Introduction
Breast cancer (BCa) is the most prevalent cancer
among women worldwide and annually accounts for 25% (1.7 million)
of new cases and 15% (more than 0.5 million) of cancer-related
deaths (1). Despite therapeutic
advances, including local interventions (mastectomy and
radiotherapy) and systemic treatments (chemo/hormonal or targeted
therapies) (2,3), thousands of women still die of BCa every
year due to relapse and metastasis (4). It has been proposed that relapse and
metastasis involve genetic and epigenetic alterations to
BCa-related oncogenes or tumor suppressors (5). Therefore, understanding the phenotypic
relevance of oncogenes reported to be associated with relapse and
metastasis of BCa is crucial.
Some studies have suggested an association between
lysine demethylase 3A (KDM3A) and BCa relapse and metastasis
(6,7).
KDM3A, also known as JMJD1, is an iron- and oxoglutarate-dependent
dioxygenase that can specifically demethylate monomethyl- and
dimethyl-H3K9 (8). Initially, it was
reported that KDM3A was upregulated during hypoxia and is required
for hypoxic-mediated gene expression (9). KDM3A has also been reported to be
involved in the chemoresistance (7),
proliferation (10,11), migration and invasion (7,12,13), angiogenesis (14) and recurrence (13) of several different cancer types, which
suggests the potential of KDM3A as a druggable target for cancer
therapy. Most studies linking KDM3A with cancer are mechanistic and
primarily use in vitro cancer cell culture systems, and
there is a paucity of data concerning KDM3A expression in terms of
clinicopathological relevance in cancer tissue.
Considering the limited information regarding KDM3A
expression in clinical BCa tissues, we explored the
clinicopathological significance of KDM3A expression via
immunohistochemistry of a BCa tissue microarray (TMA). No
significant association between KDM3A expression and any
clinicopathological variables, including demographic parameters,
clinical stage, tumor grade, lymph node metastases, and the
expression status of human epidermal growth factor receptor 2
(Her2), ER or PR was observed. Furthermore, KDM3A expression was
not significantly associated with overall prognosis. These results
suggest that KDM3A expression may not be associated with metastasis
and prognosis of BCa as previously reported.
Materials and methods
Clinical tissues
The present study was approved by the Medical Ethics
Committee at the First Affiliated Hospital of Xinjiang Medical
University (Urumqi, China). The TMA used for the immunostaining
analysis of KDM3A was commercially purchased from Shanghai Outdo
Biotech. Co. Ltd. (Shanghai, China). The array consisted of 150
individual BCa tissues. Staging and grading of the samples was
assessed in accordance with the World Health Organization
classification and grading system. None of the samples were
collected from patients who underwent chemoradiotherapy prior to
resection. Informed consent was obtained from all the subjects
involved. The corresponding clinicopathological information,
including age, clinical stage, tumor grade, estrogen receptor (ER)
status, progesterone receptor (PR) status, Her2 status, lymph node
metastasis and overall 5-year and 10-year prognoses, was available
for each BCa tissue sample.
Immunohistochemical staining
Briefly, the BCa TMA was deparaffinized and
rehydrated. Heat-induced epitope retrieval was performed using
citrate buffer (pH=6) and a microwave histoprocessor (Haier,
Qingdao, China), after which the tissue sections were incubated
with 3% hydrogen peroxide for 10 min to block endogenous peroxidase
activity. Tissue sections were then incubated with an antibody
targeting KDM3A (dilution, 1:100; TA332173; Origene, Technologies,
Inc., Rockville, MD, USA) overnight in a humidified chamber at 4°C.
Immunostaining was visualized using a labeled horseradish
peroxidase (HRP)-conjugated AffiniPure mouse Anti-rabbit IgG
antibody with 3,3′-diaminobenzidine as a chromogen (Dako Canada
Inc., Mississauga, Ontario, Canada), and the tissues were
counterstained with hematoxylin.
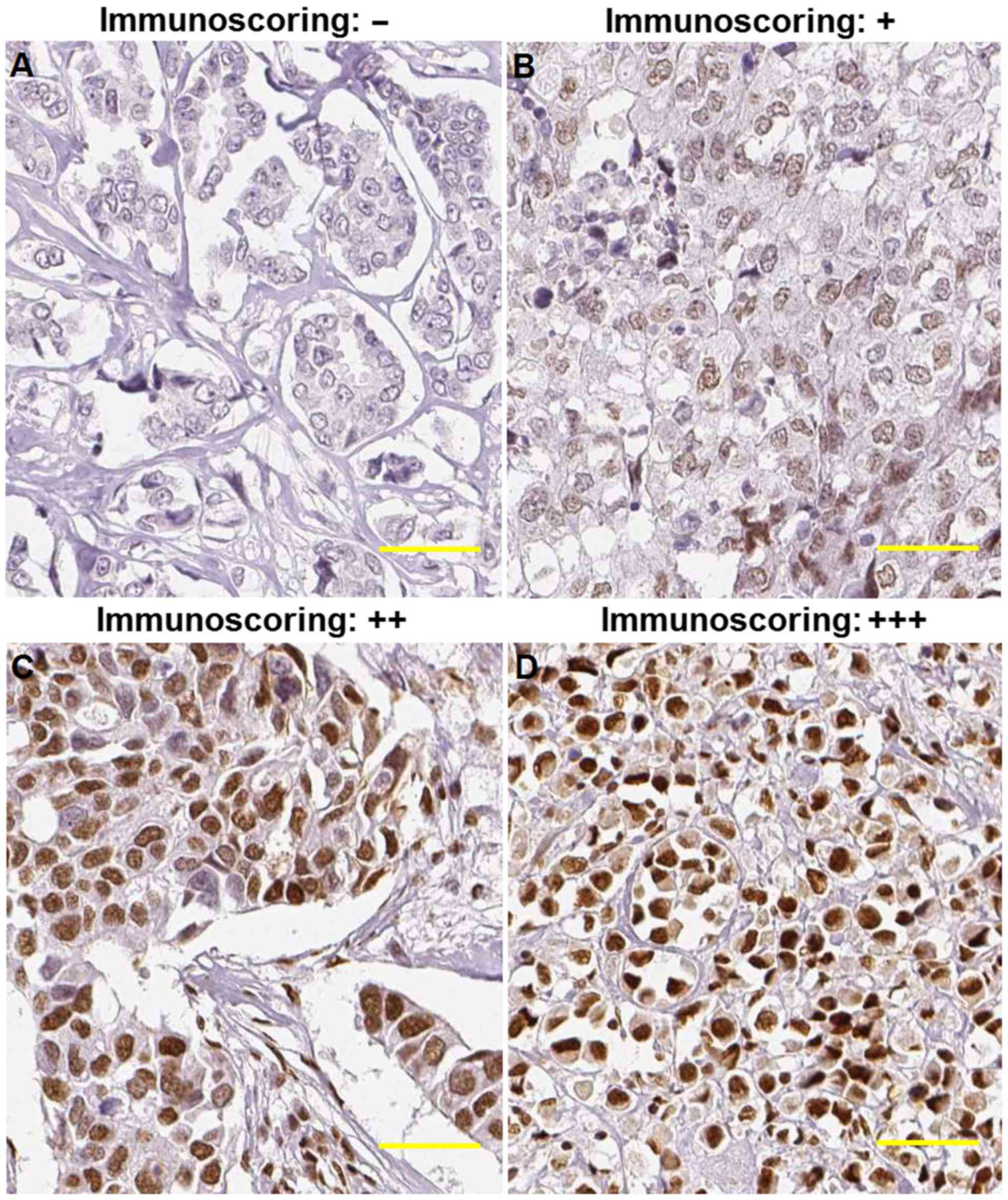
Immunoscoring
The sections were evaluated under a light
microscope, and cellular localization of the protein and the
immunostaining intensity of each section were assessed by two
pathologists. The staining patterns were scored based on the signal
intensity as follows: Negative (no positive staining), weak
(<15% of cells with positive staining), medium (>15% but
<30%) and strong (>30% of cells with positive staining). For
the clinicopathological analysis, the negative and weak samples
were recategorized as low expression, whereas medium and strong
samples were recategorized as high expression. Additionally, the
use of a general rabbit anti-human IgG in place of the primary
antibody served as a negative control as recommended by Hewitt
et al (15).
Statistical analysis
Statistical analysis was conducted using SPSS 17.0
version (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5.0. Data
are expressed as the mean ± SD and were analyzed using Student's
t-test and the χ2 test as appropriate. Kaplan-Meier
survival curves were plotted, and log-rank tests were performed.
P<0.05 was considered to indicate as statistically significant
difference, and all listed P-values (*P<0.05; **P<0.01;
***P<0.001) were calculated vs. the respective control
groups.
Results
KDM3A staining
The primary aim of our study was to investigate the
clinicopathological significance of KDM3A expression in BCa. To
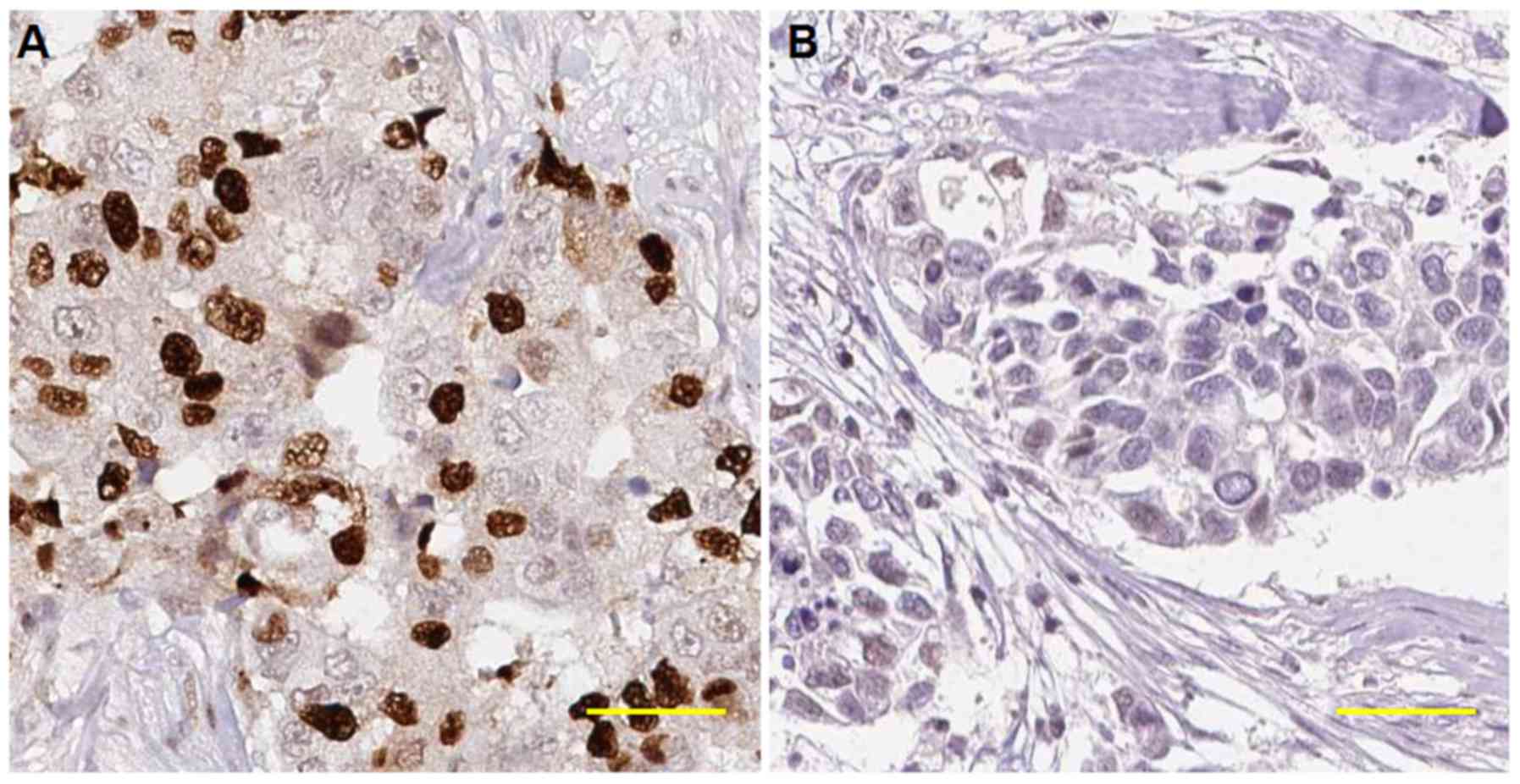
measure the expression of KDM3A in BCa tissues, IHC was performed
using a TMA consisting of 150 unique BCa samples. The specificity
of the primary antibody against KDM3A used for IHC was first
evaluated using the antigen preabsorption approach as previously
recommended (16); the results of
this trial suggested that the specificity of the primary antibody
against KDM3A was adequate for detection in our TMA (Fig. 1). KDM3A staining was primarily
nuclear, and KDM3A was heterogeneously expressed in BCa tissues,
with negative, weak, moderate or strong staining, as shown in
Fig. 2.
Association between KDM3A and
clinicopathological characteristics of BCa
We next analyzed the clinicopathological
significance of KDM3A expression. Interestingly, no significant
correlation was observed between KDM3A expression in the BCa
tissues and the clinicopathological parameters, including age,
clinical stage, tumor grade, ER status, PR status, Her2 status and
lymph node metastasis (Table I).
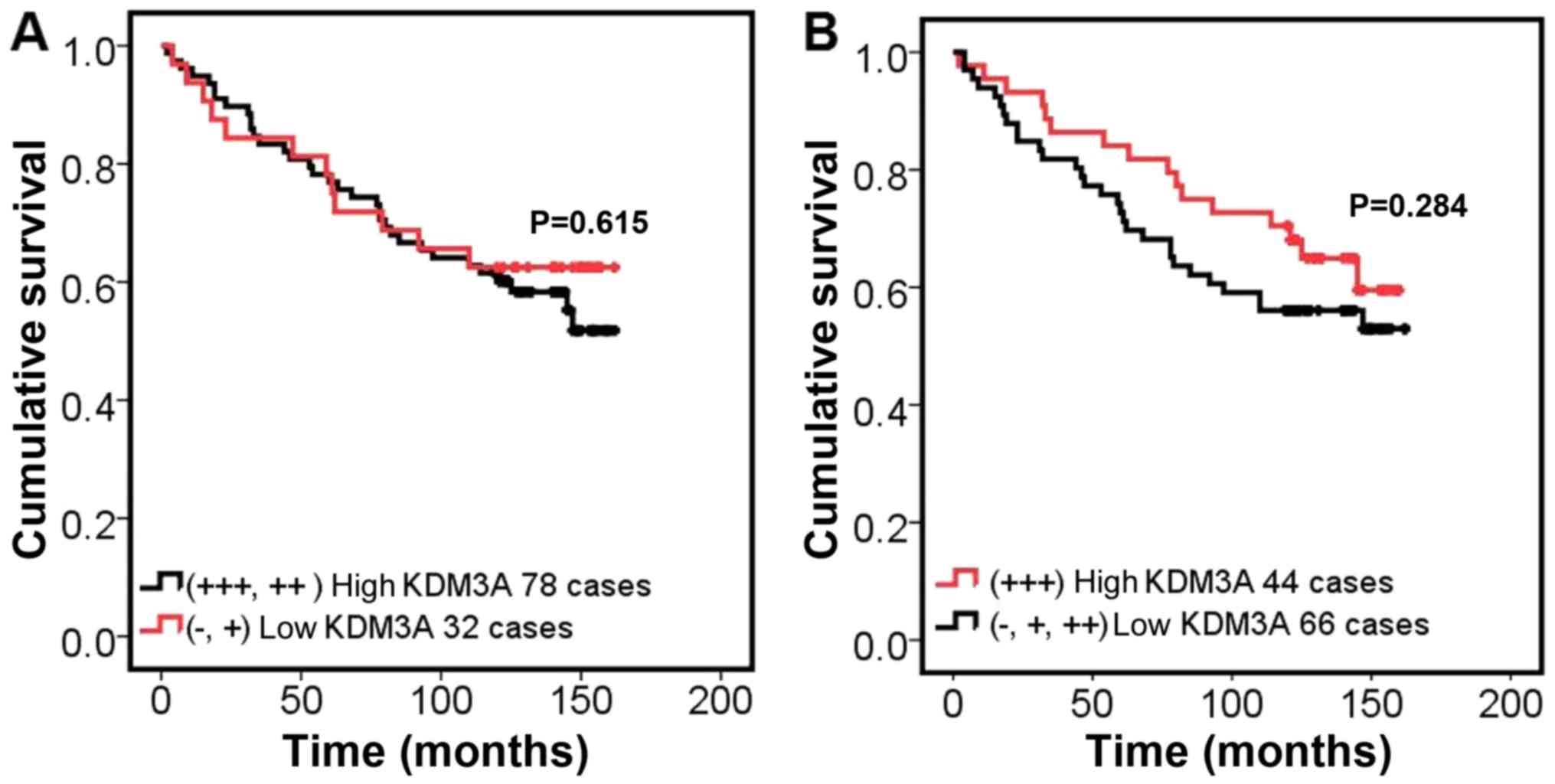
Furthermore, it was shown that there was no significant association
regarding prognosis among patients with high KDM3A expression vs.
patients with low KDM3A expression (Fig.
3). The statistical analysis suggests that KDM3A expression did
not correlate with any of the clinicopathological variables
available.
 | Table I.Analysis of the association between
KDM3A expression and clinicopathological variables in BCa
(n=150). |
Table I.
Analysis of the association between
KDM3A expression and clinicopathological variables in BCa
(n=150).
|
|
| KDM3A expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
variables | No. | Low | High | χ2 | P-value |
|---|
| Age, years |
|
|
|
|
|
| ≤50 | 72 | 43 | 29 | 1.079 | 0.299 |
|
>50 | 78 | 40 | 38 |
|
|
| Clinical stage |
|
|
|
|
|
| I | 11 | 6 | 5 | 0.03 | 0.985 |
| II | 86 | 49 | 37 |
|
|
| III | 50 | 28 | 22 |
|
|
| Pathological
grade |
|
|
|
|
|
| I | 38 | 25 | 13 | 2.102 | 0.147 |
| II | 111 | 58 | 53 |
|
|
| Diameter, cm |
|
|
|
|
|
|
<2 | 14 | 7 | 7 | 0.388 | 0.824 |
|
2–5 | 111 | 61 | 50 |
|
|
|
>5 | 25 | 15 | 10 |
|
|
| Lymph nodes
metastases |
|
|
|
|
|
| 0 | 54 | 31 | 23 | 0.103 | 0.950 |
|
1–3 | 47 | 27 | 20 |
|
|
| ≥4 | 44 | 24 | 20 |
|
|
| ER |
|
|
|
|
|
| − | 38 | 21 | 17 | 0.117 | 0.733 |
| + | 63 | 37 | 26 |
|
|
| PR |
|
|
|
|
|
| − | 46 | 25 | 21 | 0.327 | 0.567 |
| + | 55 | 33 | 22 |
|
|
| HER2 |
|
|
|
|
|
| − | 76 | 45 | 31 | 0.400 | 0.527 |
| + | 25 | 13 | 12 |
|
|
Association between KDM3A, and Her2,
ER and PR expression
To analyze whether KDM3A expression is correlated
with Her2, ER or PR expression, the Spearman correlation was used.
The results indicated that there was no significant correlation
between KDM3A and Her2, ER or PR expression (Table II), suggesting that there is no
causal relationship between KDM3A and the status of Her2, ER or
PR.
 | Table II.Analysis of the association between
KDM3A and Her2, ER and PR expression. |
Table II.
Analysis of the association between
KDM3A and Her2, ER and PR expression.
| Protein | KDM3A | ER | PR | HER2 |
|---|
| KDM3A | 1.000 | – | – | – |
| ER | −0.034 | 1.000 | – | – |
| PR | −0.057 | 0.767 | 1.000 | – |
| HER2 | 0.063 | −0.218 | −0.305 | 1.000 |
Discussion
In the present study, to the best of our knowledge,
we showed for the first time that there was no significant
association between KDM3A expression and metastasis and prognosis
of BCa, which suggests that KDM3A might not be involved with
metastasis and prognosis in BCa.
Originally, KDM3A was reported to be involved in
H3K9 demethylation and transcriptional activation of the androgen
receptor (8) as well as in
spermatogenesis (17) and hypoxia
(18,19). In the context of cancer, KDM3A has
been implicated in lung cancer carcinogenesis (20) and has been shown to be required for
growth of tumor xenografts (21,22);
furthermore, KDM3A can induce migration and invasion in
neuroblastoma (12), hepatocarcinoma
(13) and BCa (7). The literature therefore supports a
potential role for KDM3A in cancer growth and metastasis. To assess
the role of KDM3A on tumor progression and metastasis in the
context of BCa, we investigated KDM3A expression using a BCa TMA
and focused on the clinicopathological significance of its
expression.
Unexpectedly, we observed no significant
associations between KDM3A expression in BCa tissues and
clinicopathological variables, including demographic parameters,
TNM stage, tumor size, and Her2, ER and PR expression.
Additionally, no significant association between KDM3A expression
and overall prognosis was found after statistical analysis.
However, in other types of cancer such as gastric cancer, elevated
JMJD1A expression (an analog of KDM3A) was associated with its
prognosis and metastasis (23). In
our study of BCa, we did not observe this type of correlation. In
addition, increased JMJD1A levels have been purported to be
associated with the progression of renal cell carcinoma.
Nevertheless, we did not find a similar association in our own
study compared to the results by Guo et al (14). One study (13) of hepatocellular carcinoma did not
identify an association between elevated JMJD1A expression and any
clinicopathological characteristics using multivariate Cox
regression analysis (similar to the results of our study) but found
that JMJD1A was an independent predictor of recurrence. However,
another study by Suikki et al (24) detected the mRNA levels of JHDM2A
(another name of KDM3A) in prostate cancer tissues and found that
despite significant increases in JHDM2A mRNA expression in prostate
cancer than in benign prostate hyperplasia, the authors claimed
that JMJD2 was unlikely contributing a major role in the
progression of prostate cancer after statistical analysis.
Consequently, the abovementioned studies together with our own
results support the suggestion that the role of KDM3A and its
expression levels appear to vary in different types of cancer.
In a recent functional study performed in BCa
(25), KDM3A expression was observed
to gradually increase during BCa transformation and was elevated in
BCa tissues compared to paired control tissues. Consequently, the
authors deemed increased KDM3A expression levels as an important
event during BCa transformation. In consideration of this, it would
be difficult to compare the data from our study with previous
studies of KDM3A in BCa because most of the data are derived from
in vitro models of BCa, whereas our study focused on the
relationship between KDM3A expression in BCa tissue and
clinicopathological variables. Another recent study (26) of note showed a significant association
between elevated JMJD1A expression and poor overall prognosis of
patients with BCa. This result appears to contradict the
observation in our setting that despite no significant association,
there exists a small trend toward a better overall prognosis of
patients with BCa and elevated JMJD1A expression.
Because there were no paired normal control tissues
available with the BCa TMA, we were unable to assess the relative
expression of KDM3A in BCa tissues compared with corresponding
normal control tissues. In addition, we only explored the
correlation between KDM3A expression and the status of Her2, ER and
PR expression. It was determined that in our experimental setting,
there was no significant correlation between KDM3A expression and
the Her2, ER or PR status. This result leads to the suggestion that
there might be no explicitly causal relation between KDM3A and
Her2, ER or PR; however, this appears to be inconsistent with the
observation made by Wade et al, who, using mechanistic
investigations, reported that KDM3A was required for ER signaling
in BCa (6). Thus, more studies are
necessary to determine whether KDM3A plays any causal role in BCa
with a different Her2, ER or PR status. BCa can be classified as
either triple-negative or non-triple-negative based on the
expression of Her2, ER and PR. In our analysis, we were unable to
further stratify the results based on the Her2, ER and PR status.
Consequently, whether there exists an association between KDM3A and
Her2, ER or PR in triple-negative and non-triple-negative BCa
remains unknown and should be investigated in future studies.
However, Ramadoss et al (7)
discovered that KDM3A played a dual role in the invasion and
apoptosis of triple-negative BCa by demethylating histones and the
non-histone protein p53, respectively. Whether KDM3A plays a dual
role in the invasion and apoptosis of non-triple-negative BCas
remains unknown.
Several technical factors could potentially account
for the discrepancy between our findings and other relevant
reports. First and foremost, given the importance of the accuracy
and specificity of primary antibodies (27), the primary antibody against KDM3A used
in our study was distinctly different from that employed by
previous studies (14,28). Second, considering the inherent
limitations of TMAs (29,30), the BCa TMA we used could contribute
underlying bias. Third, the influence of slide aging (31) as well as the immunoscoring criteria
adopted (32) also cannot be
neglected in the analysis. Finally, in the absence of a functional
analysis of KDM3A in vitro cell culture systems, we cannot
measure the basic biological roles it mediates regarding the
proliferation, migration and invasion of BCa cells.
Taking our findings together, we showed, for the
first time to our knowledge, that KDM3A expression was not
associated with metastasis and prognosis in BCa as assessed using a
TMA, suggesting that KDM3A may not play a key role in the
progression of BCa.
Acknowledgements
The authors would like to thank clinical pathologist
Dr Wenli Cui, in the department of Pathology, the First Affiliated
Hospital of Xinjiang Medical University, for her contributions to
the study.
Funding
The study was supported by the National Natural
Science Foundation of China (grant no. 81660305) and the Natural
Science Foundation of Xinjiang Uygur Autonomous Region (grant no.
2015211C097).
Availability of data and materials
All data generated or analyzed during this study has
been included in this published article.
Authors' contributions
JY was involved in data collection. SZ interpreted
the data and performed statistical analysis. BL and XL provided the
biochemical reagents and performed immunohistochemistry. WL
conceived the whole study design and provided funding.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee at the First Affiliated Hospital of Xinjiang Medical
University. Informed consent was obtained from all the subjects
involved.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Bozorgi A, Khazaei M and Khazaei MR: New
findings on breast cancer stem cells: A review. J Breast Cancer.
18:303–312. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim SJ, Kim YS, Jang ED, Seo KJ and Kim
JS: Prognostic impact and clinicopathological correlation of CD133
and ALDH1 expression in invasive breast cancer. J Breast Cancer.
18:347–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weigelt B, Peterse JL and van't Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wade MA, Jones D, Wilson L, Stockley J,
Coffey K, Robson CN and Gaughan L: The histone demethylase enzyme
KDM3A is a key estrogen receptor regulator in breast cancer.
Nucleic Acids Res. 43:196–207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramadoss S, Guo G and Wang CY: Lysine
demethylase KDM3A regulates breast cancer cell invasion and
apoptosis by targeting histone and the non-histone protein p53.
Oncogene. 36:47–59. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamane K, Toumazou C, Tsukada Y,
Erdjument-Bromage H, Tempst P, Wong J and Zhang Y: JHDM2A, a
JmjC-containing H3K9 demethylase, facilitates transcription
activation by androgen receptor. Cell. 125:483–495. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wellmann S, Bettkober M, Zelmer A, Seeger
K, Faigle M, Eltzschig HK and Bührer C: Hypoxia upregulates the
histone demethylase JMJD1A via HIF-1. Biochem Biophys Res Commun.
372:892–897. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parrish JK, Sechler M, Winn RA and
Jedlicka P: The histone demethylase KDM3A is a
microRNA-22-regulated tumor promoter in Ewing Sarcoma. Oncogene.
34:257–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cho HS, Toyokawa G, Daigo Y, Hayami S,
Masuda K, Ikawa N, Yamane Y, Maejima K, Tsunoda T, Field HI, et al:
The JmjC domain-containing histone demethylase KDM3A is a positive
regulator of the G1/S transition in cancer cells via
transcriptional regulation of the HOXA1 gene. Int J Cancer.
131:E179–E189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tee AE, Ling D, Nelson C, Atmadibrata B,
Dinger ME, Xu N, Mizukami T, Liu PY, Liu B, Cheung B, et al: The
histone demethylase JMJD1A induces cell migration and invasion by
up-regulating the expression of the long noncoding RNA MALAT1.
Oncotarget. 5:1793–1804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamada D, Kobayashi S, Yamamoto H,
Tomimaru Y, Noda T, Uemura M, Wada H, Marubashi S, Eguchi H,
Tanemura M, et al: Role of the hypoxia-related gene, JMJD1A, in
hepatocellular carcinoma: Clinical impact on recurrence after
hepatic resection. Ann Surg Oncol. 3 Suppl 19:S355–S364. 2012.
View Article : Google Scholar
|
|
14
|
Guo X, Shi M, Sun L, Wang Y, Gui Y, Cai Z
and Duan X: The expression of histone demethylase JMJD1A in renal
cell carcinoma. Neoplasma. 58:153–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hewitt SM, Baskin DG, Frevert CW, Stahl WL
and Rosa-Molinar E: Controls for immunohistochemistry: The
Histochemical Society's standards of practice for validation of
immunohistochemical assays. J Histochem Cytochem. 62:693–697. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burry RW: Controls for
immunocytochemistry: An update. J Histochem Cytochem. 59:6–12.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okada Y, Scott G, Ray MK, Mishina Y and
Zhang Y: Histone demethylase JHDM2A is critical for Tnp1 and Prm1
transcription and spermatogenesis. Nature. 450:119–123. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beyer S, Kristensen MM, Jensen KS,
Johansen JV and Staller P: The histone demethylases JMJD1A and
JMJD2B are transcriptional targets of hypoxia-inducible factor HIF.
J Biol Chem. 283:36542–36552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pollard PJ, Loenarz C, Mole DR, McDonough
MA, Gleadle JM, Schofield CJ and Ratcliffe PJ: Regulation of
Jumonji-domain-containing histone demethylases by hypoxia-inducible
factor (HIF)-1alpha. Biochem J. 416:387–394. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou X, Sun H, Ellen TP, Chen H and Costa
M: Arsenite alters global histone H3 methylation. Carcinogenesis.
29:1831–1836. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krieg AJ, Rankin EB, Chan D, Razorenova O,
Fernandez S and Giaccia AJ: Regulation of the histone demethylase
JMJD1A by hypoxia-inducible factor 1 alpha enhances hypoxic gene
expression and tumor growth. Mol Cell Biol. 30:344–353. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Osawa T, Tsuchida R, Muramatsu M,
Shimamura T, Wang F, Suehiro J, Kanki Y, Wada Y, Yuasa Y, Aburatani
H, et al: Inhibition of histone demethylase JMJD1A improves
anti-angiogenic therapy and reduces tumor-associated macrophages.
Cancer Res. 73:3019–3028. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang H, Liu Z, Yuan C, Zhao Y, Wang L, Hu
J, Xie D, Wang L and Chen D: Elevated JMJD1A is a novel predictor
for prognosis and a potential therapeutic target for gastric
cancer. Int J Clin Exp Pathol. 8:11092–11099. 2015.PubMed/NCBI
|
|
24
|
Suikki HE, Kujala PM, Tammela TL, van
Weerden WM, Vessella RL and Visakorpi T: Genetic alterations and
changes in expression of histone demethylases in prostate cancer.
Prostate. 70:889–898. 2010.PubMed/NCBI
|
|
25
|
Zhao QY, Lei PJ, Zhang X, Zheng JY, Wang
HY, Zhao J, Li YM, Ye M, Li L, Wei G and Wu M: Global histone
modification profiling reveals the epigenomic dynamics during
malignant transformation in a four-stage breast cancer model. Clin
Epigenetics. 8:342016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Chang J, Varghese D, Dellinger M,
Kumar S, Best AM, Ruiz J, Bruick R, Peña-Llopis S, Xu J, et al: A
small molecule modulates Jumonji histone demethylase activity and
selectively inhibits cancer growth. Nat Commun. 4:20352013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baker M: Reproducibility crisis: Blame it
on the antibodies. Nature. 521:274–276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sar A, Ponjevic D, Nguyen M, Box AH and
Demetrick DJ: Identification and characterization of demethylase
JMJD1A as a gene upregulated in the human cellular response to
hypoxia. Cell Tissue Res. 337:223–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khouja MH, Baekelandt M, Sarab A, Nesland
JM and Holm R: Limitations of tissue microarrays compared with
whole tissue sections in survival analysis. Oncol Lett. 1:827–831.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Merseburger AS, Kuczyk MA, Serth J,
Bokemeyer C, Young DY, Sun L, Connelly RR, McLeod DG, Mostofi FK,
Srivastava SK, et al: Limitations of tissue microarrays in the
evaluation of focal alterations of bcl-2 and p53 in whole mount
derived prostate tissues. Oncol Rep. 10:223–228. 2003.PubMed/NCBI
|
|
31
|
Mirlacher M, Kasper M, Storz M, Knecht Y,
Dürmüller U, Simon R, Mihatsch MJ and Sauter G: Influence of slide
aging on results of translational research studies using
immunohistochemistry. Mod Pathol. 17:1414–1420. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dressler LG, Geradts J, Burroughs M, Cowan
D, Millikan RC and Newman B: Policy guidelines for the utilization
of formalin-fixed, paraffin-embedded tissue sections: The UNC SPORE
experience. University of North Carolina Specialized Program of
Research Excellence. Breast Cancer Res Treat. 58:31–39. 1999.
View Article : Google Scholar : PubMed/NCBI
|