Introduction
Hepatocellular carcinoma (HCC) is one of the most
malignant types of cancer and is associated with a poor prognosis.
Although surgical resection is potentially curative, the
postoperative recurrence rate is >50% (1,2). Due to
receiving a HCC diagnosis at an advanced stage, a number of
patients with HCC are not suited for surgical treatment. At
present, radiotherapy (RT) is recommended as the treatment for
patients with locally advanced HCC (3,4), and
certain patients can then be treated by surgical resection,
post-RT. An important aspect of HCC therapy is identifying which
patients will benefit from post-RT surgical resection.
Toll-like receptor 4 (TLR4) is an initiator of the
innate immune response that recognizes molecules derived from
pathogens (PAMPs) and endogenous danger signals (DAMPs) (5). The excessive activation of TLR4
signaling, as may be stimulated by DAMPs derived from distress or
injury in tissues following RT, may induce liver damage (6), and further influence the apoptosis,
proliferation, invasion and metastasis of HCC (7,8). Our
previous studies identified that the TLR4-dependant immune response
may promote radiation-induced liver diseases (RILDs) via enhancing
the expression of certain proteins in mice, including tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL) and
vascular endothelial growth factor receptor 2 (VEGFR2) (9,10).
However, the clinical value of these potentially influencing
factors in estimating the prognosis for patients with HCC treated
by surgical resection post-RT remains unclear.
The purpose of the present study was to evaluate the
effect of TLR4-associated proteins on the response of HCC to RT
followed by surgery. Therefore, the individual characteristics and
prognoses of patients with HCC treated by surgical resection
post-RT were considered, and the expression levels of TLR4, TRAIL
and VEGFR2 in HCC and peritumoral liver tissue samples from the
patients were assessed using immunohistochemistry (IHC) assays on
liver resection specimens.
Materials and methods
Patients
Between July 2005 and October 2013, 20 patients with
HCC who underwent surgical resection post-RT at Zhongshan Hospital
(Shanghai, China) were selected for this study. All the recruited
patients exhibited stage III disease, including 19 males and 1
female, with ages ranging from 36–69 and a mean age of 54 years.
Tumor staging was performed according to the 2002 International
Union Against Cancer system (11).
The study was approved by the Zhongshan Hospital Research Ethics
Committee, and all patients provided written consent for their
inclusion in research.
The selection criteria for the patients recruited
into the study were as follows: i) Patients were diagnosed with
stage III HCC prior to RT, confirmed by histology or a serum
α-fetoprotein level ≥400 ng/ml, with a typical clinical
presentation; ii) surgery had been judged to be unsuitable as a
primary treatment; iii) intrahepatic tumors were treated with RT;
iv) as assessed by the Response Evaluation Criteria In Solid Tumors
(12), the tumor response to RT had
been judged as a partial response (PR) prior to surgery; and v)
intrahepatic tumors underwent surgical resection following RT. In
addition, as transarterial chemoembolization (TACE) is typically
used for the palliation of unresectable HCC and numerous patients
had been treated with TACE, patients that had been previously
treated with TACE were not excluded from this retrospective
research.
Therapeutic strategy
Intrahepatic tumors were treated with external beam
RT (EBRT) using a linear accelerator or helical tomotherapy. The
plans for RT were produced using a treatment planning system (TPS)
computer. The tumor dose was 46–53.5 Gy in 10–25 fractions. To
assess the tumor response to RT, abdominal enhanced computed
tomography (CT) or magnetic resonance imaging (MRI) was performed
pre-and post-RT for each patient. Prior to the surgery, patients
included in this study were all judged to be operable by their
surgeons. Hepatectomy was then applied for the further treatment of
intrahepatic tumors subsequent to RT. In addition, patients could
be treated with or without TACE pre- or post-surgery. TACE could
also serve as abridging therapy prior to RT in some patients with
HCC, especially for those with large tumors, because the minimum
normal liver volume during RT (minus the gross tumor volume) should
be more than 700 ml to avoid RILD. Following treatment with TACE,
significant tumor regression may occur, and some patients could be
further treated with RT. Furthermore, the complete tumor response
rate of TACE is unsatisfactory (13,14),
meaning that HCC may not be cured by TACE alone. In order to
improve efficacy, some HCC patients may switch to RT following TACE
treatment. Therefore, each patient's treatment history, with or
without TACE, would be analyzed in the present study.
For example (Fig. 1),
a patient with HCC presented with a large mass, which was unsuited
for primary treatment by surgical resection (Fig. 1A), and was therefore treated with RT.
The RT dose to the tumor was 53.5 Gy in 25 fractions (Fig. 1B and C). At 2 months after RT, the
intrahepatic tumor had markedly regressed and was judged to be
suitable for surgical treatment (Fig.
1D). The surgical resection of the intrahepatic tumors was
performed at Zhongshan Hospital (Fig.
1E). A postoperative CT scan was then performed (Fig. 1F).
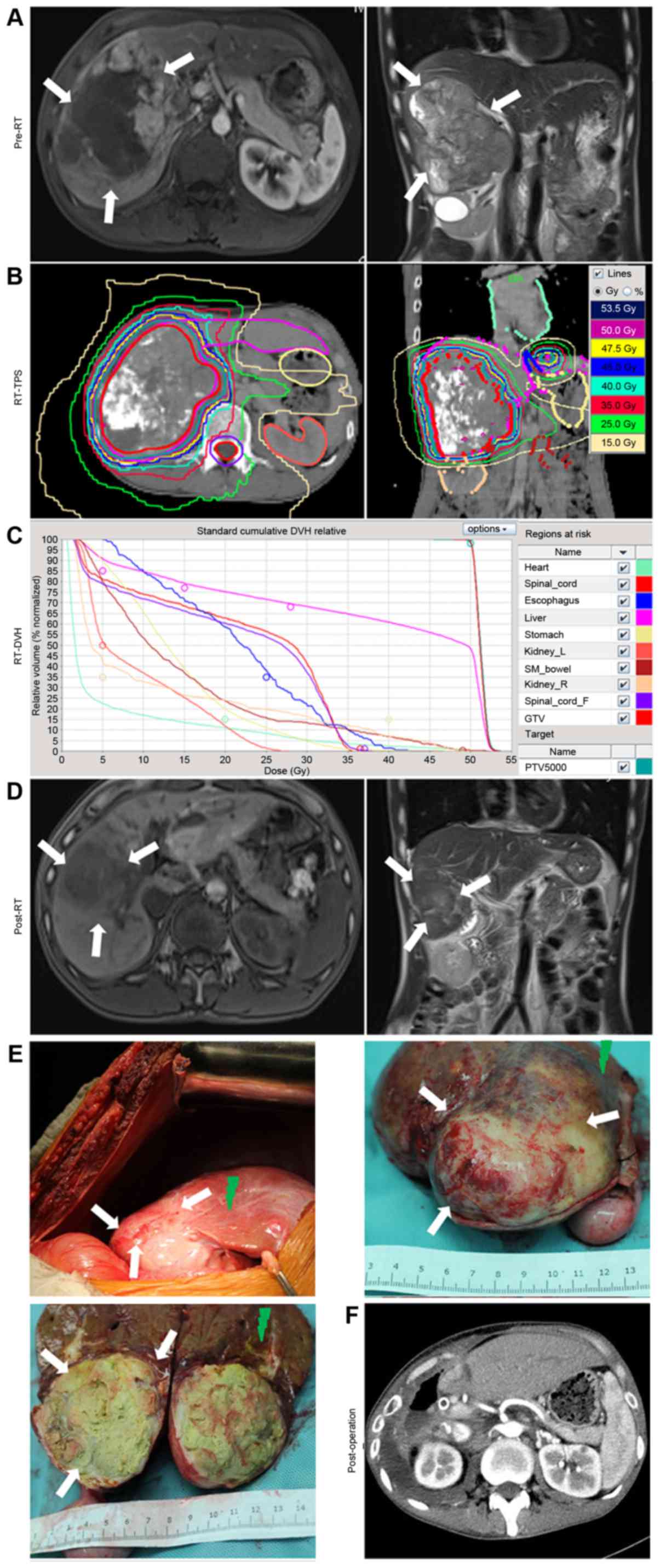 | Figure 1.Example of the treatment of a patient
with locally advanced hepatocellular carcinoma using RT followed by
resective surgery. (A) Pre-RT MRI revealed a large mass signal on
the enhanced T1-W1. (B) Isodose distribution graphs for RT, as
produced by the RT-TPS computers. The tumor, liver, stomach,
esophagus, heart, kidneys and spinal cord were delineated. (C)
RT-DVH graph of the tumor, liver, stomach, esophagus, heart,
kidneys and spinal cord, as calculated using the TPS. (D)
Pre-operative MRIs revealed that the tumor had regressed post-RT,
and was now suited for further treatment with surgery. (E) During
surgery, it was identified that the remaining tumor had been almost
eliminated by RT, with a distinct boundary (white arrows).
Radiation-induced liver diseases occurred in the swelling and
bleeding zone of the peritumoral liver tissues (green arrows). (F)
Computed tomography scans revealed the absence of the right liver
lobe and tumor tissue following surgery. RT, radiotherapy; MRI,
magnetic resonance imaging; TPS, treatment planning system; DVH,
dose volume histogram. |
Tissue microarray (TMA)
The TMAs were constructed as previously described
(9). Briefly, slides with hematoxylin
and eosin (H&E)-stained sections were screened for optimal
tumor and peritumoral liver tissues for the construction of TMAs.
Two tissue cores of 2.0 mm diameter were punched from the
non-necrotic areas of the peritumoral liver tissue adjacent to the
tumor and tumor foci in formalin-fixed, paraffin-embedded samples.
Slides containing 4-µm sections were constructed from the resulting
TMA blocks. In order to identify the irradiation dose of each
tissue section selected for the TMA, the process was directed by
the RT isodose distribution graphs from the TPS computers.
Histological evaluation (IHC and
H&E)
TMA sections were stained with H&E, or subjected
to IHC with antibodies against TLR4 (dilution, 1:100), TRAIL
(dilution, 1:50) or VEGFR2 (dilution, 1:50; Abcam; Cambridge, UK),
and were visualized with GT Vision III Detection System/Mo and Rb
kit (Gene Tech Biotechnology Co., Ltd., Shanghai, China), according
to the manufacturer's instructions. H&E-stained or IHC-stained
TMA sections (magnification, ×100 or ×400) were evaluated by three
of the authors, who were blinded to the patient outcomes.
H&E-stained TMA sections were used to compare the severity of
RILDs in patients, scored with the Fudan University Acute RILD
Histological system (9). Five
high-power fields were randomly selected to evaluate the expression
levels of TLR4, TRAIL and VEGFR2.
Based on the staining percentage and intensity of
positive cells counted in each core (9,15), the
final IHC staining scores were defined as (−), (+), (++) and (+++),
which represented a negative, weak, moderate and strong
immunoreactivity, respectively. For statistical analysis, the
staining scores of cells expressing TLR4, TRAIL and VEGFR-2 were
categorized as follows: For TLR4, (−) or (+), low expression; (++)
or (+++), high expression; for TRAIL and VEGFR-2, (−), low
expression; (+), (++) or (+++), high expression. The patients were
divided into high- and low-TLR4, TRAIL and VEGFR-2 expression
groups based on the tumor and peritumoral liver tissue
expression.
Follow-up
All patients were followed up at the outpatient
clinic or via telephone interviews following surgery. Follow-up
continued until October 2014. No patient mortality was attributed
to surgical complications. All patients underwent the surgical
resection of HCC, which was defined as the complete macroscopic
removal of the tumor. Clinical information was collected from the
computerized data base of Zhongshan Hospital. The overall survival
(OS) time was defined as the time from the initial diagnosis date
of HCC to the date of mortality or the last follow-up. The
disease-free survival (DFS) time was defined as the interval
between the date of surgery and the first diagnosis of
postoperative HCC recurrence or metastasis, or the last
follow-up.
Statistical analysis
Statistical analyses were performed with SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). OS and DFS are presented
as the median ± standard error of the mean (SEM); other values are
presented as the means ± SEM. DFS and OS were analyzed using the
Kaplan-Meier method. Differences in survival were assessed with the
log-rank test. Differences in quantitative data, including the RILD
score, were evaluated by t-tests. Differences in frequency data,
including the high TLR4 expression ratio of patients, were
evaluated by Fisher's exact test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical features and potential
biomarker expression in TMA
Following resective surgery, there was typically a
distinct tumor boundary (white arrows, Fig. 1E). RILDs typically occurred in the
swelling and bleeding zone of peritumoral liver tissues (green
arrows, Fig. 1E).
Clinical features, including the TACE treatment
history and the DFS and OS time of each patient, are included in
Table I. A total of 18 (90%) of the
20 patients were treated with TACE pre- or post-RT. In addition, 4
(27%) of the 15 patients who experienced postoperative disease
progression were treated with TACE. The median OS time was 39±3.17
months (range, 13–91 months) for the 20 patients. The OS rates at
24 and 36 months were 83.1 and 58.8%, respectively.
 | Table I.Clinical features of the 20 HCC
patients in stage III undergoing hepatectomy post-RT. |
Table I.
Clinical features of the 20 HCC
patients in stage III undergoing hepatectomy post-RT.
|
|
|
| TACE, n | Tumor TMA IHC
score | Liver TMA IHC
score |
|
| Survival time,
months |
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| No. | Sex | Age | Pre-RT | Post-RT | PO | TLR4 | VEGFR2 | TRAIL | TLR4 | VEGFR2 | TRAIL | RILD score | PO recurrence/ | metastasis
sites | DFS | OS |
|---|
| 1 | M | 44 | 1 | 0 | 2 | (++) | (+) | (+) | (−) | (−) | (−) | 1 | Liver/lymph
nodes | 17 | 24 |
| 2 | M | 46 | 1 | 0 | 0 | (−) | (+) | (−) | (−) | (−) | (−) | 1 | Lung | 13 | 48 |
| 3 | M | 53 | 1 | 2 | 0 | (−) | (−) | (−) | (+) | (−) | (−) | 2 | – | 24 | 35a |
| 4 | M | 78 | 4 | 1 | 0 | (+) | (+) | (+) | (+) | (−) | (−) | 2 | Liver | 9 | 15 |
| 5 | M | 59 | 0 | 1 | 0 | (+) | (−) | (+) | (++) | (−) | (+) | 4 | Liver | 40 | 45 |
| 6 | M | 60 | 2 | 0 | 0 | (++) | (−) | (+) | (+) | (++) | (++) | 3 | Bone | 8 | 13 |
| 7 | M | 49 | 2 | 1 | 0 | (++) | (+) | (++) | (++) | (++) | (−) | 4 | Lymph
nodes/lung/bone | 7 | 24 |
| 8 | M | 55 | 0 | 0 | 0 | (++) | (+) | (++) | (++) | (−) | (+) | 3 | Liver | 5 | 41 |
| 9 | M | 62 | 0 | 0 | 1 | (+) | (−) | (−) | (+) | (−) | (−) | 2 | Liver/lymph
nodes/bone | 59 | 91 |
| 10 | M | 59 | 1 | 0 | 0 | (++) | (−) | (+) | (++) | (+) | (++) | 4 | Lung | 33 | 36 |
| 11 | M | 55 | 3 | 2 | 2 | (+) | (−) | (+) | (++) | (+++) | (+) | 2 | Liver/lung | 8 | 38 |
| 12 | M | 40 | 1 | 1 | 0 | (+) | (−) | (−) | (+) | (+) | (−) | 1 | – | 69 | 72a |
| 13 | M | 56 | 1 | 0 | 0 | (++) | (++) | (+) | (++) | (++) | (−) | 3 | Liver | 26 | 29 |
| 14 | M | 52 | 3 | 0 | 0 | (+) | (−) | (−) | (+) | (−) | (++) | 3 | Liver | 40 | 50 |
| 15 | M | 48 | 4 | 0 | 0 | (+) | (++) | (+) | (++) | (+) | (++) | 4 | Liver | 17 | 50 |
| 16 | M | 65 | 3 | 1 | 5 | (+) | (+) | (++) | (+) | (−) | (++) | 1 | Liver/lung | 2 | 31 |
| 17 | M | 69 | 3 | 0 | 0 | (+) | (−) | (−) | (−) | (−) | (−) | 2 | Lymph nodes | 31 | 39 |
| 18 | M | 47 | 2 | 0 | 0 | (++) | (−) | (−) | (+) | (−) | (−) | 3 | – | 15 | 21a |
| 19 | F | 36 | 1 | 0 | 0 | (++) | (−) | (++) | (++) | (+) | (++) | 4 | Lung | 11 | 15a |
| 20 | M | 38 | 1 | 2 | 0 | (++) | (−) | (++) | (++) | (+) | (++) | 4 | – | 3 | 15a |
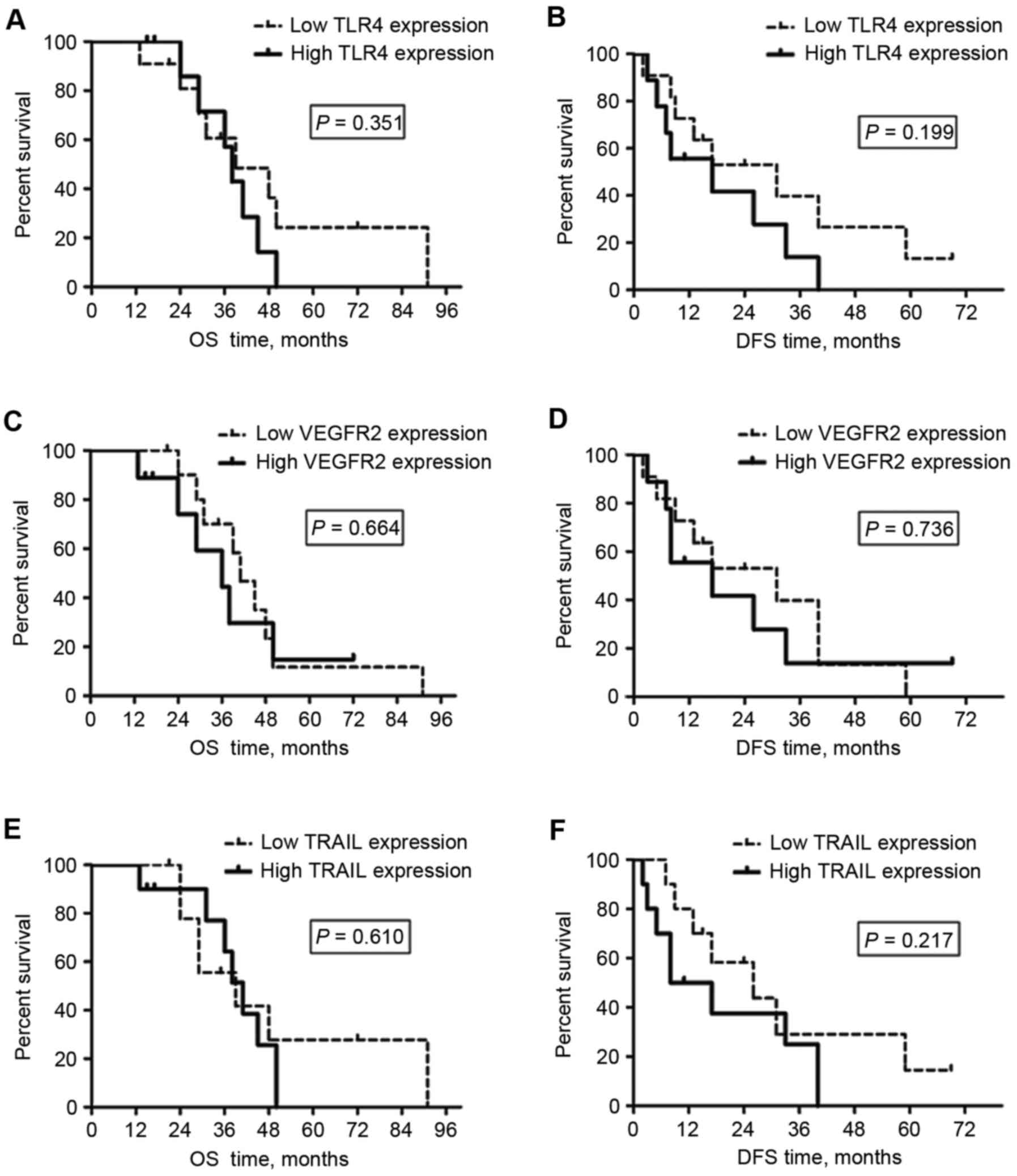
According to the results of IHC, TLR4 expression was
primarily observed on the membrane and in the cytoplasm of tumor
cells and hepatocytes, whereas VEGFR-2 and TRAIL expression were
identified in the cytoplasm and on the nuclear membrane (black
arrows; all areas of positive staining, Figs. 2 and 3).
Of the 20 specimens, 9 (45%), 8 (40%) and 13 (65%) of the 20
patients exhibited high TLR4, VEGFR2 and TRAIL expression in tumor
tissue, respectively; whereas high TLR4, VEGFR2 and TRAIL
expression of the peritumoral liver tissue was identified in 9
(45%), 9 (45%) and 10 (50%) of the specimens, respectively.
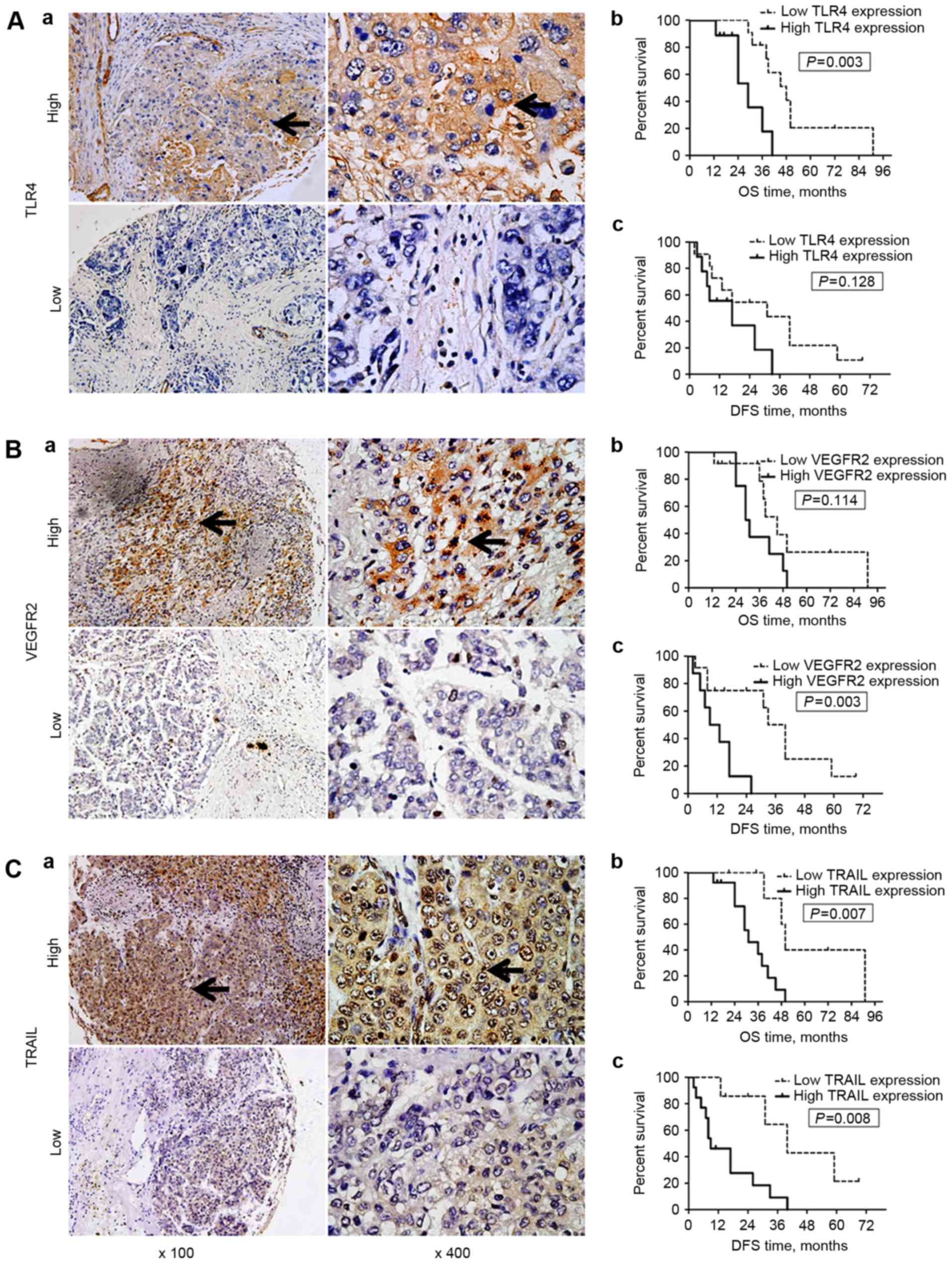 | Figure 2.Expression of TLR4, VEGFR2 and TRAIL
expression in hepatocellular carcinoma tissue samples, and
associated OS/DFS curves. TLR4 expression was primarily observed on
the membrane and in the cytoplasm of cells, whereas VEGFR-2 and
TRAIL expression were identified in the cytoplasm and on the
nuclear membrane (black arrows; all areas of positive IHC
staining). (A) TLR4 and OS/DFS. (a) Representative images of high
(n=9) and low (n=11) TLR4 expression in tumors. (b) OS and (c) DFS
curves for the patients stratified by the tumor TLR4 expression
level. Patients with low tumor TLR4 expression exhibited a
significantly longer OS (P=0.003), but not DFS (P=0.128) time than
those with high TLR4 expression. (B) VEGFR2 and OS/DFS. (a)
Representative images of high (n=8) and low (n=12) VEGFR2
expression in tumors. (b) OS and (c) DFS curves for the patients
stratified by the tumor VEGFR2 expression level. Patients with low
tumor VEGFR2 expression exhibited a significantly longer DFS
(P=0.003), but not OS (P=0.114) time than those with high VEGFR2
expression. (C) TRAIL and OS/DFS. (a) Representative images of high
(n=13) and low TRAIL (n=7) expression in tumors. (b) OS and (c) DFS
curves for the patients stratified by the tumor TRAIL expression
level. Patients with low tumor TRAIL expression exhibited
significantly longer DFS (P=0.008) and OS (P=0.007) times than
those with high TRAIL expression. Magnification, ×100 or ×400.
TLR4, Toll-like receptor 4; VEGFR2, vascular endothelial growth
factor receptor 2; TRAIL, tumor necrosis factor-related
apoptosis-inducing ligand; OS, overall survival; DFS, disease-free
survival; IHC, immunohistochemistry. |
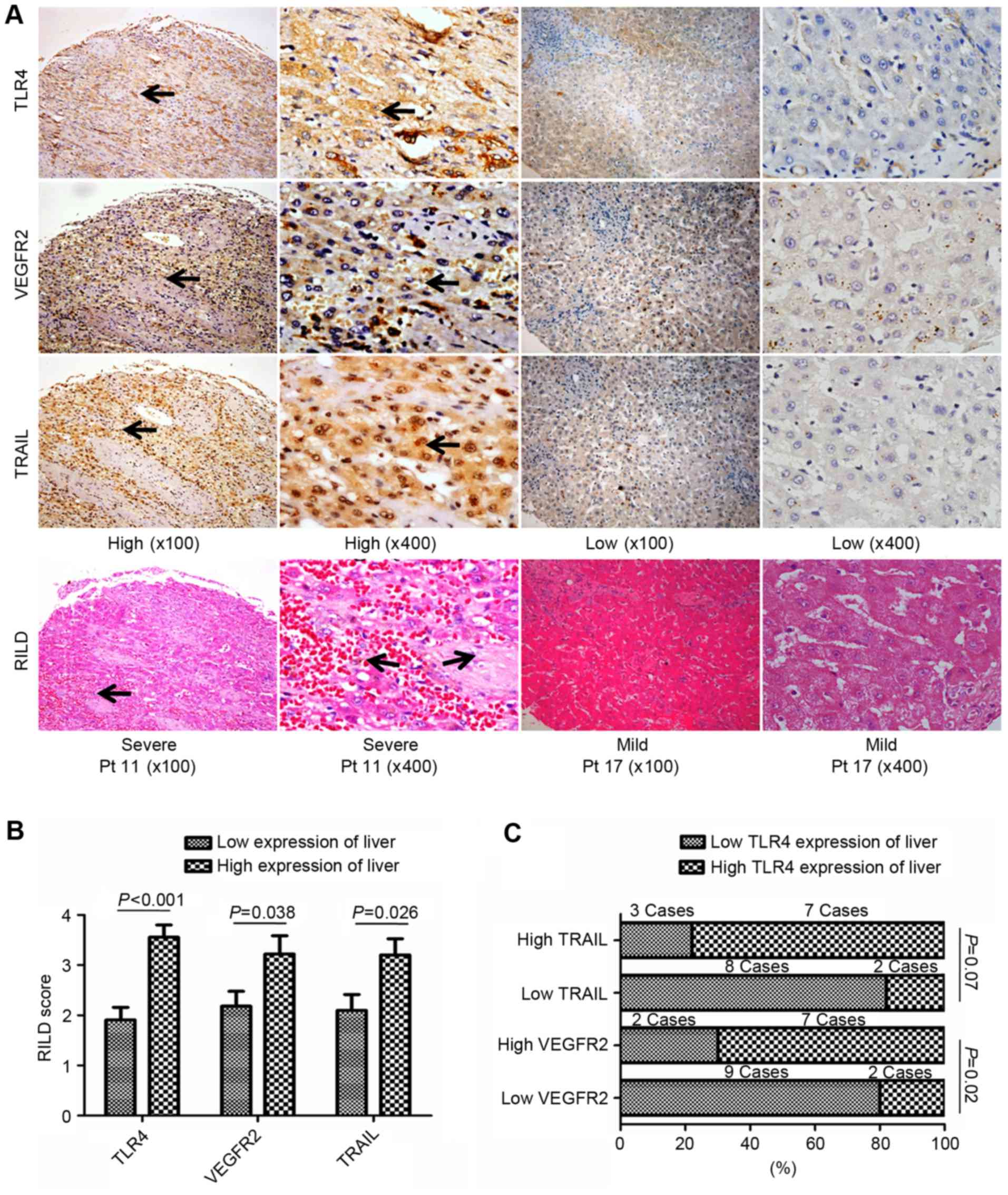 | Figure 3.Association of the non-tumor liver
tissue expression of TLR4, VEGFR2 and TRAIL with RILDs in patients
with HCC treated with RT. (A) Representative images of IHC and
H&E-stained tissue microarrays from 20 patients with HCC. High
TLR4, VEGFR2 or TRAIL expression in liver tissue following RT was
associated with more severe RILDs. TLR4 expression was primarily
observed on the membrane and in the cytoplasm of cells, whereas
VEGFR-2 and TRAIL expression were identified in the cytoplasm and
on the nuclear membrane (black arrows; all areas of positive
IHC-staining). Magnification, ×100 or ×400. (B) Comparing RILD
scores by TLR4, VEGFR2 and TRAIL expressions levels. The mean RILD
scores of patients with high TLR4, VEGFR2 or TRAIL expression were
significantly higher than the patients with the corresponding low
expression level in non-tumor liver tissue (P<0.05). (C)
Association between TLR4 and VEGFR2 or TRAIL expression in the
liver post-RT. The rate of high TLR4 expression tended to be higher
in patients with high TRAIL expression in the non-tumor liver
tissue, whereas significantly more patients with high TLR4
expression were identified in the group with high VEGFR2 expression
in the non-tumor liver tissue post-RT. TLR4, Toll-like receptor 4;
VEGFR2, vascular endothelial growth factor receptor 2; TRAIL, tumor
necrosis factor-related apoptosis-inducing ligand; RILD,
radiation-induced liver disease; HCC, hepatocellular carcinoma; RT,
radiotherapy; IHC, immunohistochemistry; H&E, hematoxylin and
eosin. |
Association of OS and DFS with TLR4,
VEGFR2 and TRAIL expression in tumor tissue
The OS and DFS times of the patients from the
present study are listed in Table I.
Kaplan-Meier survival curves are included in Fig. 2. No significant differences were
identified between the OS times of patients with high and low tumor
tissue VEGFR2 expression (P=0.114). However, patients with low TLR4
or TRAIL expression in tumor tissue had significantly improved
overall survival outcomes vs. those with high TLR4 (P=0.003) or
TRAIL (P=0.007) expression (Table
II).
 | Table II.Correlations between
TLR4/VEGFR2/TRAIL expression and survival results of HCC patients
undergoing hepatectomy post-RT. |
Table II.
Correlations between
TLR4/VEGFR2/TRAIL expression and survival results of HCC patients
undergoing hepatectomy post-RT.
|
| Median OS time | Median DFS
time |
|---|
|
|
|
|
|---|
| Expression | Low, months | High, months | χ2 | P-value | Low, months | High, months | χ2 | P-value |
|---|
| TLR4 |
|
|
|
|
|
|
|
|
|
Tumor | 48±6.86 (n=11) | 29±5.98 (n=9) | 8.854 | 0.003 | 31±13.93 | 17±9.10 | 2.321 | 0.128 |
|
Liver | 39±11.51
(n=11) | 38±2.62 (n=9) | 0.872 | 0.351 | 31±12.32 | 17±11.21 | 1.648 | 0.199 |
| VEGFR2 |
|
|
|
|
|
|
|
|
|
Tumor | 45±4.67 (n=12) | 29±3.30 (n=8) | 2.497 | 0.114 | 33±4.00 | 9±4.24 | 8.585 | 0.003 |
|
Liver | 41±4.26 (n=11) | 36±8.92 (n=9) | 0.189 | 0.664 | 31±12.32 | 17±11.21 | 0.114 | 0.736 |
| TRAIL |
|
|
|
|
|
|
|
|
|
Tumor | 50±2.91 (n=7) | 31±5.71 (n=13) | 7.348 | 0.007 | 40±9.42 | 9±3.33 | 7.04 | 0.008 |
|
Liver | 39±12.45
(n=10) | 41±3.37 (n=10) | 0.26 | 0.61 | 26±10.78 | 8±5.83 | 1.526 | 0.217 |
There was no significant difference in DFS time
between the patients with high and low tumor tissue TLR4 expression
(P=0.128), whereas the DFS of patients with low tumor VEGFR2 or
TRAIL expression was significantly longer than those with high
tumor VEGFR2 (P=0.003) or TRAIL (P=0.008) expression (Fig. 2; Table
II).
In summary, longer OS times were observed in
patients with tumors with low TLR4 or TRAIL expression; whereas
longer DFS times were observed in those with tumors with low VEGFR2
or TRAIL expression (Fig. 2; Table II). These results demonstrate that
the level of TLR4 and TRAIL expression in HCC tumor tissues may
have a prognostic value for OS, whereas the VEGFR2 or TRAIL
expression of tumor tissues were associated with the DFS of the
patients with HCC treated by surgical resection post-RT.
TLR4, VEGFR2 and TRAIL expression in
the peritumoral liver tissues are associated with RILDs, but not
with survival
As demonstrated in Fig.
4 and Table II, the median OS
and DFS times for the patients with low TLR4, VEGFR2 or TRAIL
expression in peritumoral liver tissue were not significantly
different from the times for the patients with high peritumoral
TLR4, VEGFR2 or TRAIL expression. These results demonstrate that
the levels of TLR4, VEGFR2 or TRAIL expression in peritumoral liver
tissues may not be suitable as biomarkers for survival outcomes for
patients with HCC receiving surgical resection following RT.
RILDs resulting from the irradiation of involved
normal liver tissue were assessed and scored in the H&E-stained
liver TMA slides. Patients with higher TLR4 expression developed
more severe RILDs (RILD score, 3.56±0.24 vs. 1.91±0.25;
P<0.001), which indicated that severe RILDs may have been
induced by the TLR4-dependent response to the RT treatment of HCC.
This was also observed in patients with high peritumoral TRAIL or
VEGFR2 expression (RILD scores, 3.20±0.33 and 3.22±0.36,
respectively) compared to those with low expression (RILD scores,
2.10±0.31 and 2.18±0.30, respectively; P<0.05; Fig. 3A and B).
There were 2 patients with high TLR4 expression that
also presented with low VEGFR2 expression (2/11), whereas 7
patients with high VEGFR2 expression also exhibited high TLR4
expression (7/9) in the peritumoral liver tissues post-RT (P=0.02;
Fig. 3C). Although no significant
differences were identified between the patients with low and high
TRAIL expression (P=0.07), the number of patients with high TLR4
expression was reduced in the group of patients with low TRAIL
expression in the peritumoral liver tissue post-RT (Fig. 3C). These findings demonstrated that
high TLR4 expression maybe associated with severe RILDs and high
VEGFR2 or TRAIL expression in post-RT peritumoral liver
tissues.
Discussion
Novel therapeutic strategies continue to be trialed
at Zhongshan Hospital, with the aim of improving the outcome for
patients with inoperable and/or locally advanced HCC. Assuring
clinical target volume coverage and performing advanced RT delivery
techniques, including intensity modulated RT and helical
tomotherapy, can minimize toxicity to the normal liver tissue while
continuing to deliver an effective treatment for HCC (16,17). In a
previous study at Zhongshan Hospital, OS rates for patients with
HCC treated by RT were 42.3 and 24.0% at 2 and 3 years,
respectively, whereas patients that did not receive RT exhibited OS
rates of 26.5 and 11.1% at 2 and 3 years, respectively (18). Furthermore, certain patients with
inoperable HCC gained a secondary opportunity for surgical
resection when the tumor size had reduced subsequent to RT.
Improved survival outcomes were also identified in patients with
HCC treated by surgical resection post-RT (OS rates, 83.1 and 58.8%
at 2 and 3 years, respectively) compared with those treated by RT
without surgery, which may be due to the RT-mediated effect of the
elimination of cancer cells that had spread into the liver tissue
around tumors. Although survival benefits were demonstrated in our
previous studies, not every patient with inoperable HCC was
benefited by post-RT surgical resection. The outcome for certain
patients remains unsatisfactory; for example, in the present study,
the lowest OS and DFS times for the patients were 13 and 2 months,
respectively. Therefore, it is important to identify predictors to
estimate the prognosis of patients with HCC that are treated with
surgery post-RT.
The TLR4-dependent immune response is a critical
constituent of innate immunity (19,20). TLR4
maybe expressed not only in healthy liver cells, including
hepatocytes and hepatic stellate cells (6), but also in certain types of tumor cells,
including in HCC. For example, it has been observed that TLR4 may
be stimulated by DAMPs to promote the growth of HCC (21). Yu et al (22) hypothesized that blocking the
TLR4-mediated signaling pathway may inhibit HCC invasion and
metastasis. By examining the TLR4 expression level in tumors from
30 patients with HCC, Eiró et al (23) identified that positive TLR4
immunostaining was associated with a relatively poor prognosis. In
the present study, it was identified that the OS time of patients
with low tumor TLR4 expression was significantly longer than those
with high tumor TLR4 expression. Therefore, the upregulation of
TLR4 in HCC tissue may contribute to the poor prognosis of certain
patients with HCC treated with post-RT surgery.
A number of studies have identified that distinct
cytokine responses are associated with radiation-induced
inflammation (24,25). The inhibition of TRAIL has been
demonstrated to specifically eliminate malignant cells, which can
be combined with RT to further enhance the anticancer effect
(26). Similarly, a significantly
improved OS and DFS time was observed in patients with low tumor
TRAIL expression compared with patients with high tumor TRAIL
expression in the present study.
VEGFR2 is one of the most critical receptors for
VEGF (27); tumor angiogenesis can be
inhibited by blocking the activity of VEGFR2 (27,28). Due
to escalating the rate of vascular repair, radiation-induced
upregulation of VEGFR2 in cancer cells may contribute to RT failure
(29). In the present study, it was
identified that the DFS time was significantly longer in patients
with low VEGFR2 expression in HCC tumor tissue following post-RT
surgery. Therefore, the expression levels of TRAIL or VEGFR2 in HCC
tissue may be suitable for use as predictive factors for patients
with HCC treated by post-RT surgery.
The excessive activation of TLR4 signaling may
induce liver damage (8–10). Although the expression levels of TLR4,
VEGFR2 and TRAIL in liver tissue were not identified as predictors
for survival outcomes in patients with HCC treated by post-RT
surgery, the results of the present study indicated that they were
associated with the severity of RILDs, potentially fatal
complications that restrict radiotherapeutic efficacy against HCC,
including liver piecemeal necrosis, inflammation, hepatic
veno-occlusive disease and sinusoidal obstruction syndrome
(30,31). In the present study, patients with low
TLR4 expression presented with milder RILDs than those with high
TLR4 expression in the peritumoral liver tissue subsequent to liver
RT, which was consistent with the preliminary data of another study
of ours, in mice (10). TRAIL not
only promotes the malignant behavior of cancer cells, but is also
associated with hepatocyte toxicity when combined with RT (26,32).
VEGFR2 is widely distributed throughout human tissue, including
tumors (33). According to the IHC
and H&E histological findings from the present study, higher
VEGFR-2 and TRAIL expression tended to be associated with high TLR4
expression in liver tissue. More severe RILDs in the surrounding
liver tissue were also identified in patients with high VEGFR-2 and
TRAIL expression in the liver compared with those with low
expression.
In addition, 90% of the patients in the present
study were treated with TACE pre- or post-RT. TACE is typically
used for the palliation of unresectable HCC, with a significant
tumor response rate of 17–61.9%, although the complete tumor
response rate to TACE is unsatisfactory (0–4.8%) (13). TACE can also serve as abridging
therapy prior to RT. In order to avoid RILD, the minimum normal
liver volume (minus the gross tumor volume) is defined as 700 ml.
HCC patients with particularly large tumors can only be treated
with RT when significant tumor regression has occurred, and normal
liver volume is sufficient, post-TACE (34,35). A
previous study has also demonstrated that TACE with RT, compared
with TACE alone or RT alone, improved the survival outcomes of
patients with unresectable HCC (36).
Shim et al (37) reported that
the 2-year survival rate of HCC patients treated by TACE and RT was
significantly higher than patients treated by TACE alone (36.8 vs.
14.3%, P=0.001), particularly in cases of tumors ≥8 cm diameter.
Both of these rates are lower than the 2-year survival rate of
patients treated by surgical resection post-RT in the present study
(83.1%), despite 18/20 of the patients receiving TACE treatment,
which indicates that TACE may not have affected the survival
outcome for patients in the present study. Statistical analysis of
the data from the present study demonstrated that there were no
differences in the TACE treatment status between patients with high
and low TLR4, VEGFR2 or TRAIL expression in liver or tumor tissues
in the present study, respectively (all P>0.05). This further
illustrated that pre- or post-RT TACE treatment did not affect the
survival outcomes of the patients in the present study.
In summary, patients with stage III HCC,
particularly patients with low tumor TLR4, VEGFR2 or TRAIL
expression, may benefit from treatment with surgical resection
post-RT. In addition, the high expression of TLR4, VEGFR2 or TRAIL
in the peritumoral liver tissue was associated with more severe
RILDs, but not with the prognosis of patients with HCC treated by
post-RT surgery. Therefore, therapeutic approaches of inhibiting
TLR4, VEGFR2 or TRAIL in liver and HCC tissue may prevent or lessen
RILDs, and improve the survival outcomes of patients with HCC
treated by post-RT surgery in the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. U1505229), the
Doctoral Foundation of Education Department of China (grant no.
20120071110065) and the Science and Technology Innovation Plan of
Shanghai (grant no. 14140902303).
Availability of data and materials
The data and materials of the current study are
available from the corresponding author on reasonable request.
Authors' contributions
ZFW performed the histological examination of the
tissue microarrays. The overall and disease-free survival outcomes
for each patient were assessed by YW. PY applied the resected HCC
tissues and peritumoral liver tissues to construct TMAs. They were
major contributors in writing the manuscript. The severity of RILDs
was detected by JZH. JYZ analyzed and interpreted the patient data
regarding the intrahepatic tumors treated with EBRT. All patients
were followed up by YH. ZCZ made contributions to conception and
design of the research, and gave final approval of the version to
be published. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study followed international and national
regulations in accordance with the Declaration of Helsinki, and was
approved by the Zhongshan Hospital Research Ethics Committee. All
patients provided written consent for their inclusion in
research.
Consent for publication
Identifying information of patients in our
manuscript is essential for scientific purposes and will be treated
confidentially. Patients or guardians have provided written
informed consent for publication of any associated data and
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
TLR4
|
Toll-like receptor 4
|
|
RT
|
radiotherapy
|
|
TMA
|
tissue microarray
|
|
OS
|
overall survival
|
|
DFS
|
disease-free survival
|
|
RILD
|
radiation-induced liver disease
|
|
TRAIL
|
tumor necrosis factor-related
apoptosis-inducing ligand
|
|
VEGFR2
|
vascular endothelial growth factor
receptor 2
|
References
|
1
|
Stroescu C, Dragnea A, Ivanov B, Pechianu
C, Herlea V, Sgarbura O, Popescu A and Popescu I: Expression of
p53, Bcl-2, VEGF, Ki67 and PCNA and prognostic significance in
hepatocellular carcinoma. J Gastrointestin Liver Dis. 17:411–417.
2008.PubMed/NCBI
|
|
2
|
Price TR, Perkins SM, Sandrasegaran K,
Henderson MA, Maluccio MA, Zook JE, Tector AJ, Vianna RM, Johnstone
PA and Cardenes HR: Evaluation of response after stereotactic body
radiotherapy for hepatocellular carcinoma. Cancer. 118:3191–3198.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Law AL, Ng WT, Lee MC, Chan AT, Fung KH,
Li F, Lao WC and Lee AW: Treatment of primary liver cancer using
highly-conformal radiotherapy with kV-image guidance and
respiratory control. Radiother Oncol. 102:56–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen D, Wang R, Meng X, Yan H, Jiang S,
Feng R, Zhu K, Xu X, Dou X and Jin L: Prognostic value of serum
γ-glutamyl transferase in unresectable hepatocellular carcinoma
patients treated with transcatheter arterial chemoembolization
combined with conformal radiotherapy. Oncol Lett. 8:2298–2304.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Apetoh L, Ghiringhelli F, Tesniere A,
Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E,
Saulnier P, et al: Toll-like receptor 4-dependent contribution of
the immune system to anticancer chemotherapy and radiotherapy. Nat
Med. 13:1050–1059. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seki E and Brenner DA: Toll-like receptors
and adaptor molecules in liver disease: Update. Hepatology.
48:322–335. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hiratsuka S, Watanabe A, Sakurai Y,
Akashi-Takamura S, Ishibashi S, Miyake K, Shibuya M, Akira S,
Aburatani H and Maru Y: The S100A8-serum amyloid A3-TLR4 paracrine
cascade establishes a pre-metastatic phase. Nat Cell Biol.
10:1349–1355. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang B, Zhao J, Li H, He KL, Chen Y, Chen
SH, Mayer L, Unkeless JC and Xiong H: Toll-like receptors on tumor
cells facilitate evasion of immune surveillance. Cancer Res.
65:5009–5014. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu ZF, Zhou XH, Hu YW, Zhou LY, Gao YB,
Peng XH, Yang XH, Zhang JY, Hu Y and Zeng ZC: TLR4-dependant immune
response, but not hepatitis B virus reactivation, is important in
radiation-induced liver disease of liver cancer radiotherapy.
Cancer Immunol Immunother. 63:235–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhi-Feng W, Le-Yuan Z, Xiao-Hui Z, Ya-Bo
G, Jian-Ying Z, Yong H and Zhao-Chong Z: TLR4-dependent immune
response promotes radiation-induced liver disease by changing the
liver tissue interstitial microenvironment during liver cancer
radiotherapy. Radiat Res. 182:674–682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramacciato G, Mercantini P, Cautero N,
Corigliano N, Di Benedetto F, Quintini C, Ercolani G, Varotti G,
Ziparo V and Pinna AD: Prognostic evaluation of the new American
Joint Committee on Cancer/International Union Against Cancer
staging system for hepatocellular carcinoma: Analysis of 112
cirrhotic patients resected for hepatocellular carcinoma. Ann Surg
Oncol. 12:289–297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hodi FS, Ballinger M, Lyons B, Soria JC,
Nishino M, Tabernero J, Powles T, Smith D, Hoos A, McKenna C, et
al: Immune-modified response evaluation criteria in solid tumors
(imRECIST): Refining guidelines to assess the clinical benefit of
cancer immunotherapy. J Clin Oncol. 36:850–858. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma S, Jiao B and Liu X, Yi H, Kong D, Gao
L, Zhao G, Yang Y and Liu X: Approach to radiation therapy in
hepatocellular carcinoma. Cancer Treat Rev. 36:157–163. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Galle PR, Tovoli F, Foerster F, Wörns MA,
Cucchetti A and Bolondi L: The treatment of intermediate stage
tumours beyond TACE: From surgery to systemic therapy. J Hepatol.
67:173–183. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu H, Wu Q, Dang S, Jin M, Xu J, Cheng Y,
Pan M, Wu Y, Zhang C and Zhang Y: Alteration of CXCR7 expression
mediated by TLR4 promotes tumor cell proliferation and migration in
human colorectal carcinoma. PLoS One. 6:e273992011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Long Z, Wang B, Tao D, Liu Y, Zhang J, Tan
J, Luo J, Shi F and Tao Z: Clinical research on alternating
hyperfraction radiotherapy for massive hepatocellular carcinoma.
Oncol Lett. 10:523–527. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van der Schaaf A, Xu CJ, van Luijk P,
Van't Veld AA, Langendijk JA and Schilstra C: Multivariate modeling
of complications with data driven variable selection: Guarding
against overfitting and effects of data set size. Radiother Oncol.
105:115–121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng ZC, Tang ZY, Fan J, Zhou J, Qin LX,
Ye SL, Sun HC, Wang BL, Yu Y, Wang JH and Guo W: A comparison of
chemoembolization combination with and without radiotherapy for
unresectable hepatocellular carcinoma. Cancer J. 10:307–316. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamamoto M, Sato S, Hemmi H, Hoshino K,
Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K and
Akira S: Role of adaptor TRIF in the MyD88-independent toll-like
receptor signaling pathway. Science. 301:640–643. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmidt M, Raghavan B, Müller V, Vogl T,
Fejer G, Tchaptchet S, Keck S, Kalis C, Nielsen PJ, Galanos C, et
al: Crucial role for human Toll-like receptor 4 in the development
of contact allergy to nickel. Nat Immunol. 11:814–819. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu FH, Yuan Y, Li D, Liao SJ, Yan B, Wei
JJ, Zhou YH, Zhu JH, Zhang GM and Feng ZH: Extracellular HSPA1A
promotes the growth of hepatocarcinoma by augmenting tumor cell
proliferation and apoptosis-resistance. Cancer Lett. 317:157–164.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu P, Cheng X, Du Y, Huang L and Dong R:
TAK-242 can be the potential agents for preventing invasion and
metastasis of hepatocellular carcinoma. Med Hypotheses. 81:653–655.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eiró N, Altadill A, Juárez LM, Rodríguez
M, González LO, Atienza S, Bermúdez S, Fernandez-Garcia B,
Fresno-Forcelledo MF, Rodrigo L and Vizoso FJ: Toll-like receptors
3, 4 and 9 in hepatocellular carcinoma: Relationship with
clinicopathological characteristics and prognosis. Hepatol Res.
44:769–778. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Varnum SM, Springer DL, Chaffee ME, Lien
KA, Webb-Robertson BJ, Waters KM and Sacksteder CA: The effects of
low-dose irradiation on inflammatory response proteins in a 3D
reconstituted human skin tissue model. Radiat Res. 178:591–599.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gallet P, Phulpin B, Merlin JL, Leroux A,
Bravetti P, Mecellem H, Tran N and Dolivet G: Long-term alterations
of cytokines and growth factors expression in irradiated tissues
and relation with histological severity scoring. PLoS One.
6:e293992011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Cheung RM, Komaki R, Fang B and
Chang JY: Radiotherapy sensitization by tumor-specific TRAIL gene
targeting improves survival of mice bearing human non-small cell
lung cancer. Clin Cancer Res. 11:6657–6668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schenone S, Bondavalli F and Botta M:
Antiangiogenic agents: An update on small molecule VEGFR
inhibitors. Curr Med Chem. 14:2495–2516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Paz K and Zhu Z: Development of
angiogenesis inhibitors to vascular endothelial growth factor
receptor 2. Current status and future perspective. Front Biosci.
10:1415–1439. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Solberg TD, Nearman J, Mullins J, Li S and
Baranowska-Kortylewicz J: Correlation between tumor growth delay
and expression of cancer and host VEGF, VEGFR2, and osteopontin in
response to radiotherapy. Int J Radiat Oncol Biol Phys. 72:918–926.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng JC, Liu HS, Wu JK, Chung HW and Jan
GJ: Inclusion of biological factors in parallel-architecture
normal-tissue complication probability model for radiation-induced
liver disease. Int J Radiat Oncol Biol Phys. 62:1150–1156. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang BS, Tsang NM, Lin SM, Lin DY, Lien
JM, Lin CC, Chen WT, Chen WY and Hong JH: High-dose
hypofractionated X-ray radiotherapy for hepatocellular carcinoma:
Tumor responses and toxicities. Oncol Lett. 6:1514–1520. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lawrence D, Shahrokh Z, Marsters S,
Achilles K, Shih D, Mounho B, Hillan K, Totpal K, DeForge L, Schow
P, et al: Differential hepatocyte toxicity of recombinant
Apo2L/TRAIL versions. Nat Med. 7:383–385. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stewart M, Turley H, Cook N, Pezzella F,
Pillai G, Ogilvie D, Cartlidge S, Paterson D, Copley C, Kendrew J,
et al: The angiogenic receptor KDR is widely distributed in human
tissues and tumors and relocates intracellularly on
phosphorylation. An immunohistochemical study. Histopathology.
43:33–39. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hawkins MA and Dawson LA: Radiation
therapy for hepatocellular carcinoma: From palliation to cure.
Cancer. 106:1653–1663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pan CC, Kavanagh BD, Dawson LA, Li XA, Das
SK, Miften M and Ten Haken RK: Radiation-associated liver injury.
Int J Radiat Oncol Biol Phys. 76 3 Suppl:S94–S100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kondo Y, Kimura O and Shimosegawa T:
Radiation therapy has been shown to be adaptable for various stages
of hepatocellular carcinoma. World J Gastroenterol. 21:94–101.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shim SJ, Seong J, Han KH, Chon CY, Suh CO
and Lee JT: Local radiotherapy as a complement to incomplete
transcatheter arterial chemoembolization in locally advanced
hepatocellular carcinoma. Liver Int. 25:1189–1196. 2005. View Article : Google Scholar : PubMed/NCBI
|