Introduction
Multidrug resistance (MDR) is a major obstacle to
successful cancer chemotherapy. MDR is often associated with the
overexpression of the ATP binding cassette subfamily B member 1
(ABCB1, also known as MDR1) gene, which encodes
P-glycoprotein (P-gp) (1,2). P-gp expression enhances drug efflux pump
activity, depleting the intracellular concentration of anticancer
drugs, driving drug resistance. Various environmental stimuli,
including an inverted CCAAT box, can activate the MDR1
promoter (3–5), which directly interacts with
Y-box-binding protein (YB-1) (5–9). Assays
into MDR1 promoter activity using reporter genes
demonstrated that the nuclear translocation of YB-1 was able to
activate the MDR1 promoter (5,7),
contributing to the expression of MDR1 and to drug
resistance (5,9). In addition, the expression of
MDR1 was also observed in tumor specimens derived from
cancer patients (9–11). Therefore, the nuclear localization of
the YB-1 is a notable marker of disease progression (6). Moreover, multidrug resistance-associated
protein 1 (MRP1) is a protein encoded by the ABCC1 gene in human,
which may interacted with MDR1 to regulate the drug resistant as
well (12). However, a previous study
revealed that the amount of nuclear YB-1 was not associated with
MDR1 expression (13). The
mechanism of YB-1 nuclear translocation is presently unclear,
meaning that further research is required.
The sesquiterpene lactone 6-O-angeloylplenolin, a
bioactive component of Centipeda minima (L.) A (14), has been reported to have various
biological activities, including antiprotozoal and antibacterial
activities (15). It was previously
reported that 6-O-angeloylplenolin induced apoptosis through a
mitochondrial/caspase pathway in acute promyelocytic leukemia HL-60
cells (16); it was also reported to
trigger mitotic arrest and caspase-dependent apoptosis in human
multiple myeloma cells (17). The
result of proteome microarray analysis revealed that
6-O-angeloylplenolin inhibited S-phase kinase-associated protein 1
(Skp1) and signal transducer and activator of transcription 3 to
repress Skp2, exhibiting inhibitory effects on lung cancer cell
proliferation (18). In addition, it
was reported that a number of sesquiterpene lactones could bind
specifically to human P-gp and reverse cellular multidrug
resistance (19). However, the
detailed mechanism behind the 6-O-angeloylplenolin-dependent rescue
of drug resistance in cancer was not revealed. The present study
demonstrated that 6-O-angeloylplenolin exhibited MDR-reversing
activity in colon carcinoma cells and attempted to investigate the
underlying mechanisms behind this reversal.
Materials and methods
Chemicals, cell lines and cell
culture
6-O-angeloylplenolin (also known as brevifolin or
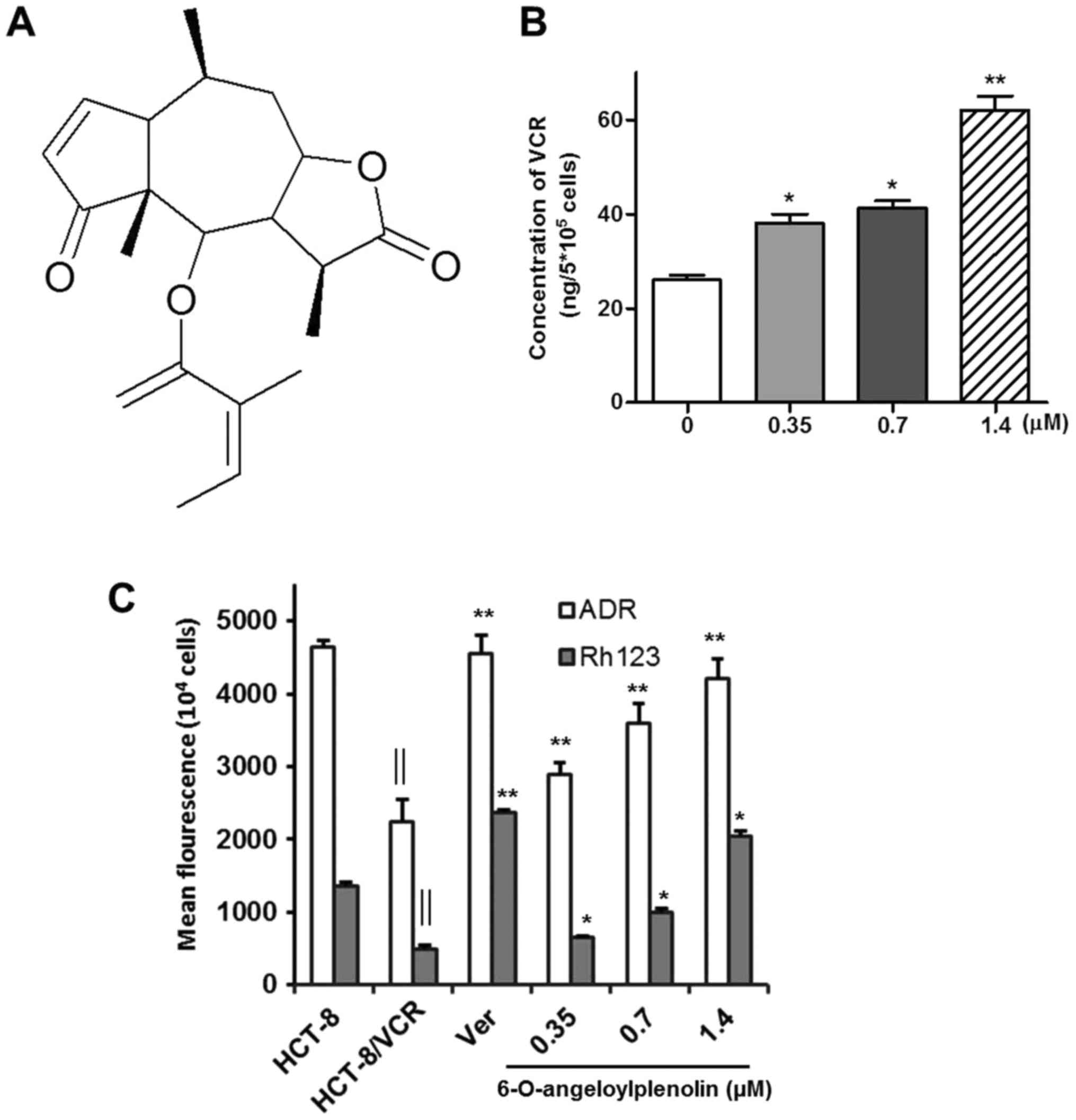
brevilin-A) (Fig. 1A) was isolated
from C. minima in Capital Medical University (Beijing
China), as described previously (17). The purity of 6-O-angeloylplenolin was
>97% (HPLC). The human colon carcinoma HCT-8 cell line and its
MDR variant HCT-8/VCR were obtained from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
Cells were maintained in RPMI 1640 (Life Technologies; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (Life Technologies; Thermo
Fisher Scientific, Inc.), penicillin (100 ng/ml) and streptomycin
(100 ng/ml) in a humidified atmosphere with 5% CO2 at
37°C. HCT-8/VCR cells were grown under the same conditions, but the
medium contained 2 mg/l vincristine (Sigma Aldrich; Merck KGaA,
Darmstadt, Germany), which was removed for 1 week prior to assay
commencement. In terms of cell proliferation and cell cycle
distribution, there was no significant difference between these two
cell lines.
MTT assay
The MTT colorimetric assay was performed as
described by Mosman et al (20). HCT-8 or HCT-8/VCR cells were seeded
into 96-well plates at a density of 1×104 cells/well.
The cytotoxicity of 6-O-angeloylplenolin, vincristine, mitomycin
(Sigma Aldrich; Merck KGaA), hydroxycamptothecin (Sigma Aldrich;
Merck KGaA) or the compounds in combinations in HCT-8 and HCT-8/VCR
cells was analyzed by MTT assay following incubation of cells with
these compounds for 24 h. The concentrations of vincristine,
mitomycin or hydroxycamptothecin were 1, 3, 10, 30, 100 and 300
µg/ml. The cytotoxicity was expressed as the half-maximal
inhibitory concentration (IC50), which was extrapolated
from linear regression analysis of experimental data.
Detection of intracellular vincristine
accumulation
High-performance liquid chromatography (HPLC) was
used to measure the accumulation of drugs in cells and tissue, as
described previously (21). HCT-8/VCR
cells were incubated with 6-O-angeloylplenolin for 24 h and then
the cells were treated with 80 µg/ml vincristine for 12 h. A total
of 1×106 cells were collected and centrifuged at 4°C for
10 min at 15,996 × g. The supernatant was discarded. The cell
pellet was resuspended and lysed (Cell Signaling Technology, Inc.
Danvers, MA, USA) for 10 min at 4°C. A total of 50 µl cell lysate
samples were taken for HPLC evaluation. The concentrations of
vincristine were determined by HPLC as described by Chinese
pharmacopoeia (22). Briefly,
separation was performed on a C18 column (Eclipse
C18, 150 by 4.6 mm, with a particle size of 5 µm;
Agilent Technologies, Inc., Santa Clara, CA, USA) at 20°C. The
mobile phase was a 55:45 (v/v) mixture of methanol and 60 mM sodium
phosphate buffer (pH 3.0). The UV detector was set at wavelength of
297 nm. Data were expressed as the ratio of the peak area to that
of the internal standard.
Adriamycin and Rh123 accumulation
assay
Accumulation of the fluorescent compounds adriamycin
(Sigma-Aldrich; Merck KGaA) and Rh123 (0.5 mg/ml; Sigma-Aldrich;
Merck KGaA) were used as a functional index of P-gp, as described
previously (5,23). HCT-8/VCR cells (1×104
cells/well) were first treated with 0.35, 0.7 and 1.4 µM
6-O-angeloylplenolin for 24 h. Next, one group of cells were
treated with 12 µM verapamil for 12 h, which was used as the
positive control (24). Following
this, the cells were incubated in medium containing 50 µM
adriamycin and 10 µM Rh123 for 3 h. Finally, the cells were washed
twice with PBS prior to measurement. The fluorescence intensity of
were detected by fluorospectrophotometer (Synergy 2, BioTek
Instruments, Inc., Winooski, VT, USA) for Rh123 at 530 nm
(excitation wavelength, 500 nm) and adriamycin at 530 nm
(excitation wavelength, 485 nm).
Determination of gene expression by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
HCT-8/VCR cells were treated with 1.4 µM
6-O-angeloylplenolin for 12 h. Total RNA was isolated by RNeasy
Mini kit 250 (cat. no. 74106; Qiagen GmbH, Hilden, Germany) from
HCT-8 or HCT-8 cells. QuantiTect Reverse Transcription kit 200
(cat. no. 205313; Qiagen GmbH) was used to synthesize complementary
DNA (cDNA), according to manufacturer's protocol. The synthesized
cDNA was subjected to RT-qPCR using QuantiTect SYBR®
Green RT-PCR kit (Qiagen GmbH) on a Bio-Rad CFX Connect real-time
PCR detection system (Bio-Rad Laboratories, Hercules, CA, USA) with
conditions of 30 cycles of 40 sec at 95°C, 40 sec at 60°C and 30
sec at 72°C. The primers used for PCR were shown below: MDR1
forward, 5′-CGAAACCGTATCAGTCCTCG-3′ and reverse,
5′-CTTGAGTCTGAGAGACCACC-3′ (25);
MRP1 forward, 5′-GTGGAATTCCGGAACTAC-3′ and reverse,
5′-CGGAGGTCGTGCAGGCCG-3′ (24);
YBX1 forward, 5′-CGGAGGCAGCAAATGTTA-3′ and reverse,
5′-CACCCTGGTTGTCAGCAC-3′ (4); and
GAPDH forward, 5′-GTCAACGGATTTGGTCGTATT-3′ and reverse,
5′-AGTCTTCTGGGTGGCAGTGAT-3′. The PCR products were separated by
electrophoresis on an 1.5% agarose gel and stained with 0.1 µg/ml
ethidium bromide. Differential gene expression was quantified using
the Image Analysis system, version 3 (FR-980A gel Image Analysis
System, Shanghai Furi Science and Technology Co., Ltd., Shanghai,
China).
Flow cytometry assay
HCT-8/VCR cells were treated with 0.35, 0.7 or 1.4
µM 6-O-angeloylplenolin for 24 h, and flow cytometry was performed.
A total of 1×105 cells/ml cells were washed in PBS with
0.1% sodium azide (PBS-azide). The cells were firstly blocked with
3% Bovine Serum Albumin (BSA) at room temperature for 1 h. Then,
HCT-8/VCR cells were incubated with 2 µg monoclonal antibodies
against P-gp (Aviva Systems Biology, Corp., San Diego, CA, USA;
cat. no. APR51326_P050) at a dilution of 1:100 for 2h at room
temperature. Next, cells were washed twice with PBS and incubated
with 1 µg goat anti-rabbit secondary antibodies (1:10,000)
conjugated with fluorescein isothiocyanate (FITC) (Rockland
Immunochemicals, Inc., Limerick, PA, USA; cat. no. 611-1102) at
room temperature for 30 min in dark. Over 2×104 events
were acquired and analyzed by a FACScan flow cytometer with
CellQuest software (version 5.1; BD Biosciences, Franklin Lakes,
NJ, USA).
Western blot analysis
Following treatment with 0.35, 0.7 or 1.4 µM
6-O-angeloylplenolin for 12 h, the cytosolic and nuclear proteins
in HCT-8/VCR cells were extracted described previously (26). A total of 20 µg total cytosolic and
nuclear proteins were separated by 12% SDS-PAGE. Following
electrophoresis, the proteins were transferred to a polyvinylidene
difluoride (PVDF) membrane, which were blocked with 3% BSA at room
temperature for 1 h. The PVDF membranes were incubated with
anti-YB-1 (monoclonal rabbit IgG; Cell Signaling Technology, Inc.)
or rabbit anti-GAPDH antibody (dilution, 1:5,000; Sigma-Aldrich,
Merck KGaA; cat. no. PLA0125) antibodies at 4°C for overnight. The
primary antibodies were detected with anti-rabbit IgG conjugated to
alkaline phosphatase (1:3,000 dilutions, Bio-Rad Laboratories,
Inc., Hercules, CA, USA; cat. no. 170-6518) to generate a BCIP/NBT
(Promega Corporation, Madison, WI, USA; cat. no. S3771) signal. Gel
analysis was performed with Image Lab 3.0 software (Bio-Rad
Laboratories, Inc.).
Transient transfection and luciferase
assay
Plasmid construction was performed as described
previously (7,27). HCT-8/VCR cells were seeded into
six-well plates (3×105 cells/well) and incubated for 24
h before transfection. Using Lipofect Transfection reagent (Beijing
Tiangen Biotech Co., Ltd., Beijing, China), the cells were
co-transfected with 4 µg/well plasmid pGL3-MDR1 (Promega
Corporation, Madison, WI, USA). The sequence for MDR1 promoter was:
Forward, 5′-CTAGAGAGGTGCAACGGA-3′ (−198 to −181) and reverse,
5′-GCGGCCTCTGCTTCTTTGA-3′ (+25 to +43). Next, the cells were
treated with 0.35, 0.7 or 1.4 µM 6-O-angeloylplenolin for 24 h.
Firefly and Renilla luciferase activities in
1×104 cells/well were measured using the Dual-Luciferase
Assay system (Promega Corporation) using a Microplate Luminometer
(Berthold Technologies GmbH, Bad Wildbad, Germany).
Electrophoretic mobility shift assay
(EMSA)
HCT-8/VCR (3×105 cells/well) cells were
treated with 0.35, 0.7 or 1.4 µM 6-O-angeloylplenolin for 12 h and
the EMSA was performed as described by Han et al (24). The DNA sequence of the ds-oligomer
used was a CAAT-like motif (5′-ATCAGCATTCAGTCAATCCGGGCC-3′)
(5,7).
The gels were stained using the EMSA kit (Invitrogen; Thermo Fisher
Scientific, Inc.) as described by the manufacturer and images were
captured using an Olympus Standard fluorescence microscope (Olympus
Corporation, Tokyo, Japan). The magnification used was ×100.
Animal study
Nude mice (BALB/c; female; age, 6–8 week; 20–22 g;
n=5) were provided by Vital River Laboratories Co., Ltd. (Beijing,
China), which were housed in barrier facilities on a 12-h
light/dark cycle at 23–25°C with free access to food and water.
Each mouse was subcutaneously injected with 5×106
HCT-8/VCR cells in the left dorsal flank. When the tumor reached
about 150 mm3, the mice were randomly assigned to 5
groups and received the following treatments for 12 days: Control
(PBS), 6-O-angeloylplenolin (7 mg/kg per day, oral), VCR (2.5 mg/kg
per day, oral), VCR + 6-O-angeloylplenolin (3.5 mg/kg per day,
oral), VCR + 6-O-angeloylplenolin (7 mg/kg per day, oral).
Following 12 days, the tumors were isolated and weighed. The tumor
growth inhibitory rate (IR) was calculated as follows: IR
(%)=1-(TWtreated/TWcontrol)×100. The protocol
was approved by the Committee on the Ethics of Animal Experiments
of the Capital Medical University.
Statistical analysis
Statistical analysis was performed using one-way
analysis of variance followed by Dunnett's test (version 5.0;
GraphPad Software, Inc., La Jolla, CA, USA). All results are
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
MDR-reversing effect of
6-O-angeloylplenolin
HCT-8 and HCT-8/VCR cells were incubated with
vincristine, mitomycin and hydroxycamptothecin for 24 h and
subjected to MTT assays to assess cell viability. HCT-8/VCR cells
were significantly more resistant to vincristine,
6-O-angeloylplenolin, mitomycin and hydroxycamptothecin than HCT-8
cells (Table I). Treatment with 1.4
µM 6-O-angeloylplenolin had no significant effect on the viability
of HCT-8/VCR cells. Therefore, the concentration of
6-O-angeloylplenolin was applied to indicate a reversal of
resistance. HCT-8/VCR cells were co-incubated with 1.4 µM
6-O-angeloylplenolin and one of three anti-cancer drugs
vincristine, mitomycin or hydroxycamptothecin for 24 h. The
concentrations of vincristine, mitomycin or hydroxycamptothecin
were 1, 3, 10, 30, 100 and 300 µg/ml. The results of the MTT assay
revealed that 1.4 µM 6-O-angeloylplenolin significantly increased
the cytotoxicity of each of the three anticancer drugs on HCT-8/VCR
cells (Table I).
 | Table I.Cytotoxicity of drugs in HCT-8 and
HCT-8/VCR cells by MTT assay. |
Table I.
Cytotoxicity of drugs in HCT-8 and
HCT-8/VCR cells by MTT assay.
|
|
IC50 |
|---|
|
|
|
|---|
| Cell line | VCR, µg/ml | HEPT, µg/ml | MIT, µg/ml |
6-O-angeloylplenolin, µM |
|---|
| HCT-8 | 12.7±0.59 | 23.3±0.47 | 1.1±0.11 | 5.97±0.47 |
| HCT-8/VCR |
250±8.45a |
40±0.35a |
3.9±0.15a |
24.21±0.64a |
| HCT-8/VCR +
6-O-angeloylplenolin |
13.8±0.78b |
18.5±0.15b |
0.48±0.02b | − |
Treatment with 6-O-angeloylplenolin
increases the intracellular accumulation of vincristine, adriamycin
and Rh123
Inhibiting drug efflux and increasing the
intracellular accumulation of drugs is an effective way of
overcoming drug resistance (24). To
investigate whether 6-O-angeloylplenolin increased the
intracellular accumulation of vincristine, adriamycin and Rh123 in
HCT-8/VCR cells, HPLC and fluorescence intensity assays were
performed. The results indicated that 6-O-angeloylplenolin
significantly increased the intracellular accumulation of
vincristine, adriamycin and Rh123 in HCT-8/VCR cells following
treatment with 6-O-angeloylplenolin for 24 h (Fig. 1B and C; P<0.01).
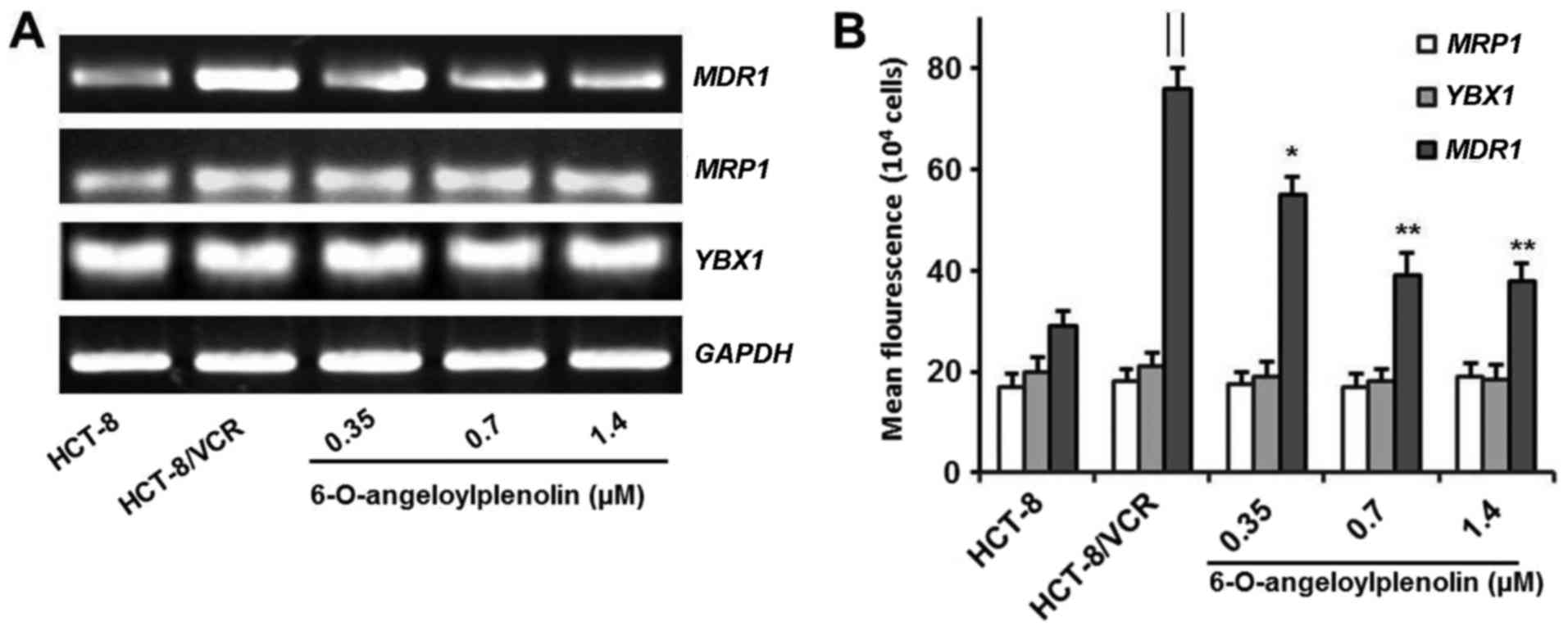
Treatment with 6-O-angeloylplenolin
does not alter the expression of MRP1 and YB-1
It is reported that vincristine is susceptible to
common mechanisms of multidrug resistance, including the
overexpression of P-gp (28). Here,
6-O-angeloylplenolin did not affect the expression of MRP1
in colon cancer cells. This result indicated that
6-O-angeloylplenolin reversed vincristine resistance by suppressing
MDR1 expression. Moreover, 6-O-angeloylplenolin had no
effect on the expression of YBX1, which encodes YB-1
(Fig. 2A and B). These results
indicated that 6-O-angeloylplenolin suppressed YB-1 nuclear
translocation, but not YB-1 expression.
Treatment with 6-O-angeloylplenolin
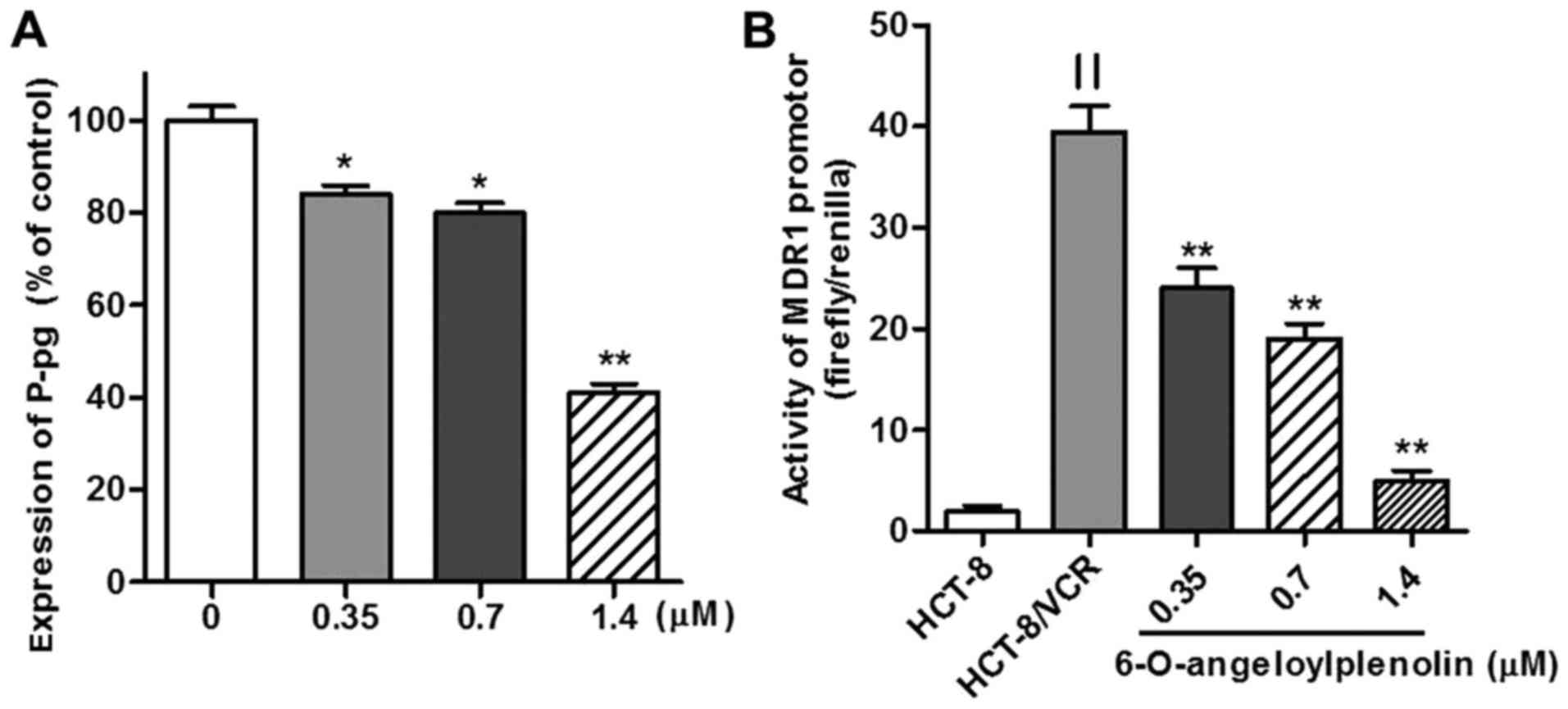
downregulates expression of MDR1 mRNA and protein
The overexpression of MDR1 and its protein
product have been associated with the MDR phenotype (1). Therefore, the expression of MDR1
mRNA was assessed by RT-PCR and flow cytometry analysis. Whether
6-O-angeloylplenolin could downregulate the expression of
MDR1 in HCT-8/VCR cells was examined. The results of this
analysis revealed that 6-O-angeloylplenolin decreased MDR1
expression in HCT-8/VCR cells (Fig.
2), in parallel with a reduction of the protein expression of
MDR1 (Fig. 3A).
Treatment with 6-O-angeloylplenolin
suppresses MDR1 promoter activity
To determine whether the CAAT segment of the
MDR1 promoter was regulated by 6-O-angeloylplenolin or not,
the wild-type MDR1 promoter (residues −198 to +43,241 bp)
DNA fragment was cloned into a luciferase-expressing pGL3-basic
vector to construct pGL3-MDR, as described previously (27). The data revealed that there was an
18-fold increase in MDR1 promoter activity in HCT-8/VCR
cells transiently transfected with dual-reporter gene vectors,
compared with the level in HCT-8 (Fig.
3B). MDR1 promoter activity was significantly decreased
in HCT-8/VCR cells following incubation with 6-O-angeloylplenolin
for 24 h (Fig. 3B).
6-O-angeloylplenolin suppresses YB-1
nuclear translocation
Whether the expression and localization of YB-1 is
associated with the expression of MDR1 gene is key to
understanding the mechanism of P-gp action. To confirm the effect
of 6-O-angeloylplenolin on YB-1 nuclear translocation, western blot
analysis was performed. Compared with HCT-8 cells, nuclear
translocation of YB-1 was enhanced in HCT-8/VCR cells. The data
revealed that 6-O-angeloylplenolin effectively inhibited the
nuclear translocation of YB-1 in HCT-8/VCR cells (Fig. 4A-C).
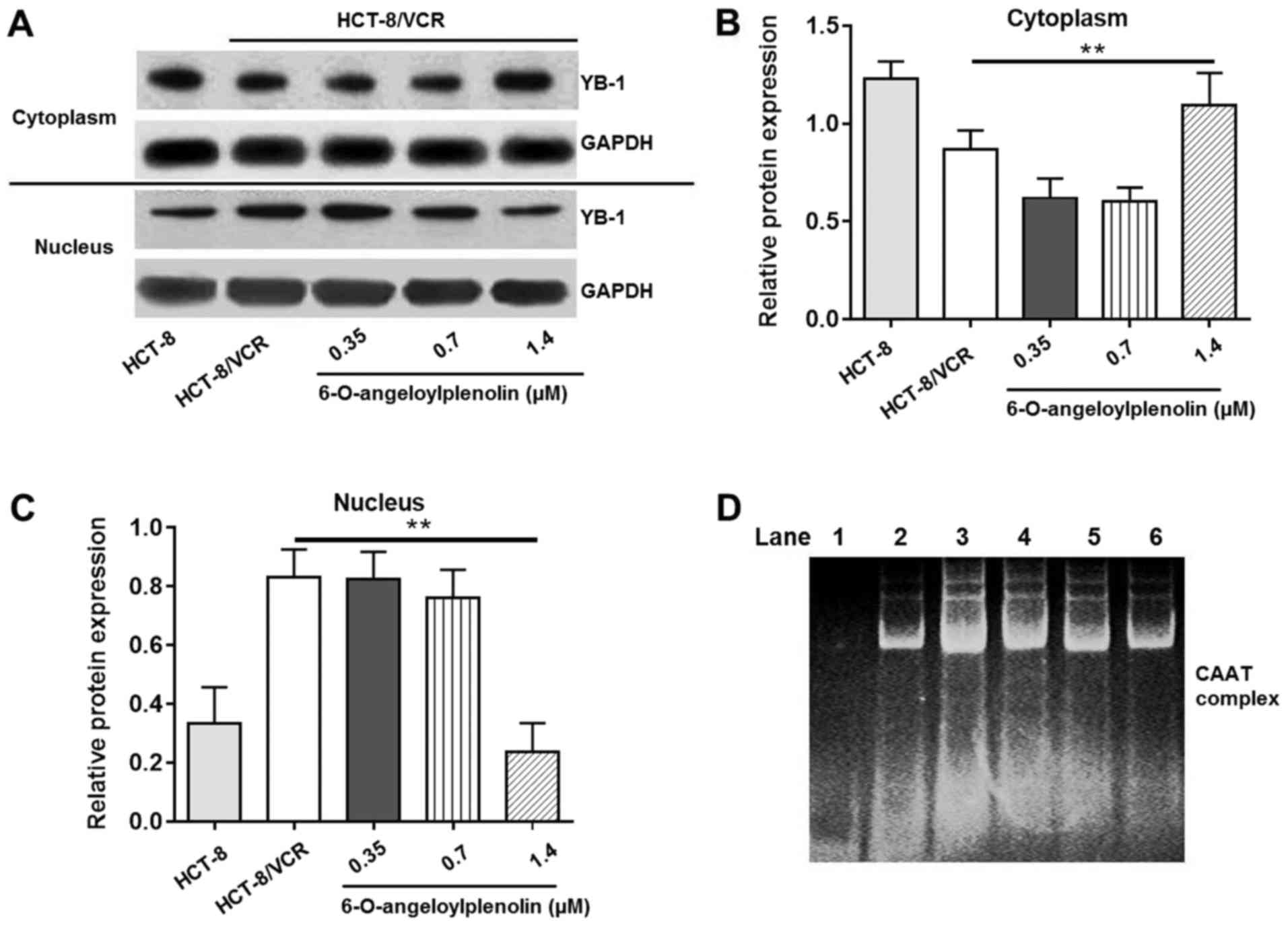 | Figure 4.Effect of 6-O-angeloylplenolin on
YB-1 nuclear translocation and interaction between the nuclear
protein and the CAAT region of the MDR1 promoter. (A) The
cytoplasm and nucleolus protein were extracted following incubation
with 6-O-angeloylplenolin for 12 h, resolved using SDS-PAGE, and
western blot analysis was performed with the indicated antibodies.
GAPDH was used as the loading control. (B) The quantification of
the YB-1 protein expression in the cytoplasm. (C) Quantification of
the YB-1 protein expression in cytoplasm. **P<0.01 vs. untreated
HCT-8/VCR cells. (D) The nucleolar protein was extracted following
incubation with 6-O-angeloylplenolin for 12 h, and the amount of
complexed nuclear protein and DNA were measured using an
electrophoretic mobility shift assay. Lane 1, only nuclear
extracts; lane 2, probe incubated with nuclear extracts of HCT-8
cells; lane 3, probe incubated with nuclear extracts of HCT-8/VCR
cells; lane 4, probe incubated with nuclear extracts of HCT-8/VCR
cells following treatment with 0.35 µM 6-O-angeloylplenolin; lane
5, probe incubated with nuclear extracts of HCT-8/VCR cells
following treatment with 0.7 µM 6-O-angeloylplenolin; lane 6, probe
incubated with nuclear extracts of HCT-8/VCR cells following
treatment with 1.4 µM 6-O-angeloylplenolin. YB-1, Y-box binding
protein 1. |
6-O-angeloylplenolin downregulates
MDR1 expression by decreasing binding of MDR1 promoter with nuclear
transcription factors
A number of studies (29,30) have
provided evidence implicating complex mechanisms for the
transcriptional regulation of MDR1 in human cancer cells.
Since the present study demonstrated that 6-O-angeloylplenolin
could inhibit the expression of MDR1 mRNA, it was necessary
to examine whether 6-O-angeloylplenolin had any effect on
MDR1 promoter. An EMSA was performed using a probe from the
MDR1 promoter sequence, −86 to −67 bp. The amount of
specific protein complex interacting with the probe was lower in
the HCT-8 cells than in HCT-8/VCR cells (Fig. 4D; lanes 2–3). Treatment with
6-O-angeloylplenolin decreased the number of protein/DNA complexes
in HCT-8/VCR cells (Fig. 4B, lanes
3–6). This result indicated that 6-O-angeloylplenolin inhibited the
binding of nuclear proteins to the CAAT region of the MDR1
promoter in HCT-8/VCR cells, which could result in the inhibition
of MDR1 expression.
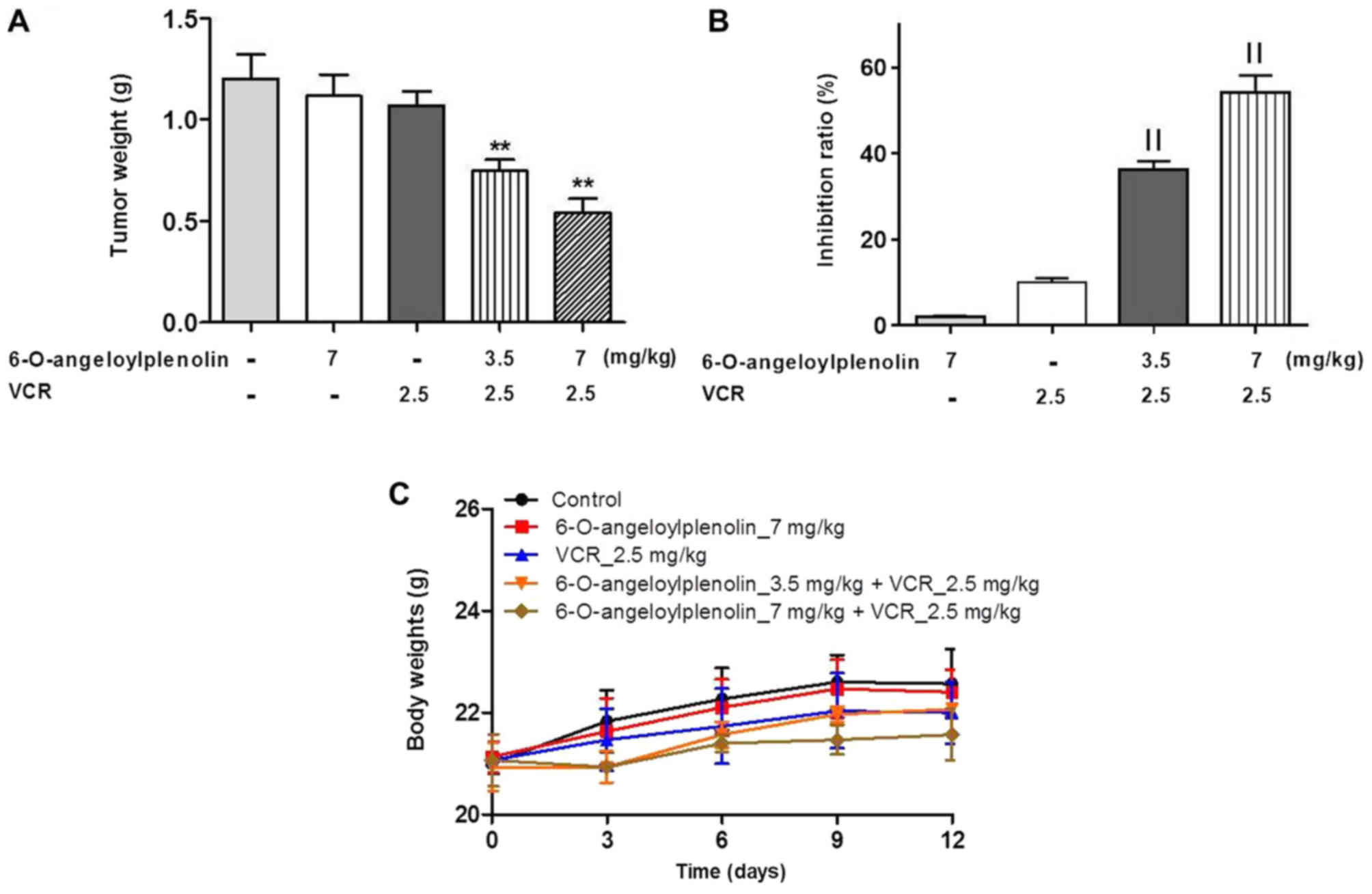
6-O-angeloylplenolin reverses VCR
resistance in HCT/VCR xenograft model
To evaluate whether 6-O-angeloylplenolin could
reverse vincristine resistance in vivo, a HCT/VCR xenograft
model was established by injecting HCT/VCR cells into the left
dorsal flanks of nude mice. The data demonstrated that
6-O-angeloylplenolin or vincristine treatment alone had no effect
on tumor growth. However, the combination of 6-O-angeloylplenolin
with vincristine significantly inhibited tumor growth (Fig. 5A and B). In addition, the body weights
of mice were stable, indicating the combination treatments were
tolerable (Fig. 5C).
Discussion
Resistance to chemotherapeutics remains a major
cause of cancer treatment failure. Thus, in addition to
investigating more efficient therapeutic drugs, there is a
requirement to develop compounds to inhibit MDR activity or to
synergize with existing treatments. The current study revealed that
6-O-angeloylplenolin treatment reversed vincristine resistance in
HCT-8/VCR cells, increasing the intracellular accumulation. MDR is
often associated with the overexpression of MDR1, which
causes the enhancement of drug efflux pump activity and drug
resistance (29). Treatment with
6-O-angeloylplenolin inhibited the expression or function of P-gp,
data that were further supported by RT-PCR, flow cytometry and
promoter activity analysis.
Several approaches to overcoming MDR have been
proposed (12,28,29); of
them, inhibition of MDR-associated genes has promise. P-gp is
encoded by MDR1, which was investigated in the present
study. The expression of MDR1 was depleted following
6-O-angeloylplenolin administration. Besides P-gp, MRP1 is another
protein that is important to MDR; it is encoded by MRP1. The
results of the present study indicated that the mRNA level of
MRP1 was unchanged following 6-O-angeloylplenolin treatment
(Fig. 2) and that
6-O-angeloylplenolin exerts its main effect via regulating the
expression of MDR1 in vitro.
Previous reports have demonstrated that YB-1
activity is closely associated with the expression of MDR1 in
vivo and in vitro (5–9). Treatment
with 6-O-angeloylplenolin decreased the level of YB-1 in nucleus
and complexes of this nuclear protein with MDR1 promoter in
a dose-dependent manner. However, 6-O-angeloylplenolin regulating
the expression of YBX1 on mRNA level was not observed.
Therefore, 6-O-angeloylplenolin regulated the expression of MDR1 by
inhibiting YB-1 nuclear translocation, not by depressing its
expression. These findings differ from previous reports (9,12), which
might be due to the different tumor type. Therefore, these novel
results shed light on the mechanism of chemotherapy resistance in
colon cancer. These results provide evidence for the combination
use of 6-O-angeloylplenolin with vincristine in patients with
refractory colon cancer. However, there were certain limitations to
the current study. The detailed interaction between YB1 and MDR1
following 6-O-angeloylplenolin treatment in colon cancer was not
clarified. Future studies should focus on the protein kinase
B-nuclear factor-κB-YB-1-MDR1 and tumor protein p53-YB-1-MDR1
signaling pathways in the future.
Taken together, the results of the present study
indicated that 6-O-angeloylplenolin displayed a significant
antitumor activity by reserving drug resistance. The effect of
6-O-angeloylplenolin was exerted via inhibition of the
intracellular accumulation of YB-1 and the expression of
MDR1, resulting in a decrease in efflux pump activity. These
results demonstrated that 6-O-angeloylplenolin may represent a
potential anticancer drug adjuvant with the potential to reverse
drug resistance.
Acknowledgements
Not applicable.
Funding
The present study was funded by: Key Projects in the
National Science & Technology Pillar Program (grant no.
2015BAI09B01), National Science Foundation of China (grant no.
31572348) and Support Project of High-level Teachers in Beijing
Municipal Universities in Period of 13th Five-year Plan (grant no.
IDHT20170516).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL was mainly responsible for study design, data
analysis, manuscript development and conduction of experiments,
including cell culture, MTT assay, RT-PCR, and the accumulation
assay of vincristine, adriamycin and Rh123. HW performed flow
cytometry assay of HCT-8/VCR cells, western blot analysis, and
assisted with statistical analysis. YY helped with transient
transfection and luciferase assay. JL was involved in
electrophoretic mobility shift assay and animal study. ZC performed
experiments and reviewed and revised the manuscript.
Ethics approval and consent to publish
Ethics approval for animal study was given by the
local research ethics committee at East China University of Science
and Technology.
Consent for publication
There are no human participants, human data or human
tissue involved in this manuscript.
Competing interests
The authors declare no competing financial
interests.
References
|
1
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ambudkar SV, Dey S, Hrycyna CA,
Ramachandra M, Pastan I and Gottesman MM: Biochemical, cellular,
and pharmacological aspects of the multidrug transporter. Annu Rev
Pharmacol Toxicol. 39:361–398. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kohno K, Sato S, Uchiumi T, Takano H,
Tanimura H, Miyazaki M, Matsuo K, Hidaka K and Kuwano M: Activation
of the human multidrug resistance 1 (MDR1) gene promoter in
response to inhibitors of DNA topoisomerases. Int J Oncol. 1:73–77.
1992.PubMed/NCBI
|
|
4
|
Uchiumi T, Kohno K, Tanimura H, Matsuo K,
Sato S, Uchida Y and Kuwano M: Enhanced expression of the human
multidrug resistance 1 gene in response to UV light irradiation.
Cell Growth Differ. 4:147–157. 1993.PubMed/NCBI
|
|
5
|
Stein U, Jürchott K, Walther W, Bergmann
S, Schlag PM and Royer HD: Hyperthermia-induced nuclear
translocation of transcription factor YB-1 leads to enhanced
expression of multidrug resistance-related ABC transporters. J Biol
Chem. 276:28562–28569. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuwano M, Oda Y, Izumi H, Yang SJ, Uchiumi
T, Iwamoto Y, Toi M, Fujii T, Yamana H, Kinoshita H, et al: The
role of nuclear Y-box binding protein 1 as a global marker in drug
resistance. Mol Cancer Ther. 3:1485–1492. 2004.PubMed/NCBI
|
|
7
|
Ogretmen B and Safa AR: Identification and
characterization of the MDR1 promoter-enhancing factor 1 (MEF1) in
the multidrug resistant HL60/VCR human acute myeloid leukemia cell
line. Biochemistry. 39:194–204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vaiman AV, Stromskaya TP, Rybalkina EY,
Sorokin AV, Guryanov SG, Zabotina TN, Mechetner EB, Ovchinnikov LP
and Stavrovskaya AA: Intracellular localization and content of YB-1
protein in multidrug resistant tumor cells. Biochemistry (Mosc).
71:146–154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bargou RC, Jürchott K, Wagener C, Bergmann
S, Metzner S, Bommert K, Mapara MY, Winzer KJ, Dietel M, Dörken B
and Royer HD: Nuclear localization and increased levels of
transcription factor YB-1 in primary human breast cancers are
associated with intrinsic MDR1 gene expression. Nat Med. 3:447–450.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujita T, Ito K, Izumi H, Kimura M, Sano
M, Nakagomi H, Maeno K, Hama Y, Shingu K, Tsuchiya S, et al:
Increased nuclear localization of transcription factor Y-box
binding protein 1 accompanied by up-regulation of P-gp in breast
cancer pretreated with paclitaxel. Clin Cancer Res. 11:8837–8844.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saji H, Toi M, Saji S, Koike M, Kohno K
and Kuwano M: Nuclear expression of YB-1 protein correlates with
P-glycoprotein expression in human breast carcinoma. Cancer Lett.
190:191–197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cole SP, Bhardwaj G, Gerlach JH, Mackie
JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM and
Deeley RG: Overexpression of a transporter gene in a
multidrug-resistant human lung cancer cell line. Science.
258:1650–1654. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaszubiak A, Kupstat A, Müller U, Hausmann
R, Holm PS and Lage H: Regulation of MDR1 gene expression in
multidrug-resistant cancer cells is independent from YB-1. Biochem
Biophys Res Commun. 357:295–301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan CO, Jin DP, Dong NP, Chen SB and Mok
DK: Qualitative and quantitative analysis of chemical constituents
of Centipeda minima by HPLC-QTOF-MA& HPLC-DAD. J Pharm
Biomed Anal. 125:400–407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taylor RS and Towers GH: Antibacterial
constituents of the Nepalese medicinal herb, Centipeda
minima. Phytochemistry. 47:631–634. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li CL, Wu HZ, Huang YP, Yang YF, Liu YW
and Liu JW: Brevilin A induces apoptosis through a
mitochondrial/caspase and NF-κB pathway in human leukemia HL-60
cells. Biomed Pharmacother. 62:401–409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu YQ, Wang XL, Cheng X, Lu YZ, Wang GZ,
Li XC, Zhang J, Wen ZS, Huang ZL, Gao QL, et al: Skp1 in lung
cancer: Clinical significance and therapeutic efficacy of its small
molecule inhibitors. Oncotarget. 6:34953–34967. 2015.PubMed/NCBI
|
|
18
|
Liu Y, Chen XQ, Liang HX, Zhang FX, Zhang
B, Jin J, Chen YL, Cheng YX and Zhou GB: Small compound
6-O-angeloylplenolin induces mitotic arrest and exhibits
therapeutic potentials in multiple myeloma. PLoS One. 6:e219302011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Muñoz-Martínez F, Lu P, Cortés-Selva F,
Pérez-Victoria JM, Jiménez IA, Ravelo AG, Sharom FJ, Gamarro F and
Castanys S: Celastraceae sesquiterpenes as a new class of
modulators that bind specifically to human P-glycoprotein and
reverse cellular multidrug resistance. Cancer Res. 64:7130–7138.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mosman T: Rapid colorimetric assay for
cellular growth and survival, application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Q, Wei DZ and Liu JW: In vivo
reversal of doxorubicin resistance by (−)-epigallocatechin gallate
in a solid human carcinoma xenograft. Cancer Lett. 208:179–186.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Pharmacopoeia Committee: National
Pharmacopoeia Committee Pharmacopoeia of the People's Republic of
China. 1. Chemical Industry Press; pp. 2721Beijing: 2015
|
|
23
|
Wei D, Mei Y and Liu J: Quantification of
doxorubicin and validation of reversal effect of tea polyphenols on
multidrug resistance in human carcinoma cells. Biotechnol Lett.
25:291–294. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han SO, Inui M and Yukawa H: Expression of
Corynebacterium glutamicum glycolytic genes varies with carbon
source and growth phase. Microbiology. 153:2190–2202. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Constable PA, Lawrenson JG, Dolman DE,
Arden GB and Abbott NJ: P-Glycoprotein expression in human retinal
pigment epithelium cell lines. Exp Eye Res. 83:24–30. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Braga F, Ayres-Saraiva D, Gattass CR and
Capella MA: Oleanolic acid inhibits the activity of the multidrug
resistance protein ABCC1 (MRP1) but not of the ABCB1
(P-glycoprotein): Possible use in cancer chemotherapy. Cancer Lett.
248:147–152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu ST, Chen TM, Tseng SY and Chen YH:
Tryptanthrin inhibits MDR1 and reverses doxorubicin resistance in
breast cancer cells. Biochem Biophys Res Commun. 358:79–84. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boháčová V, Sulová Z, Dovinová I, Dovinová
I, Poláková E, Barančík M, Uhrík B, Orlický J and Breier A: L1210
cells cultivated under the selection pressure of doxorubicin or
vincristine express common mechanisms of multidrug resistance based
on the overexpression of P-glycoprotein. Toxicol In Vitro.
20:1560–1568. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H, Jing X, Wu X, Hu J, Zhang X, Wang
X, Su P, Li W and Zhou G: Suppression of multidrug resistance by
rosiglitazone treatment in human ovarian cancer cells through
downregulation of FZD1 and MDR1 genes. Anticancer Drugs.
26:706–715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yun M, Lee D, Park MN, Kim EO, Sohn EJ,
Kwon BM and Kim SH: Cinnamaldehyde derivative (CB-PIC) sensitizes
chemo-resistant cancer cells to drug-induced apoptosis via
suppression of MDR1 and its upstream STAT3 and AKT signaling. Cell
Physiol Biochem. 35:1821–1830. 2015. View Article : Google Scholar : PubMed/NCBI
|