to be partial methylation of the gene. When the
methylated primer amplification reaction was negative and the
non-methylated primer amplification reaction was positive, this was
determined to indicate negative gene methylation.
Statistical analysis
All data were analyzed using SPSS 18.0 statistical
software (SPSS, Inc., Chicago, IL, USA) and expressed as the mean ±
standard deviation. Comparisons between groups were performed using
one-way analysis of variance, followed by a Tukey's post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Primary culture and identification of
glioma stem cells
After 24 h culture in serum-containing medium,
glioma tissue cells began to adhere to the glass surface,
exhibiting varied morphologies, including star-like and spindle
shaped cells, prominent cell processes and nuclear abnormalities.
After 3–5 days, the cells grew in a single layer, presenting as
spindle-shaped in a fence-like pattern into a network. When the
cells covered the bottom, small bulges appeared.
Immunocytochemistry detecting CD133 and nestin markers of the
glioma stem cells indicated that CD133 appeared in the cell
membrane, while nestin was expressed in the cytoplasm. In addition,
observation by fluorescence microscopy demonstrated that the area
exhibiting CD133 positive-expression was green, the nestin
positive-expression area was red and the nucleus was blue with DAPI
staining. Following fixation with paraformaldehyde, the cell
morphology became irregular, but the cells expressed CD133 and
nestin. Finally, the 3 cell types expressing CD133 and nestin were
named G1, G2 and G3 by immunofluorescence staining.
Expression of MGMT protein in glioma
stem cells
The presence of brown-colored particles in the
nucleus or cytoplasm was identified as MGMT-positive expression by
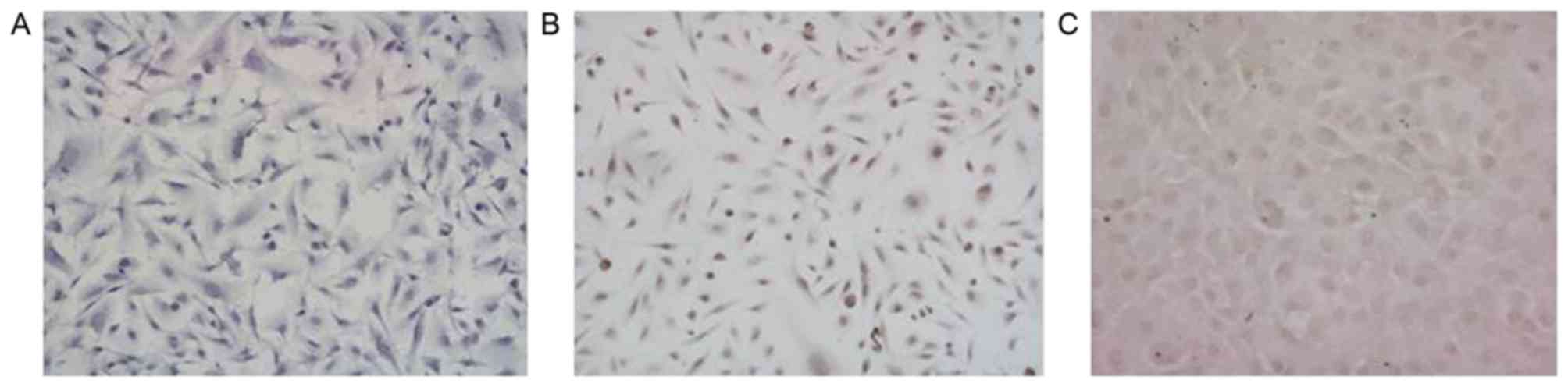
immunocytochemical detection. As indicated in Fig. 1, in the 3 glioma stem cell populations
(G1, G2, G3), the immunocytochemistry results demonstrated that
MGMT was negatively expressed in G1, but positively expressed in G2
and G3 (Fig. 1).
Detection of glioma cell
viability
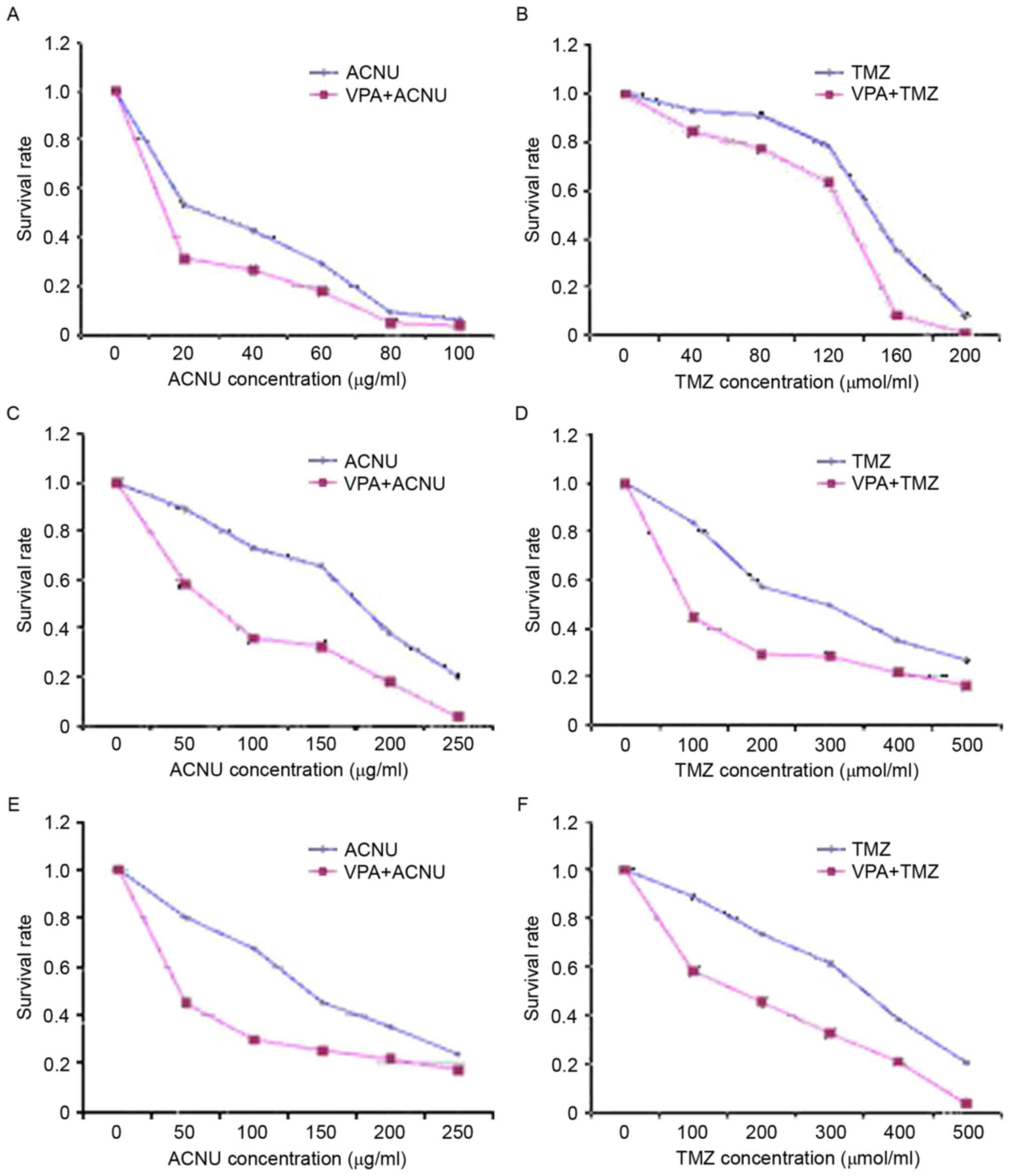
The absorbance values of each group were measured by
ELISA to calculate the viability of the glioma stem cells. As
indicated in Fig. 2, the survival
rate of the 3 glioma cells exposed to the various concentrations of
TMZ or ACNU in VPA + TMZ/ACNU groups was decreased compared with
that of the TMZ or ACNU alone group (P <0.05). There was a
particularly significant difference in cells exposed to lower
concentrations of TMZ or ACNU compared with cells exposed to higher
TMZ/ACNU concentrations (P<0.05), which indicated that VPA at
various concentrations enhanced the inhibitory effects of TMZ and
ACNU on the growth of MGMT-negative/positive cells (Fig. 2), in particular in the MGMT-positive
cells (G2 and G3) (Fig. 2C-F).
VPA combination with TMZ/ACNU
increases the apoptotic rate of glioma stem cells
In the MGMT-negative glioma cells (G1) or
MGMT-positive cell (G2, G3), the apoptotic rate of the VPA alone
group was increased compared with that of the control group
(P<0.05), indicating that VPA induced the apoptosis of glioma
stem cells. Additionally, the apoptotic rate in glioma cells
exposed to VPA combined with TMZ or ACNU was increased compared
with that of cells treated with TMZ or ACNU alone (P<0.05), and
there was a significant difference in the MGMT-positive cells,
compared with the MGMT-negative cells (G2, G3) (P<0.05), which
suggested that VPA combined with TMZ or ACNU may increase
TMZ/ACNU-induced apoptosis of glioma stem cells, particularly in
MGMT-positive cells (Table II).
 | Table II.Percentage of apoptotic cells in each
group. |
Table II.
Percentage of apoptotic cells in each
group.
| Glioma cells | Control group, % | VPA, % | TMZ, % | VPA+TMZ, % | ACNU, % | VPA+ACNU, % |
|---|
| G1 | 16.86±2.31 |
21.14±1.01a | 27.72±2.29 | 35.16±0.45 | 26.33±0.65 | 28.51±1.06 |
| G2 |
7.02±2.84 |
17.49±1.54a | 18.42±1.47 |
43.44±0.93b | 15.99±1.29 |
30.42±1.64b |
| G3 |
8.77±0.66 |
21.69±0.79a | 45.47±1.00 |
59.44±0.76b | 27.29±0.76 |
40.28±1.05b |
VPA downregulates the expression of
MGMT protein
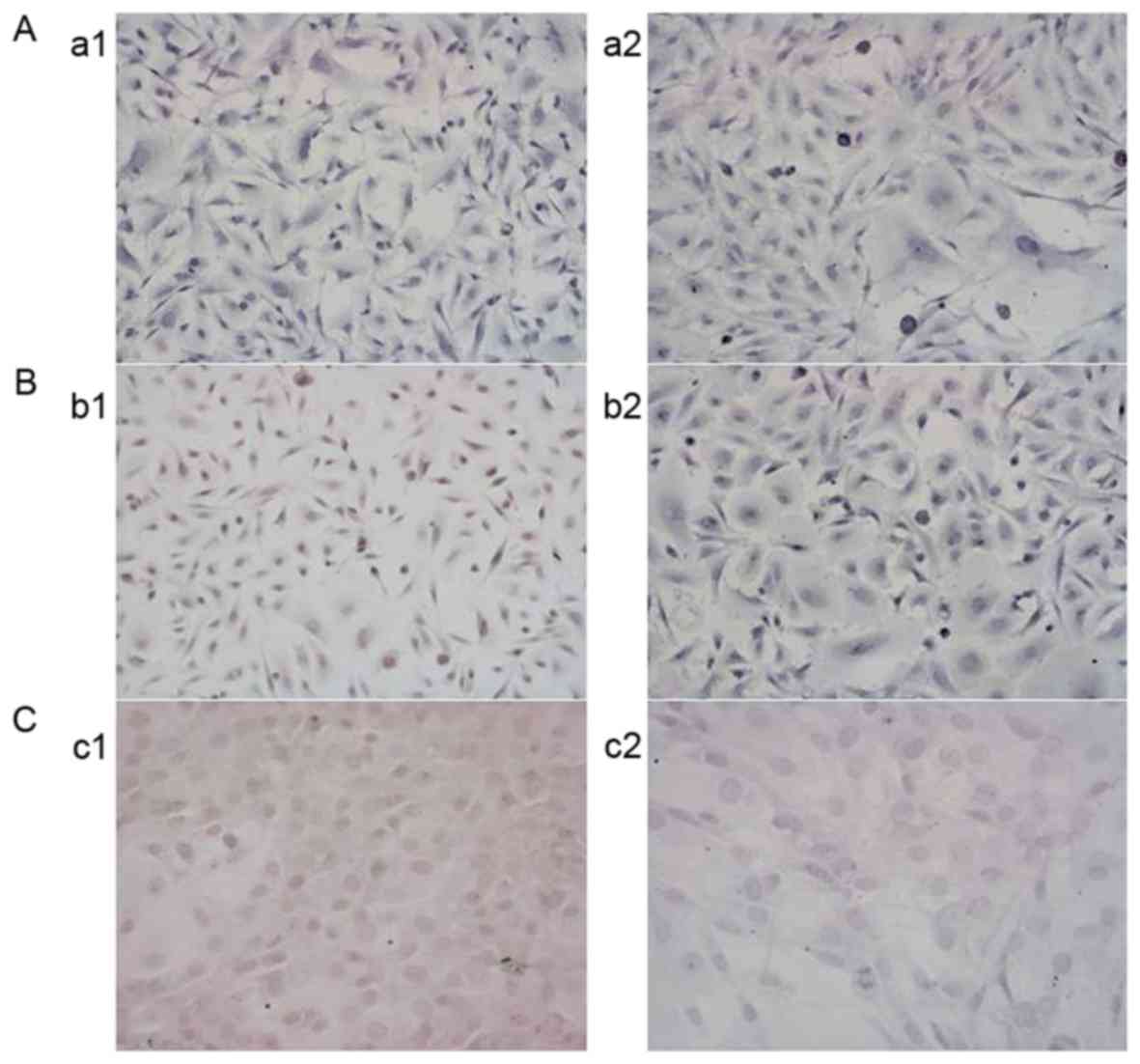
As demonstrated in Fig.
3, the MGMT-negative G1 cells were cultured in medium
containing 1 mmol/l VPA for 3 days. MGMT remained negatively
expressed in the G1 line, but MGMT expression appeared negative in
the 2 MGMT-positive cell populations (G2 and G3) following exposure
to 1 mmol/l VPA for 3 days, indicating that VPA may downregulate
the expression of MGMT in MGMT-positive cell populations.
VPA promotes the methylation of MGMT
promoter
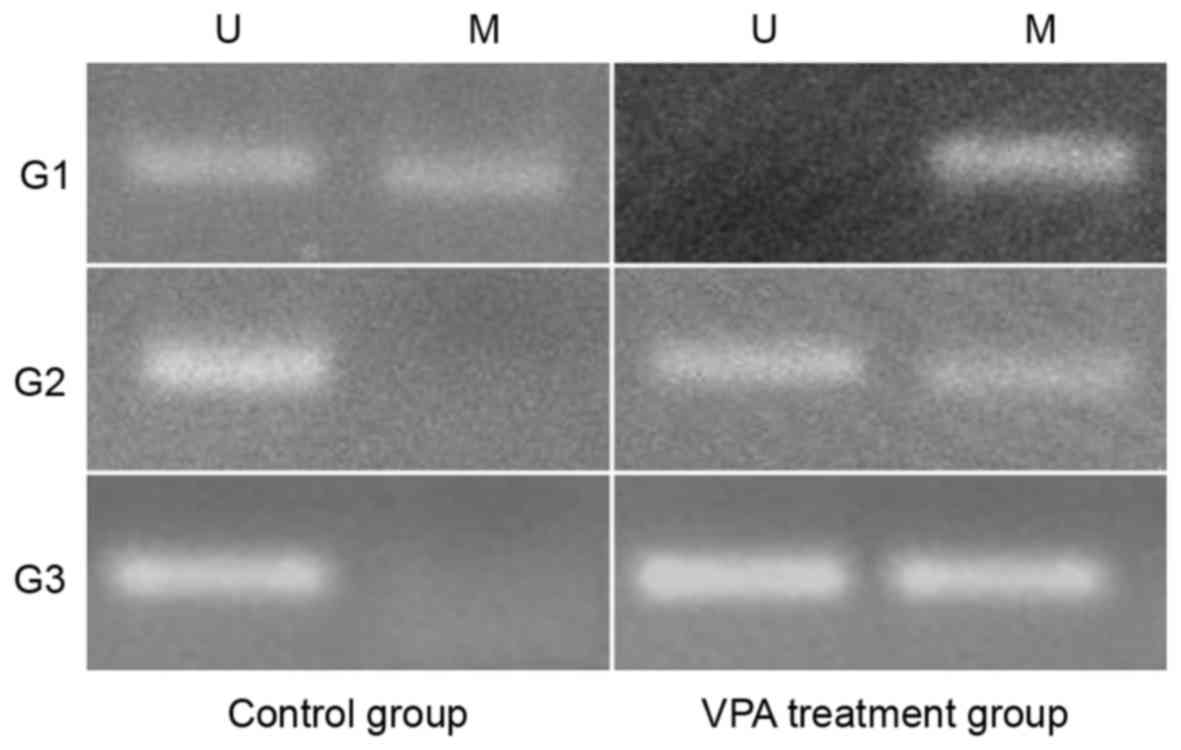
As indicated in Fig.
4, the MGMT promoter methylation assays revealed that the MGMT
promoter of G1 was partially methylated in the control group, but
that the promoter became fully methylated in the VPA treatment
group following VPA treatment. Concurrently, the promoters of G2
and G3 were unmethylated in the control groups, but were partially
methylated in the VPA treatment groups, which indicated that VPA
may promote the methylation of the MGMT promoter and silence MGMT
expression in glioma cells.
Discussion
TMZ and ACNU are components of standard
chemotherapeutic schedules for the first-line treatment of
malignant gliomas. However, these alkylating agents do not exhibit
the same levels of efficacy in all glioblastomas, and certain
glioma cells exhibit alkylating agent resistance, which has been
demonstrated to be correlated with DNA repair protein
O6-methylguanine-DNA methyltransferase (MGMT) expression (10). Alkylating agents may also modify the
O6 position of DNA guanine with methyl groups, eventually leading
to cell death dues to failure of mitosis (7,8). In
addition, MGMT is able to remove the methyl group with toxic and
mutagenic effects from the O6 position of guanine for
O6-methylguanine repair, therefore protecting the cells against the
damage of methyl groups (9,10). CpG methylation of the MGMT promoter
may inhibit MGMT expression, and almost one-half of primary
glioblastoma samples have evidence of high promoter methylation, as
determined by methylation-specific PRC (20,21).
Furthermore, among patients with glioma treated with TMZ combined
with radiation, there is significant difference in the 2-year
survival of patients with MGMT promoter hypermethylation (46%) as
compared with those without evidence of tumor hypermethylation
(14%) (22). Therefore, a decrease in
the expression of MGMT as a result of MGMT promoter
hypermethylation in glioma cells may enhance the sensitivity of
these cells to alkylating agents, which is valuable for future
studies.
It has been documented that VPA exhibits anti-glioma
effects due to its inhibition of histone deacetylase (23). In addition, VPA may induce glioma cell
differentiation and apoptosis, and inhibit glioma proliferation,
invasion and angiogenesis (24).
However, its sensitivity varies among malignant glioma cell
populations. For example, VPA has an anti-cancer effect in U87 and
U251 cells at low dosages, while VPA has the similar effects on T98
and U138 cells at higher dosages (25). Numerous in vitro studies have
used concentrations of VPA ranging from 1–10 mM (26,27). The
present study used a dosage of 1 mM, as this concentration was
close to the concentration (0.5–1 mM) that may be achieved in
vivo. The glioma stem cells in the present study were isolated
and cultured from the tumor specimens excised from patients with
glioma, as glioma stem cells are the cause of the development and
recurrence of glioma, and cells from tumor tissues may provide an
accurate reflection of the overall biological characteristics of
the tumor.
In the present study, the results indicated that VPA
enhanced the inhibitory effects of TMZ and ACNU at various
concentrations on the growth of MGMT-negative/positive cells,
particularly in the MGMT-positive cell populations. Flow cytometry
used to measure the levels of apoptosis indicated that VPA alone
induced the apoptosis of glioma cells, and VPA combined with TMZ or
ACNU increased the rate of TMZ/ACNU-induced apoptosis of glioma
stem cells, particularly in MGMT-positive cells compared with VPA
treatment alone. The immunocytochemistry results indicated that
MGMT expression in the 2 MGMT-positive cell populations (G2 and G3)
became negative following VPA treatment, indicating that the
expression of MGMT was downregulated by VPA in MGMT-positive glioma
stem cells. It was also identified that VPA may downregulate the
expression of MGMT in T98 and U138 glioma cell lines by western
blot analysis (28).
Methylation-specific PCR detection demonstrated that VPA treatment
enhanced MGMT promoter methylation in the 3 glioma stem cell
populations, suggesting that VPA did not induce the demethylation
of MGMT gene promoter region; on the contrary, it may enhance MGMT
gene promoter methylation, thereby resulting in the downregulation
of MGMT expression, increase in TMZ and ACNU sensitivity in glioma
stem cells and enhanced inhibitory effects of TMZ and ACNU on the
proliferation of glioma stem cells, finally inducing tumor cell
apoptosis. In the MGMT-negative cells, VPA remained able to enhance
the inhibitory effects of TMZ and ACNU on cell proliferation,
indicating that VPA used other mechanisms to sensitize cells to TMZ
and ACNU.
In the clinical setting, VPA is a common an
antiepileptic drug, and used in the treatment and prevention of
seizures in patients with brain tumors. The anti-glioma effects of
VPA suggests that VPA may be the first choice for treatment in
patients with glioma to control and prevent seizures, and it may
enhance the susceptibility of glioma cells to radiation by
inhibiting histone deacetylase. In concordance with the results of
the present study, it was also demonstrated that VPA downregulated
MGMT expression by increasing the level of MGMT gene promoter
methylation in glioma stem cells, which enhanced the sensitivity of
TMZ and ACNU to glioma cells and the inhibitory effects of TMZ and
ACNU on the proliferation, finally resulting in cell apoptosis.
This process suggests that patients with glioma may receive
chemotherapy plus concomitant and adjuvant VPA. In addition, VPA
may also promote the methylation of the MGMT promoter and silence
MGMT expression in glioma cells, which may be an important
mechanism of VPA enhancing the sensitivity of TMZ and ACNU-targeted
glioma stem cells. However, a number of issues in the clinical
application of anti-glioma VPA treatment require additional
exploration, for example; whether clinical anti-epileptic
concentrations produce anti-glioma effects and sensitize glioma
cells to radiotherapy and chemotherapy, or whether an established
concentration and dosage may be identified to exhibit potential
anti-tumor effects as far as possible, without causing serious side
effects.
Acknowledgements
Not applicable.
Funding
The present study was granted by the Outstanding
Talent Project of Henan Province, China (grant no.
084200410011).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZL and XB conceived the study design and drafted the
manuscript. YX and DY participated in the study design and
coordination. YY, XG and GZ assisted in study conceptualization,
conducted the statistical analysis and contributed to the
manuscript draft. ZW and JJ supervised with the data collection and
assisted in the implementation of the study. All authors revised
and approved the final manuscript.
Ethical approval and consent to
participate
The present study was approved by the Ethics
Committee of Zhengzhou No. 7 People's Hospital (Zhengzhou, China).
Written informed consent was gained from all participants.
Consent for publication
All subjects participating in this study have
provided their consent for the publication of this data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lu B, Zhou Y, Su Z, Yan A and Ding P:
Effect of CCL2 siRNA on proliferation and apoptosis in the U251
human glioma cell line. Mol Med Rep. 16:3387–3394. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong J, Zhao Y, Huang Q, Fei X, Diao Y,
Shen Y, Xiao H, Zhang T, Lan Q and Gu X: Glioma stem/progenitor
cells contribute to neovascularization via transdifferentiation.
Stem Cell Rev. 7:141–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Audia A, Conroy S, Glass R and Bhat KPL:
The impact of the tumor microenvironment on the properties of
glioma stem-like cells. Front Oncol. 7:1432017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versusradiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wakabayashi T, Yoshida J, Mizuno M and
Kajita Y: Intratumoral microinfusion of nimustine (ACNU) for
recurrent glioma. Brain Tumor Pathol. 18:23–28. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaina B, Ziouta A, Ochs K and Coquerelle
T: Chromosomal instability, reproductive cell death and apoptosis
induced by O6-methylguanine in Mex-, Mex+ and methylation-tolerant
mismatch repair compromised cells: Facts and models. Mutat Res.
381:227–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
D'Atri S, Tentori L, Lacal PM, Graziani G,
Pagani E, Benincasa E, Zambruno G, Bonmassar E and Jiricny J:
Involvement of the mismatch repair system in temozolomide-induced
apoptosis. Mol Pharmacol. 54:334–341. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brennand J and Margison GP: Reduction of
the toxicity and mutagenicity of alkylating agents in mammalian
cell sharboring the Escherichia coli alkyltransferase gene. Proc
Natl Acad Sci USA. 83:6292–6296. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gerson SL: MGMT: Its role in cancer
aetiology and cancer therapeutics. Nat Rev Cancer. 4:296–307. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hegi ME, Diserens AC, Godard S, Dietrich
PY, Regli L, Ostermann S, Otten P, Van Melle G, de Tribolet N and
Stupp R: Clinical trial substantiates the predictive value of
O-6-methylguanine-DNAmethyltransferase promoter methylation in
glioblastoma patients treated with temozolomide. Clin Cancer Res.
10:1871–1874. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Göttlicher M, Minucci S, Zhu P, Krämer OH,
Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG
and Heinzel T: Valproic acid defines a novel class of HDAC
inhibitors inducing differentiation of transformed cells. EMBO J.
20:6969–6978. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kostrouchová M, Kostrouch Z and
Kostrouchová M: Valproic acid, a molecular lead to multiple
regulatory pathways. Folia Biol (Praha). 53:37–49. 2007.PubMed/NCBI
|
|
15
|
Michaelis M, Michaelis UR, Fleming I,
Suhan T, Cinatl J, Blaheta RA, Hoffmann K, Kotchetkov R, Busse R,
Nau H and Cinatl J Jr: Valproic acid inhibits angiogenesis in vitro
and in vivo. Mol Pharmacol. 65:520–527. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Riva G, Butta V, Cilibrasi C, Baronchelli
S, Redaelli S, Dalprà L, Lavitrano M and Bentivegna A: Epigenetic
targeting of glioma stem cells: Short-term and long-term treatments
with valproic acid modulate DNA methylation and differentiation
behavior, but not temozolomide sensitivity. Oncol Rep.
35:2811–2824. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu H, Song XD, Li Y and Dai J: The new
method of cells growing on the glass slide. Zhongguo Ying Yong
Sheng Li Xue Za Zhi. 25:283–285. 2009.(In Chinese). PubMed/NCBI
|
|
18
|
Ku JL, Jeon YK and Park JG:
Methylation-specific PCR. Methods Mol Biol. 791:23–32. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen H, Zhang JH, Li BY, Zhang XQ, Gao HQ,
Xu XQ and Wang JFL: The application of hematoxylin stain method in
determining the effect of non-monomer herbal extract on the cell
proliferation. Chin J Biochem Pharm. 31:183–185. 2010.(In
Chinese).
|
|
20
|
Paz MF, Yaya-Tur R, Rojas-Marcos I, Reynes
G, Pollan M, Aguirre-Cruz L, García-Lopez JL, Piquer J, Safont MJ,
Balaña C, et al: CpG island hypermethylation of the DNA repair
enzyme methyltransferase predicts response to temozolomide in
primary gliomas. Clin Cancer Res. 10:4933–4938. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Esteller M, Hamilton SR, Burger PC, Baylin
SB and Herman JG: Inactivation of the DNA repair gene
O6-methylguanine-DNA methyltransferase by promoter hypermethylation
is a common event in primary human neoplasia. Cancer Res.
59:793–797. 1999.PubMed/NCBI
|
|
22
|
Kim JH, Shin JH and Kim IH: Susceptibility
and radiosensitization of human glioblastoma cells to trichostatin
A, a histone deacetylase inhibitor. Int J Radiat Oncol Biol Phys.
59:1174–1180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alvarez AA, Field M, Bushnev S, Longo MS
and Sugaya K: The effects of histone deacetylase inhibitors on
glioblastoma-derived stem cells. J Mol Neurosci. 55:7–20. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oi S, Natsume A, Ito M, Kondo Y, Shimato
S, Maeda Y, Saito K and Wakabayashi T: Synergistic induction of
NY-ESO-1 antigen expression by a novel histone deacetylase
inhibitor, valproic acid, with 5-aza-2′-deoxycytidine in glioma
cells. J Neurooncol. 92:15–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Greenblatt DY, Vaccaro AM, Jaskula-Sztul
R, Ning L, Haymart M, Kunnimalaiyaan M and Chen H: Valproic acid
activates notch-1 signaling and regulates the neuroendocrine
phenotype in carcinoid cancer cells. Oncologist. 12:942–951. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rodriguez-Menendez V, Gilardini A, Bossi
M, Canta A, Oggioni N, Carozzi V, Tremolizzo L and Cavaletti G:
Valproate protective effects on cisplatin-induced peripheral
neuropathy: An in vitro and in vivo study. Anticancer Res.
28:335–342. 2008.PubMed/NCBI
|
|
27
|
Van Nifterik KA, Van den Berg J, Slotman
BJ, Lafleur MV, Sminia P and Stalpers LJ: Valproic acid sensitizes
human glioma cells for temozolomide and γ-radiation. J Neurooncol.
107:61–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ryu CH, Yoon WS, Park KY, Kim SM, Lim JY,
Woo JS, Jeong CH, Hou Y and Jeun SS: Valproic acid downregulates
the expression of MGMT and sensitizes temozolomide-resistant glioma
cells. J Biomed Biotechnol. 2012:9874952012. View Article : Google Scholar : PubMed/NCBI
|