Introduction
Thyroid carcinoma is one of the major common
malignant tumors of the endocrine system. Its incidence has grown
to become the fifth most common cancer in females globally
(1,2).
In total, >50% of thyroid cancers in women and >45% of those
in men were diagnosed at age 50+ (3).
The increased incidence rates of thyroid cancer may be accounted
for by changes in environmental risk factors, including obesity and
molecular pathways (4–6). Thyroid carcinoma is classed into four
main types, including papillary, follicular, medullary and
anaplastic carcinomas (7). Among
these four types of thyroid carcinoma, papillary thyroid carcinoma
(PTC) is the predominant type, accounting for ~70% of all thyroid
malignancies (8). Traditional
clinical treatments for thyroid carcinoma include surgery,
radioactive iodine and endocrine suppression therapy. PTC generally
has an indolent course and favorable prognosis (9). However, there remain 20–30% of patients
where PTC recrudesces and became resistant to radioactive iodine
treatment, particularly in patients with advanced thyroid
carcinoma, yielding poor prognosis and shorter survival (10,11).
Therefore, there is an urgent requirement to elucidate the
molecular mechanism underlying thyroid carcinoma progression and to
improve the survival rate of patients with thyroid carcinoma.
Previously, there has been extensive research into
the molecular alterations in thyroid carcinomas, with particular
focus on the investigation of several oncogenic pathways that
contribute to various cancer types (12,13).
Numerous molecular markers associated with the prognosis of thyroid
carcinomas have been identified, including RAS,
phosphatidylinositol-4,5-bisphosphate 3-kinase, phosphatase and
tensin homolog, tumour protein p53, anaplastic lymphoma kinase and
B-Raf proto-oncogene serine/threonine kinase (BRAF). The most
common gene alteration identified in thyroid carcinoma is BRAF
mutation, which i§s associated with ~50% of these tumors (14,15). BRAF
mutations are relatively specific for PTC and have not been
identified in other types of thyroid carcinomas. The specific
mutation that occurs in thyroid carcinomas is a threonine-to
alanine nucleotide transversion at position 1799 in exon 15, which
results in a valine-to-glutamate substitution at codon 600, named
BRAFV600E, which has been revealed to be a potential
prognostic factor in thyroid carcinoma (16,17).
BRAF belongs to the RAF protein kinase family, and
is a serine/threonine kinase which serves an important function in
the mitogen activated protein kinase (MAPK) signaling pathway
(18). The mutation in this gene may
activate the RAF/mitogen-activated protein kinase/extracellular
regulated kinase (MEK)/extracellular signal regulated kinase (ERK)
signaling pathway, promoting cell proliferation and inhibiting
apoptosis. BRAF has been revealed to be mutated in a variety of
malignant tumors. For example, BRAF mutations have been detected in
~70% of skin cancer (19), ~50% of
thyroid carcinoma (20), ~20% of
colorectal cancer (21), 14–30% of
ovarian cancer (22), ~15% of
hepatocellular carcinoma (23) and
~5% of lung cancer and breast cancer (24,25).
Notably, BRAF mutation has also been identified as a lethal factor.
Multiple studies have demonstrated that tumors with a BRAF mutation
are resistant to traditional treatment, leading to poor prognosis
(26,27). Therefore, the development of a novel
therapeutic strategy for cancer with BRAF mutation is urgently
required.
Previous studies have indicated that
BRAFV600E mutation status may predict recurrence and
prognosis in patients with thyroid carcinomas (15,28,29).
BRAFV600E contributes to tumorigenesis by the continual
activation of phospho-MAPK and the downstream Monopolar spindle 1
(Mps1) in thyroid carcinomas, leading to chromosome euploidy which
promotes tumor development (30).
Although there has been extensive research into the high frequency
of BRAF mutations in thyroid carcinomas, the exact molecular
mechanisms of BRAF mutation are yet to be fully understood. The
present study will focus on observations of the
BRAFV600E mutation in thyroid carcinomas, the
associations between the BRAFV600E mutation,
phospho-MAPK, Mps1 and clinical parameters of thyroid carcinomas
and its prognostic significance.
Materials and methods
Ethics statement and clinical
specimens
Cancer tissue samples were obtained from 161
patients with thyroid carcinomas who underwent surgical resection
at the First Hospital of Shanxi Medical University, (Shanxi, China)
and Shanxi Provincial People's Hospital, (Shanxi, China) between
January 2009 and June 2015 (Table I).
The present study was approved by the Ethics Committee of the First
Hospital of Shanxi Medical University and written informed consent
was obtained from the patients. All patients were enrolled at the
time of surgery and did not receive treatment prior to surgery,
including radiotherapy or chemotherapy. Surgically removed tissue
was fixed in 10% buffered formalin for 24 h at room temperature,
and embedded in paraffin. Hematoxylin and eosin-stained tumor
tissues (stained for 5 min and 30 sec at room temperature,
respectively) were reviewed by two pathologists. The tumor area was
marked and then manually cut into 2 tissue volumes (10-µm thick) to
collect tumor cells for DNA extraction. DNA was extracted using the
FFPE DNA kit (Omega Bio-Tek, Inc., Norcross, GA, USA) following the
manufacturer's protocol. The extracted DNA was quantified using a
spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
 | Table I.Clinicopathological features of 161
patients with thyroid carcinomas. |
Table I.
Clinicopathological features of 161
patients with thyroid carcinomas.
| Clinicopathological
feature | No. (161) |
|---|
| Sex |
|
|
Male | 34 |
|
Female | 127 |
| Age, years |
|
|
>35 | 139 |
|
≤35 | 22 |
| Smoking status |
|
|
Yes | 9 |
| No | 152 |
| Drinking
status |
|
|
Yes | 6 |
| No | 155 |
| Familial
history |
|
|
Yes | 2 |
| No | 159 |
| Tumor grade |
|
|
Papillocarcinoma | 157 |
|
Non-papillocarcinoma | 4 |
| Pathological T
stage |
|
|
T1-T2 | 133 |
|
T3-T4 | 28 |
| Pathological N
stage |
|
| N0 | 64 |
| N1 | 97 |
| Clinical M |
|
| M0 | 161 |
| M1 | 0 |
| Pathological
stage |
|
|
I–II | 106 |
|
III–IV | 55 |
BRAFV600E mutation
analysis
To screen for the BRAFV600E mutation, DNA
from tumor tissue was analyzed by polymerase chain reaction
(PCR)-direct sequencing. Exon 15 of the BRAF gene, which contains
the T1796A mutation, was amplified using the following specific
primers: Forward, 5′-CTCTTCATAATGCTTGCTCTGATAGG-3′; and reverse,
5′-CTCTTCATAATGCTTGCTCTGATAGG-3′. The cycling conditions for PCR
were as follows: Initial denaturation (98°C, 3 min) followed by 30
cycles of denaturation 98°C for 30 sec, annealing 58°C for 30 sec
and then an extension 72°C for 30 sec. All PCR products were
visualized using electrophoresis on a 2% agarose gel. Sanger
sequencing was performed by the Beijing Genomics Institute
(Shenzhen, China).
Immunohistochemistry (IHC)
Phospho-MAPK or Mps1 protein levels in thyroid
carcinomas were determined by IHC with phospho-MAPK antibody (cat.
no. 4370, Cell Signaling Technology, Inc., Danvers, MA, USA) or
Mps1 antibody (cat. no. ab1118, Abcam, Cambridge, UK). IHC was
performed as follows: In brief, sections were incubated with
primary antibodies against phospho-MAPK (1:100) or Mps1 (1:50)
overnight at 4°C, followed by detection using the PV8000 kit
(Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China)
and DAB detection kit (Fuzhou Maixin Biotech Co., Ltd., Fuzhou,
China), according to the manufacturer's protocol. Slides were
counterstained with hematoxylin for 50 sec at room temperature. All
images were captured using an Aprio automatic biopsy scanner at
×100 magnification. The percentage and intensity of positive
staining of phospho-MAPK and Mps1 were analyzed using Aperio
Cytoplasma 2.0 software (Leica Microsystems, Inc., Buffalo Grove,
IL, USA). Statistical analyses were performed using GraphPad Prism
v.6.0 software package (GraphPad Software, Inc., La Jolla, CA,
USA). Using a scoring system from (−) to (+++) respectively,
nucleus Phospho-MAPK expression was measured as negative for 0–25
scores (−), weak for 25–50 scores (+), median for 50–75 scores (++)
or strong for >130 scores (+++). Cytoplasm Mps1 expression was
measured as negative for 0–50 scores (−), weak for 50–90 scores
(+), median for 90–130 scores (++) or strong for >130 scores
(+++).
Statistical analysis
60 cases were selected for analysis, including 30
BRAFV600E-positive cases, which were randomly matched
with 30 BRAFV600E-negative cases. The expression of
phospho-MAPK and Mps1 levels between the paired samples were
compared using χ2 tests or Fisher's exact tests. This
was also used to assess the association between BRAF mutation
status and clinical parameters. Cox regression was used to measure
the association between BRAFV600E mutation and the
clinical pathological information, linear regression was used to
analyze the association between phospho-MAPK and Mps1 expression
and survival analysis was performed using Kaplan-Meier analysis and
the Log-rank test. Univariate and multivariate survival analyses
were performed using a Cox proportional hazards regression model.
P<0.05 was considered to indicate a statistically significant
difference. All statistical tests were performed using IBM SPSS
19.0 (IBM Corp., Armonk, NY, USA).
Results
BRAFV600E mutation and its
association with clinical parameters in thyroid carcinomas
Single-base substitutions were detected by Sanger
sequencing, which were presented in BRAF exon 15: T1796A, leading
to a substitution of valine to glutamic acid at position 600
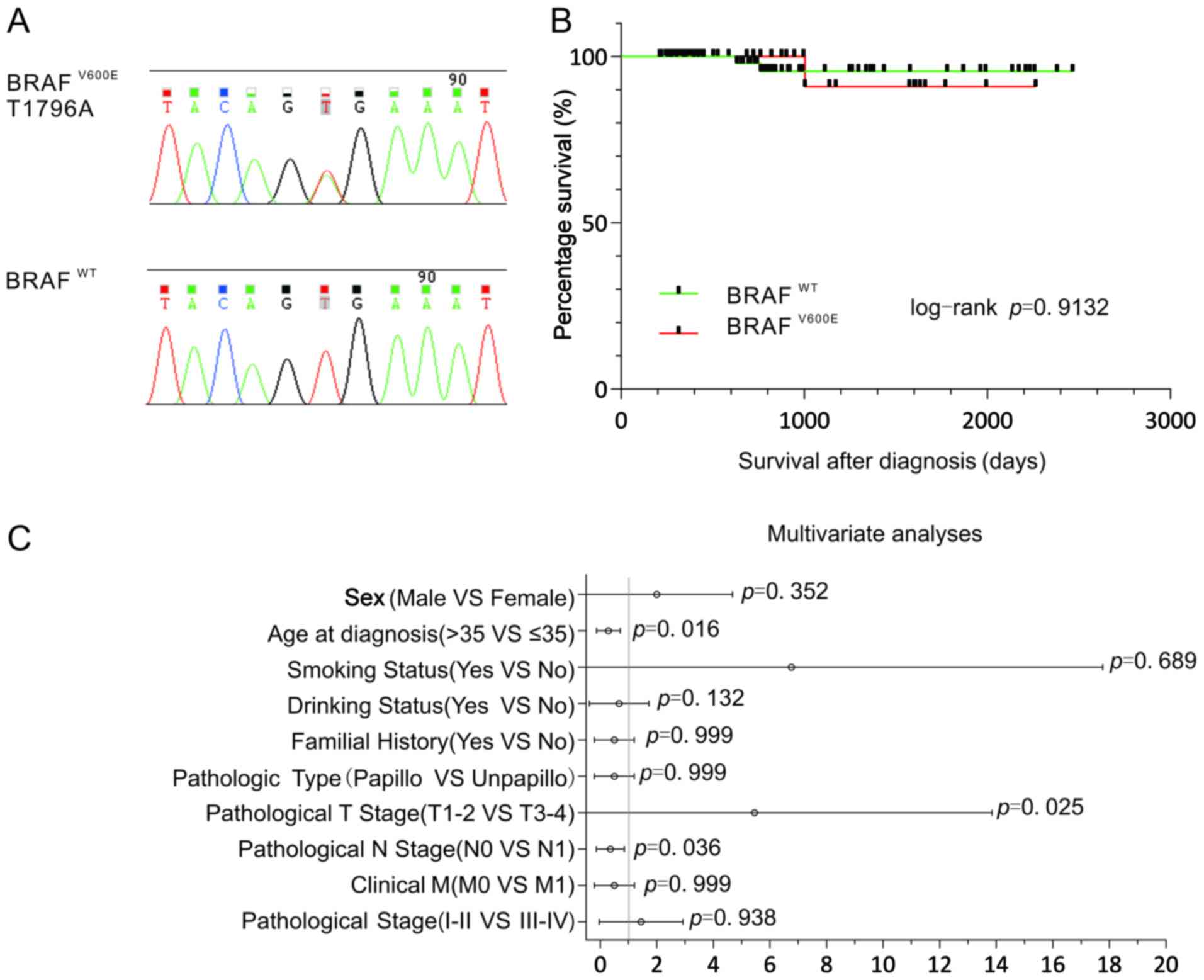
(V600E) in thyroid cancer (Fig. 1A).
In 161 cases of thyroid carcinomas, 55 cases (34%) of
BRAFV600E mutation were identified. A statistical
analysis of BRAF mutations and clinical parameters revealed that
BRAF mutations were significantly associated with age, tumor (T)
stage and lymph node (N) stage, were more prevalent in younger
patients (≤35 years), pathological T1-T2 stage patients and
pathological N0 stage patients (χ2 test or Fisher's
exact test, P<0.05, Table II; Cox
regression analysis, P<0.05, Fig.
1C). However, there was no significant association between BRAF
mutation status and other clinical parameters, including sex,
smoking status, drinking status, familial history, tumor grade,
clinical metastasis (M) and pathological stage (χ2 test
or Fisher's exact test, P>0.05, Table
II; Cox regression analysis, P>0.05, Fig. 1C). Although patients with thyroid
carcinomas with BRAFV600E mutations had a higher risk of
mortality, the association of BRAFV600E mutations with
overall survival (OS) was not statistically significant [Log-rank
(MantelCox), P=0.9132, Fig. 1B].
Furthermore, the Cox regression model revealed that age, T stage
and N stage were associated with BRAFV600E mutation in
thyroid cancer (Fig. 1C).
 | Table II.Associations between
BRAFV600E mutation/phospho-MAPK/Mps1 and
clinicopathological parameters in thyroid carcinomas. |
Table II.
Associations between
BRAFV600E mutation/phospho-MAPK/Mps1 and
clinicopathological parameters in thyroid carcinomas.
|
| B-RAFV600E mutation
(n=161) | Phospho-MAPK in
tumor (n=60) | MpS1 in tumor
(n=60) |
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | + | − | P-value | + | − | P-value | + | − | P-value |
|---|
| No. (%) | 54 (34%) | 107 (67%) |
| 2 (3%) | 58 (97%) |
| 47 (78%) | 13 (22%) |
|
| Sex |
|
| 0.514 |
|
| >0.999 |
|
| >0.999 |
|
Male | 13 | 21 |
| 1 | 29 |
| 10 | 3 |
|
|
Female | 41 | 86 |
| 1 | 29 |
| 37 | 10 |
|
| Age, years |
|
| 0.025 |
|
| 0.472 |
|
| >0.999 |
|
>35 | 42 | 97 |
| 2 | 28 |
| 38 | 11 |
|
|
≤35 | 12 | 10 |
| 0 | 30 |
| 9 | 2 |
|
| Smoking status |
|
| 0.727 |
|
| 0.472 |
|
| >0.999 |
|
Yes | 4 | 5 |
| 0 | 30 |
| 2 | 1 |
|
| No | 50 | 102 |
| 2 | 28 |
| 45 | 12 |
|
| Drinking
status |
|
| 0.190 |
|
| 0.472 |
|
| >0.999 |
|
Yes | 4 | 2 |
| 0 | 30 |
| 2 | 0 |
|
| No | 50 | 105 |
| 2 | 28 |
| 45 | 13 |
|
| Familial
history |
|
| 0.211 |
|
| 0.472 |
|
|
|
|
Yes | 2 | 0 |
| 0 | 30 |
| 0 | 0 |
|
| No | 52 | 107 |
| 2 | 28 |
| 47 | 13 |
|
| Tumor grade |
|
| 0.367 |
|
| 0.472 |
|
|
|
|
Papillocarcinoma | 54 | 103 |
| 2 | 28 |
| 47 | 13 |
|
|
Non-papillocarcinoma | 0 | 4 |
| 0 | 30 |
| 0 | 0 |
|
| Pathological T
stage |
|
| 0.018 |
|
| 0.472 |
|
| 0.629 |
|
T1-T2 | 50 | 83 |
| 2 | 28 |
| 41 | 10 |
|
|
T3-T4 | 4 | 24 |
| 0 | 30 |
| 6 | 3 |
|
| Pathological N
stage |
|
| 0.004 |
|
| 0.472 |
|
| 0.898 |
| N0 | 30 | 34 |
| 0 | 30 |
| 28 | 8 |
|
| N1 | 24 | 73 |
| 2 | 28 |
| 19 | 5 |
|
| Clinical M |
|
|
|
|
| 0.472 |
|
|
|
| M0 | 54 | 107 |
| 2 | 28 |
| 47 | 13 |
|
| M1 | 0 | 0 |
| 0 | 30 |
| 0 | 0 |
|
| Pathological
stage |
|
| 0.585 |
|
| 0.472 |
|
| >0.999 |
|
I–II | 34 | 72 |
| 0 | 30 |
| 33 | 9 |
|
|
III–IV | 20 | 35 |
| 2 | 28 |
| 14 | 4 |
|
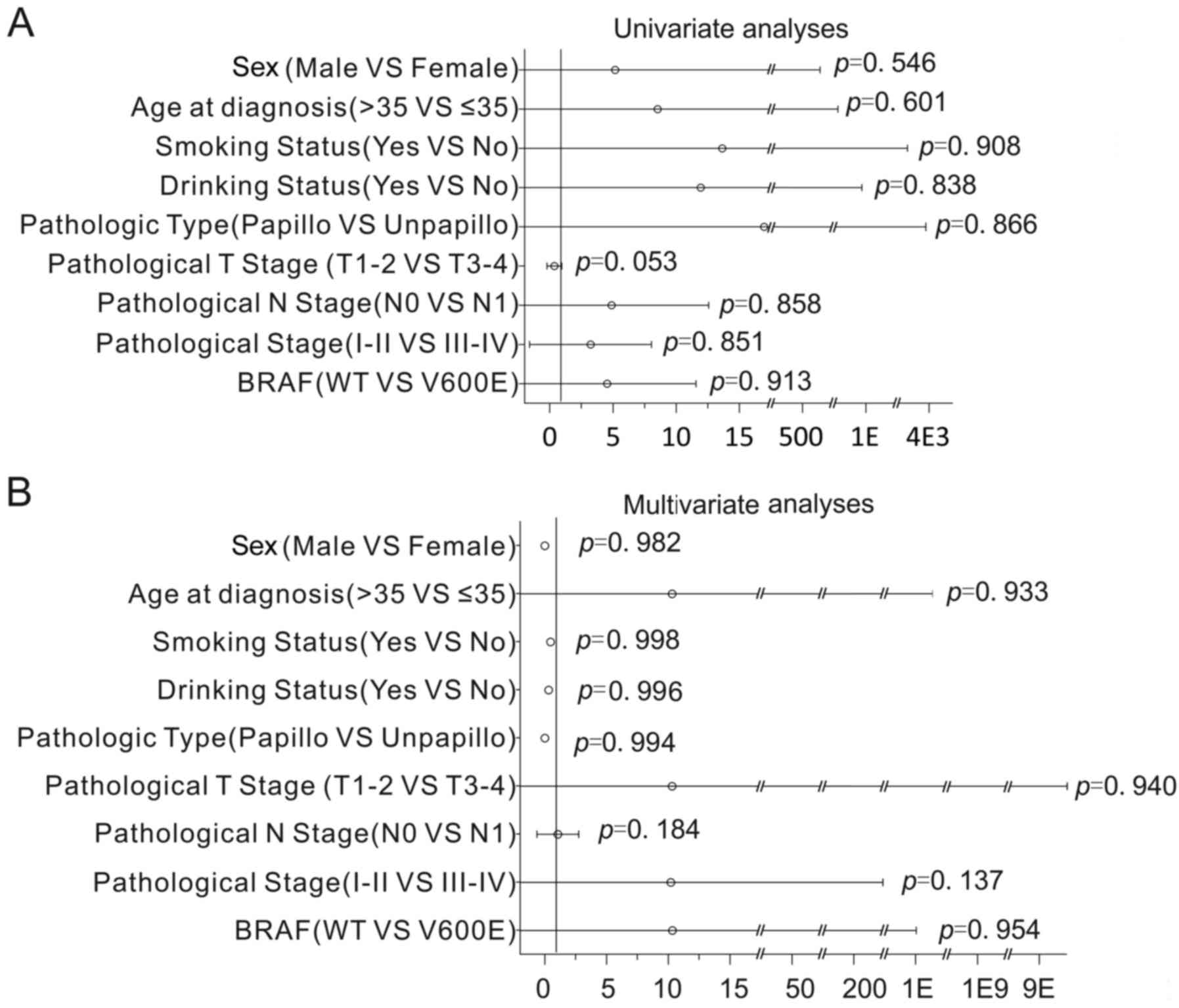
Cox regression analysis was used to assess the
impact of BRAF mutations and clinical parameters on OS. Notably,
the results revealed that the association of BRAF mutations with OS
was not statistically significant (Cox regression univariate
analyses, P=0.913, Fig. 2A; Cox
regression multivariate analyses, P=0.954; Fig. 2B). Additionally, the association of
clinical parameters with OS was not statistically significant (Cox
regression univariate analyses, P>0.05; Fig. 2A; Cox regression multivariate
analyses, P>0.05; Fig. 2B). This
data indicates that there were no significantly associated
prognostic factors in thyroid carcinoma.
IHC and evaluation of phospho-MAPK and
Mps1 in thyroid carcinomas
Aside from a number of the papillary thyroid micro
carcinoma cases, which were excluded due to the tumor tissue being
too thin to obtain, 30 BRAFV600E cases and 30
BRAFWT cases matched in age, sex, pathological type and
tumor size were selected to undergo IHC.
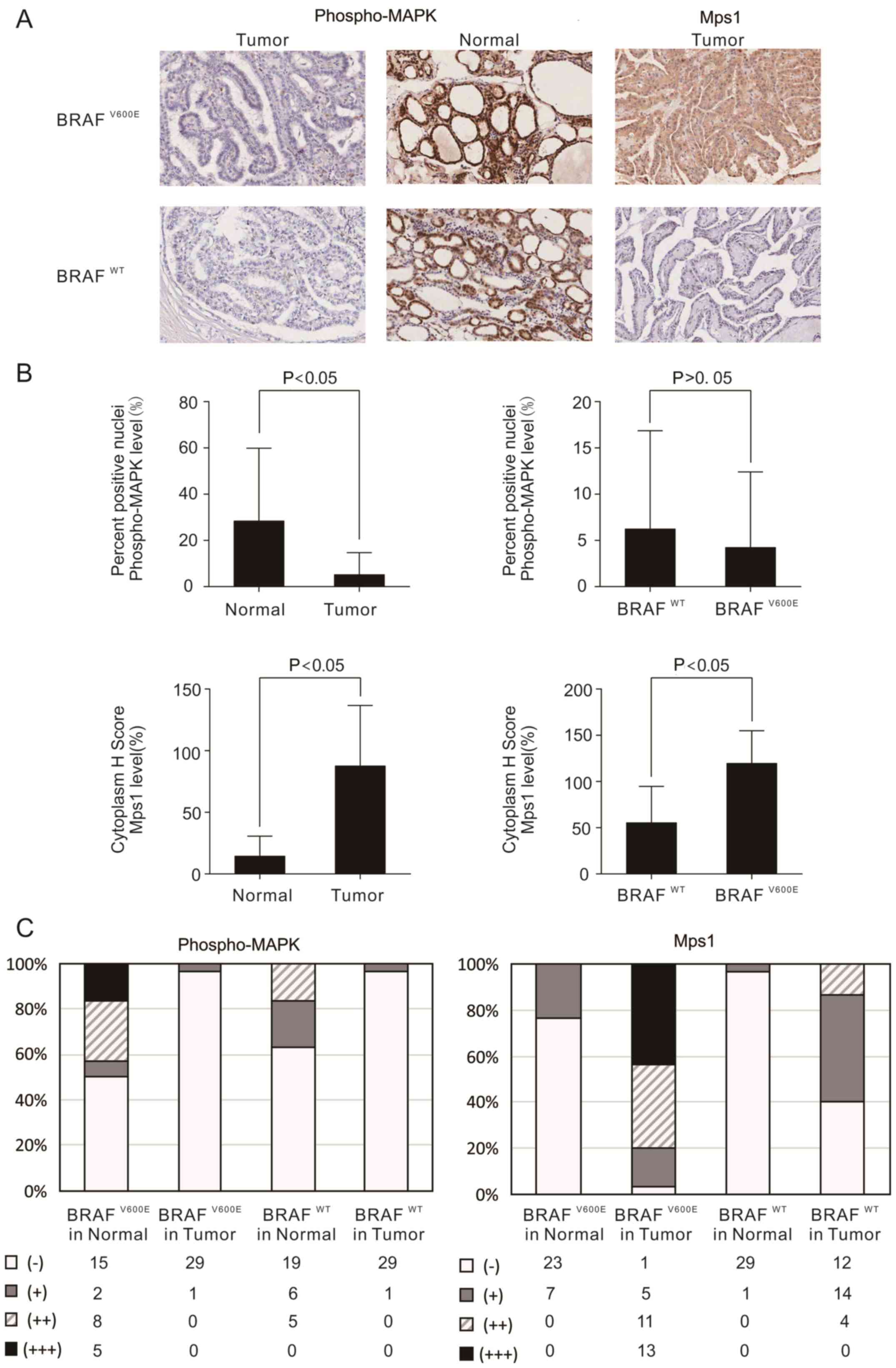
The results of IHC demonstrated that phospho-MAPK
was expressed in the nucleus of normal tissues. The expression of
phospho-MAPK was negative and had no difference between
BRAFV600E and BRAFWT thyroid carcinomas
tissues (Fig. 3A; χ2 test
or Fisher's exact test, P>0.05, Table III). However, unexpectedly,
phospho-MAPK levels were significantly decreased in tumor tissues
compared with those in matched normal thyroid tissues, particularly
in the tissues with BRAFV600E mutations (P<0.05;
Fig. 3A). In the BRAFV600E
samples, only 3.33% (1/30) demonstrated positive stained nuclei
phospho-MAPK in tumor tissues, while 50% (15/30) demonstrated
strongly stained nuclei in normal thyroid tissues (Fig. 3B and C). However, in the
BRAFWT cases, only 3.33% (1/30) had nuclei that stained
positive for phospho-MAPK in the tumor tissues, while 36.67%
(11/30) demonstrated strongly positive nuclei staining in normal
thyroid tissues (Fig. 3B and C).
 | Table III.Associations between phospho-MAPK and
Msp1 expression, and BRAF mutation status. |
Table III.
Associations between phospho-MAPK and
Msp1 expression, and BRAF mutation status.
|
| Phospho-MAPK | MpS1 |
|---|
|
|
|
|
|---|
| PTC | + (%) | − (%) | P-value | + (%) | − (%) | P-value |
|---|
|
BRAFV600E | 1 (3) | 29 (97) |
| 29 (97) | 1 (3) |
|
|
BRAFWT | 1 (3) | 29 (97) | – | 18 (60) | 12 (40) | 0.002 |
Further evaluations were made concerning the
expression of Mps1 in cytoplasm. The expression of Mps1 was
significantly increased in tumor tissues compared with that in
matched normal tissues (P<0.05; Fig.
3A), and it was significantly increased in BRAFV600E
mutation-positive tissues compared with that in BRAFWT
tissues (Fig. 3A; χ2 test
or Fisher's exact test, P<0.05, Table III). Furthermore, 71.67% (43/60) of
the samples demonstrated cytoplasmic stained Mps1 in the tumor
tissues, while 13.3% (8/60) stained positive in normal tissues
(Fig. 3B). When the BRAF status was
analyzed, 96.67% (29/30) of the samples were cytoplasmic-positive
for Mps1 in BRAFV600E samples, while 60% (18/30) were
cytoplasmic-positive in BRAFWT samples (Fig. 3B and 3C).
Association between phospho-MAPK or
Msp1 expression and clinical parameters
Analysis of phospho-MAPK expression and clinical
parameters revealed that the expression of phospho-MAPK in thyroid
carcinomas had no significant association with clinical parameters,
including sex, age, smoking status, drinking status, familial
history, tumor grade, pathological N stage, clinical M and
pathological stage (P>0.05, Table
II). The expression of Mps1 had no significant association with
clinical parameters, including sex, age, smoking status, drinking
status, familial history, tumor grade, pathological T stage,
pathological N stage, clinical M and pathological stage (P>0.05,
Table II).
Associations between phospho-MAPK or
Msp1 expression and BRAF mutation
The associations between phospho-MAPK or Msp1
expression and BRAF mutation were analyzed. No association was
identified between the expression of phospho-MAPK and BRAF mutation
(P>0.05, Table III). However,
there was significant association between the expression of Mps1
and BRAF mutation (P<0.05, Table
III). The expression of phospho-MAPK and Mps1 were not
significantly associated (Linear regression correlation coefficient
R2=0.032, P>0.05).
Discussion
The majority of patients with thyroid carcinoma are
cured routinely and have a relatively good prognosis (31). Generally, patients with thyroid
carcinomas are treated with thyroidectomy, and then radioiodine to
remove residual tumor tissue and metastasis (12,32).
However, in certain patients, thyroid cancer is diagnosed as a
poorly differentiated carcinoma or anaplastic thyroid carcinoma
rather than well-differentiated PTC. These patients also have
significantly reduced survival (32).
Therefore, it is important to identify novel therapeutic approaches
for these types of thyroid carcinomas.
Thyroid carcinomas are associated with RAF-MEK-MAPK
signaling (33). It has been revealed
that BRAFV600E causes a 500-fold increase in activation
of BRAF, and activates MEK-MAPK signaling constantly to regulate
the expression of a variety of malignant tumor-associated genes,
resulting in cell proliferation and differentiation (34–36). Thus,
it serves an important function in the occurrence and progression
of cancer.
BRAFV600E mutation appears to be the most
frequent ontogenetic event in thyroid carcinomas. Due to its high
frequency and specificity for thyroid carcinomas, the
BRAFV600E mutation serves a unique and fundamental
function in thyroid carcinomas (37).
Therefore, it is of great importance to investigate the function
and molecular mechanism of BRAFV600E in thyroid cancer,
in order to identify novel treatment strategies, and to improve the
survival rate. In the present study, 55 cases (34%) with
BRAFV600E mutation in 161 cases of thyroid carcinomas
were identified. Multiple studies have reported that the frequency
of BRAFV600E mutation is 30–50% in thyroid carcinomas,
and is associated with lymph node metastasis, extra thyroidal
extension, tumor size, recurrence and drug tolerance (38–41). Xing
(42) conducted a multicenter
retrospective study, and after a mean follow-up of 33 months, they
identified that the BRAFV600E mutation significantly
increased cancer-associated mortality. However, other research from
Japan and South Korea yielded different results, demonstrating that
B-RAFV600E was not associated with poor prognosis in
thyroid cancer (43,44). The results of the present study
revealed that BRAF mutations were significantly more prevalent in
younger patients (≤35 years), pathological T1-T2 stage patients,
and pathological N0 stage patients. The association of
BRAFV600E mutations with OS was not statistically
significant. This may be due to the good prognosis of thyroid
carcinomas, short follow-up time, difference of analysis method and
the genetic difference between those of eastern Asian and Caucasian
descent. The specific mechanisms of BRAFV600E still
require further study.
The development of specific kinase inhibitors
targeting BRAF, and the BRAFV600E allele in particular,
has been achieved. One of these is inhibitor PLX4032. It is a
highly selective inhibitor of BRAF kinase and has an
anti-proliferative effect on the A375 melanoma cell line, which is
BRAFV600E-positive. However, no differences in apoptosis
and cell cycle in the thyroid carcinoma cell line NPA and ARO were
observed, which also carries the BRAFV600E mutation.
These results may be due to the opposite direction of regulation of
p21cip1/waf1 between melanoma and thyroid cancer cells (45). Previous preclinical studies indicated
that combination with BAY43-9006 or other agents to increase its
efficacy was thought to be a novel strategy for effective clinical
therapy (46).
Previous studies have identified Mps1 to be a
downstream target of B-RAFV600E, and demonstrated that
is significantly associated with phospho-MAPK in melanoma (30). Based on the high mutation rate of
BRAFV600E in thyroid carcinoma, the present study
detected the expression of phospho-MAPK and Mps1 in thyroid
carcinoma by IHC. Compared with normal thyroid tissues,
phospho-MAPK was significantly decreased in patients with thyroid
carcinomas with the BRAFV600E mutation, which was
consistent with previous studies (47,48).
Furthermore, the expression of phospho-MAPK was not associated with
tumor size or clinical stage. The expression of Mps1 in patients
with the BRAFV600E mutation was significantly higher
than that in patients with BRAFWT, it was significantly
higher in tumor tissues than in paired normal tissue, and was not
associated with clinical factors. The present study revealed that
the expression of phospho-MAPK and Mps1 were not associated in
patients with BRAFV600E. The classical theory of
BRAFV600E, which is that it serves an important function
in thyroid carcinomas through the continuous activation of the
RAF-MEK-ERK signaling pathway, has been demonstrated in numerous
studies in vivo and in vitro (49,50).
However, the results of these studies contradict one another.
Potential reasons include the high tumor metabolic rate of thyroid
carcinomas. This suggests that the tumor specimens lost their blood
supply following operation, and thus the phosphorylation process
would be forced to stop due to the lack of ATP, which may lead to
the low-level of phospho-MAPK (47).
Therefore, the level of phospho-MAPK observed in specimens may not
represent the actual level of phospho-MAPK in vivo. In
addition, MAPK pathway signaling may be important in a
context-dependent manner (30). As
ERK phosphorylation was not associated with the presence of
activating BRAF mutations, the way in which activated BRAF
contributes to oncogenesis may be more complex than previously
studied. This has important implications for therapeutic approaches
targeting the MAP kinase pathway.
In conclusion, the results of the present study
suggested that Mps1 expression is associated with
BRAFV600E mutation while its upstream signal
phospho-MAPK has no relevance. However, as a downstream gene of
BRAF, the expression of Mps1 is affected not only by
BRAFV600E but also by BRAFWT. These results
revealed that BRAFV600E may regulate the expression of
Mps1 in MAP kinase independent ways in thyroid carcinoma. In
addition, these results demonstrated that the expression of Mps1
was not directly associated with prognosis of thyroid cancer, which
may be due to the overall positive prognosis of thyroid cancer and
limited number of samples. In future studies, sample size should be
expanded to further research and explore the unknown pathways
associated with Mps1, in order to provide a theoretical basis for
molecular treatment of thyroid cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81201956).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Grady TJ, Gates MA and Boscoe FP:
Thyroid cancer incidence attributable to overdiagnosis in the
United States 1981–2011. Int J Cancer. 137:2664–2673. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aschebrook-Kilfoy B, Grogan RH, Ward MH,
Kaplan E and Devesa SS: Follicular thyroid cancer incidence
patterns in the United States, 1980–2009. Thyroid. 23:1015–1021.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen AY, Jemal A and Ward EM: Increasing
incidence of differentiated thyroid cancer in the United States,
1988–2005. Cancer. 115:3801–3807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmid D, Ricci C, Behrens G and Leitzmann
MF: Adiposity and risk of thyroid cancer: A systematic review and
meta-analysis. Obes Rev. 16:1042–1054. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Faam B, Ghaffari MA, Ghadiri A and Azizi
F: Epigenetic modifications in human thyroid cancer. Biomed Rep.
3:3–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ulisse S, Baldini E, Sorrenti S, Barollo
S, Prinzi N, Catania A, Nesca A, Gnessi L, Pelizzo MR, Mian C, et
al: In papillary thyroid carcinoma BRAFV600E is associated with
increased expression of the urokinase plasminogen activator and its
cognate receptor, but not with disease-free interval. Clin
Endocrinol (Oxf). 77:780–786. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SH, Roh JL, Gong G, Cho KJ, Choi SH,
Nam SY and Kim SY: Differences in the recurrence and survival of
patients with symptomatic and asymptomatic papillary thyroid
carcinoma: An observational study of 11,265 person-years of
follow-up. Thyroid. 26:1472–1479. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wobker SE, Kim LT, Hackman TG and Dodd LG:
Use of BRAF v600e immunocytochemistry on FNA direct smears of
papillary thyroid carcinoma. Cancer Cytopathol. 123:531–539. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benvenga S and Koch CA: Molecular pathways
associated with aggressiveness of papillary thyroid cancer. Curr
Genomics. 15:162–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xing M: BRAF mutation in papillary thyroid
cancer: Pathogenic role, molecular bases, and clinical
implications. Endocr Rev. 28:742–762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caronia LM, Phay JE and Shah MH: Role of
BRAF in thyroid oncogenesis. Clin Cancer Res. 17:7511–7517. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soares P, Trovisco V, Rocha AS, Lima J,
Castro P, Preto A, Máximo V, Botelho T, Seruca R and
Sobrinho-Simões M: BRAF mutations and RET/PTC rearrangements are
alternative events in the etiopathogenesis of PTC. Oncogene.
22:4578–4580. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xing M, Alzahrani AS, Carson KA, Shong YK,
Kim TY, Viola D, Elisei R, Bendlová B, Yip L, Mian C, et al:
Association between BRAF V600E mutation and recurrence of papillary
thyroid cancer. J Clin Oncol. 33:42–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xing M, Haugen BR and Schlumberger M:
Progress in molecular-based management of differentiated thyroid
cancer. Lancet. 381:1058–1069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mazzaferri EL and Kloos RT: Clinical
review 128: Current approaches to primary therapy for papillary and
follicular thyroid cancer. J Clin Endocrinol Metab. 86:1447–1463.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anderson S, Bloom KJ, Vallera DU,
Rueschoff J, Meldrum C, Schilling R, Kovach B, Lee JR, Ochoa P,
Langland R, et al: Multisite analytic performance studies of a
real-time polymerase chain reaction assay for the detection of BRAF
V600E mutations in formalin-fixed, paraffin-embedded tissue
specimens of malignant melanoma. Arch Pathol Lab Med.
136:1385–1391. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim SJ, Lee KE, Myong JP, Park JH, Jeon
YK, Min HS, Park SY, Jung KC, Koo do H and Youn YK: BRAF V600E
mutation is associated with tumor aggressiveness in papillary
thyroid cancer. World J Surg. 36:310–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kalady MF, Dejulius KL, Sanchez JA, et al:
BRAF mutations in colorectal cancer are associated with distinct
clinical characteristics and worse prognosis. Dis Colon Rectum.
55:128–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wong KK, Tsang YT, Deavers MT, Mok SC, Zu
Z, Sun C, Malpica A, Wolf JK, Lu KH and Gershenson DM: BRAF
mutation is rare in advanced-stage low-grade ovarian serous
carcinomas. Am J Pathol. 177:1611–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Colombino M, Sperlongano P, Izzo F,
Tatangelo F, Botti G, Lombardi A, Accardo M, Tarantino L, Sordelli
I, Agresti M, et al: BRAF and PIK3CA genes are somatically mutated
in hepatocellular carcinoma among patients from South Italy. Cell
Death Dis. 3:e2592012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kobayashi M, Sonobe M, Takahashi T,
Yoshizawa A, Ishikawa M, Kikuchi R, Okubo K, Huang CL and Date H:
Clinical significance of BRAF gene mutations in patients with
non-small cell lung cancer. Anticancer Res. 31:4619–4623.
2011.PubMed/NCBI
|
|
25
|
Greenman C, Stephens P, Smith R, Dalgliesh
GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C,
et al: Patterns of somatic mutation in human cancer genomes.
Nature. 446:153–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weeraratna AT: RAF around the edges-the
paradox of BRAF inhibitors. N Engl J Med. 366:271–273. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Su F, Viros A, Milagre C, Trunzer K,
Bollag G, Spleiss O, Reis-Filho JS, Kong X, Koya RC, Flaherty KT,
et al: RAS mutations in cutaneous squamous-cell carcinomas in
patients treated with BRAF inhibitors. N Engl J Med. 366:207–215.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yarchoan M, LiVolsi VA and Brose MS: BRAF
mutation and thyroid cancer recurrence. J Clin Oncol. 33:7–8. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rossi M, Buratto M, Tagliati F, Rossi R,
Lupo S, Trasforini G, Lanza G, Franceschetti P, Bruni S, Uberti
Degli E and Zatelli MC: Relevance of BRAF(V600E) mutation testing
versus RAS point mutations and RET/PTC rearrangements evaluation in
the diagnosis of thyroid cancer. Thyroid. 25:221–228. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu J, Cheng X, Zhang Y, Li S, Cui H,
Zhang L, Shi R, Zhao Z, He C, Wang C, et al: Phosphorylation of
Mps1 by BRAFV600E prevents Mps1 degradation and contributes to
chromosome instability in melanoma. Oncogene. 32:713–723. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Samaan NA, Schultz PN, Hickey RC, Goepfert
H, Haynie TP, Johnston DA and Ordonez NG: The results of various
modalities of treatment of well differentiated thyroid carcinomas:
A retrospective review of 1599 patients. J Clin Endocrinol Metab.
75:714–720. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiloeches A and Marais R: Is BRAF the
Achilles' Heel of thyroid cancer? Clin Cancer Res. 12:1661–1664.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jinushi M, Chiba S, Baghdadi M, Kinoshita
I, Dosaka-Akita H, Ito K, Yoshiyama H, Yagita H, Uede T and Takaoka
A: ATM-mediated DNA damage signals mediate immune escape through
integrin-αvβ3-dependent mechanisms. Cancer Res. 72:56–65. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dhomen N, Reis-Filho JS, da Rocha Dias S,
Hayward R, Savage K, Delmas V, Larue L, Pritchard C and Marais R:
Oncogenic Braf induces melanocyte senescence and melanoma in mice.
Cancer Cell. 15:294–303. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Perera PM, Wypasek E, Madhavan S,
Rath-Deschner B, Liu J, Nam J, Rath B, Huang Y, Deschner J, Piesco
N, et al: Mechanical signals control SOX-9, VEGF, and c-Myc
expression and cell proliferation during inflammation via
integrin-linked kinase, B-Raf, and ERK1/2-dependent signaling in
articular chondrocytes. Arthritis Res Ther. 12:R1062010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jeong D, Jeong Y, Park JH, Han SW, Kim SY,
Kim YJ, Kim SJ, Hwangbo Y, Park S, Cho HD, et al: BRAF (V600E)
mutation analysis in papillary thyroid carcinomas by peptide
nucleic acid clamp real-time PCR. Ann Surg Oncol. 20:759–766. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim TH, Park YJ, Lim JA, Ahn HY, Lee EK,
Lee YJ, Kim KW, Hahn SK, Youn YK, Kim KH, et al: The association of
the BRAF(V600E) mutation with prognostic factors and poor clinical
outcome in papillary thyroid cancer: A meta-analysis. Cancer.
118:1764–1773. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Elisei R, Viola D, Torregrossa L, Giannini
R, Romei C, Ugolini C, Molinaro E, Agate L, Biagini A, Lupi C, et
al: The BRAF(V600E) mutation is an independent, poor prognostic
factor for the outcome of patients with low-risk intrathyroid
papillary thyroid carcinoma: Single-institution results from a
large cohort study. J Clin Endocrinol Metab. 97:4390–4398. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Barollo S, Pennelli G, Vianello F,
Fernando Watutantrige S, Negro I, Boschin Merante I, Pelizzo MR,
Rugge M, Mantero F, Nacamulli D, et al: BRAF in primary and
recurrent papillary thyroid cancers: The relationship with (131)I
and 2-[(18)F]fluoro-2-deoxy-D-glucose uptake ability. Eur J
Endocrinol. 163:659–663. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pelizzo MR, Boschin IM, Barollo S,
Pennelli G, Toniato A, Zambonin L, Vianello F, Piotto A, Ide EC,
Pagetta C, et al: BRAF analysis by fine needle aspiration biopsy of
thyroid nodules improves preoperative identification of papillary
thyroid carcinoma and represents a prognostic factor. A
mono-institutional experience. Clin Chem Lab Med. 49:325–329. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xing M: Prognostic utility of BRAF
mutation in papillary thyroid cancer. Mol Cell Endocrinol.
321:86–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kebebew E, Weng J, Bauer J, Ranvier G,
Clark OH, Duh QY, Shibru D, Bastian B and Griffin A: The prevalence
and prognostic value of BRAF mutation in thyroid cancer. Ann Surg.
246:466–471. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nam JK, Jung CK, Song BJ, Lim DJ, Chae BJ,
Lee NS, Park WC, Kim JS, Jung SS and Bae JS: Is the BRAF(V600E)
mutation useful as a predictor of preoperative risk in papillary
thyroid cancer? Am J Surg. 203:436–441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sala E, Mologni L, Truffa S, Gaetano C,
Bollag GE and Gambacorti-Passerini C: BRAF silencing by short
hairpin RNA or chemical blockade by PLX4032 leads to different
responses in melanoma and thyroid carcinoma cells. Mol Cancer Res.
6:751–759. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Madhunapantula SV and Robertson GP: Is
B-Raf a good therapeutic target for melanoma and other
malignancies? Cancer Res. 68:5–8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim SW, Kim HK, Lee JI, Jang HW, Choe JH,
Kim JH, Kim JS, Hur KY, Kim JH and Chung JH: ERK phosphorylation is
not increased in papillary thyroid carcinomas with BRAF(V600E)
mutation compared to that of corresponding normal thyroid tissues.
Endocr Res. 38:89–97. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zuo H, Nakamura Y, Yasuoka H, Zhang P,
Nakamura M, Mori I, Miyauchi A and Kakudo K: Lack of association
between BRAF V600E mutation and mitogen-activated protein kinase
activation in papillary thyroid carcinoma. Pathol Int. 57:12–20.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kimura ET, Nikiforova MN, Zhu Z, Knauf JA,
Nikiforov YE and Fagin JA: High prevalence of BRAF mutations in
thyroid cancer: Genetic evidence for constitutive activation of the
RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma.
Cancer Res. 63:1454–1457. 2003.PubMed/NCBI
|
|
50
|
Melillo RM, Castellone MD, Guarino V, De
Falco V, Cirafici AM, Salvatore G, Caiazzo F, Basolo F, Giannini R,
Kruhoffer M, et al: The RET/PTC-RAS-BRAF linear signaling cascade
mediates the motile and mitogenic phenotype of thyroid cancer
cells. J Clin Invest. 115:1068–1081. 2005. View Article : Google Scholar : PubMed/NCBI
|