Introduction
Chronic myelogenous leukemia (CML) is one of the
most frequent types of cancer in humans and its treatment is
limited by the development of multidrug resistance (MDR) (1). MDR is a phenomenon in which cancer cells
develop resistance to a variety of unrelated drugs following
exposure to a single chemotherapeutic (2). A number of underlying mechanisms lead to
the development of MDR; however, the increased efflux of cytotoxic
drugs by ATP binding cassette (ABC) transporters is the most well
studied mechanism (3). Multidrug
resistance protein 1 (MRP1) is a member of the ABC family of
transporters, which are primarily expressed in the plasma membrane
and remove cytotoxic agents from the cell through active transport
(4). Increased MRP1 expression leads
to a reduction in the concentration of chemotherapeutics inside
tumor cells, resulting in the decreased efficacy of these agents
(5–10). MRP1 has been associated with
resistance to various chemotherapeutic agents, including
Adriamycin® (ADR; doxorubicin), methotrexate, etoposide
and vincristine (11).
The c-Jun N-terminal kinases (JNK) belong to the
mitogen-activated protein kinase (MAPK) family (12). There are three JNK isoforms in
mammals, JNK1, JNK2 and JNK3, which are encoded by the MAPK8, MAPK9
and MAPK10 genes, respectively (13).
JNK1 and JNK2 are ubiquitously expressed, whereas JNK3 is primarily
expressed in the heart, brain and testes (14). JNK activity can be induced by a number
of stimuli, including inflammatory cytokines, environmental
stressors and therapeutic agents (15). The JNK signaling pathway has been
reported to be involved in cellular proliferation, apoptosis and
differentiation, and tumor cell migration (16,17).
Increasing evidence has indicated that JNK activity serves an
important role in the development of chemoresistance and is
associated with MRP1 expression (12–16).
Nightshade (Solanum nigrum) is used in
traditional Chinese herbal medicine, and is used to treat a number
of diseases including sores, carbuncles, swellings and other
injuries (18). Solanine, a
nightshade extract, is a glycoalkaloid belonging to the
Solanaceae family, which is reported to have
anti-inflammatory activity (19,20).
Recently, studies have demonstrated antitumorigenic effects of
solanine in various types of cancer cell, for example, solanine
suppresses proliferation and metastasis, and induces apoptosis in
pancreatic cancer cells (19,21). Solanine was demonstrated to induce
apoptosis in HepG2 cells by facilitating the opening of
mitochondrial permeability transition pores (22) and downregulating expression of
apoptosis regulator Bcl-2 (Bcl-2) (23). Previous studies have demonstrated that
solanine inhibits the invasion of human melanoma and prostate
cancer cells at non-toxic doses (18,24).
Furthermore, Kang et al (25)
reported that solanine is able to inhibit JNK activity. However,
the underlying molecular mechanisms behind the effect of solanine
on MDR cancer cells remain to be elucidated. The present study
aimed to investigate the ability of solanine to resensitize the
ADR-resistant human myelogenous leukemia cell line K562/ADM to ADR,
in addition to the mechanisms underlying its effects.
Materials and methods
Reagents
ADR (Melone Pharmaceutical Co., Ltd., Dalian, China)
was made up to a final concentration of 2 g/l using
double-distilled H2O. Solanine (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) was made up to a final concentration
of 100 µg/ml using dimethyl sulfoxide (DMSO) and diluted as
required using RPMI-1640 cell culture medium (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA). PBS buffer (Shanghai
MacLean Biochemical Technology Co., Ltd., Shanghai, China). Rabbit
polyclonal anti-MRP1, anti-JNK and anti-phosphorylated (p)-JNK
(Thr183/Thr185) antibodies were obtained from
Beijing Biosynthesis Biotechnology Co., Ltd. (Beijing, China). The
anti-JNK antibody detects all JNK isoforms. The rabbit polyclonal
anti-GAPDH antibody was obtained from Goodhere Biotechnology Co.,
Ltd. (Hangzhou, China).
Cell lines and culture
The human CML K562 cell line was obtained from the
Key Laboratory of Tumor Molecular Biology of Binzhou Medical
University (Binzhou, China) with the original source from the
Department of Pharmacology at the Institute of Hematology of
Chinese Academy of Medical Sciences (Tianjin, China). The
MDR-resistant subline, K562/ADM, was obtained from the Department
of Pharmacology at the Institute of Hematology of Chinese Academy
of Medical Sciences. Cells were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum (Hyclone; GE Healthcare
Life Sciences) at 37°C in a humidified atmosphere containing 5%
CO2. K562/ADM cells were maintained in medium containing
4 mg/l ADR, but cultured in drug-free medium for one week prior to
experimentation.
Determination of MDR accumulation in
K562/ADM cells
The Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Shanghai, China) assay was used to determine
the viability of CML cells. The CML cells (1×105
cells/well) were incubated (humidified atmosphere, 37°C, 5%
CO2 for 24 h) with ADR at the following concentrations:
0.2–1.6 mg/l for K562 cells; and 16–128 mg/l for K562/ADM cells.
RPMI-1640 medium was used to dissolve ADR. A total of 10 µl CCK-8
solution was added to each well 24 h following incubation. The
plates were incubated for 1–4 h at 37°C in a humidified atmosphere
containing 5% CO2. Absorbance was subsequently measured
at 570 nm using an F-7000 Fluorescence Spectrophotometer (Hitachi,
Ltd., Tokyo, Japan). A blank well containing RPMI-1640 medium and
ADR alone was used as a control. The half-maximal inhibitory
concentration (IC50) of cell growth was calculated as
described previously (26).
Solanine cytotoxicity assay
Solanine cytotoxicity was evaluated using the CCK-8
assay as described above. K562/ADM cells (1×105) were
seeded into a 96-well plate and incubated with between 5 and 10
µg/ml solanine at 37°C for 24 h. Cells in the control group were
incubated with DMSO alone. The number of viable cells was
determined using the CCK-8 assay, as described above.
Evaluating the MDR reversal efficacy
of solanine
K562/ADM cells (1×105) were seeded into
96-well plates and treated with 0–128 µg/ml ADR alone or 0–128
µg/ml ADR combined with 5–10 µg/ml solanine for 24 h at 37°C. Cell
viability was subsequently assessed using the CCK-8 assay, which
was performed five times. IC50 values were calculated
and untreated cells were used as the negative control. Reversal
fold (RF) values were used to quantify sensitivity reversal and
were obtained using the following formula: RF=IC50 of
ADR treatment alone/IC50 of combined ADR and solanine
treatment.
Evaluating intracellular ADR
accumulation
K562/ADM cells were incubated for 1 h at 37°C with 3
mg/l ADR alone or in combination with 5–10 µg/ml solanine. Cells
were subsequently harvested using centrifugation (room temperature)
at 150 × g for 5 min and washed twice with ice-cold PBS to remove
the unbound ADR. The cell-associated mean fluorescence intensity of
ADR-treated cells was detected using an FC500 Flow Cytometry
Analyzer (Beckman Coulter, Inc., Brea, CA, USA), and the respective
excitation and emission wavelengths were 485 and 580 nm.
Western blot analysis
K562/ADM cells were incubated with 5–10 µg/ml
solanine at 37°C in a humidified atmosphere containing 5%
CO2 for 24 h prior to harvesting. Cells were lyzed in
100 µl lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) and the total protein concentration was determined using a
BCA Protein Assay kit (Beyotime Institute of Biotechnology). A
total of 50 µg total protein was separated using SDS-PAGE on 6–8%
gels depending on the molecular mass of the protein (initial
voltage, 80 V; initial duration, 30 min; final voltage, 100 V;
final duration, 1.5 h). Resolved proteins were transferred to a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA) and blocked at room temperature with 5% skimmed milk for 2 h.
Membranes were subsequently incubated overnight at 4°C with rabbit
polyclonal anti-MRP1 (1:500), anti-JNK (1:200), anti-p-JNK (1:500)
and anti-GADPH (1:1,000) antibodies. Membranes were incubated with
horseradish peroxidase-labeled goat anti-rabbit immunoglobulin G
(1:5,000; Beijing Biosynthesis Biotechnology Co., Ltd., Beijing
China) for 2 h at room temperature. Images were captured using a
FluorChem FC2 Imaging system (Alpha Innotech, San Leandro, CA,
USA). The intensity of each band was normalized to GADPH and
quantified using ImageJ software 2× v.2.1.4.7 (National Institutes
of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 13.0; SPSS, Inc., Chicago, IL, USA). Values are
presented as the mean ± standard deviation. Statistical comparisons
were evaluated using one-way analysis of variance followed by
Student-Newman-Keuls test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparing the ADR resistance of K562
and K562/ADM cells
K562/ADM cells exhibited significant resistance to
ADR compared with the non-ADR resistant K562 cell line (P<0.05;
Table I). As shown in Table I, a 37.56115 fold increase in ADR
resistance was observed in the K562/ADM cells compared with the
K562 cells (P<0.05).
 | Table I.Determination of ADR IC50
values in the K562 and K562/ADM cell lines. |
Table I.
Determination of ADR IC50
values in the K562 and K562/ADM cell lines.
|
| IC50
(µg/ml) |
|
|---|
|
|
|
|
|---|
| Treatment | K562/ADM | K562 | Fold increase in
resistance |
|---|
| ADR |
52.4579±1.0874a | 1.3966±0.01526 | 37.56115 |
Effect of solanine on K562/ADM cell
proliferation
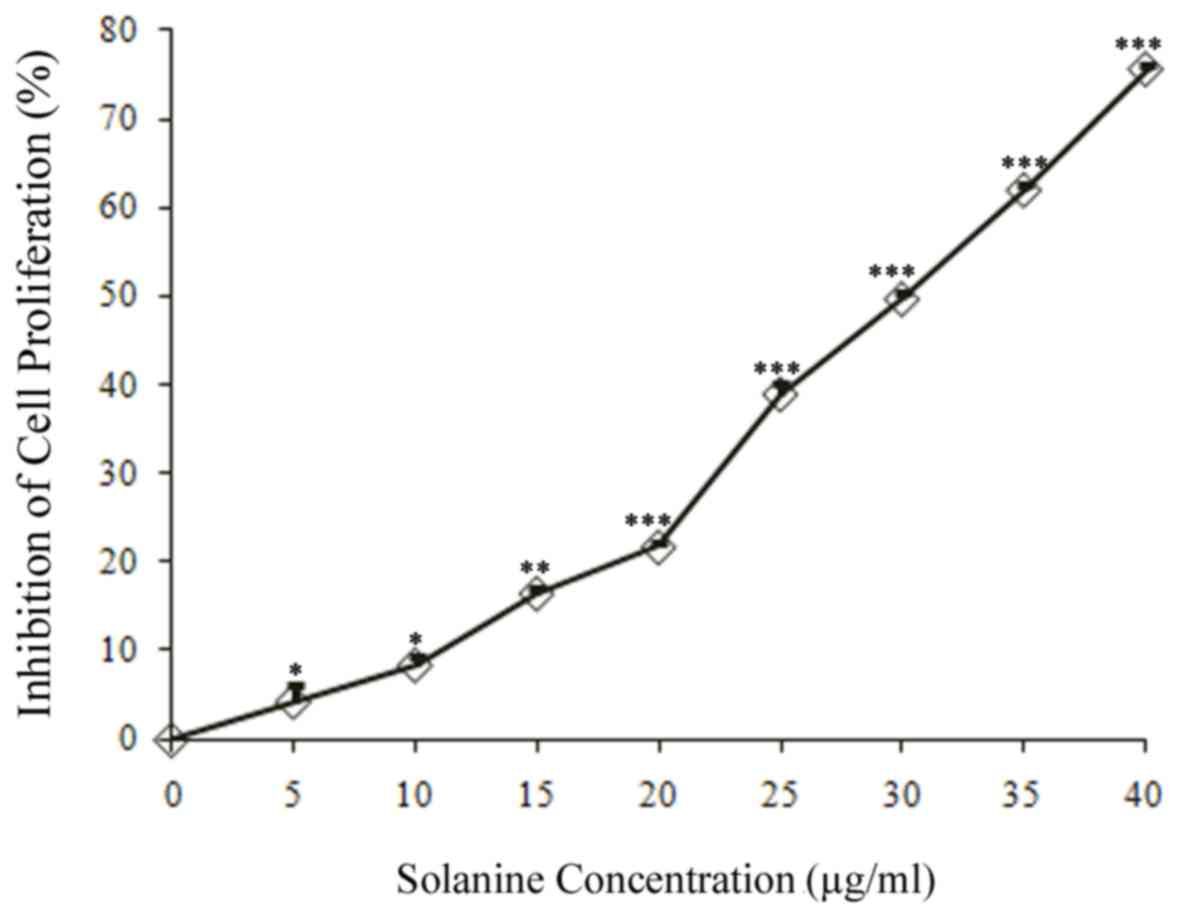
Treatment with solanine inhibited K562/ADM cell
proliferation in a dose-dependent manner (Fig. 1). Treatment with 5 or 10 µg/ml
solanine had no marked inhibitory effect on cell proliferation
(<5 and <10% vs. the control group, respectively). Therefore,
these concentrations were selected for subsequent experiments, as
they had little effect on K562/ADM cell proliferation.
Effect of combined treatment with ADR
and solanine on K562/ADM cell sensitivity to ADR
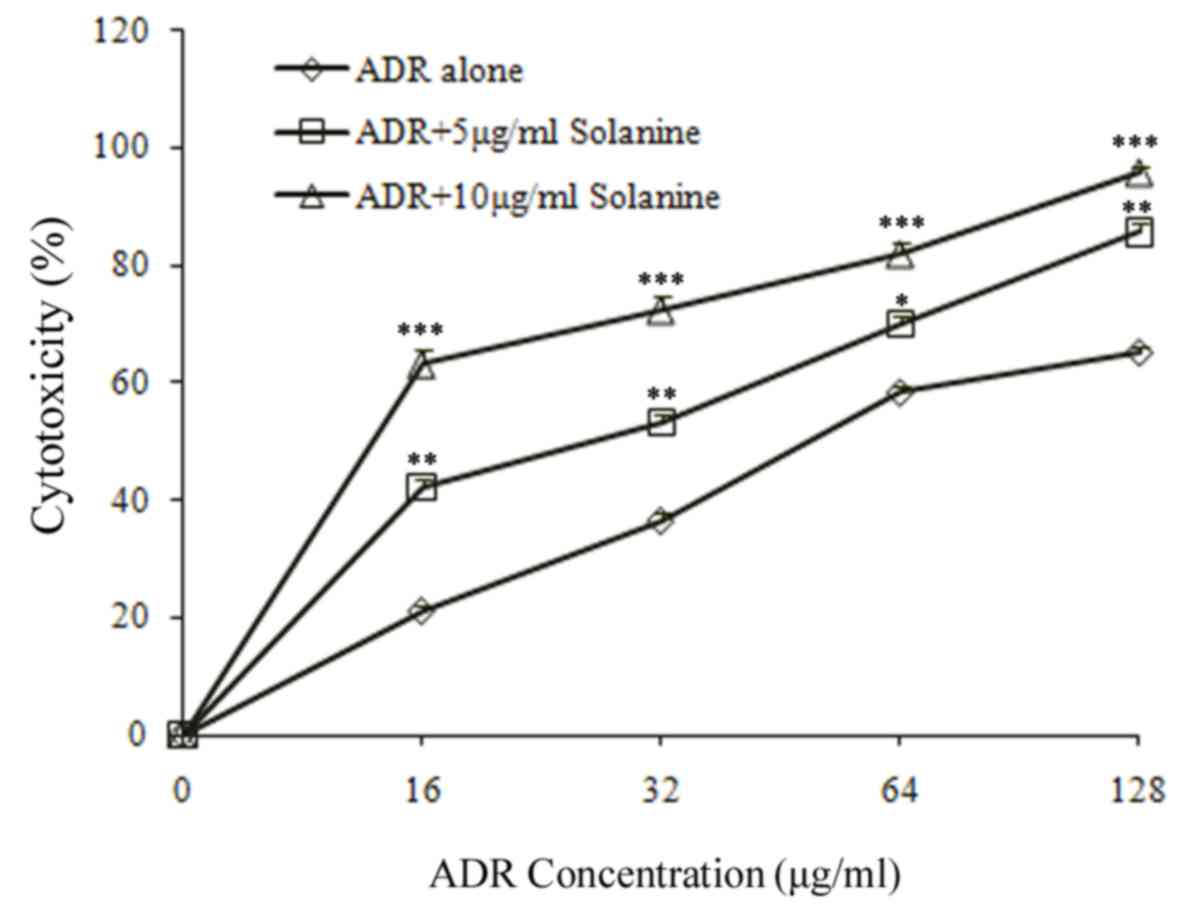
To determine whether combined treatment with ADR
(range, 0–128 µg/ml) and 5 or 10 µg/ml solanine had an effect on
drug resistance in vitro, cytotoxicity was assessed using
the CCK-8 assay (Fig. 2; Table II). The results demonstrated a
significantly decreased rate of proliferation in K562/ADM cells 24
h following combined treatment with ADR and solanine, compared with
treatment with ADR alone (P<0.05; Fig.
2; Table II). This suggests that
combined treatment with solanine increases ADR cytotoxicity in
K562/ADM cells. Combined treatment with 5 and 10 µg/ml solanine led
to a 1.68 and 2.64 fold increase in K562/ADM sensitivity to ADR,
respectively (P<0.05 vs. ADR treatment alone; Table II).
 | Table II.Effect of treatment with solanine on
the ADR IC50 values in K562/ADM cells. |
Table II.
Effect of treatment with solanine on
the ADR IC50 values in K562/ADM cells.
| Treatment | IC50
(µg/ml) | Fold reversal vs.
ADR alone |
|---|
| ADR alone |
54.7985±1.71941 | N/A |
| ADR + 5 µg/ml
solanine |
32.6094±0.66628a | 1.68 |
| ADR + 10 µg/ml
solanine |
14.7863±0.61516a | 3.71 |
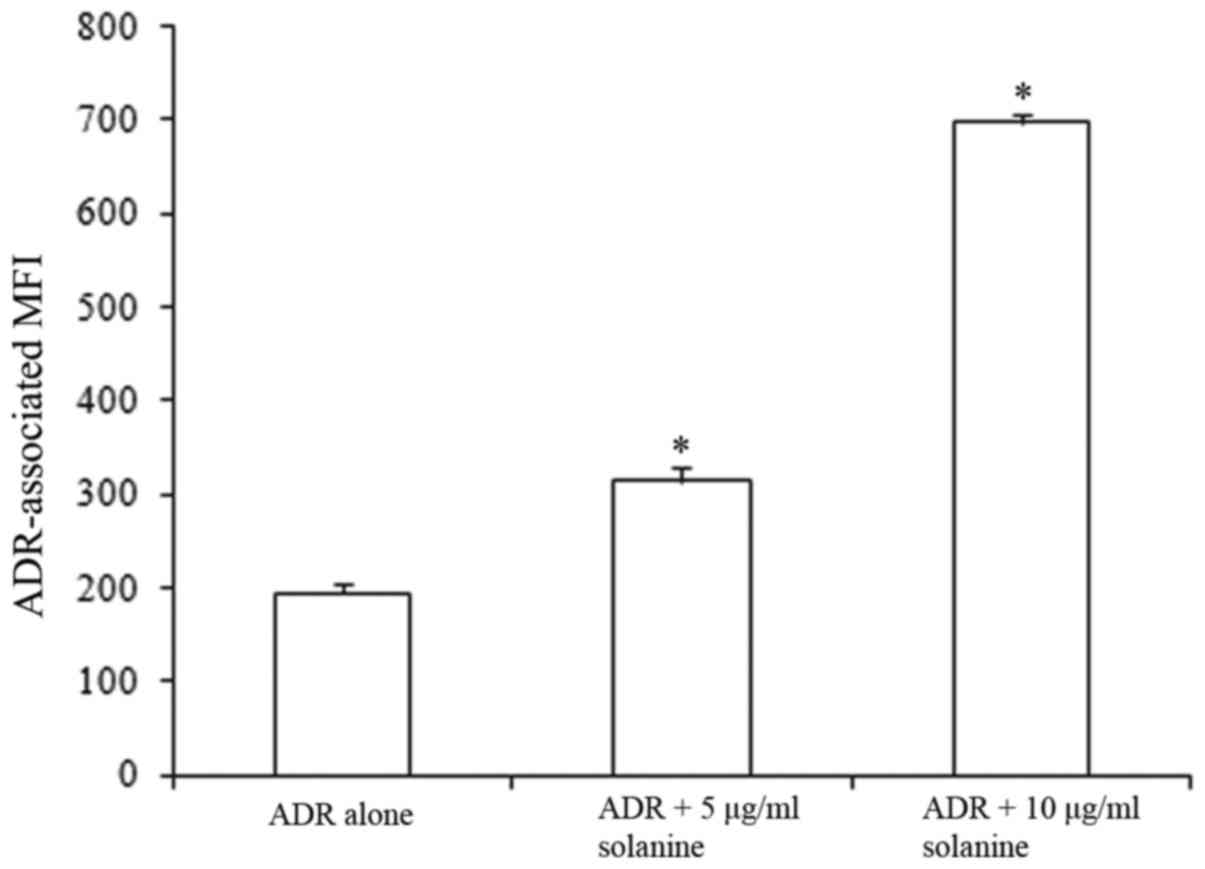
Solanine increases the intracellular
accumulation of ADR
In a previous study, intracellular accumulation of
ADR was reported to be significantly decreased in K562/ADM cells
compared with K562 cells (7). In the
present study, it was determined that solanine significantly
increased the intracellular accumulation of ADR in K562/ADM cells
(P<0.05 vs. the control group; Fig.
3). These results indicate that solanine increases the
sensitivity of K562/ADM cells to ADR by increasing intracellular
ADR accumulation.
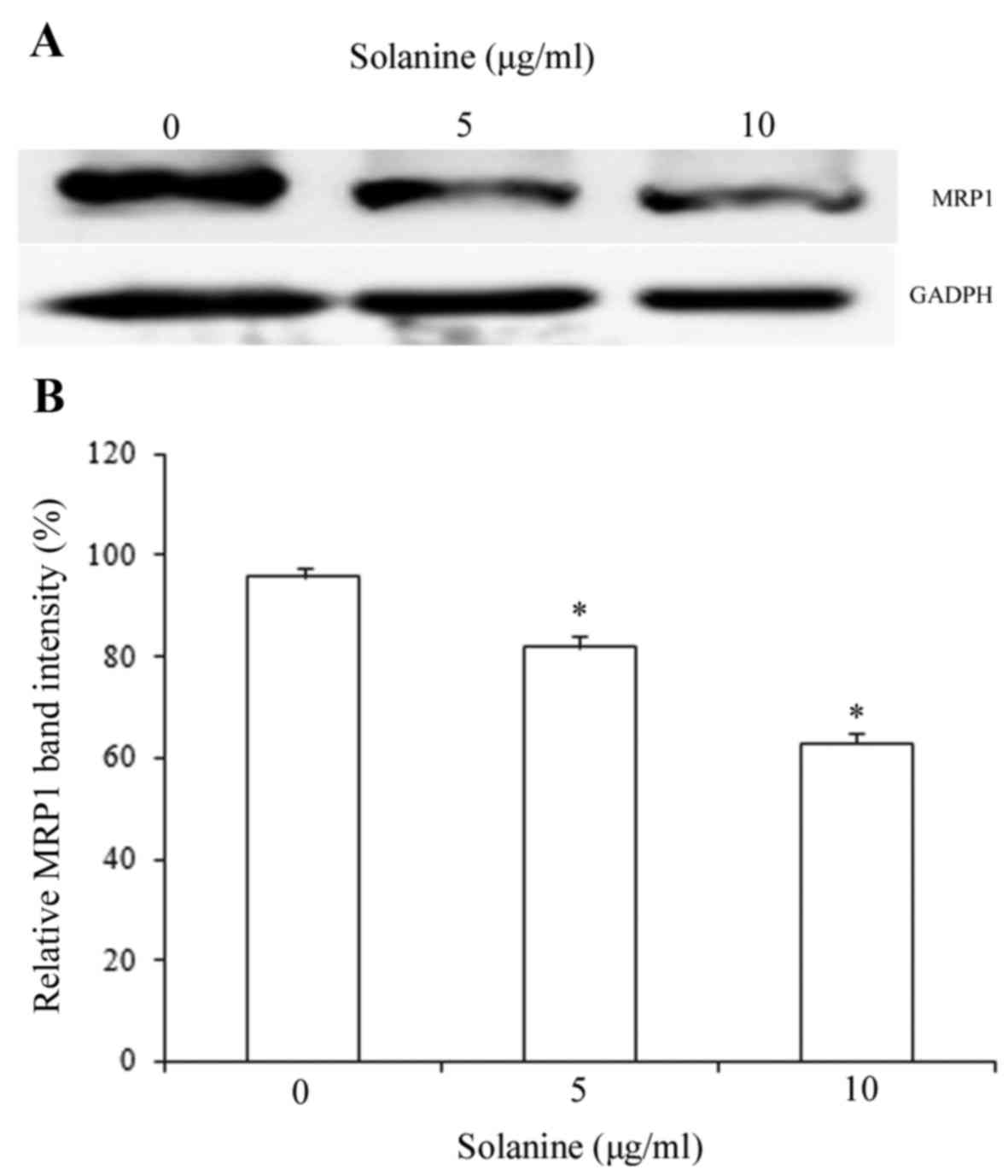
Treatment with solanine decreases MRP1
protein expression in K562/ADM cells
MRP1 is an ABC transporter, which is overexpressed
in numerous drug-resistant cell lines, including K562/ADM cells,
compared with the corresponding non-resistant cell lines (11). K562/ADM cells were identified to
express MRP1 protein at high levels (11). Western blot analysis demonstrated that
MRP1 protein expression was significantly decreased in K562/ADM
cells following treatment with 5 or 10 µg/ml solanine compared with
the untreated cells (P<0.05; Fig.
4). These results indicate that solanine increases MRP1 protein
expression, which likely increases intracellular ADR
accumulation.
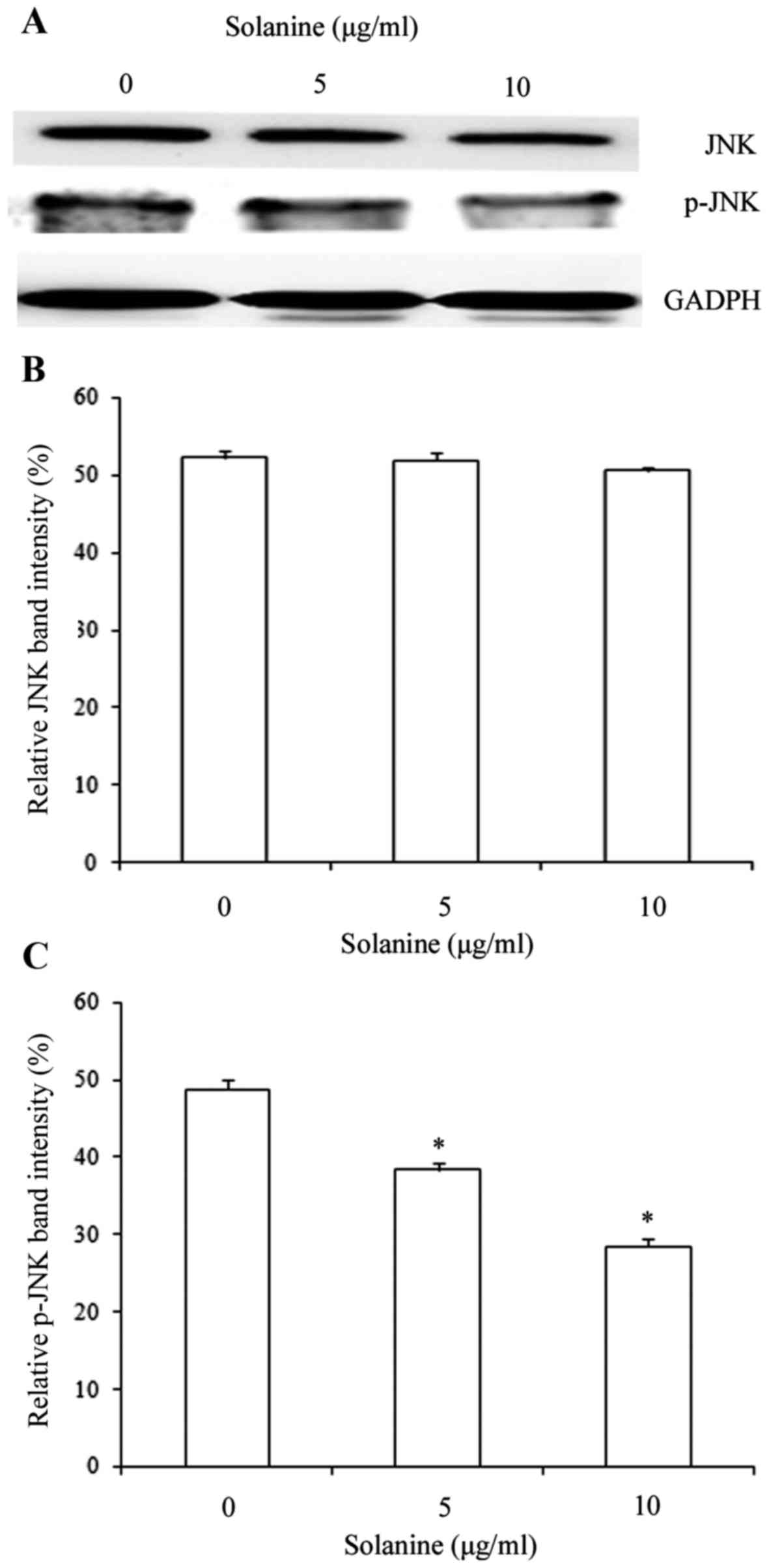
Treatment with solanine decreases JNK
phosphorylation
JNK serves an essential role in the development of
MDR, therefore the phosphorylation pattern of JNK in MDR K562/ADM
cells was analyzed using western blotting. As shown in Fig. 5, total JNK expression did not change
significantly 24 h following treatment with solanine compared with
the control group (P>0.05; Fig. 5A and
B); however, the expression of p-JNK was significantly
decreased in a dose-dependent manner compared with the untreated
cells (P<0.05; Fig. 5A and C). The
effect of treatment with solanine on JNK phosphorylation was
consistent with its effect on MRP1 expression. These results
suggest that the JNK signaling pathway participates in the
modulation of MRP1 protein expression.
Discussion
CML is a stem-cell disorder characterized by chronic
and blast crisis phases (27).
Chemotherapy serves an essential role in CML treatment; however, it
is frequently accompanied by the development of MDR, which results
in treatment failure. MDR is the ability of tumors to exhibit
simultaneous resistance to a number of structurally and
functionally unrelated chemotherapeutic agents (28). There are multiple underlying molecular
mechanisms of MDR, including increased expression of ABC
transporter proteins, and abnormalities in a number of enzymatic
and apoptotic signaling pathways (28). ADR is an effective chemotherapeutic
that has been used extensively to treat CML, but is limited by the
development of MDR (1). MRP1
overexpression is one of the well-known causes of MDR (11). Significant efforts have been made to
identify novel MDR-inhibiting agents.
Glycoalkaloids are secondary plant metabolites that
contain nitrogen, are found in solanaceous plants and possess
anticarcinogenic activity (29).
Nightshade is a plant used in traditional Chinese herbal medicine
(24), which contains the
glycoalkaloid solanine (21).
Increasing evidence has demonstrated that solanine possesses
antitumor activity, for example, solanine has been demonstrated to
inhibit the growth of U937 cells (30). In addition, previous studies revealed
that solanine has antitumor activity in other types of cancer
(18–19,21). A
previous study suggested that Solanine suppressed proliferation of
mouse breast cancer cells by inducing expression of the apoptosis
regulator Bcl2 associated X (Bax) and decreasing expression of
Bcl-2 (30). Solanine was
demonstrated to inhibit the activity of matrix metalloproteinase
(MMP)-2 and −9 by suppressing the phosphatidylinositol
4,5-bisphosphate 3-kinase/protein kinase B and JNK signaling
pathways at non-toxic doses, which resulted in the inhibition of
melanoma cell migration and invasion (18). In addition, it was reported that
treatment with solanine suppressed MMP-2 and 9 expression in
pancreatic cancer cells at toxic doses, which resulted in the
suppression of pancreatic cancer cell migration and invasion
(19,21). Furthermore, treatment with solanine
decreased the Bcl-2/Bax ratio and induced activation of the
capase-3 zymogen to facilitate pancreatic cancer cell apoptosis
(19). However, the effect of
treatment with solanine on the reversal of MDR in K562/ADM cells
was unclear. The present study aimed to investigate the effects and
underlying mechanisms of solanine action in K562/ADM cells.
In the present study, the efficacy of solanine as a
reverser of ADR resistance in K562/ADM cells was examined. The
results suggested that treatment with 5 or 10 µg/ml solanine alone
exhibited no significant antiproliferative effect on K562/ADM cells
compared with the control group. The effect of treatment with
solanine on cellular functions in K562/ADM cells was subsequently
investigated. The CCK-8 assay demonstrated that treatment with 5
and 10 µg/ml solanine enhanced the cytotoxicity of ADR to K562/ADM
cells, suggesting that solanine reverses MDR in K562/ADM cells. The
results of the flow cytometric assay demonstrated that treatment
with solanine increased the intracellular accumulation of ADR,
which suggests that this is the mechanism through which solanine
reverses MDR. To further examine the mechanism by which solanine
elevates K562/ADM intracellular ADR accumulation, MRP1 protein
expression was evaluated.
MRP1, an ATP-dependent molecular pump, is a member
of the ABC transporter protein family and is able to decrease
intracellular drug accumulation, thus decreasing the cellular
toxicity of various chemotherapeutic agents, including ADR,
daunorubicin, epirubicin, mitoxantrone, bisantrene, vincristine,
vinblastine, etoposide and paclitaxel (11,31). In
the present study, western blot analysis demonstrated that
treatment with solanine significantly decreased MRP1 protein
expression compared with treatment with ADR alone in K562/ADM
cells. Decreased expression of MRP1 protein was associated with
increased intracellular accumulation of ADR in K562/ADM cells.
It has been reported that the JNK signaling pathway
is involved in the development of MDR in a number of tumor cell
types. Decreased JNK activity has been observed in drug-resistant
hepatocellular carcinoma cells, while increased JNK activity was
demonstrated to attenuate chemoresistance in R-HepG2 cells by
suppressing P-glycoprotein (P-gp) expression (32,33).
Increased JNK signaling pathway activation was identified to
downregulate P-gp expression and reverse P-gp-mediated MDR in
gastric and pancreatic cancer cells (17). However, decreased JNK phosphorylation
has been demonstrated to inhibit P-gp expression during hypoxia
(14,34). Increasing evidence has demonstrated
that JNK activity is associated with MRP1 activity and expression.
Suppression of the JNK signaling pathway led to a decrease in MRP1
activity and expression in glial cells (35). However, MRP1 expression was negatively
associated with JNK activity in another study (36). Cripe et al (37) reported that JNK activity and MRP1
expression were upregulated in HL60/ADR cells compared with HL60
cells and furthermore, that inhibition of JNK activity decreased
MRP1-efflux and MRP1 protein expression. These results indicate
that JNK activity serves an important role in the development of
MDR and MRP1 expression. Cripe et al (37) also reported that JNK activity was
upregulated in K562 cells, and in tumor cells exposed to ADR in a
time- and concentration-dependent manner. As expected, in the
present study treatment with solanine led to inhibited JNK activity
while having no significant impact on total JNK expression. The
results of the present study indicate that decreased JNK activity
is associated with the inhibition of MRP1 expression in K562/ADM
cells, suggesting that solanine is able to reverse MDR by
modulating the expression of MRP1, through regulation of the JNK
signaling pathway.
In conclusion, treatment with solanine enhanced the
sensitivity of K562/ADM cells to ADR as a result of increased
intracellular ADR accumulation, which was associated with the
downregulation of MRP1 expression. Downregulated MRP1 expression
was likely a result of decreased JNK phosphorylation, a marker of
JNK activity. The results of the present study suggest that
solanine is a novel therapeutic agent for the treatment of cancer,
which reverses MDR in human myelogenous leukemia K562/ADM
cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shandong
Science and Technology Committee (grant no. 2010GSF10264), the
Committee of Shandong Educational Foundation (grant nos. J10LC60
and J11LC01), the Natural Science Foundation of Shandong Province
(grant no. ZR2014HL032) and the Medical and Health Technology
Development Program of Shandong Province (grant no.
2014WS0183).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJY was responsible for the main experiments and
writing the manuscript. XHJ analyzed experimental data and modified
the manuscript. CZ, JYW, JRC, HW conducted some parts of the
experiments. YJL designed and guided the experiments. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ren J, Xu Y, Huang Q, Yang J, Yang M, Hu K
and Wei K: Chabamide induces cell cycle arrest and apoptosis by the
Akt/MAPK pathway and inhibition of P-glycoprotein in K562/ADR
cells. Anticancer Drugs. 26:498–507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li H, Guo K, Wu C, Shu L, Guo S, Hou J,
Zhao N, Wei L, Man X and Zhang L: Controlled and targeted drug
delivery by a UV-responsive liposome for overcoming
chemo-resistance in non-hodgkin lymphoma. Chem Biol. Drug Des.
86:783–794. 2015.
|
|
3
|
Nabekura T, Hiroi T, Kawasaki T and Uwai
Y: Effects of natural nuclear factor-kappa B inhibitors on
anticancer drug efflux transporter human P-glycoprotein. Biomed
Pharmacother. 70:140–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wen X, Zhang HD, Zhao L, Yao YF, Zhao JH
and Tang JH: Ginsenoside Rh2 differentially mediates microRNA
expression to prevent chemoresistance of breast cancer. Asian Pac J
Cancer. 16:1105–1109. 2015. View Article : Google Scholar
|
|
5
|
Bao W, Zhu F, Duan Y, Yang Y and Cai H:
HtrA1 resensitizes multidrug-resistant hepatocellular carcinoma
cells by targeting XIAP. Biomed Pharmacother. 70:97–102. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Z, Yang L, Xia Y, Guo C and Kong L:
Icariin enhances cytotoxicity of doxorubicin in human
multidrug-resistant osteosarcoma cells by inhibition of ABCB1 and
down-regulation of the PI3K/Akt pathway. Biol Pharm Bull.
38:277–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song YN, Guo XL, Zheng BB, Liu XY, Dong X,
Yu LG and Cheng YN: Ligustrazine derivate DLJ14 reduces multidrug
resistance of K562/A02 cells by modulating GSTp activity. Toxicol
In Vitro. 25:937–943. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma J, Wang T, Guo R, Yang X, Yin J, Yu J,
Xiang Q, Pan X, Tang H and Lei X: Involvement of miR-133a and
miR-326 in ADM resistance of HepG2 through modulating expression of
ABCC1. J Drug Target. 25:519–524. 2015. View Article : Google Scholar
|
|
9
|
Yang X, Iyer AK, Singh A, Choy E, Hornicek
FJ, Amiji MM and Duan Z: MDR1 siRNA loaded hyaluronic acid-based
CD44 targeted nanoparticle systems circumvent paclitaxel resistance
in ovarian cancer. Sci Rep. 5:85092015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fantappiè O, Sassoli C, Tani A, Nosi D,
Marchetti S, Formigli L and Mazzanti R: Mitochondria of a human
multidrug-resistant hepatocellularcarcinoma cell line
constitutively express inducible nitric oxide synthase in the inner
membrane. J Cell Mol Med. 19:1410–1417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu X, Zhang Y, Li W, Miao H, Zhang H, Zhou
Y, Li Z, You Q, Zhao L and Guo Q: Wogonin reverses multi-drug
resistance of human myelogenous leukemia K562/A02 cells via
downregulation of MRP1 expression by inhibiting Nrf2/ARE signaling
pathway. Biochem Pharmacol. 92:220–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han L, Wang Y, Guo X, Zhou Y, Zhang J,
Wang N, Jiang J, Ma F and Wang Q: Downregulation of MDR1 Gene by
cepharanthine hydrochloride is related to the activation of
c-Jun/JNK in K562/ADR cells. Biomed Res Int. 2014:1643912014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seki E, Brenner DA and Karin M: A liver
full of JNK: Signaling in regulation of cell function and disease
pathogenesis, and clinical approaches. Gastroenterology.
143:307–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang W, Chen BA, Jin JF, He YJ and Niu
YQ: Involvement of c-Jun N-terminal kinase in reversal of multidrug
resistance of human leukemia cells in hypoxia by
5-bromotetrandrine. Leuk Lymphoma. 54:2506–2516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu MM, Tong JL, Xu Q, Nie F, Xu XT, Xiao
SD and Ran ZH: Increased JNK1 Signaling pathway is responsible for
ABCG2-mediated multidrug resistance in human colon cancer. PLoS
One. 7:e417632012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhan X, Feng X, Kong Y, Chen Y and Tan W:
JNK signaling maintains the mesenchymal properties of multi-drug
resistant human epidermoid carcinoma KB cells through snail and
twist1. BMC Cancer. 13:1802013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou J, Liu M, Aneja R, Chandra R, Lage H
and Joshi HC: Reversal of P-glycoprotein-mediated multidrug
resistance in cancer cells by the c-Jun NH2-terminal kinase. Cancer
Res. 66:445–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu MK, Shih YW, Chien Chang TT, Fang LH,
Huang HC and Chen PS: α-Solanine inhibits human melanoma cell
migration and invasion by reducing matrix metalloproteinase-2/9
activities. Biol Pharm Bull. 33:1685–1691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun H, Lv C, Yang L, Wang Y, Zhang Q, Yu
S, Kong H, Wang M, Xie J, Zhang C and Zhou M: Solanine induces
mitochondria-mediated apoptosis in human pancreatic cancer cells.
Biomed Res Int. 2014:8059262014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kenny OM, McCarthy CM, Brunton NP, Hossain
MB, Rai DK, Collins SG, Jones PW, Maguire AR and O'Brien NM:
Anti-inflammatory properties of potato glycoalkaloids in stimulated
Jurkat and Raw 264.7 mouse macrophages. Life Sci. 92:775–782. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lv C, Kong H, Dong G, Liu L, Tong K, Sun
H, Chen B, Zhang C and Zhou M: Antitumor Efficacy of α-Solanine
against pancreatic cancer in vitro and in vivo. PLoS One.
9:e878682014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao SY, Wang QJ and Ji YB: Effect of
solanine on the membrane potential of mitochondria in HepG2 cells
and [Ca2+]i in the cells. World J Gastroenterol. 12:3359–3367.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ji YB, Gao SY, Ji CF and Zou X: Induction
of apoptosis in HepG2 cells by solanine and Bcl-2 protein. J
Ethnopharmacol. 115:194–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen KH, Liao AC, Hung JH, Lee WJ, Hu KC,
Lin PT, Liao RF and Chen PS: α-Solanine inhibits invasion of human
prostate cancer cell by suppressing epithelial-mesenchymal
transition and MMPs expression. Molecules. 19:11896–11914. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang H, Jeong HD and Choi HY: The
chloroform fraction of Solanum nigrum suppresses nitric oxide and
tumor necrosis factor-α in LPS-stimulated mouse peritoneal
macrophages through inhibition of p38, JNK and ERK1/2. Am J Chin
Med. 39:1261–1273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang JY, Jia XH, Xing HY, Li YJ, Fan WW,
Li N and Xie SY: Inhibition of Forkhead box protein M1 by
thiostrepton increases chemosensitivity to doxorubicin in T-cell
acute lymphoblastic leukemia. Mol Med Rep. 12:1457–1464. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sengupta A, Banerjee D, Chandra S, Banerji
SK, Ghosh R, Roy R and Banerjee S: Deregulation and cross talk
among Sonic hedgehog, Wnt, Hox and Notch signaling in chronic
myeloid leukemia progression. Leukemia. 21:949–955. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abdallah HM, Al-Abd AM, El-Dine RS and
El-Halawany AM: P-glycoprotein inhibitors of natural origin as
potential tumor chemo-sensitizers: A review. J Adv Res. 6:45–62.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Friedman M: Chemistry and anticarcinogenic
mechanisms of glycoalkaloids produced by eggplants, potatoes, and
tomatoes. J Agric Food Chem. 63:3323–3337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Friedman M, Lee KR, Kim HJ, Lee IS and
Kozukue N: Anticarcinogenic effects of glycoalkaloids from potatoes
against human cervical, liver, lymphoma, and stomach cancer cells.
J Agric Food Chem. 53:6162–6169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang XB, Wang SS, Zhang QF, Liu M, Li HL,
Liu Y, Wang JN, Zheng F, Guo LY and Xiang JZ: Inhibition of
tetramethylpyrazine on P-gp, MRP2, MRP3 and MRP5 in multidrug
resistant human hepatocellular carcinoma cells. Oncol Rep.
23:211–215. 2010.PubMed/NCBI
|
|
32
|
Yan F, Wang XM, Liu ZC, Pan C, Yuan SB and
Ma QM: JNK1, JNK2, and JNK3 are involved in P-glycoprotein-mediated
multidrug resistance of hepatocellular carcinoma cells.
Hepatobiliary Pancreat Dis Int. 9:287–295. 2010.PubMed/NCBI
|
|
33
|
Tang PM, Zhang DM, Xuan NH, Tsui SK, Waye
MM, Kong SK, Fong WP and Fung KP: Photodynamic therapy inhibits
p-glycoprotein mediated multidrug resistance via JNK activation in
human hepatocellular carcinoma using the photosensitizer
pheophorbide a. Mol Cancer. 8:562009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Comerford KM, Cummins EP and Taylor CT:
c-Jun NH2-terminal kinase activation contributes to
hypoxia-inducible factor 1alpha-dependent P-glycoprotein expression
in hypoxia. Cancer Res. 64:9057–9061. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ronaldson PT, Ashraf T and Bendayan R:
Regulation of multidrug resistance protein 1 by tumor necrosis
factor α in cultured glial cells: Involvement of nuclear
factor-kappaB and c-Jun N-terminal kinase signaling pathways. Mol
Pharmacol. 77:644–659. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Findlay VJ, Townsend DM, Saavedra JE,
Buzard GS, Citro ML, Keefer LK, Ji X and Tew KD: Tumor cell
responses to a novel glutathione S-transferase-activated nitric
oxide-releasing prodrug. Mol Pharmacol. 65:1070–1079. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cripe LD, Gelfanov VM, Smith EA, Spigel
DR, Phillips CA, Gabig TG, Jung SH, Fyffe J, Hartman AD, Kneebone
P, et al: Role for c-jun N-terminal kinase in treatment-refractory
acute myeloid leukemia (AML): Signaling to multidrug-efflux and
hyperproliferation. Leukemia. 16:799–812. 2002. View Article : Google Scholar : PubMed/NCBI
|