Introduction
In addition to the implementation of tumor
cytoreductive surgery, the majority of patients with ovarian cancer
require regular chemotherapy to remove residual small lesions.
Though initially sensitive to chemotherapy, up to 80% of patients
with ovarian cancer produce endogenous or acquired chemoresistance
(1). The recurrence and metastasis of
ovarian cancer results in poor prognosis. The 5-year survival rate
for patients with advanced ovarian cancer is 34–39% (2).
Previous studies have demonstrated that epithelial
ovarian cancer treatment improves with platinum-based combination
chemotherapy compared with nonplatinum-based chemotherapy (3–5). Cisplatin
is commonly used by gynecologists to kill tumor cells by inducing
DNA damage, promoting cell cycle arrest and increasing the rate of
apoptosis (6,7). Previous studies have reported the
following mechanisms underlying chemotherapy resistance: Certain
tumor cells exhibit enhanced DNA repair functions and a weakened
apoptotic process, with the transformation to epithelial stroma
facilitating the secretion of matrix protein enzymes, including
matrix metalloproteinase (MMP)-2 and MMP-9, which enable these
tumor cells to break through the basement membrane, metastasize and
invade. Therefore, chemoresistance and invasion are associated in
tumor cells (8–12).
Tumor protein p53 binding protein 1 (53BP1), an
adaptor/mediator protein, is a cytologic marker of endogenous
doublestranded DNA damage (13–15). 53BP1
is primarily associated with DNA damage response activation, which
maintains the stability of genetic information within the cell.
However, Lai et al (11)
studied the clinical and biological significance of 53BP1 and
demonstrated that increased expression of 53BP1 was associated with
increased resistance to cisplatin and worsened prognosis in lung
adenocarcinomas. To the best of our knowledge, no research has
focused on the effects of 53BP1 on migration and chemoresistance in
ovarian cancer. The present study assessed the modulation of 53BP1
expression level in the ovarian cancer cells SKOV3, and recorded
alterations in cell metastasis and chemotherapy sensitivity.
Materials and methods
Reagents
Lipofectamine 2000, RPMI-1640 medium and puromycin
were purchased from Invitrogen; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). N-Myc-proto-oncogene (MYC)-53BP1-wild type (WT)
pLPC-Puro plasmids were obtained from Addgene, Inc. (Cambridge, MA,
USA). The N-MYC-WT pLPC-Pruo plasmid was obtained from the
Laboratory of Cell Biology and Genetics of Rockefeller University
(New York, NY, USA). The enhanced chemiluminescence (ECL) kit used
for western blot analysis and the gel electrophoresis device were
acquired from GE Healthcare Bio-Sciences (Pittsburgh, PA, USA). The
antibodies against protein kinase B (Akt; 1:1,000; cat. no. 9272),
phosphorylated (p)-Akt (1:1,000; cat. no. 4060), B cell lymphoma
(Bcl)-2 (1:1,000; cat. no. 2872), Bcl-2 associated X (Bax; 1:1,000;
cat. no. 2772), p21 (1:1,000; cat. no. 2947), cyclin dependent
kinase 2 (CDK2; 1:1,000; cat. no. 2546) and β-actin (1:1,000; cat.
no. 4970) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Cisplatin was purchased from Sigma-Aldrich;
Merck KGaA.
Cell culture and transfection
The human ovarian cancer cell line SKOV3 was
obtained from the American Type Culture Collection (Manassas, VA,
USA). The cells were cultured in RPMI-1640 supplemented with 10%
fetal bovine serum (FBS; Tianjin Haoyang Biological Products
Technology Co., Ltd., Tianjin, China) in accordance with the
supplier's protocol. The cells were transfected with
N-MYC-proto-oncogene-53BP1-WT pLPC-Puro and N-MYC-WT pLPC-Pruo
using Lipofectamine 2000 according to the manufacturer's protocol.
Endogenous 53BP1 overexpression was induced in SKOV3 cells using
N-MYC-53BP1 WT PLPC-Puro plasmids. Cells were selected for
overexpression in complete RPMI-1640 medium containing 1.5 µg/ml
puromycin at 37°C. Cells that survived for 10 days were named
SKOV3/pLPC-53BP1 (as the experimental group) or SKOV3/pLPC-vector
(as the control group). The efficiency of transfection was
confirmed using western blot analysis as subsequently
described.
Transwell migration assay
SKOV3/pLPC-53BP1 and SKOV3/pLPC-vector cells
(1×107 cells/ml, 100 µl) were resuspended in RPMI-1640
medium without FBS and placed into the coated membrane of the upper
chamber of Transwell plates. RPMI-1640 supplemented with 20% FBS
was used as an attractant in the lower chamber. Following
incubation at 37°C for 24 h, migratory cells located on the lower
side of the chamber were fixed in methanol (at room temperature for
15 min) and then stained with 0.2% crystal violet (at room
temperature for 15 min). The stained cell images were captured
using a light microscope and 10 random fields at magnification, ×10
were counted. Results represented the average of triplicate samples
from three independent experiments.
Gelatin zymography analysis
Gelatinolytic activity was induced in the cell
culture supernatant of SKOV3/pLPC-53BP1 and SKOV3/pLPC-vector cells
via SDS-PAGE with a 10% gel containing 1 mg/ml gelatin (Invitrogen;
Thermo Fisher Scientific, Inc.). Following incubation in
renaturation buffer (2.5% Triton X-100 at room temperature for 45
min) and development buffer (pH 7.6, Tris. HCl 6.06 g,
CaCl2 0.56 g, NaCl 11.688 g, ZnCl2 0.136 mg,
ddH2O up to 1,000 ml) at 37°C for 18 h, the gels were
stained using Coomassie Brilliant Blue R-250 (Bio-Rad Laboratories,
Inc.), and destained in a mixture of acetic acid and methanol at
room temperature every 5 min until a colorless enzyme band showed.
Relative quantities of protein were measured using a densitometer
(ImageJ software v1.48, National Institutes of Health, Bethesda,
ML, USA). All procedures were performed in duplicate.
Drug sensitivity assay
An MTT assay was performed to assess the sensitivity
of SKOV3/pLPC-53BP1 cells to cisplatin; SKOV3/pLPC-vector cells
served as the control. Cells were seeded onto 96-well plates in
RPMI-1640 medium with 10% FBS (4.0×103 cells/well; final
volume=200 µl). Following attachment to the plates, cells were
exposed to 0, 0.4, 0.8, 1.6, 3.2, 6.4, 12.8 or 25.6 µg/ml cisplatin
for 72 h at 37°C in a 5% CO2 incubator. Subsequently, 20
µl MTT (5 mg/ml; Sigma-Aldrich; Merck KGaA) was added to each well
and wells were incubated for a further 4 h at 37°C. The medium was
then removed and 150 µl dimethyl sulfoxide was added to each well.
Absorbance of each well at 490 nm was read using a microplate
reader. The half maximal inhibitory concentration (IC50)
of each drug was estimated from the relative survival curves.
Independent experiments were performed three times in 5 duplicate
wells. Mitochondrial activity, which may reflect cellular growth
and viability, was evaluated by measuring optical density (OD) at
490 nm on a microtiter plate reader. The relative survival rate was
calculated as follows:
Relative survival
rate(%)=(ODtreated-ODzero setting)/(ODcontrol-ODzero
setting)x100%.
Western blot analysis
The SKOV3/pLPC-53BP1 cells that were maintained in
0, 0.4, 0.8 or 1.6 µg/ml cisplatin for 72 h were harvested.
Whole-cell lysates were prepared by incubating cells in RIPA buffer
(Shennengbocai, Shanghai, China; 1% NP-40, 0.1% SDS, 5 mM EDTA,
0.5% sodium deoxycholate, 1 mM sodium vandate) containing protease
inhibitors (1 mM phenylmethane sulfonyl fluoride and 1 mM sodium
fluoride) on ice for 30 min. Cell lysates were centrifuged at 8,000
× g for 15 min at 4°C. The supernatant was subsequently collected
and the protein concentration was measured using a BCA Protein
Assay kit (Merck KGaA). A total of 50 µg proteins were separated
using 8% SDS-PAGE and transferred onto polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked using 5% non-fat milk for 1 h at room temperature and then
incubated with primary antibodies, including Akt, p-Akt, Bcl-2,
Bax, p21, CDK2 and β-actin overnight at 4°C. The membranes were
then washed three times with TBST and subsequently incubated with
horseradish peroxidase-conjugated secondary antibodies (1:5,000;
cat. no. 5210-0174; Kirkegaard and Perry Laboratories Inc.,
Gaithersburg, MD, USA) at room temperature for 2 h. Bands were
detected with an enhanced chemiluminescence detection system (cat.
no. NEL102001EA; PerkinElmer Inc., Waltham, MA, USA). ImageJ
software (version 1.48; National Institutes of Health, Bethesda,
ML, USA) was used for the densitometric analysis of western
blotting.
Statistical analysis
Data were expressed as the mean ± standard
deviation. Statistical comparisons between groups of normally
distributed data were performed using the Student's t-test via SPSS
version 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
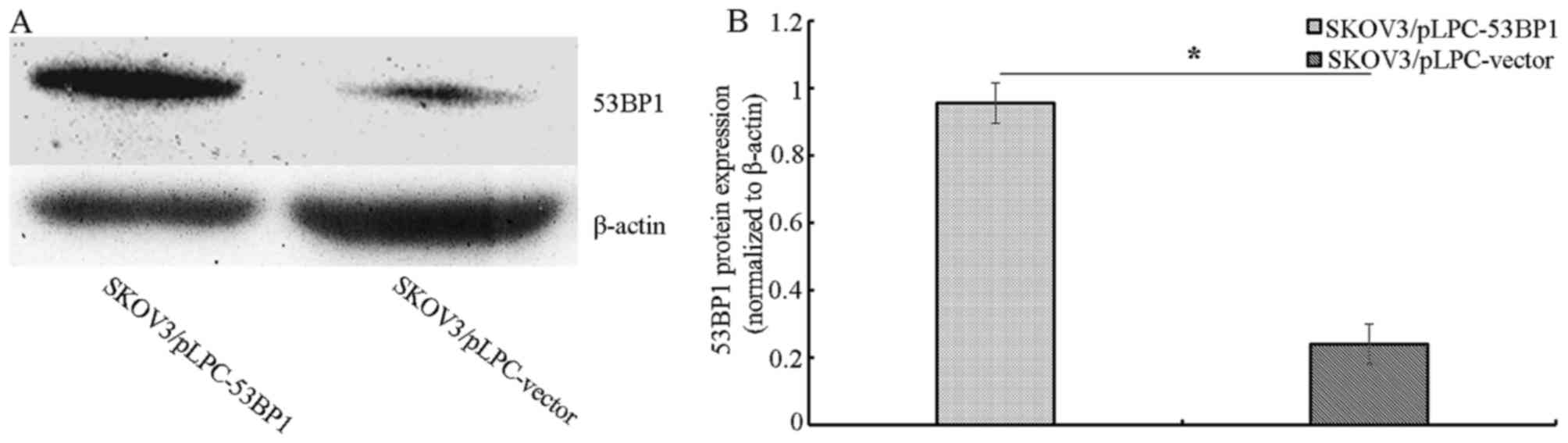
Evaluation of 53BP1 protein expression
in ovarian cancer cells following transfection with
53BP1-overexpressing plasmids
Stable transfected cell lines were generated by
expanding the resistant colonies, including SKOV3/pLPC-53BP1 and
SKOV3/pLPC-vector transfected cell lines. The expression of 53BP1
in these cell lines was assessed using western blot analysis.
SKOV3/pLPC-53BP1 cells exhibited increased 53BP1 expression
compared with SKOV3/pLPC-vector cells (Fig. 1A and B).
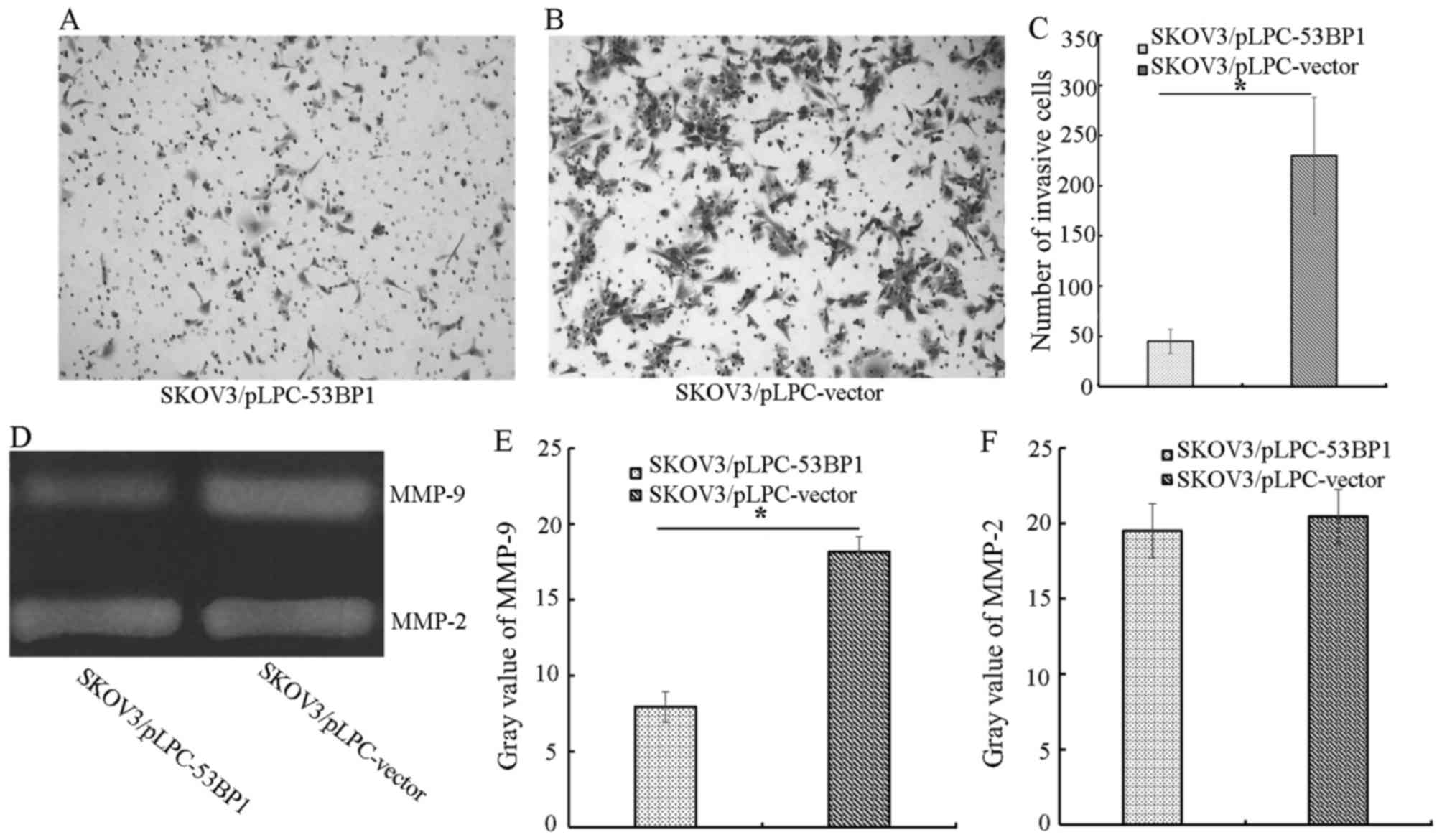
53BP1 ectopic expression decreases
migration in SKOV3-derived cell lines
To determine the effect of 53BP1 on SKOV3 migration,
Transwell assays were performed. SKOV3/pLPC-53BP1 cells exhibited a
significant decrease in migration compared with SKOV3/pLPC-vector
cells (P<0.05). The number of migratory SKOV3/pLPC-53BP1 cells
was 45.00±12.00, compared with 230.00±58.00 migratory
SKOV3/pLPC-vector cells (P<0.05; Fig.
2A-C). Therefore, the present study demonstrated that 53BP1
significantly decreased migration in SKOV3 cells.
53BP1 decreases MMP-associated
proteolytic activity
Using gelatin zymography, the effect of 53BP1 on
MMPs was evaluated. SKOV3/pLPC-vector cells (gray value=18.17±2.13)
exhibited increased MMP-9 activity compared with SKOV3/pLPC-53BP1
cells (gray value=7.94±1.12; Fig.
2D-F). However, the two types of cell exhibited no difference
in the activity of MMP-2.
Evaluation of cisplatin-associated
antitumor activity using an MTT assay
The cytotoxicity of cisplatin in SKOV3/pLPC-53BP1
and SKOV3/pLPC-vector cells was evaluated using an MTT assay.
SKOV3/pLPC-vector cells (IC50=2.98±0.27 µg/ml) were
demonstrated to be more sensitive to 0.4–6.4 µg/ml cisplatin
compared with SKOV3/pLPC-53BP cells (IC50=17.58±0.51
µg/ml; Fig. 3A).
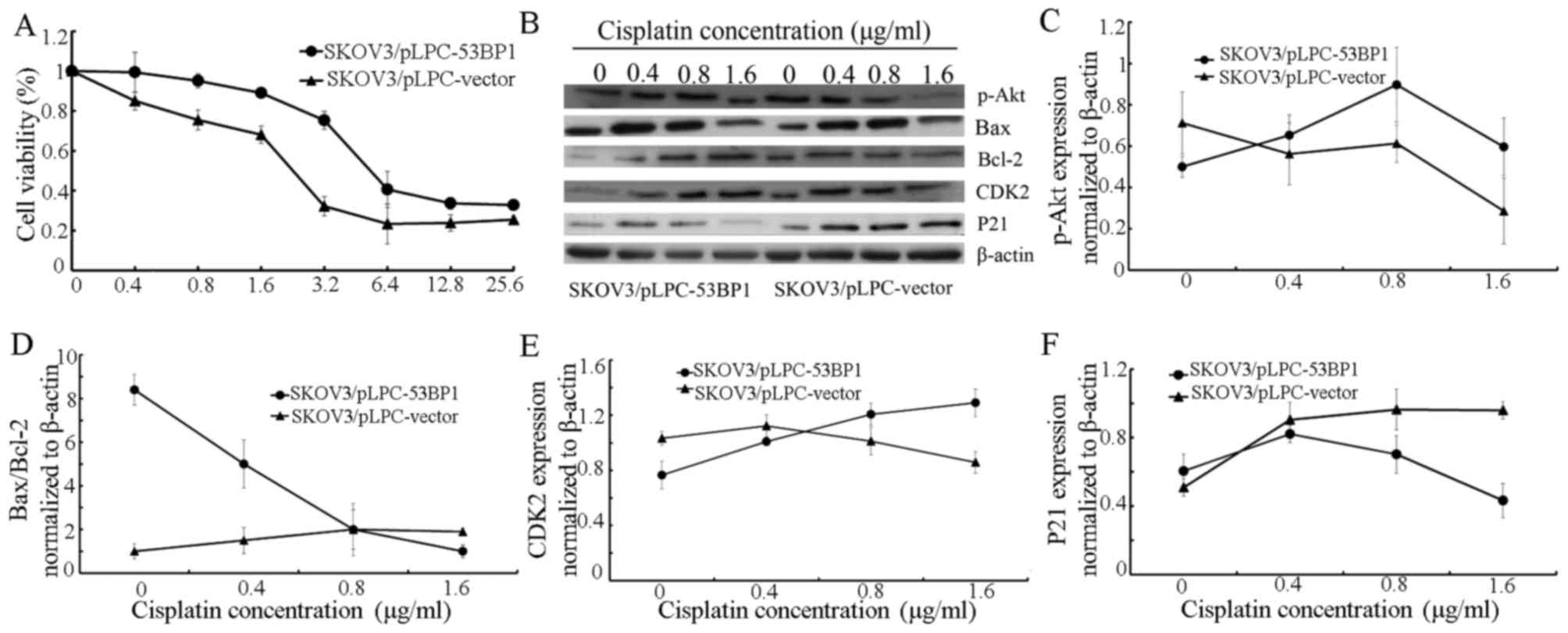 | Figure 3.Expression of 53BP1 is associated with
cisplatin chemoresistance. (A) Cell viability was assayed following
treatment with an increasing concentration of cisplatin for 72 h.
(B) Expression of p-Akt, Bax, Bcl-2, CDK2 and p21 in
SKOV3/pLPC-53BP1 and SKOV3/pLPC-vector cells following treatment
with 0, 0.4, 0.8, 1.6 µg/ml cisplatin was assessed using western
blot analysis. β-actin served as a loading control. The relative
expression of (C) p-Akt, (D) Bax/Bcl-2, (E) CDK2 and (F) p21 in
SKOV3/pLPC-53BP1 and SKOV3/pLPC-vector cells. β-actin was a
normalization control. Data were represented as the mean ± standard
deviation. 53BP1, tumor protein p53 binding protein 1; p-Akt,
phosphorylated protein kinase B; Bax, Bcl-2 associated X; Bcl-2, B
cell lymphoma-2; CDK2, cyclin dependent kinase 2. |
Western blot analysis
When culturing cells without cisplatin, increased
53BP1 expression was associated with the upregulation of proteins
associated with the inhibition of apoptosis, including Bax, p21 and
the Bax/Bcl-2 ratio, However, with the downregulation of
proliferation-promoting proteins, including p-Akt, Bcl-2 and CDK2
(Fig. 3B-F), SKOV3/pLPC-53BP1 cells
exposed to 0.4–1.6 µg/ml cisplatin exhibited a decrease in the
protein expression of Bax/Bcl-2 and p21 compared with
SKOV3/pLPC-vector cells (Fig. 3B, D and
F, respectively). For treatment with 0.8–1.6 µg/ml cisplatin,
the expression of p-Akt and CDK2 was increased in SKOV3/pLPC-53BP1
compared with SKOV3/pLPC-vector cells (Fig. 3C and E, respectively).
Discussion
Despite the progress in cancer treatment and the
understanding of tumor biology, ovarian cancer remains one of the
most lethal gynecologic malignancies (16). Further study is required to resolve
aggressive behavior and drug resistance in ovarian cancer, which
are major challenges to developing more effective therapies. The
BRCA1 C-Terminal domain-containing 53BP1, a p53-binding protein, is
associated with the regulation of the cell cycle and DNA damage
response (17–19). Previous studies have demonstrated that
following knockdown of 53BP1, mice exhibited growth retardation,
radiotherapy sensitivity and tumor susceptibility (20,21).
Furthermore, 53BP1 may attenuate the expression of p-Akt and Bcl-2,
increase the protein expression of Bax and the Bax/Bcl-2 ratio, and
induce cell cycle arrest and apoptosis, resulting in decreased
proliferation, in SKOV3 cells (22).
To further assess the effects of 53BP1 on SKOV3 cells, the present
study evaluated migration and chemosensitivity to cisplatin in
53BP1-overexpressing SKOV3 cells.
During metastasis, cancer cells receive signals from
the tumor microenvironment and undergo an epithelial-mesenchymal
transition, which decreases tumor polarity and cell-cell adhesion
(23). Furthermore, cancer cells may
secret proteinases to degrade multiple components of the
extracellular matrix and thereby enhance migration and metastasis
(24). The extracellular matrix and
basement membrane are barriers inhibiting metastasis. The
degradation of the extracellular matrix by metastatic cancer cells
is associated with multiple proteolytic enzymes, including MMPs and
cathepsins (25,26). Typically, increased cancer aggression
is associated with increased MMP secretion by the cancer (27).
In the present study, MMP-9 expression was
downregulated by 53BP1 overexpression compared with the control,
resulting in the inhibition of migration in SKOV3 cells (Fig. 2). However, no statistically
significant difference in MMP-2 expression was demonstrated between
groups. These results suggested that 53BP1 decreased the metastatic
potential of SKOV3 cells by inducing the downregulation of MMP-9.
Further study is required to determine the 53BP1-associated pathway
that facilitated MMP-9 attenuation.
Certain tumor invasion-regulating genes are
associated with numerous characteristics of tumor biology,
including drug sensitivity and the induction of angiogenesis
(28). Drug resistance remains a
substantial challenge to successfully treating ovarian cancer.
Cisplatin is an effective chemotherapeutic agent for the treatment
of ovarian cancer. The present study evaluated the effect of 53BP1
on the chemosensitivity of SKOV3 cells to cisplatin. SKOV3 cells
overexpressing 53BP1 and cultured without cisplatin exhibited
decreased viability compared with control cells cultured without
cisplatin, a result consistent with those of a previous study
(22). However, increased 53BP1
expression was associated with increased cisplatin resistance in
SKOV3 cells. With particular respect to the 0.4–1.6 µg/ml cisplatin
treatment, 53BP1 expression was associated with resistance to
cisplatin.
Resistance to cisplatin is associated with multiple
complex system properties, and the underlying mechanism remains to
be fully understood. Previously, Bcl-2, p-Akt, p21, Bax and CDK2
have been demonstrated to be associated with chemotherapy
resistance in multiple types of tumor (22,29). The
present study therefore proposed that these proteins may also be
associated with 53BP1-induced chemotherapy resistance in SKOV3
cells. The present study demonstrated that treatment with 0.4–1.6
µg/ml cisplatin resulted in a decreasing trend in the protein
expression of Bax/Bcl-2 and p21; an increasing tendency in the
expression of p-Akt and CDK2 in SKOV3/pLPC-53BP1 was also observed
at 0.4–1.6 µg/ml cisplatin, compared with SKOV3/pLPC-vector cells.
The Akt signaling pathway is associated with Bax/Bcl-2-mediated
cell survival and the phosphorylation of the Thr145 and Ser146
residues of p21, which renders the protein incapable of entering
the nucleus from the cytoplasm (30).
Increased active CDK2 expression promotes p21 degradation and
thereby facilitates p21 repression. Thus, decreased expression of
p21, a tumor suppressor, may combine with increased CDK2 expression
to promote cell survival (31).
However, a consensus on the effects of 53BP1 on
cellular responses to chemotherapeutic agents has yet to be
reached, with multiple studies having demonstrated increased or
decreased drug sensitivity with 53BP1 overexpression (11,32,33). These
conflicting reports suggest that the association between 53BP1 and
chemosensitivity may be more complex than previously assumed.
To conclude, the present study revealed that 53BP1
suppressed SKOV3 cell migration by decreasing MMP-9 expression.
However, the present study also suggested that 53BP1 promoted
cisplatin chemoresistance, a function associated with decreased
Bax/Bcl-2 and p21 expression and increased p-Akt and CDK2
expression. Although the mechanism underlying 53BP1-induced
chemoresistance remains to be understood, a potential underlying
mechanism for future studies to evaluate is DNA repair.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Projects of
Medical and Health Technology Development Program (grant no.
2015WS0242).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
BK designed the study. SH performed experiments,
participated in statistical analysis and drafted the manuscript.
SH, XL, YZ and QY assisted with experiments and manuscript
preparation. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li J, Yang S, Su N, Wang Y, Yu J, Qiu H
and He X: Overexpression of long non-coding RNA HOTAIR leads to
chemoresistance by activating the Wnt/β-catenin pathway in human
ovarian cancer. Tumour Biol. 37:2057–2065. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Musella A, Marchetti C, Palaia I, Perniola
G, Giorgini M, Lecce F, Vertechy L, Iadarola R, De Felice F, Monti
M, et al: Secondary cytoreduction in Platinum-resistant recurrent
ovarian cancer: A Single-institution experience. Ann Surg Oncol.
22:4211–4216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gabra H: Introduction to managing patients
with recurrent ovarian cancer. EJC Suppl. 12:2–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du XL, Parikh RC, Lairson DR, Giordano SH
and Cen P: Comparative effectiveness of platinum-based chemotherapy
versus taxane and other regimens for ovarian cancer. Med Oncol.
30:4402013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pinato DJ, Graham J, Gabra H and Sharma R:
Evolving concepts in the management of drug resistant ovarian
cancer: Dose dense chemotherapy and the reversal of clinical
platinum resistance. Cancer Treat Rev. 39:153–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vendetti FP, Lau A, Schamus S, Conrads TP,
O'Connor MJ and Bakkenist CJ: The orally active and bioavailable
ATR kinase inhibitor AZD6738 potentiates the anti-tumor effects of
cisplatin to resolve ATM-deficient non-small cell lung cancer in
vivo. Oncotarget. 6:44289–44305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu S, Pabla N, Tang C, He L and Dong Z:
DNA damage response in cisplatin-induced nephrotoxicity. Arch
Toxicol. 89:2197–2205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Mosel AJ, Oakley GG and Peng A:
Deficient DNA damage signaling leads to chemoresistance to
cisplatin in oral cancer. Mol Cancer Ther. 11:2401–2409. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schiewer MJ, Goodwin JF, Han S, Brenner
JC, Augello MA, Dean JL, Liu F, Planck JL, Ravindranathan P,
Chinnaiyan AM, et al: Dual roles of PARP-1 promote cancer growth
and progression. Cancer Discov. 2:1134–1149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johannessen TC, Prestegarden L, Grudic A,
Hegi ME, Tysnes BB and Bjerkvig R: The DNA repair protein ALKBH2
mediates temozolomide resistance in human glioblastoma cells. Neuro
Oncol. 15:269–278. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lai TC, Chow KC, Lin TY, Chiang IP, Fang
HY, Chen CY and Ho SP: Expression of 53BP1 as a cisplatin-resistant
marker in patients with lung adenocarcinomas. Oncol Rep.
24:321–328. 2010.PubMed/NCBI
|
|
12
|
Zhu B, Block NL and Lokeshwar BL:
Interaction between stromal cells and tumor cells induces
chemoresistance and matrix metalloproteinase secretion. Ann N Y
Acad Sci. 878:642–646. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fukuda T, Wu W, Okada M, Maeda I, Kojima
Y, Hayami R, Miyoshi Y, Tsugawa K and Ohta T: Class I histone
deacetylase inhibitors inhibit the retention of BRCA1 and 53BP1 at
the site of DNA damage. Cancer Sci. 106:1050–1056. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J and Xu X: DNA double-strand break
repair: A tale of pathway choices. Acta Biochim Biophys Sin
(Shanghai). 48:641–646. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zimmermann M and de Lange T: 53BP1: Pro
choice in DNA repair. Trends Cell Biol. 24:108–117. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schultz LB, Chehab NH, Malikzay A and
Halazonetis TD: p53 binding protein 1 (53BP1) is an early
participant in the cellular response to DNA double-strand breaks. J
Cell Biol. 151:1381–1390. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rappold I, Iwabuchi K, Date T and Chen J:
Tumor suppressor p53 binding protein 1 (53BP1) is involved in DNA
damage-signaling pathways. J Cell Biol. 153:613–620. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chai YL, Cui J, Shao N, Shyam E, Reddy P
and Rao VN: The second BRCT domain of BRCA1 proteins interacts with
p53 and stimulates transcription from the p21WAF1/CIP1 promoter.
Oncogene. 18:263–268. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Markova E, Vasilyev S and Belyaev I: 53BP1
foci as a marker of tumor cell radiosensitivity. Neoplasma.
62:770–776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morales JC, Xia Z, Lu T, Aldrich MB, Wang
B, Rosales C, Kellems RE, Hittelman WN, Elledge SJ and Carpenter
PB: Role for the BRCA1 C-terminal repeats (BRCT) protein 53BP1 in
maintaining genomic stability. J Biol Chem. 278:14971–14977. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong S, Li X, Zhao Y, Yang Q and Kong B:
53BP1 suppresses tumor growth and promotes susceptibility to
apoptosis of ovarian cancer cells through modulation of the Akt
pathway. Oncol Rep. 27:1251–1257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fischer KR, Durrans A, Lee S, Sheng J, Li
F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al:
Epithelial-to-mesenchymal transition is not required for lung
metastasis but contributes to chemoresistance. Nature. 527:472–476.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nicolson GL: Tumor and host molecules
important in the organ preference of metastasis. Semin Cancer Biol.
2:143–154. 1991.PubMed/NCBI
|
|
26
|
Dass K, Ahmad A, Azmi AS, Sarkar SH and
Sarkar FH: Evolving role of uPA/uPAR system in human cancers.
Cancer Treat Rev. 34:122–136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pârvănescu V, Georgescu M, Georgescu I,
Surlin V, Pâtrascu S, Picleanu AM and Georgescu E: The role of
matrix metalloproteinase-9 (MMP-9) as a prognostic factor in
epithelial and lymphatic neoplasia. Chirurgia (Bucur). 110:506–510.
2015.PubMed/NCBI
|
|
28
|
Lee JH, Chiang SY, Nam D, Chung WS, Lee J,
Na YS, Sethi G and Ahn KS: Capillarisin inhibits constitutive and
inducible STAT3 activation through induction of SHP-1 and SHP-2
tyrosine phosphatases. Cancer Lett. 345:140–148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Michaud WA, Nichols AC, Mroz EA, Faquin
WC, Clark JR, Begum S, Westra WH, Wada H, Busse PM, Ellisen LW and
Rocco JW: Bcl-2 blocks cisplatin-induced apoptosis and predicts
poor outcome following chemoradiation treatment in advanced
oropharyngeal squamous cell carcinoma. Clin Cancer Res.
15:1645–1654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou BP, Liao Y, Xia W, Spohn B, Lee MH
and Hung MC: Cytoplasmic localization of p21Cip1/WAF1 by
Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat
Cell Biol. 3:245–252. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghanem L and Steinman R: A Proapoptotic
function of p21 in differentiating granulocytes. Leuk Res.
29:1315–1323. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li X, Kong X, Kong X, Wang Y, Yan S and
Yang Q: 53BP1 sensitizes breast cancer cells to 5-fluorouracil.
PLoS One. 8:e749282013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pan S, Cheng L, White JT, Lu W, Utleg AG,
Yan X, Urban ND, Drescher CW, Hood L and Lin B: Quantitative
proteomics analysis integrated with microarray data reveals that
extracellular matrix proteins, catenins, and p53 binding protein 1
are important for chemotherapy response in ovarian cancers. OMICS.
13:345–354. 2009. View Article : Google Scholar : PubMed/NCBI
|