Introduction
The human brain glioblastoma is one of the most
common malignant tumors in the central nervous system, and the
median survival time of patients with glioblastoma is typically
<1 year (1). Although considerable
progress has been made in the biological research and treatment of
cancer, the study of glioblastoma has not been thoroughly explored,
and its exact pathogenesis remains unclear. The main treatment for
glioblastoma is surgery, in combination with postoperative
radiation, chemotherapy and immunotherapy. However, the overall
treatment effect is poor with a high tumor recurrence rate
(2). Therefore, there is an urgent
requirement to investigate the underlying molecular mechanisms of
glioblastoma progression.
Chicken ovalbumin upstream promoter transcription
factor II (COUP-TFII) is a member of the steroid receptor
superfamily and is an orphan nuclear receptor (3). COUP-TFII regulates numerous key
biological processes, including cell fate determination and organ
development (4). COUP-TFII is
expressed in multiple tissues and is important for organogenesis
(5). There is a growing number of
studies that demonstrate that COUP-TFII is dysregulated in multiple
cancer types and is crucial for cancer initiation and progression
(6–9).
However, the role of COUP-TFII in glioma remains unknown.
Pyruvic acid is one of the most important
metabolites in eukaryotic cells, which is transported into the
mitochondria of differentiated mammalian cells. Pyruvic acid then
undergoes oxidative phosphorylation for ATP production. However,
cancer cells exhibit an increased dependence on the glycolytic
pathway for ATP generation (10),
referred to as the Warburg effect (11). Pyruvate metabolism and synthesis have
a key role in the Warburg effect,
The mitochondrial pyruvate carrier (MPC), in polymer
form, has an important role in mitochondrial pyruvate metabolism
(12,13). The MPC gene encodes two proteins-MPC1
and MPC2-and existing research indicates that the MPC gene is
downregulated in a variety of tumors, including colon, kidney
(14) and prostate cancer (7), and is closely associated with prognosis.
MPC serves an important function in the initiation and progression
of tumor development; however, its function in glioma remains to be
fully elucidated.
In the present study, it was observed that MPC1 was
downregulated in glioblastoma and the PROMO TRANSFAC software,
predicted that transcriptional binding sites are present in MPC1
for COUP-TFII. Therefore, the aim of the present study was to
investigate the COUP-TFII-mediated regulation of MPC1 in
glioblastoma. The data indicated that downregulating COUP-TFII
expression was able to inhibit glioblastoma growth in vitro
and in vivo, which may be mediated by targeting MPC1. These
findings support the possibility that COUP-TFII may be a novel
therapeutic target for glioblastoma treatment.
Materials and methods
Cell culture and reagents
The U87 human glioblastoma cell line and normal
brain cell line (HEB) were obtained from the Basic Medical
Institute, Chinese Academy of Medical Sciences (Beijing, China).
U87 cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; Hyclone; GE Healthcare, Chicago, IL, USA) supplemented with
10% heat-inactivated fetal bovine serum (FBS; Hyclone) at 37°C in a
humidified atmosphere with 5% CO2. The U87 cell line is
known to be misidentified/cross-contaminated with an unknown cell
line. Despite the reported misidentification/contamination of the
U87 cells used in the present study, this cell line is suitable for
this current research project, as it is likely a glioblastoma cell
line, and therefore exhibits a similar biological nature (15).
Cell transfection
U87 cells were seeded in a 6-well plate at a density
of 4.8×105 cells/well and cultured for 24 h (37°C) until
the cells obtained 40% confluence. The cells were transfected with
lentiviruses, with a small interfering-COUP-TFII (100 nM) sequence
and scrambled siRNA negative control (siNC, 100 nM), (Shanghai
GenePharma Co, Ltd., Shanghai, China) using
Lipofectamine® 2,000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from U87 cells or tumors at
48 h post-transfection using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. RT-qPCR
was performed to detect the expression levels of COUP-TFII and MPC1
mRNA. Total RNA was reverse transcribed using a one-step RT-PCR kit
(Takara Bio, Inc., Otsu, Japan) according to the manufacturer's
protocol. qPCR of COUP-TFII and MPC1 were performed with 5 µl
COUP-TFII and MPC1 cDNA, using SYBR green (Invitrogen; Thermo
Fisher Scientific, Inc.). The reaction conditions were as follows:
93°C for 1 min, 55°C for 1 min, and 72°C for 1 min, for 40 cycles.
The primer sequences for COUP-TFII and MPC1 were as follows:
COUP-TFII forward, 5′-CGGGTGGTCGCCTTTATGG-3′; and reverse
5′-ACAGGCATCTGAGGTGAACAG-3′; MPC1 forward,
5′-ATTTGCCTACAAGGTACAGCC-3′; and reverse
5′-AGTCATCTCGTGTTTGATAAGCC-3′, (Shanghai GenePharma Co., Ltd.). The
relative expression levels of COUP-TFII and MPC1 were quantified
using the 2−ΔΔCq method (16). Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) served as the internal control.
The primer sequences of GAPDH were as follows:
Forward, 5′-GGTGAAGGTCGGAGTCAACG-3′, and reverse,
5′-CAAAGTTGTCATGGATGHACC-3′.
Luciferase reporter assay
The U87 cells were cultured at a density of 10,000
cells/well in 96-well plates, and infected with 5 µl lenti-si
COUP-TFII or lenti-siNC (6 µg/ml), and incubated with 2 µl
Lipofectamine® 2,000 and 0.2 µg MPC1 luciferase reporter
vectors (Thermo Fisher Scientific, Inc.). Following 48 h of
incubation, luciferase activity was measured using a
Dual-Luciferase Reporter system (Promega Corporation, Madison, WI,
USA). The luciferase enzyme activity was normalized to the Renilla
luciferase enzyme activity, All controls used were untreated
cells.
Cell proliferation
U87 cells were digested by trypsin (Thermo Fisher
Scientific, Inc.) after 48 h post-transfection and seeded at a
density of 5×103 cells/well in a 12-well plate. The
cells were dived into three groups: Blank control group, si
COUP-TFII group and siNC group. The cell cultures were maintained
(37°C) and counted for 6 days under a light microscope (×100
magnification, Olympus Corporation, Tokyo, Japan).
Xenografts
U87 cells (3×106), transfected with si
COUP-TFII or siNC and mixed with Matrigel were subcutaneously
injected into the abdomen of 4-week-old 12 g male SCID mice
(Institute of Zoology, Chinese academy of Sciences, Beijing,
China). A total of 20 mice were maintained in a specific pathogen
free facility with a constant humidity (40–70%) and temperature
(21±2°C), in a 12/12 h light/dark cycle with free access to food
and water. The tumor size was measured using a caliper 2 weeks
later until the termination of the experiment. All data was
recorded, and the formula v=0.5xaxb2 (v, tumor volume;
a, major diameter of the tumor; b, minor diameter) was used to
calculate tumor volume. The mice were sacrificed at the end of the
experiment, or if tumor size reached 15 mm diameter, and tumor
tissue was removed from mice for further examination. A portion of
the tumor tissue was collected, embedded in paraffin and cut into
sections. All animal experiments were approved by the Institutional
Animal Care and Use Committee (Nanchang University, Jiangxi,
China).
Immunohistochemistry
The tissue sections of 5-µm thickness were routinely
deparaffinized. Antigen retrieval was performed in 1 mM EDTA buffer
(pH 8.0) for 10 min at 95–100°C. The sections were rinsed three
times in phosphate-buffered saline (PBS) and then blocked in PBS
containing 3% normal horse serum (Thermo Fisher Scientific, Inc.)
for 30 min. Subsequently, the sections were incubated with an
anti-Ki-67 primary antibody (1:100; catalog no. ab66155; Abcam,
Cambridge, UK) overnight at room temperature. The following day,
the slides were rinsed in PBS and incubated with a biotinylated
goat anti-mouse IgG (dilution, 1:100; catalog no. sc-2039; Santa
Cruz Biotechnology, Inc., TX, USA) secondary antibody at 37°C for
40 min. The slides were then incubated with ABC-peroxidase reagents
(Santa Cruz Biotechnology, Inc.) at 37°C for 1 h. Antigen-antibody
complexes were detected using a 3,3′-diaminobenzidine horseradish
peroxidase color development kit (Beyotime Institute of
Biotechnology, Shanghai, China) and evaluated under a light
microscope (CX21BIM-SET6; Olympus Corporation, Tokyo, Japan).
Statistical analysis
The data are presented as the mean ± standard
deviation. Data were statistically analyzed by χ2-test
and one-way analysis of variance using SPSS software (version 11.5;
SPSS Inc., Chicago, IL, USA), The Student-Newman-Keuls post-hoc
test was used following the analysis of variance for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference. Each experiment was repeated 3 times.
Results
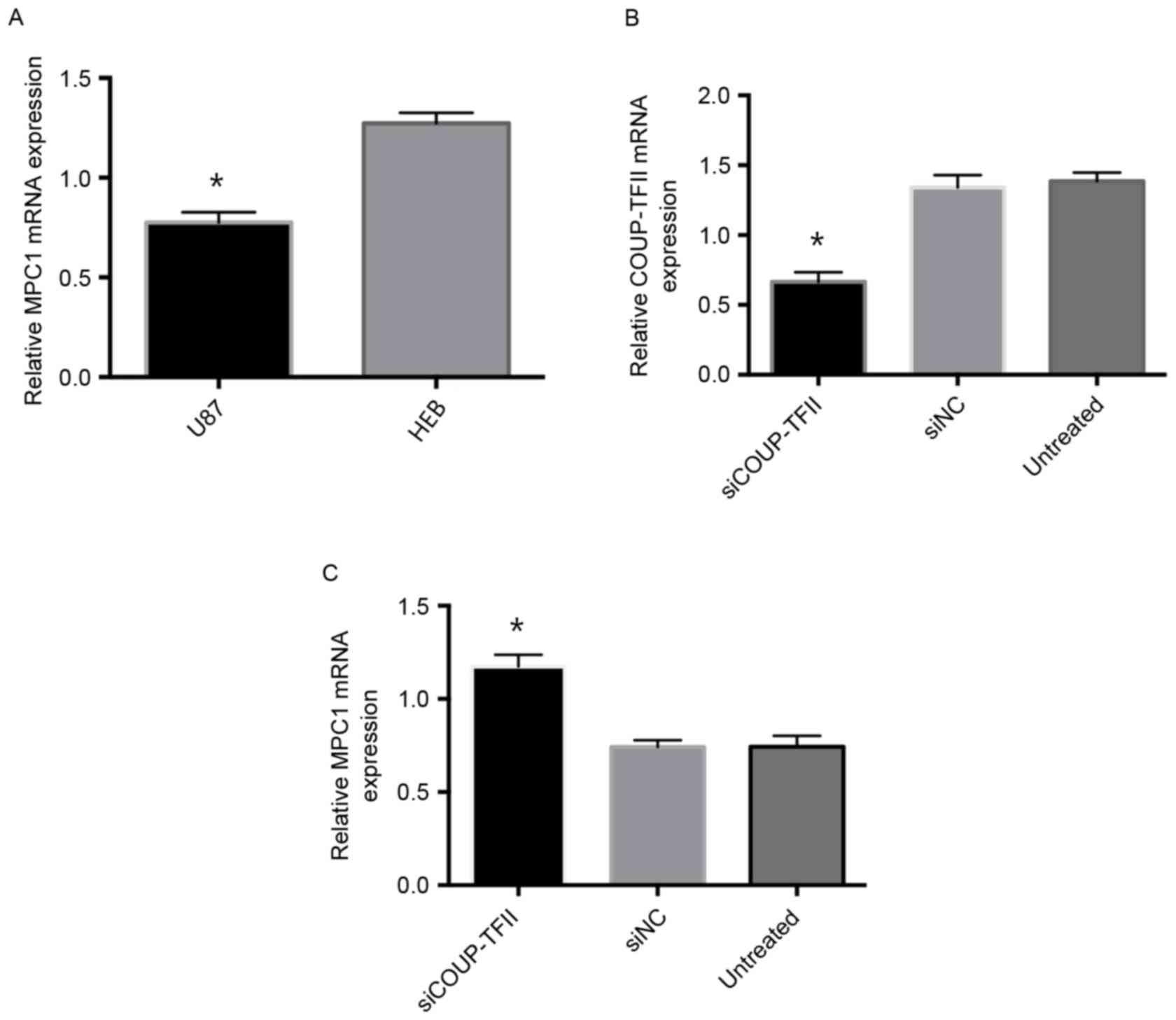
MPC1 is downregulated in U87 cells and
upregulated when COUP-TFII is suppressed
The expression of MPC1 in U87 cells was examined by
RT-qPCR, and MPC1 mRNA expression was significantly decreased in
U87 cells compared with normal brain cells (Fig. 1A). Following knockdown of COUP-TFII by
siCOUP-TFII in U87 cells, COUP-TFII mRNA expression (Fig. 1B) was significantly decreased in
siCOUP-TFII-transfected cells compared with siNC and untreated
cells. Also, RT-qPCR analysis revealed that MPC1 mRNA levels were
significantly increased in siCOUP-TFII-transfected U87 cells
compared with siNC and untreated cells (Fig. 1C). These results indicated that MPC1
was downregulated in U87 cells, and transfection with siCOUP-TFII
was able to effectively inhibit COUP-TFII expression. Furthermore,
MPC1 mRNA expression was upregulated in response to COUP-TFII
suppression.
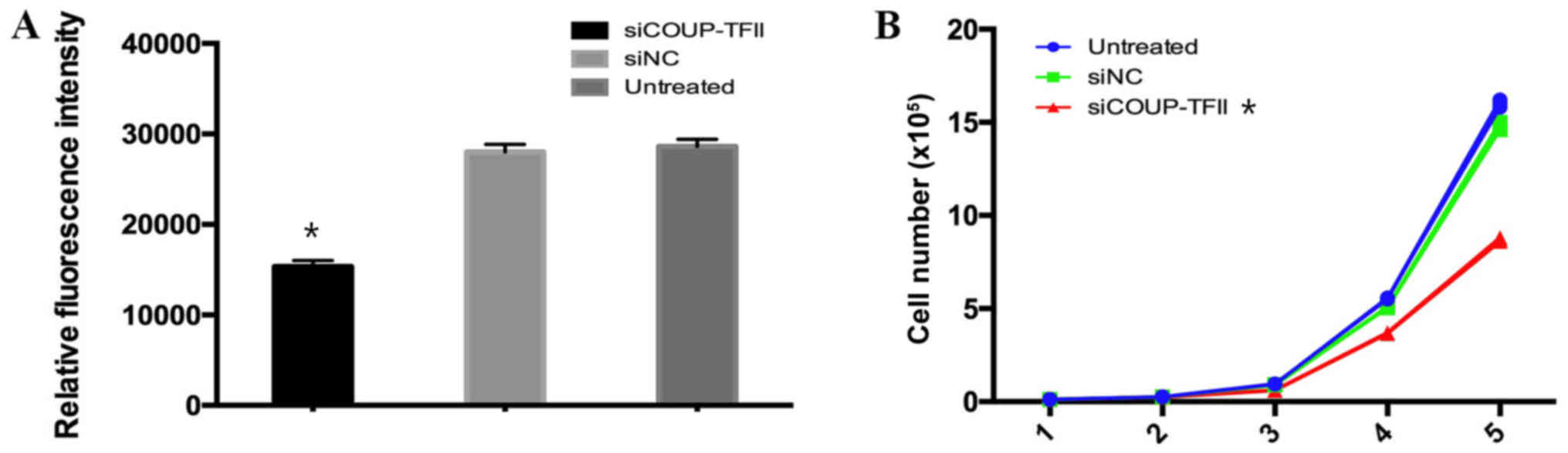
Downregulation of COUP-TFII suppresses
cell proliferation via targeting MPC1
PROMO TRANSFAC predicted that MPC1 has
transcriptional binding sites for COUP-TFII (17). To determine whether the prediction was
correct, U87 cells were infected with siCOUP-TFII and transfected
with MPC1 luciferase reporter vectors. Luciferase activity was
measured using a dual-luciferase reporter system. The luciferase
activity was significantly reduced in U87 cells that were infected
with siCOUP-TFII compared with untreated cells or cells that were
infected with siNC (Fig. 2A). This
result indicated that MPC1 might be a direct target of COUP-TFII.
Furthermore, the effect of COUP-TFII on cell proliferation was
investigated, and it was demonstrated that U87 cell proliferation
was inhibited by siCOUP-TFII compared with siNC and the untreated
group (Fig. 2B). Therefore, these
findings indicated that the downregulation of COUP-TFII suppresses
U87 cell proliferation via targeting MPC.
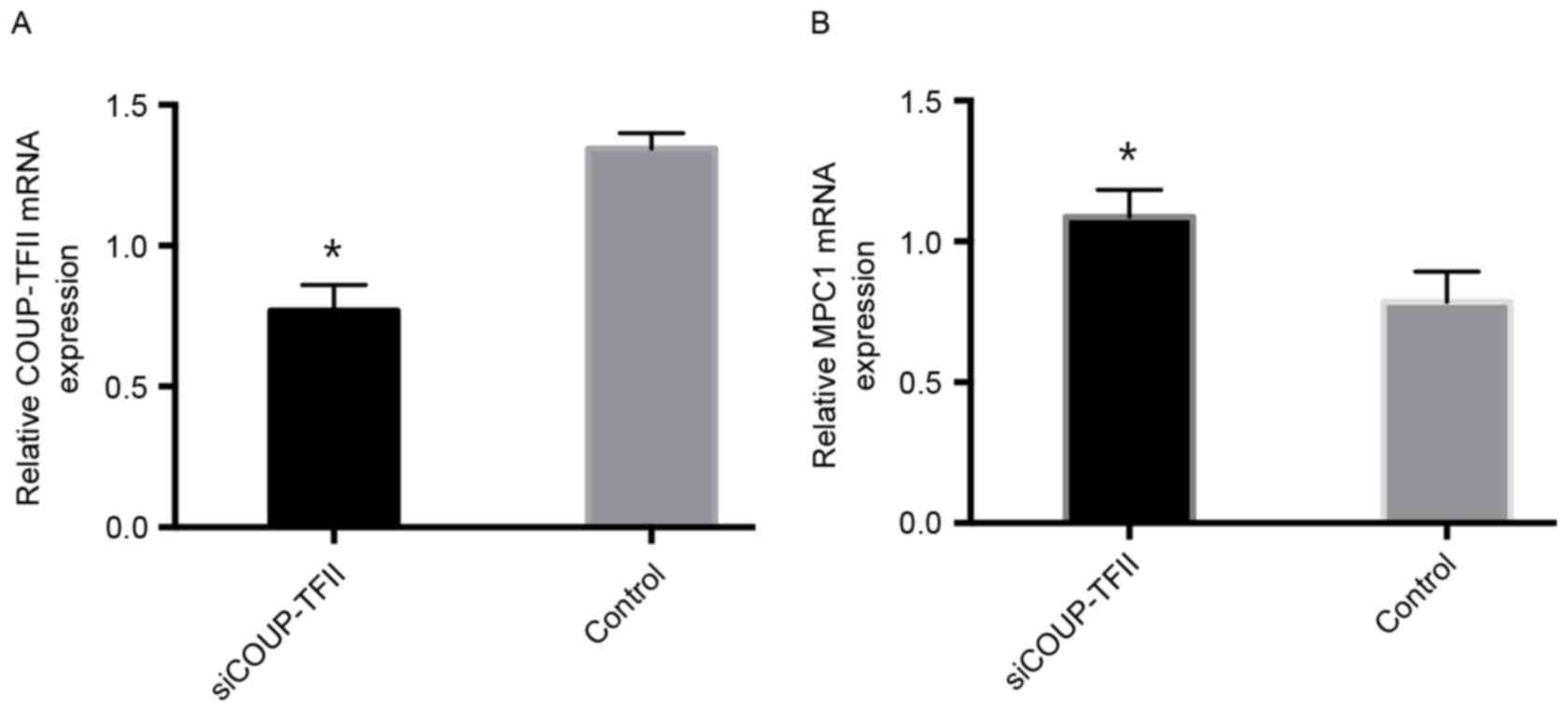
SiCOUP-TFII works efficiently in an in
vivo animal model
To test the efficiency of COUP-TFII knockdown in a
mouse model, siCOUP-TFII and si-NC-transfected U87 cells were
injected into the abdomen of SCID mice. The efficiency of
siCOUP-TFII was good. COUP-TFII mRNA expression was significantly
downregulated by siCOUP-TFII compared with the control group as
evaluated by RT-qPCR (Fig. 3A).
However, MPC1 mRNA expression was significantly increased by
siCOUP-TFII compared with the control group (Fig. 3B). These findings indicated that the
injection of siCOUP-TFII-transfected U87 cells was able to
downregulate COUP-TFII expression and upregulate MPC1 in
vivo.
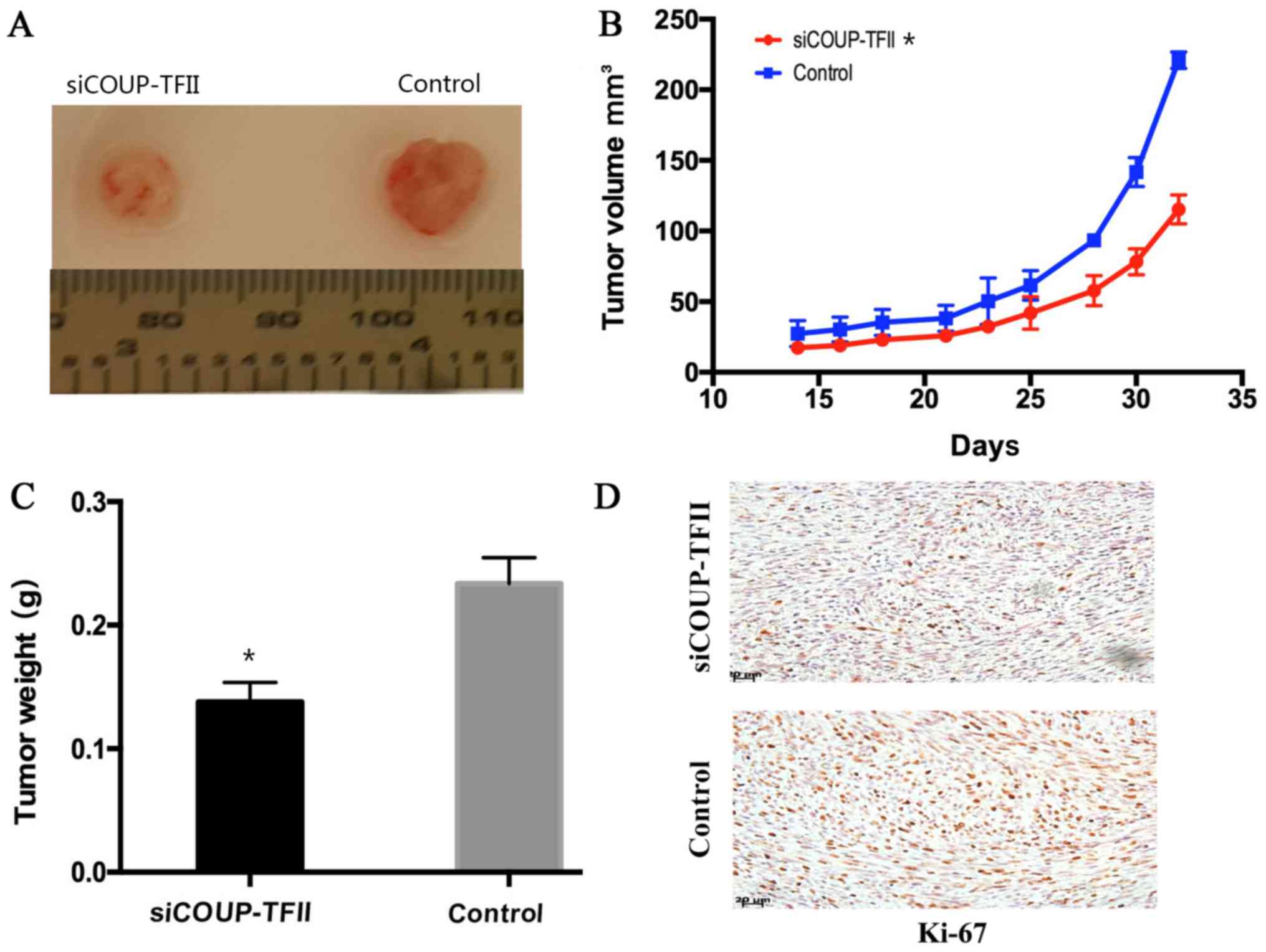
Downregulation of COUP-TFII suppresses
tumor growth in vivo
To assess the effect of siCOUP-TFII knockdown on
tumor volume, xenograft assays in SCID mice were performed. In U87
cell lines, siCOUP-TFII was able to slow down tumor growth
throughout the experiment. The size (Fig.
4A), volume (Fig. 4B) and weight
(Fig. 4C) of the tumors in the
siCOUP-TFII group were markedly smaller compared with the control
group. Tumor cell proliferation was then assessed by
immunohistochemical detection of Ki-67, and the results were
consistent with the in vitro findings. Ki-67 expression was
more evident in the control group (Fig.
4D), which indicated that the proliferation of U87 cells in
xenograft was inhibited by siCOUP-TFII. These results indicated
that the downregulation of COUP-TFII was able to suppress tumor
growth in vivo.
Discussion
In previous years, multiple studies have been
conducted concerning COUP-TFII, which has been identified to
contribute an essential role in the initiation and progression of
cancer. Therefore, COUP-TF11 has been indicated to be a potential
therapeutic target for cancer treatment. Wang et al
(14) reported that COUP-TFII was
upregulated in patients with prostate cancer, which can facilitate
a metabolic switch to enhance glycolysis and promote prostate
cancer progression. COUP-TFII cooperates with phosphatase and
tensin homolog deletion to promote prostate metastasis and
progression (15). This observation
provides a possibility of targeting COUP-TFII to regulate cancer
cell metabolism for prostate cancer therapy.
A growing number of studies support an important
role for COUP-TFII in cell fate determination (4,18,19); however, the underlying mechanism, by
which COUP-TFII regulates these processes, remains unclear. During
differentiation, previous studies have demonstrated that COUP-TFII
is regulated by microRNA (miR)-302a, miR-194, sonic hedgehog and
Wnt family member 3A (20–23). Lee et al (18) observed that fibroblast growth factor 2
is an inducer of COUP-TFII expression and may suppress the
osteogenic potential of mesenchymal cells by inducing COUP-TFII
expression. However, the role of COUP-TFII in glioma remains
unknown.
COUP-TFII modulates a large number of target genes
in various cell types (24),
including several genes in the glycolysis pathway. In the present
study, bioinformatics analysis predicted that the MPC1 promoter
contains the binding site of COUP-TFII, which indicated that MPC1
is regulated by COUP-TFII, and MPC1 was downregulated in U87
glioblastoma cells.
Previously, MPC1 and MPC2 were known as BRP44L and
BRP44. It was later reported that MPC1 and MPC2 form a complex that
transports pyruvate into mitochondria for further ATP production
(7,12,25).
Previous studies have indicated that MPC gene was downregulated in
a variety of tumors, including colon, kidney (13) and prostate cancer (14), and was closely associated with
prognosis. MPC has an important role in the initiation and
progression of tumor development, over-expression of MPC was able
to significantly inhibit colon cancer xenograft growth, colony
formation in soft agar and spheroid formation, indicating that MPC
may become a novel tumor therapy target (26). In the present study, in response to
COUP-TFII inhibition, MPC1 mRNA expression was increased, and the
proliferation of U87 cells was suppressed, The xenograft assay and
Ki-67 immunohistochemistry also demonstrated that, in response to
COUP-TFII inhibition, tumor growth was inhibited in
vivo.
In conclusion, the present study demonstrated that
MPC1 was downregulated in glioblastoma and regulated by COUP-TFII.
The inhibition of COUP-TFII may suppress the proliferation of
glioblastoma in vitro and in vivo; therefore,
COUP-TFII may be a novel therapeutic target of glioblastoma.
However, the mechanism underlying the effects of COUP-TFII in
glioblastoma remains to be elucidated and more in-depth research is
required.
Acknowledgements
The authors wish to thank the Central laboratory of
Second Affiliated Hospital of Nanchang University for lab
support.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81660420), the
Construction Plan of the Superior Science and Technology Innovation
Team of Jiangxi Province (grant no. 20152BCB24009), and the Foreign
Science and Technology Cooperation Plan of Jiangxi Province (grant
no. 20151BDH80009).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XZ and MW conceived and designed the study. BXi, YF,
MY, SL, BXu and YC performed the experiments. BXi and YF wrote the
paper, MW reviewed and edited the manuscript. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee (Nanchang University,
Jiangxi, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krex D, Klink B, Hartmann C, von Deimling
A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger
G, et al: Long-term survival with glioblastoma multiforme. Brain.
130:2596–2606. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao B, Zhou X, Ye M, Lv S, Wu M, Liao C,
Han L, Kang C and Zhu X: MicroRNA-566 modulates vascular
endothelial growth factor by targeting Von Hippel-Landau in human
glioblastoma in vitro and in vivo. Mol Med Rep. 13:379–385. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin FJ, Qin J, Tang K, Tsai SY and Tsai
MJ: Coup d'Etat: An orphan takes control. Endocr Rev. 32:404–421.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mi NL, Kim JW, Oh SH, Jeong BC, Hwang YC
and Koh JT: FGF2 stimulates COUP-TFII expression via the MEK1/2
pathway to inhibit osteoblast differentiation in C3H10T1/2 Cells.
PLoS One. 11:e01592342016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pereira FA, Tsai MJ and Tsai SY: COUP-TF
orphan nuclear receptors in development and differentiation. Cell
Mol Life Sci. 57:1388–1398. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eboli ML, Paradies G, Galeotti T and Papa
S: Pyruvate transport in tumour-cell mitochondria. Biochim Biophys
Acta. 460:183–187. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bricker DK, Taylor EB, Schell JC, Orsak T,
Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N,
et al: A mitochondrial pyruvate carrier required for pyruvate
uptake in yeast, Drosophila, and humans. Science. 337:96–100. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Herzig S, Raemy E, Montessuit S, Veuthey
JL, Zamboni N, Westermann B, Kunji ER and Martinou JC: Identi
cation and functional expression of the mitochondrial pyruvate
carrier. Science. 337:93–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang C, Ko B, Hensley CT, Jiang L, Wasti
AT, Kim J, Sudderth J, Calvaruso MA, Lumata L, Mitsche M, et al:
Glutamine oxidation maintains the TCA cycle and cell survival
during impaired mitochondrial pyruvate transport. Mol Cell.
56:414–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Warburg O, Wind F and Negelein E: The
metabolism of tumors in the body. J Gen Physiol. 8:519–530. 1927.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herzig S, Raemy E, Montessuit S, Veuthey
JL, Zamboni N, Westermann B, Kunji ER and Martinou JC:
Identification and functional expression of the mitochondrial
pyruvate carrier. Science. 337:93–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schell J, Olson K, Jiang L, Hawkins AJ,
Van Vranken JG, Xie J, Egnatchik RA, Earl EG, DeBerardinis RJ and
Rutter J: A Role for the mitochondrial pyruvate carrier as a
repressor of the warburg effect and colon cancer cell growth. Mol
Cell. 56:400–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang L, Xu M, Qin J, Lin SC, Lee HJ, Tsai
SY and Tsai MJ: MPC1, a key gene in cancer metabolism, is regulated
by COUPTFII in human prostate cancer. Oncotarget. 7:14673–14683.
2016.PubMed/NCBI
|
|
15
|
Qin J, Wu SP, Creighton CJ, Dai F, Xie X,
Cheng CM, Frolov A, Ayala G, Lin X, Feng XH, Ittmann MM, et al:
COUP-TFII inhibits TGF-beta-induced growth barrier to promote
prostate tumorigenesis. Nature. 493:236–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allen M, Bjerke M, Edlund H, Nelander S
and Westermark B: Origin of the U87MG glioma cell line: Good news
and bad news. Sci Transl Med. 8:354re32016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee S, Kang J, Yoo J, Ganesan SK, Cook SC,
Aguilar B, Ramu S, Lee J and Hong YK: Prox1 physically and
functionally interacts with COUP-TFII to specify lymphatic
endothelial cell fate. Blood. 113:1856–1859. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu SP, Yu CT, Tsai SY and Tsai MJ: Choose
your destiny: Make a cell fate decision with COUP-TFII. J Steroid
Biochem Mol Biol. 157:7–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu Z, Yu S, Hsu CH, Eguchi J and Rosen ED:
The orphan nuclear receptor chicken ovalbumin upstream
promoter-transcription factor II is a critical regulator of
adipogenesis. Proc Natl Acad Sci USA. 105:2421–2426. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okamura M, Kudo H, Wakabayashi KI, Tanaka
T, Nonaka A, Uchida A, Tsutsumi S, Sakakibara I, Naito M, Osborne
TF, et al: COUP-TFII acts down-stream of Wnt/beta-catenin signal to
silence PPARgamma gene expression and repress adipogenesis. Proc
Natl Acad Sci USA. 106:5819–5824. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeong BC, Kang IH, Hwang YC, Kim SH and
Koh JT: MicroRNA-194 reciprocally stimulates osteogenesis and
inhibits adipogenesis via regulating COUP-TFII expression. Cell
Death Dis. 5:e15322014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang IH, Jeong BC, Hur SW, Choi H, Choi
SH, Ryu JH, Hwang YC and Koh JT: MicroRNA-302a stimulates
osteo-blastic differentiation by repressing COUP-TFII Expression. J
Cell Physiol. 230:911–921. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsai SY and Tsai MJ: Chick ovalbumin
upstream pro-moter-transcription factors (COUP-TFs): Coming of age.
Endocr Rev. 18:229–240. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trachtulec Z and Forejt J: Synteny of
orthologous genes conserved in mammals, snake, fly, nematode, and
ssion yeast. Mamm Genome. 12:227–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vacanti NM, Divakaruni AS, Green CR,
Parker SJ, Henry RR, Ciaraldi TP, Murphy AN and Metallo CM:
Regulation of substrate utilization by the mitochondrial pyruvate
carrier. Mol Cell. 56:4252014. View Article : Google Scholar : PubMed/NCBI
|