Introduction
Thyroid cancer is the most common malignancy of the
endocrine organs. The incidence of thyroid cancer is increasing,
having increased more than three-fold during the last three decades
(1), mainly as a result of the
widespread use of diagnostic imaging and surveillance. At present,
in women in the USA, thyroid cancer is the fifth most common type
of cancer. In 2015, there were 62,000 new cases in women and men
(2). Papillary thyroid carcinoma
(PTC) is the most common histological type of thyroid cancer, which
is more common than other types, including follicular carcinoma,
medullary carcinoma, poorly differentiated carcinoma and anaplastic
carcinoma. Different types of thyroid cancer exhibit different
clinical behaviors from indolent tumors, with low mortality rates
in the majority of cases to aggressive malignancies, for example,
anaplastic thyroid cancer. Therefore, a proper and accurate
diagnosis is critical to provide an appropriate treatment approach
(3).
In order to provide accurate diagnoses and
personalized treatment, it is important to understand the molecular
mechanisms underlying thyroid cancer occurrence and progression
(4). As next generation sequence
(NGS) has developed, numerous studies have examined the mechanism
of thyroid cancer using NGS. There are several characteristic
genetic alterations, including point mutations in proto-oncogenes
(BRAF, NRAS, HRAS and KRAS) and chromosomal rearrangements
(RET/PTC1, RET/PTC3 and PAX8/PPARG), which vary with histologic
subtype (5).
Despite substantial progress in genetic research,
the molecular mechanism of PTC remains to be fully elucidated. In
order to further understand the occurrence and progression of PTC,
the present study performed whole transcriptome sequencing of 19
pairs of primary thyroid cancer samples with matched adjacent
normal thyroid tissues (6).
Subsequently, by application of bioinformatics, it was found that
upregulated Cbp/p300-interacting transactivators with glutamic acid
[E] and aspartic acid [D]-rich C-terminal domain 1 (CITED1) may be
a potential gene associated with PTC.
It is well known that several genes are
significantly overexpressed in PTC, which are potential markers in
molecular diagnosis (7–9). However, the mechanism involved in the
overexpression of these genes remains to be fully elucidated.
CITED1 is one of these genes (10,11). The
CITED1 family of transcriptional cofactors consists of four
members, which regulate diverse CBP/p300-dependent transcriptional
responses (12–14). In adults, the expression of CITED1 is
upregulated in PTC (11,15), malignant melanoma (16,17), and
Wilms tumor (18), which indicates
that CITED1 may effectively induce tumorigenesis and progression.
Other studies have found that there is a possible link between the
BRAF mutation and upregulated CITED1; however, knockdown of the
expression of BRAF by small interfering (si)RNA in a cell line with
the BRAF V600E mutation did not induce the suppression of CITED1
(7,19). Therefore, the biological function and
mechanism of CITED1 in PTC remain to be elucidated.
Although studies have demonstrated that the
expression level of CITED1 is associated with tumorigenesis, the
biological function of CITED1 in PTC has not been examined. In the
present study, transcriptome sequence analysis showed that the
CITED1 gene may be significantly involved in PTC. The present study
aimed to investigate the association between the expression of
CITED1 and clinicopathological features, and examine the biological
function of CITED1 in PTC cell lines.
Materials and methods
Patients and tissue collection
Fresh PTC tissue samples with adjacent normal
thyroid tissue samples were obtained from patients with PTC at the
First Affiliated Hospital of Wenzhou Medical University (Wenzhou,
China) between September 2015 and September 2016. A total of 47
pairs of fresh PTC tissues with normal tissues were frozen in
liquid nitrogen until further use. All tumor tissues were
histologically reviewed by two pathologists. Patients signed
informed consent and study protocols for the use of human tissues
were approved by and performed in accordance with the ethical
standards of the Ethics Committee of the First Affiliated Hospital
of Wenzhou Medical University. The CITED1 Reads Per Kilobase per
Million reads (RPKM) expression value was obtained via The Caner
Genome Atlas (TCGA) portal (https://cancergenome.nih.gov/). In total, 375 PTC
sequence data with complete clinical features and 58 pairs of
thyroid cancer with matched normal tissues were selected.
Cell lines and cell culture
The human thyroid cancer cell lines (TPC1 and BCPAP)
were provided by Professor Mingzhao Xing of Johns Hopkins
University School of Medicine (Baltimore, MA, USA). The TPC1 and
BCPAP cell lines were cultured in RPMI 1640 (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.), 1X MEM nonessential amino acids and 1X sodium pyruvate. All
cells were maintained in a humidified incubator at 37°C with 5%
CO2.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated from the tissues or cell
lines using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to manufacturer's protocol. Reverse
transcription was performed with ReverTra Ace® qPCR RT
Kit (Toyobo Co., Ltd., Osaka, Japan) according to the
manufacturer's protocol (20 µl reaction: 14 µl RNA+ 4 µl 5xRT
Buffer+ 1 µl Primer Mix+ 1 µl EnzymeMix, step 1 16°C for 5 min,
step 2 42°C for 30 min, step 3 98°C for 5 min). The RT-qPCR
analysis was performed using the Applied Biosystems QuantStudio 5
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with THUNDERBIRD SYBR qPCR mix (Toyobo Co., Ltd.) according
to the manufacturer's protocol (20 µl reaction: 1 µl cDNA+ 7 µl
RNA-free water+ 0.8 µl forward primer+ 0.8 µl reverse primer+ 0.4
µl ROX+ 10 µl Thunderbird SYBR qPCR Mix, the concentration of
primer was 0.2 µM, step 1 95°C for 2 min, step 2 95°C for 15 sec,
step 3 60°C for 60 sec, repeat step 2 and step 3 for 40 cycles,
final step 72°C for 5 min). GAPDH was used as internal control. The
relative expression levels were calculated using the
2−ΔΔCq equation (20). The
primer sequences for PCR were as follows: CITED1, forward
5′-AGGATGCCAACCAAGAGATG-3′ and reverse 5′-GTTTAGTGGGAGGGGTGGTT-3′;
GAPDH, forward 5′-GGTCGGAGTCAACGGATTTG-3′ and reverse
5′-ATGAGCCCCAGCCTTCTCCAT-3′.
Transfection
A siRNA against CITED1 was purchased from Shanghai
Gene Pharma (Shanghai, China). The TPC1 (6×104 cells)
and BCPAP (12×108 cells) cell lines were inoculated in
6-well plates 24 h prior to transfection to achieve 50% confluence.
RNAiMAX (Thermo Fisher Scientific, Inc.) was used to transfect
cells with the constructs. After 24 h, the cells were harvested,
and the knockdown efficiency was verified using RT-qPCR analysis.
The siRNA sequences were as follows: siRNA1, forward
5′-GGCCUGCACUUGAUGUCAATT-3′ and reverse
5′-UUGACAUCAAGUGCAGGCCTT-3′; siRNA2, forward
5′-GAGCCCUGCUAUCAUCGAUTT-3′ and reverse
5′-AUCGAUGAUAGCAGGGCUCTT-3′; siRNA3, forward
5′-GGGAUCUCCAAUAGGCUCUTT-3′ and reverse
5′-AGAGCCUAUUGGAGAUCCCTT-3′. All assays were performed in
triplicate.
Cell proliferation assay
The thyroid cancer TPC1 (2×103 cells) and
BCPAP cells (3×103 cells) were plated into a 96-well
plate and then transfected with siRNA. The plates were harvested
daily. A total of 20 µl MTS (Solution Cell Proliferation Assay;
Promega, Fitchburg, WI, USA) was added to each well with 100 µl
aforementioned medium and the plate was placed at room temperature
for 2 h. The absorbance was read at 490 nm using a Spectramax M5
microplate reader (Molecular Devices, Sunnyvale, CA, USA). All
assays were performed in triplicate.
Colony formation assay
At 48 h post-transfection, the two transfected cell
lines or control cells (2×103 cells for TPC1 and
4×103 cells for BCPAP) were initially seeded into each
well of a six-well plate, and maintained in medium containing 10%
FBS, which was refreshed every 2 days. Following incubation of the
cells for 10 days at 37°C in 5% CO2, their colonies were
visible to the naked eye. The cells were fixed with 4%
paraformaldehyde (PFA; Sigma; Merck Millipore, Darmstadt, Germany)
for 30 min and stained with 0.01% crystal violet for 30 min. The
colony numbers were counted using ImageJ 1.5 software (National
Institutes of Health, Bethesda, MD, USA). All assays were performed
in triplicate.
Migration and invasion assays
The migration and invasion assays were performed
using Transwell chambers with membranes (Corning, Inc., Corning,
NY, USA). The membranes were uncoated for the migration assays and
were coated with 25 µg Matrigel® (BD Biosciences,
Franklin Lakes, NJ, USA) for the invasion assays. The membranes
were incubated with PBS (migration) or Matrigel® for 4 h
at 37°C in a 5% CO2 atmosphere. The transfected cells
and control cells (migration assays: 3×104 cells for
TPC1 and 5×104 for BCPAP; invasion assays:
6×104 cells for TPC1, 8×104 cells for BCPAP)
were suspended in culture medium containing 5% FBS and plated in
the upper chamber; the lower chamber contained culture medium with
10% FBS. After 24 h at 37°C in 5% CO2, the non-migrating
cells on the top chamber were removed using a cotton swab, and
those cells that migrated through the membrane were fixed (4% PFA
in PBS) and stained with 0.5% crystal violet. Images of the cells
were captured using a light microscope.
Statistical analysis
Data are presented as the mean ± standard deviation.
Student's t-test (unpaired) was used to compare two groups. Two-way
analysis of variance were used with an LSD post hoc test to
identify significant differences among multiple groups. Categorical
variables are expressed as a percentage and were compared with a
χ2 test or Fisher's exact test, as appropriate.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was performed with SPSS software
version 22.0 (IBM SPSS, Armonk, NY, USA). GraphPad Prism 5
(GraphPad Software, Inc., La Jolla, CA, USA) was used for
graphs.
Results
CITED1 is upregulated in PTC
In order to assess the results from the whole
transcriptome sequence analysis (Fig.
1A, P<0.001), the mRNA expression of CITED1 in 58 pairs of
thyroid cancer with matched normal thyroid tissues was analyzed in
TCGA and it was found that CITED1 was significantly upregulated in
the PTC tissues, compared with that in the normal thyroid tissues
(Fig. 1B, P<0.001). Using RT-qPCR
analysis, the mRNA expression of CITED1 was then evaluated among 47
pairs of PTC samples with matched adjacent normal thyroid tissues.
The expression of CITED1 was significantly upregulated in the tumor
samples, compared with that in the normal tissues (Fig. 1C, P<0.001), which was in accordance
with the transcriptome sequence and TCGA datasets. In addition,
receiver operating characteristic analysis was used to assess the
potential diagnostic value of CITED1. For the TCGA cohort, the
expression of CITED1 to distinguish thyroid cancer tissues from
normal tissues had an area under the curve (AUC) value of 0.913
with a sensitivity/specificity of 81.5/98.3% (Fig. 1D). For the validated cohort, CITED1
identified thyroid cancer with an AUC value of 0.853, a sensitivity
of 80.9%, and a specificity of 80.9% (Fig. 1E).
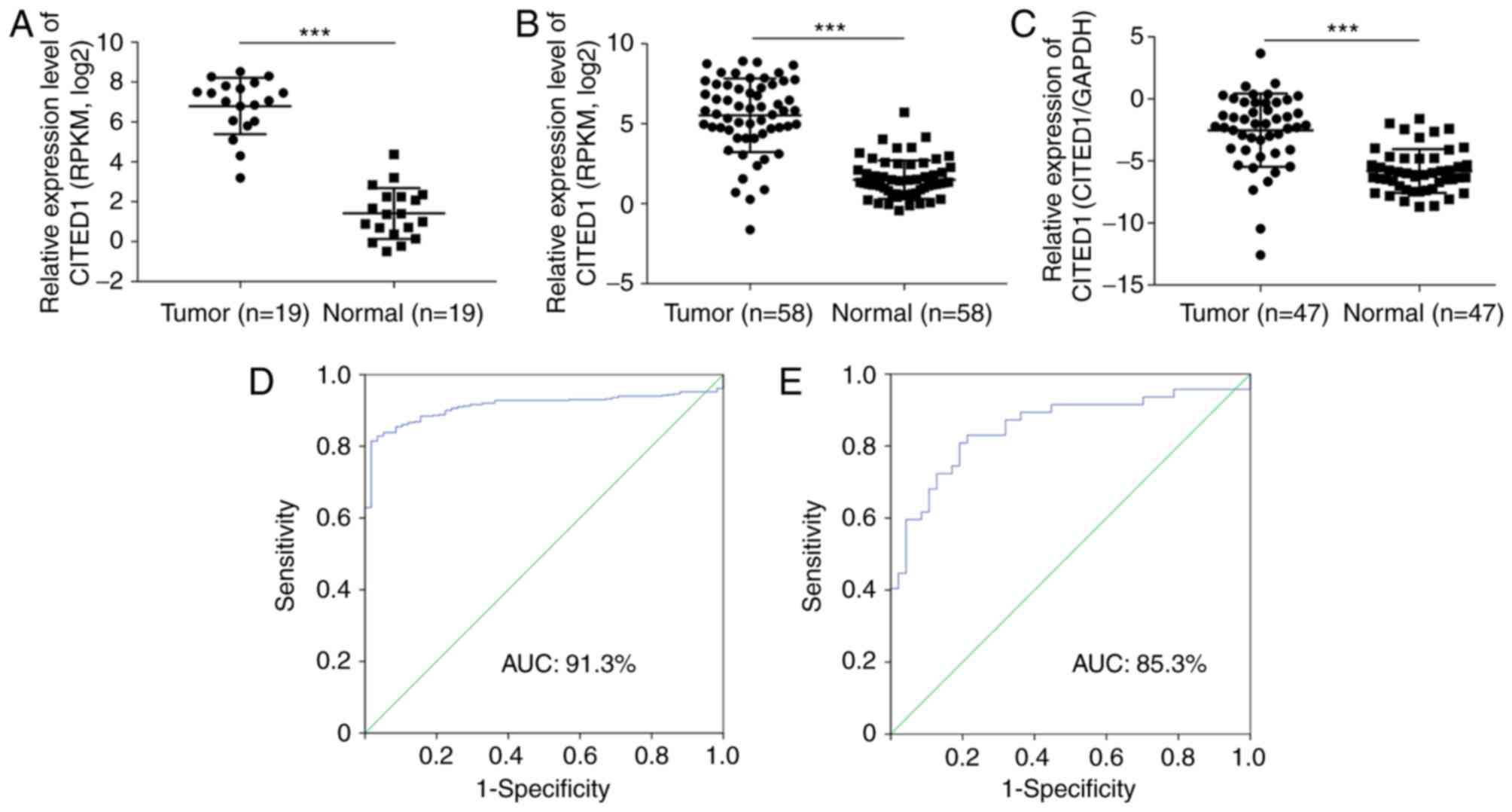 | Figure 1.CITED1 expression of sequence
datasets, TCGA cohort and validated cohort in thyroid cancer. (A)
Sequence datasets contained 19 tumor tissues and matched adjacent
normal thyroid tissues. (B) TCGA cohort contained 58 tumor tissues
and matched adjacent normal thyroid tissues. RPKM was represented
by the expression of UNC5B-as1. (C) Expression of CITED1 of the
validated cohort, 47 tumor tissues and matched adjacent normal
thyroid tissues by reverse transcription-quantitative polymerase
chain reaction analysis. (D) ROC curve for expression of CITED1 to
diagnose PTC in TCGA cohort. The AUC was 91.3%, with 81.5%
sensitivity and 98.3% specificity. (E) ROC curve for expression of
CITED1 to diagnose PTC in the validated cohort. The AUC was 85.3%,
with 80.9% sensitivity and 80.9% specificity. Data are presented as
the mean ± standard deviation ranges of UNC5B-as1 expression.
***P<0.001, compared with the normal control group using
Student's t-test. PTC, papillary thyroid carcinoma; CITED1,
Cbp/p300-interacting transactivators with glutamic acid [E] and
aspartic acid [D]-rich C-terminal domain 1; RPKM, Reads Per
Kilobase per Million reads; TGCA, The Cancer Genome Atlas; ROC,
receiver operating characteristic; AUC, area under the curve. |
Association between the expression of
CITED1 and clinicopathological features
To investigate the role of CITED1 in the
tumorigenesis and progression in PTC, the present study analyzed
the association between the expression of CITED1 and
clinicopathological features. The PTC patient group was divided
into two groups by the expression of CITED1, based on the median
value, as a low expression group and high expression group. In the
TCGA cohort, there was a significant association between a high
expression of CITED1 and lymph node metastasis (P=0.006) and
clinical stage (P=0.003), as shown in Table I. However, the expression of CITED1
was not associated with age (P=0.214), gender (P=228), tumor size
(P=0.835) or histological type (P=0.935) in either cohort
(P>0.05).
 | Table I.Association between the expression of
CITED1 and clinicopathological features in The Cancer Genome Atlas
cohort. |
Table I.
Association between the expression of
CITED1 and clinicopathological features in The Cancer Genome Atlas
cohort.
| Clinicopathological
features | Low CITED1 expression
(n=187), n (%) | High CITED1
expression (n=188), n (%) | P-value |
|---|
| Age (years) |
|
| 0.214 |
| Mean ±
SD | 45.5±15.4 | 47.5±15.3 |
|
| ≤45 | 94 (50.3) | 82 (43.6) |
|
|
>45 | 93 (49.7) | 106 (56.4) |
|
| Gender |
|
| 0.228 |
| Male | 54 (28.9) | 44 (23.4) |
|
|
Female | 133 (71.1) | 144 (76.6) |
|
| Tumor size (cm) |
|
| 0.835 |
|
Mean | 3.03±1.77 | 2.79±1.64 |
|
| ≤1 | 15 (8.0) | 14 (7.4) |
|
|
>1 | 172 (92.0) | 174 (92.6) |
|
| Lymph node
metastasis |
|
| 0.006 |
| No | 95 (50.8) | 69 (36.7) |
|
|
Yes | 92 (49.2) | 119 (63.3) |
|
| Clinical stage |
|
| 0.003 |
|
I+II | 131 (70.1) | 104 (55.3) |
|
|
III+IV | 56 (29.9) | 84 (44.7) |
|
| Histological
type |
|
| 0.935 |
|
Classical | 134 (71.7) | 134 (71.3) |
|
|
Other | 53 (28.3) | 54 (28.7) |
|
Upregulated expression of CITED1
increases the risk of lymph node metastasis in patients with
PTC
In order to investigate whether the expression of
CITED1 is a major risk factor for lymph node metastasis, logistic
regression was performed. Univariate logistic regression analysis
demonstrated that the significant variables for lymph node
metastasis were expression of CITED1 (OR 1.781, 95% CI 1.179–2.69,
P=0.006), age (OR 0.646, 95% CI 0.428–0.976, P=0.038), gender (OR
0.501, 95% CI 0.308–0.815, P=0.005), histological type (OR 0.358,
95% CI 0.226–0.569, P<0.001) and tumor size (OR 2.242, 95% CI
1.028–4.889, P=0.042), as shown in Table
II. Multivariate logistic analysis also showed that the
expression of CITED1 (OR 2.007, 95% CI 1.295–3.109, P=0.002), age
(OR 0.639, 95% CI 0.412–0.989, P=0.045), gender (OR 0.473, 95% CI
0.284–0.786, P=0.004) and histological type (OR 0.371, 95% CI
0.229–0.600, P<0.001) were positively correlated with increased
lymph node metastasis, whereas lymph node metastasis status was not
associated with tumor size (OR 2.171 95% CI 0.962–4.900, P=0.062;
Table III). Therefore, upregulated
CITED1 increased the risk of lymph node metastasis in patients with
PTC.
 | Table II.Univariate logistic regression
analysis for the risk of lymph node metastasis. |
Table II.
Univariate logistic regression
analysis for the risk of lymph node metastasis.
| Factor | OR | 95% CI | P-value |
|---|
| CITED1 expression
(high, vs. low) | 1.781 | 1.179–2.69 |
0.006 |
| Age, years (≤45 vs.
>45) | 0.646 | 0.428–0.976 |
0.038 |
| Gender (male vs.
female) | 0.501 | 0.308–0.815 |
0.005 |
| Histological
type | 0.358 | 0.226–0.569 | <0.001 |
| Tumor size
(cm) | 2.242 | 1.028–4.889 |
0.042 |
 | Table III.Multivariate logistic regression
analysis for risk of lymph node metastasis. |
Table III.
Multivariate logistic regression
analysis for risk of lymph node metastasis.
| Factor | OR | 95% CI | P-value |
|---|
| CITED1 expression
(high, vs. low) | 2.007 | 1.295–3.109 |
0.002 |
| Age (≤45 vs.
>45) | 0.639 | 0.412–0.989 |
0.045 |
| Gender (male vs.
female) | 0.473 | 0.284–0.786 |
0.004 |
| Histological
type | 0.371 | 0.229–0.600 | <0.001 |
| Tumor size
(cm) | 2.171 | 0.962–4.900 |
0.062 |
Downregulated expression of CITED1
inhibits cell proliferation in PTC cell lines
As the CITED1 gene is commonly upregulated in PTC,
it was hypothesized that this gene is important in tumorigenesis
and progression. Therefore, the present study selected effective
siRNA1 and siRNA2 to significantly knock down the expression of
CITED1 in the cell lines, and the expression level was evaluated
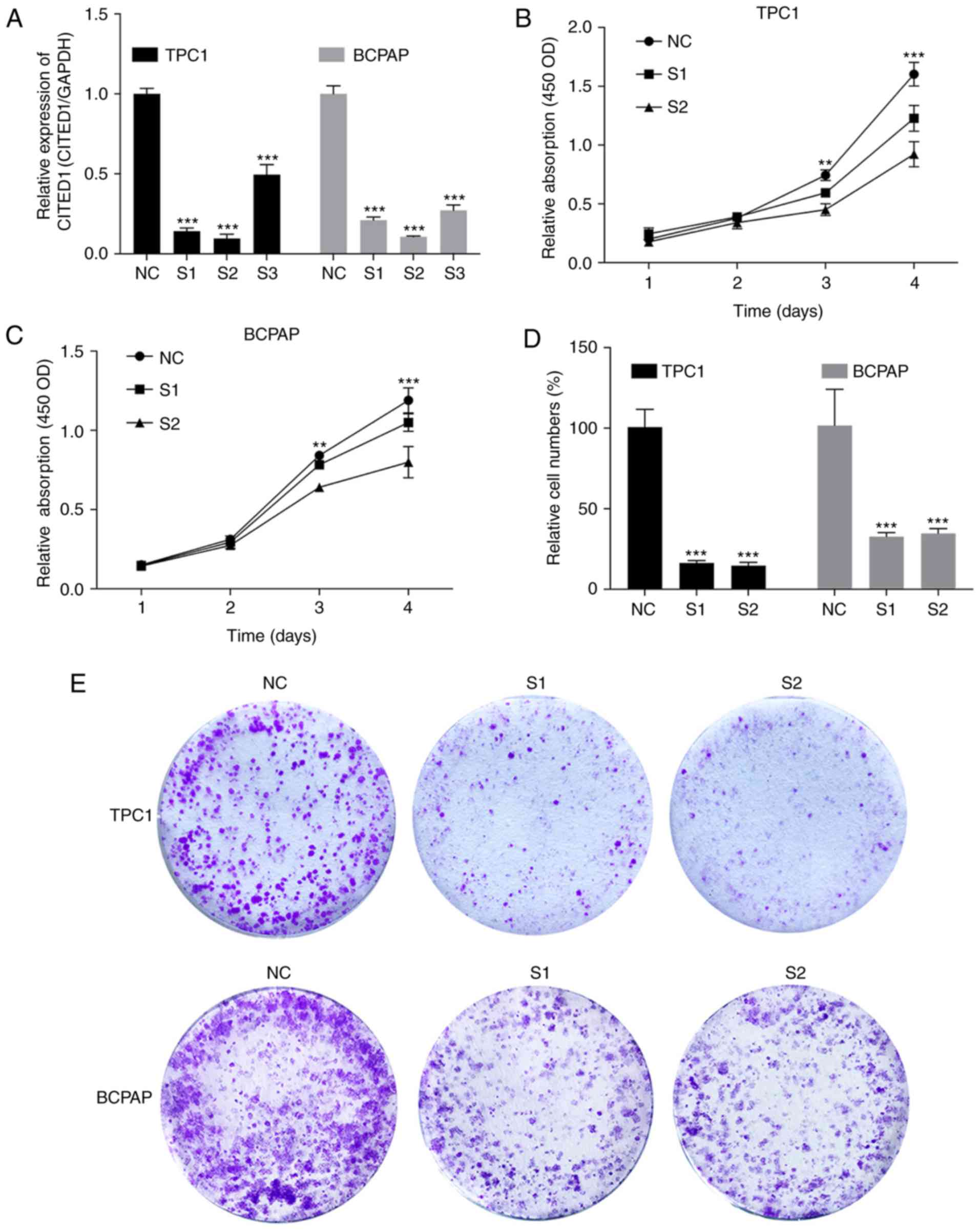
using RT-qPCR analysis (Fig. 2A).
Cell proliferation assays and colony formation assays were then
performed. The results revealed that the downregulation of CITED1
effectively inhibited thyroid cell line proliferation (Fig. 2B and C, P<0.001) and colony
formation (Fig. 2D and E,
P<0.001), compared with the control group.
Downregulated of CITED1 inhibits
migration and invasion in PTC cell lines
As the increased expression level of CITED1 was
associated with lymph node metastasis in the clinical feature
analysis, the role of CITED1 in PTC was demonstrated using
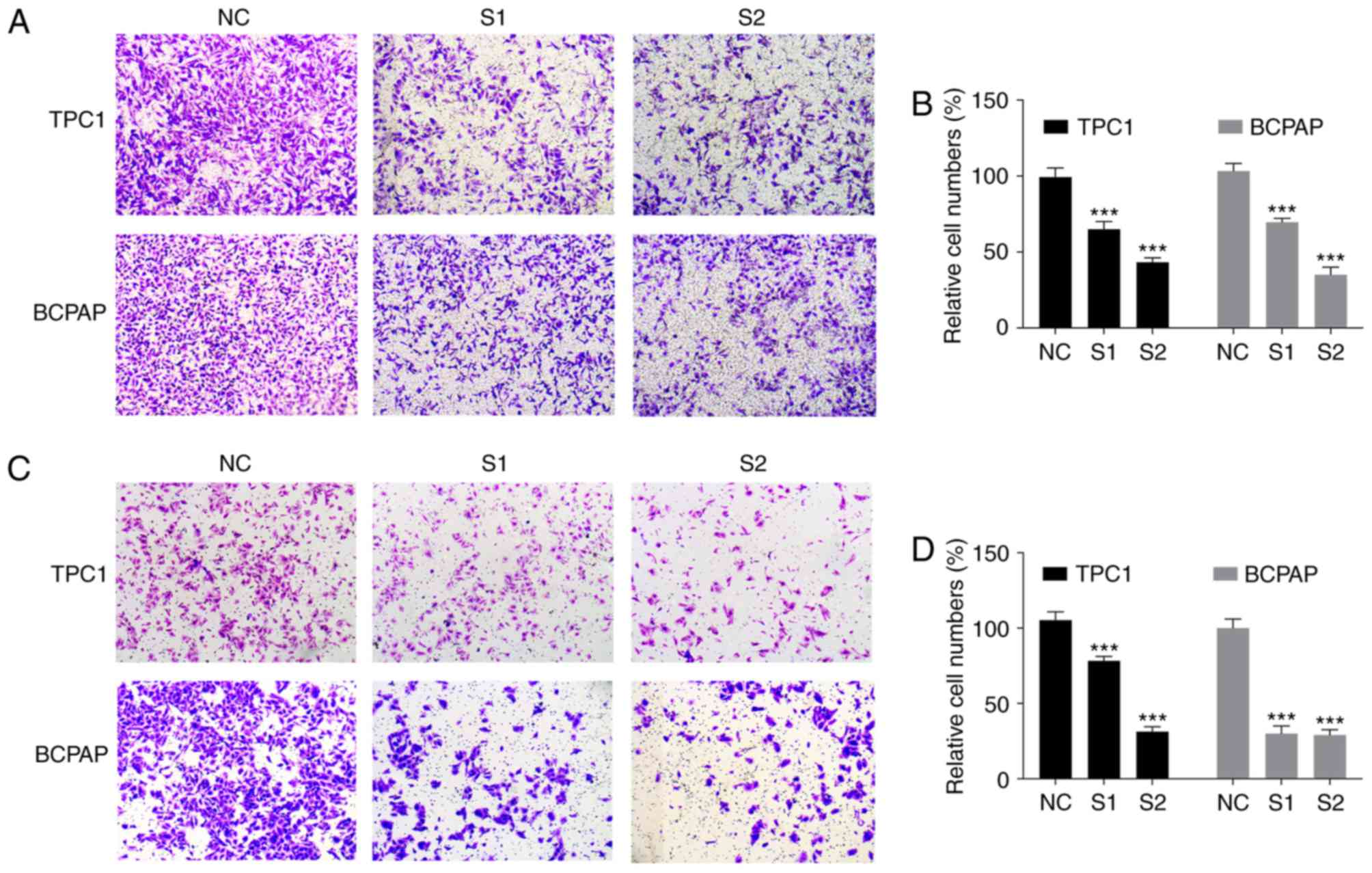
migration and invasion assays. The data revealed that downregulated
CITED1 significantly inhibited the PTC cell migration capacity,
compared with that in the control group (P<0.001, Fig. 3A and B). The Transwell invasion assays
also showed that downregulated CITED1 effectively inhibited the
invasion capacity of the PTC cells (P<0.001, Fig. 3C and D). The biological function of
CITED1 in these experiments was consistent with the results of the
clinical feature analysis, revealing that CITED1 is a potential
risk factor for metastasis in PTC.
Discussion
PTC is the most common type of thyroid malignancy,
accounting for >85% of all cases (21). PTC has a high propensity for lymph
node metastasis, with over one-third of patients having clinically
detectable lymph node involvement on initial presentation (22). Even in patients with no detectable
nodal disease on examination, ~80% of patients present with
micrometastatic lymph node disease on postoperative pathologic
examination (23). Several studies
have identified that lymph node metastasis can increase the risk of
local recurrence, which is a poor prognostic factor (24–26). In
order to avoid unnecessary lymph node dissection, it is important
to identify a gene associated with PTC as a molecular marker, which
can predict the progression and lymph node metastasis status of
PTC.
Although thyroid cancer has been investigated in
previous decades, ~4% of PTC cases are without a known oncogenetic
driver or epigenetic alterations (27). In the present study, it was found that
CITED1 may be a potentially important PTC-associated gene, which
was significantly upregulated on analysis of the sequencing
dataset. In addition, the expression level of CITED1 was confirmed
in TCGA data sets and by relative expression by RT-qPCR
analysis.
It is known that CITED1 is important in several
types of tumor. In breast cancer, it has been found that expression
level of CITED1 parallels that of estrogen receptor (ER)α, which
resulted in a good outcome. This suggested that potential
maintenance of the ERα-CITED1 co-regulated signaling pathway in
breast tumors can indicate good prognosis (28). However, in other types of tumor,
CITED1 is a gene associated with tumorigenesis. The mRNA expression
of CITED1 is associated with CXXC finger protein 4 and Kringle
containing transmembrane protein 1, which indicated that it as a
potential marker in embryonic liver tumors (29). In papillary thyroid cancer,
hypomethylation of the CpGs in the promoter region of CITED1 is
associated with a higher mRNA expression of CITED1, indicating that
epigenetic regulation is involved in the overexpression of CITED1
(30). The expression of CITED1 has
also been identified as an independent risk factor for lymph node
metastasis in patents with T3 colorectal cancer, and CITED1 has the
potential ability to predict the presence of lymph node metastasis
in patients with T1 colorectal cancer (31). These studies are consistent with the
results of the present study that the CITED1 was a gene associated
with PTC and increased the risk of lymph node metastasis in
PTC.
In the present study, the mRNA expression level of
CITED1 was evaluated by RT-qPCR analysis, which was consistent with
the bioinformatics analysis. In addition, logistic regression
analysis indicated that a high expression of CITED1 was a risk
factor for lymph node metastasis in patients with PTC. In PTC cell
lines, it was demonstrated that downregulated CITED1 inhibited cell
proliferation and colony formation, and decreased migration and
invasion, which was observed using cellular and molecular
approaches. These results were consistent with clinicopathological
features that showed CITED1 was associated with lymph node
metastasis and increased risk of metastasis.
The present study, as with others, had several
limitations. First, the function of CITED1 was not verified in
vivo. Second, the mechanism of CITED1 in tumorigenesis and
metastasis remains to be elucidated and requires further
investigation. Finally, the number of patients recruited was
limited and additional cases are required to provide more rigorous
results.
In conclusion, the present study investigated the
association between CITED1 and lymph node metastasis, and the
function of CITED1 in vitro. It was found that CITED1 was
significantly associated with lymph node metastasis, and that
CITED1 resulted in increased tumorigenesis and metastasis. These
findings provide a potential diagnostic and therapeutic molecular
marker for PTC.
Acknowledgements
The authors would like to thank Ms.Jizhao Niu
(Division of Cardiothoracic Surgery, The First Affiliated Hospital
of Wenzhou Medical University) for their support with the
study.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81372380).
Availability of data and materials
The data sets supporting the conclusions of this
article are included in this article. Raw data are available on the
main electronic data storage system of the First Affiliated
Hospital of Wenzhou Medical University and access is available from
the corresponding author on reasonable request.
Ethical approval and consent to
participate
Written informed consent was obtained from each
individual participant. Ethical approval for this study was
obtained from the Ethics Committee of the First Affiliated Hospital
of Wenzhou Medical University.
Consent for publication
Written informed consent was obtained from each
individual participant for the publication of their data.
Authors' contributions
AB and FY analyzed the raw data and wrote the
original draft. YW and EX investigated and interpreted the data. ZY
and JN collected the raw data. OW conceptualized and designed the
study and provided supervision. All authors read and approved the
final manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith RA, Manassaram-Baptiste D, Brooks D,
Doroshenk M, Fedewa S, Saslow D, Brawley OW and Wender R: Cancer
screening in the United States, 2015: A review of current American
cancer society guidelines and current issues in cancer screening.
CA Cancer J Clin. 65:30–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cabanillas ME, McFadden DG and Durante C:
Thyroid cancer. Lancet. 388:2783–2795. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cha YJ and Koo JS: Next-generation
sequencing in thyroid cancer. J Transl Med. 14:3222016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hunt J: Understanding the genotype of
follicular thyroid tumors. Endocr Pathol. 16:311–321. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang QX, Chen ED, Cai YF, Li Q, Jin YX,
Jin WX, Wang YH, Zheng ZC, Xue L, Wang OC and Zhang XH: A panel of
four genes accurately differentiates benign from malignant thyroid
nodules. J Exp Clin Cancer Res. 35:1692016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watanabe R, Hayashi Y, Sassa M, Kikumori
T, Imai T, Kiuchi T and Murata Y: Possible involvement of BRAFV600E
in altered gene expression in papillary thyroid cancer. Endocr J.
56:407–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nikiforova MN and Nikiforov YE: Molecular
diagnostics and predictors in thyroid cancer. Thyroid.
19:1351–1361. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang KT and Lee CH: BRAF mutation in
papillary thyroid carcinoma: Pathogenic role and clinical
implications. J Chin Med Assoc. 73:113–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang Y, Prasad M, Lemon WJ, Hampel H,
Wright FA, Kornacker K, LiVolsi V, Frankel W, Kloos RT, Eng C, et
al: Gene expression in papillary thyroid carcinoma reveals highly
consistent profiles. Proc Natl Acad Sci USA. 98:15044–15049. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prasad ML, Pellegata NS, Kloos RT,
Barbacioru C, Huang Y and de la Chapelle A: CITED1 protein
expression suggests Papillary Thyroid Carcinoma in high throughput
tissue microarray-based study. Thyroid. 14:169–175. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shioda T, Fenner MH and Isselbacher KJ:
MSG1, a novel melanocyte-specific gene, encodes a nuclear protein
and is associated with pigmentation. Proc Natl Acad Sci USA.
93:12298–12303. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yahata T, Shao W, Endoh H, Hur J, Coser
KR, Sun H, Ueda Y, Kato S, Isselbacher KJ, Brown M and Shioda T:
Selective coactivation of estrogen-dependent transcription by
CITED1 CBP/p300-binding protein. Genes Dev. 15:2598–2612. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shioda T, Fenner MH and Isselbacher KJ:
MSG1 and its related protein MRG1 share a transcription activating
domain. Gene. 204:235–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prasad ML, Pellegata NS, Huang Y, Nagaraja
HN, de la Chapelle A and Kloos RT: Galectin-3, fibronectin-1,
CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful
for the differential diagnosis of thyroid tumors. Mod Pathol.
18:48–57. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li H, Ahmed NU, Fenner MH, Ueda M,
Isselbacher KJ and Shioda T: Regulation of expression of MSG1
melanocyte-specific nuclear protein in human melanocytes and
melanoma cells. Exp Cell Res. 242:478–486. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sedghizadeh PP, Williams JD, Allen CM and
Prasad ML: MSG-1 expression in benign and malignant melanocytic
lesions of cutaneous and mucosal epithelium. Med Sci Monit.
11:BR189–BR194. 2005.PubMed/NCBI
|
|
18
|
Lovvorn HN III, Boyle S, Shi G, Shyr Y,
Wills ML, Perantoni AO and de Caestecker M: Wilms' tumorigenesis is
altered by misexpression of the transcriptional co-activator,
CITED1. J Pediatr Surg. 42:474–481. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schweppe RE, Klopper JP, Korch C,
Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow LA, Copland
JA, Smallridge RC and Haugen BR: Deoxyribonucleic acid profiling
analysis of 40 human thyroid cancer cell lines reveals
cross-contamination resulting in cell line redundancy and
misidentification. J Clin Endocrinol Metab. 93:4331–4341. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuo SF, Lin SF, Chao TC, Hsueh C, Lin KJ
and Lin JD: Prognosis of multifocal papillary thyroid carcinoma.
Int J Endocrinol. 2013:8093822013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sancho JJ, Lennard TW, Paunovic I,
Triponez F and Sitges-Serra A: Prophylactic central neck disection
in papillary thyroid cancer: A consensus report of the European
society of endocrine surgeons (ESES). Langenbecks Arch Surg.
399:155–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pereira JA, Jimeno J, Miquel J, Iglesias
M, Munné A, Sancho JJ and Sitges-Serra A: Nodal yield, morbidity,
and recurrence after central neck dissection for papillary thyroid
carcinoma. Surgery. 138:1095–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Machens A, Hinze R, Thomusch O and Dralle
H: Pattern of nodal metastasis for primary and reoperative thyroid
cancer. World J Surg. 26:22–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zaydfudim V, Feurer ID, Griffin MR and
Phay JE: The impact of lymph node involvement on survival in
patients with papillary and follicular thyroid carcinoma. Surgery.
144:1070–1078. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leboulleux S, Rubino C, Baudin E, Caillou
B, Hartl DM, Bidart JM, Travagli JP and Schlumberger M: Prognostic
factors for persistent or recurrent disease of papillary thyroid
carcinoma with neck lymph node metastases and/or tumor extension
beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol
Metab. 90:5723–5729. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cancer Genome Atlas Research Network:
Integrated genomic characterization of papillary thyroid carcinoma.
Cell. 159:676–690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McBryan J, Howlin J, Kenny PA, Shioda T
and Martin F: ERalpha-CITED1 co-regulated genes expressed during
pubertal mammary gland development: Implications for breast cancer
prognosis. Oncogene. 26:6406–6419. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murphy AJ, de Caestecker C, Pierce J,
Boyle SC, Ayers GD, Zhao Z, Libes JM, Correa H, Walter T, Huppert
SS, et al: CITED1 expression in liver development and
hepatoblastoma. Neoplasia. 14:1153–1163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sassa M, Hayashi Y, Watanabe R, Kikumori
T, Imai T, Kurebayashi J, Kiuchi T and Murata Y: Aberrant promoter
methylation in overexpression of CITED1 in papillary thyroid
cancer. Thyroid. 21:511–517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nasu T, Oku Y, Takifuji K, Hotta T,
Yokoyama S, Matsuda K, Tamura K, Ieda J, Yamamoto N, Takemura S, et
al: Predicting lymph node metastasis in early colorectal cancer
using the CITED1 expression. J Surg Res. 185:136–142. 2013.
View Article : Google Scholar : PubMed/NCBI
|