Introduction
With the rapid development of industrialization,
lung cancer has become the most common type of malignant tumor,
with high rates of morbidity and mortality (1). It has been reported that >730,000
cases were diagnosed, and ~610,000 mortalities due to lung cancer
occurred in China in 2015 (2).
Non-small cell lung cancer (NSCLC) accounts for ~85% lung cancer
cases, and the majority of patients are diagnosed at a late stage
of NSCLC, and thus have a poor prognosis (3). Lung adenocarcinoma is the most common
subtype of NSCLC, with a high recurrence rate and short survival
time (4). According to previous
research, oncogenes serve an important role in the occurrence and
development of lung adenocarcinomas, and may be potential
therapeutic targets (4–6).
Epidermal growth factor receptor (EGFR) is an
important driving gene in lung adenocarcinoma, and it has been
reported that EGFR mutations are more common in Asian patients,
non-smokers and females (6). Previous
studies have demonstrated that mutation of EGFR is a positive
predictor of prognosis for patients with lung adenocarcinoma
(7–10). Patients with EGFR mutations have been
indicated to respond well to EGFR tyrosine kinase inhibitors
(EGFR-TKIs) (11). However, it has
been demonstrated that patients with different EGFR mutation
subtypes experience different outcomes following EGFR-TKI treatment
(8,12,13).
Therefore, the association between EGFR mutations and survival time
of patients with lung adenocarcinoma requires further
investigation. Furthermore, in developing countries with limited
economic conditions, including China, EGFR mutations of patients
with lung adenocarcinoma often go undetected (14). This highlights the importance of
characterizing the significance of EGFR mutations in lung
adenocarcinoma and the associated clinicopathological
characteristics. The International Association for the Study of
Lung Cancer, American Thoracic Society and European Respiratory
Society (IASLC/ATS/ERS) classification system (2011 version) is
often used to classify lung adenocarcinoma (13,15).
However, there is some controversy over its effectiveness (15–17).
Using IASLC/ATS/ERS classification, EGFR mutation
detection was performed using tissues from 219 patients with lung
adenocarcinoma. The associations between EGFR mutation status and
clinical characteristics were analyzed, and the significance was
evaluated in the context of survival time to provide empirical and
theoretical foundations for the improvement of the clinical
treatment of lung cancer.
Materials and methods
Patients and clinical data
A total of 435 patients with primary lung
adenocarcinoma, who underwent surgical resection between October
2012 and March 2013 at the Affliated Hospital of Binzhou Medical
University (Yantai, China) or the Yuhuangding Hospital (Yantai,
China), were invited to participate in the present study. However,
216 patients were excluded due to the lack of follow-up information
or accurate classification following surgery. The final 219
participants included 105 females and 114 males, with a mean age of
60 years (range, 30–88 years).
Biopsy materials were selected in accordance with
the National Comprehensive Cancer Network guidelines of 2011
(18). The tissues were classified by
2 experienced pathologists of the Affliated Hospital of Binzhou
Medical University (Yantai, China) and the Yuhuangding Hospital
(Yantai, China), using the IASLC/ATS/ERS system (19). Pathological Tumor-Node-Metastasis
(pTNM) classification was performed according to the international
lung cancer staging system (20).
Adenocarcinoma subtypes included minimally invasive adenocarcinoma
(MIA), invasive adenocarcinoma (IA) and invasive adenocarcinoma
variant (IAV).
The present study was approved by the Medical
Research Ethics Committee of Binzhou Medical University (Yantai,
China), and all patients provided written informed consent for
their participation in the present study. No patients had received
prior radiotherapy or chemotherapy. The postoperative treatment was
as follows: Pemetrexed and cisplatin for patients without EGFR
mutations or with mutations in exon 20; the first-line TKI,
gefitinib, for patients with 19-Del and L858R mutations, and the
second-line TKI, afatinib, for patients with other EGFR mutations.
All patients were followed-up by telephone or hospital appointment,
including a computed tomography scan of the chest and upper
abdomen. Tumor-enlargement or identification of distant metastasis
was considered indicative of disease recurrence. Non-smokers were
defined as smoking <100 cigarettes in lifetime. The final
follow-up took place on April 30th, 2017. The overall survival (OS)
time was defined as the period from surgery to the last day of
follow-up, or the occurrence of mortality.
EGFR mutation detection
All surgical specimens were fixed in formalin and
embedded in paraffin. The sample DNA was obtained using a paraffin
tissue DNA Extraction kit (cat. no. 56404; Qiagen GmbH, Hilden,
Germany). The concentration of DNA was adjusted to 1 ng/µl, and
EGFR mutations were detected using the amplification refractory
mutation system (ARMS) with human EGFR Mutations Detection kit
(cat. no. ADx-EG01; Amoy Diagnostics, Co., Ltd., Xiamen, China),
and the assay was performed according to the manufacturer's
protocol and as previously described (21). In brief, the ARMS-PCR assay was
performed in a 50-µl volume containing 5 µl PCR buffer, 10 pM
forward and reverse primers, 20 pM probe and 12.5 µM dNTPs. The
thermocycling conditions were as follows: 95°C for 5 min, then 15
cycles of 95°C for 25 sec, 64°C for 20 sec and 72°C for 20 sec,
followed by 31 cycles of 93°C for 25 sec, 60°C for 35 sec and 72°C
for 20 sec. The human EGFR Mutations Detection kit is able to
detect 29 EGFR mutations from exon 18 to exon 21, including 3-point
mutations in exon 18 (G719X), 19 19-Del mutations, 3 insertion
mutations in exon 20 (20-Ins), T790M, S768I, L858R, and another
base-pair substitution mutation in exon 21 (L861Q).
Statistical analysis
The associations between EGFR mutations and clinical
characteristics were analyzed using χ2. OS curves, which
were constructed using the Kaplan-Meier method, and further
evaluation was performed using the log-rank test. The association
between clinical characteristics and OS time was analyzed using Cox
regression. All statistical analyses were performed using SPSS
software (version 17.0; SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Descriptive statistics
The patients' clinical data are presented in
Table I. Of the 219 lung
adenocarcinoma patients enrolled in the present study, 54 patients
(24.7%) were current or former smokers, 171 patients (78.1%) had
pTNM stage I tumors, 170 patients (77.6%) had T1 stage tumors, 184
patients (84%) were classified with N0 stage lung adenocarcinoma,
and 202 patients (92.2%) were classified with M0 stage lung
adenocarcinoma. A total of 29 patients (13.2%) exhibited nerve
invasion, 33 patients (15.1%) exhibited vascular invasion and 209
patients (95.4%) were diagnosed with invasive adenocarcinoma.
Following surgery, 110 patients received chemotherapy and 109 cases
underwent TKI targeted therapy (Table
II).
 | Table I.Associations between EGFR mutation
status and clinical characteristics. |
Table I.
Associations between EGFR mutation
status and clinical characteristics.
|
|
| EGFR, number
(%) |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | No. (%) | Wild type | Mutation | P-value |
|---|
| Sex |
|
|
|
|
|
Female | 105 (47.9) | 31 (29.5) | 74 (70.5) | <0.001 |
|
Male | 114 (52.1) | 77 (67.5) | 37 (32.5) |
|
| Age, years |
|
|
|
|
|
<60 | 99 (45.2) | 44 (44.4) | 55 (55.6) | 0.190 |
|
≥60 | 120 (54.8) | 64 (53.3) | 56 (46.7) |
|
| Smoking status |
|
|
|
|
|
Non-smoker | 165 (75.3) | 72 (43.6) | 93 (56.4) | 0.003 |
|
Smoker | 54 (24.7) | 36 (66.7) | 18 (33.3) |
|
| T stage |
|
|
|
|
| T1 | 170 (77.6) | 77 (45.3) | 93 (54.7) | 0.027 |
| T2 | 49 (22.4) | 31 (63.3) | 18 (36.7) |
|
| N stage |
|
|
|
|
| N0 | 184 (84.0) | 88 (47.8) | 96 (52.2) | 0.540 |
| N1 | 27 (12.3) | 16 (59.3) | 11 (40.7) |
|
| N2 | 8 (3.7) | 4 (50.0) | 4 (50.0) |
|
| M stage |
|
|
|
|
| M0 | 202 (92.2) | 100 (49.5) | 102 (50.5) | 0.856 |
|
M1a | 3 (1.4) | 1 (33.3) | 2 (66.7) |
|
|
M1b | 14 (6.4) | 7 (50.0) | 7 (50.0) |
|
| pTNM stage |
|
|
|
|
| I | 171 (78.1) | 82 (48.0) | 89 (52.0) | 0.607 |
| II | 30 (13.7) | 17 (56.7) | 13 (43.3) |
|
|
III | 1 (0.5) | 1 (100.0) | 0 (0.0) |
|
| IV | 17 (7.8) | 8 (47.1) | 9 (52.9) |
|
| Nerve invasion |
|
|
|
|
| No | 190 (86.8) | 89 (46.8) | 101 (53.2) | 0.061 |
|
Yes | 29 (13.2) | 19 (65.5) | 10 (34.5) |
|
| Vascular
invasion |
|
|
|
|
| No | 186 (84.9) | 86 (46.2) | 100 (53.8) | 0.031 |
|
Yes | 33 (15.1) | 22 (66.7) | 11 (33.3) |
|
| Recurrence |
|
|
|
|
| No | 197 (90.0) | 99 (50.3) | 98 (49.7) | 0.406 |
|
Yes | 22 (10.0) | 9 (40.9) | 13 (59.1) |
|
| Histologic
subtypes |
|
|
|
|
|
MIA | 3 (1.4) | 0 (0.0) | 3 (100.0) | <0.001 |
| IA | 209 (95.4) | 101 (48.3) | 108 (51.7) |
|
|
IAV | 7 (3.2) | 7 (100.0) | 0 (0.0) |
|
 | Table II.EGFR mutation types and treatment of
219 patients. |
Table II.
EGFR mutation types and treatment of
219 patients.
| Mutation type | Total, no. (%) | Treatment |
|---|
| Wild type | 108 (49.3) |
Chemotherapya |
| G719X | 3 (2.7) | Second-line
TKIc |
| 19-Del | 40 (36.0) | First-line
TKIb |
| L858R | 61 (55.0) | First-line
TKIb |
| L861Q | 5 (4.5) | Second-line
TKIc |
| 20-Ins | 2 (1.8) |
Chemotherapya |
Association between EGFR mutations and
clinicopathological characteristics
Of the 219 patients, EGFR mutations were identified
in 111 patients (50.7%), including 61 cases of L858R mutations
(55%), 40 cases of 19-Del (36%), 5 cases of L861Q (4.5%), 3 cases
of G719X (2.7%) and 2 cases of 20-Ins (1.8%). Double mutations were
not detected. EGFR mutations were more common in females compared
with males (70.5% vs. 32.5%; P<0.001), and the mutation rate was
increased in non-smokers compared with smokers (56.4% vs. 33.3%;
P=0.003). The EGFR mutation rate in MIA cases was significantly
increased compared with IA and IAV (100% vs. 51.7% and 0%,
respectively; P<0.001). EGFR mutations were more common in
patients with T1 stage tumors compared with other stages (54.7%;
P=0.027) and in patients without vascular invasion compared with
patients exhibiting vascular invasion (53.8%; P=0.031).
Association between
clinicopathological characteristics and survival time
A total of 151 mortalities occurred prior to March
30th, 2017. The mean follow-up time was 30.9 months (range,
4.7–53.8 months). The 1-, 2- and 3-year survival rates of the
patients were 83.6, 54.8 and 42.9%, respectively, and the median
survival time (MST) was 27.2 months (data not shown). The survival
time was significantly increased in female patients, patients
<60 years-old and non-smokers compared with male patients,
patients ≥60 years-old and smokers (Fig.
1A-C). Patients with pTNM stage I tumors were associated with
increased OS time compared with those with stage II or III tumors
(39.6 months vs. 11.3 months and 10.4 months, respectively;
P<0.001; Fig. 1D). The survival
time was significantly increased in patients without nerve invasion
or vascular invasion or clinical recurrence compared with patients
with nerve invasion or vascular invasion or clinical recurrence,
respectively (Fig. 1E-G). There was
no significant difference in OS time among adenocarcinoma subtypes
(P=0.112; Fig. 1H). The survival time
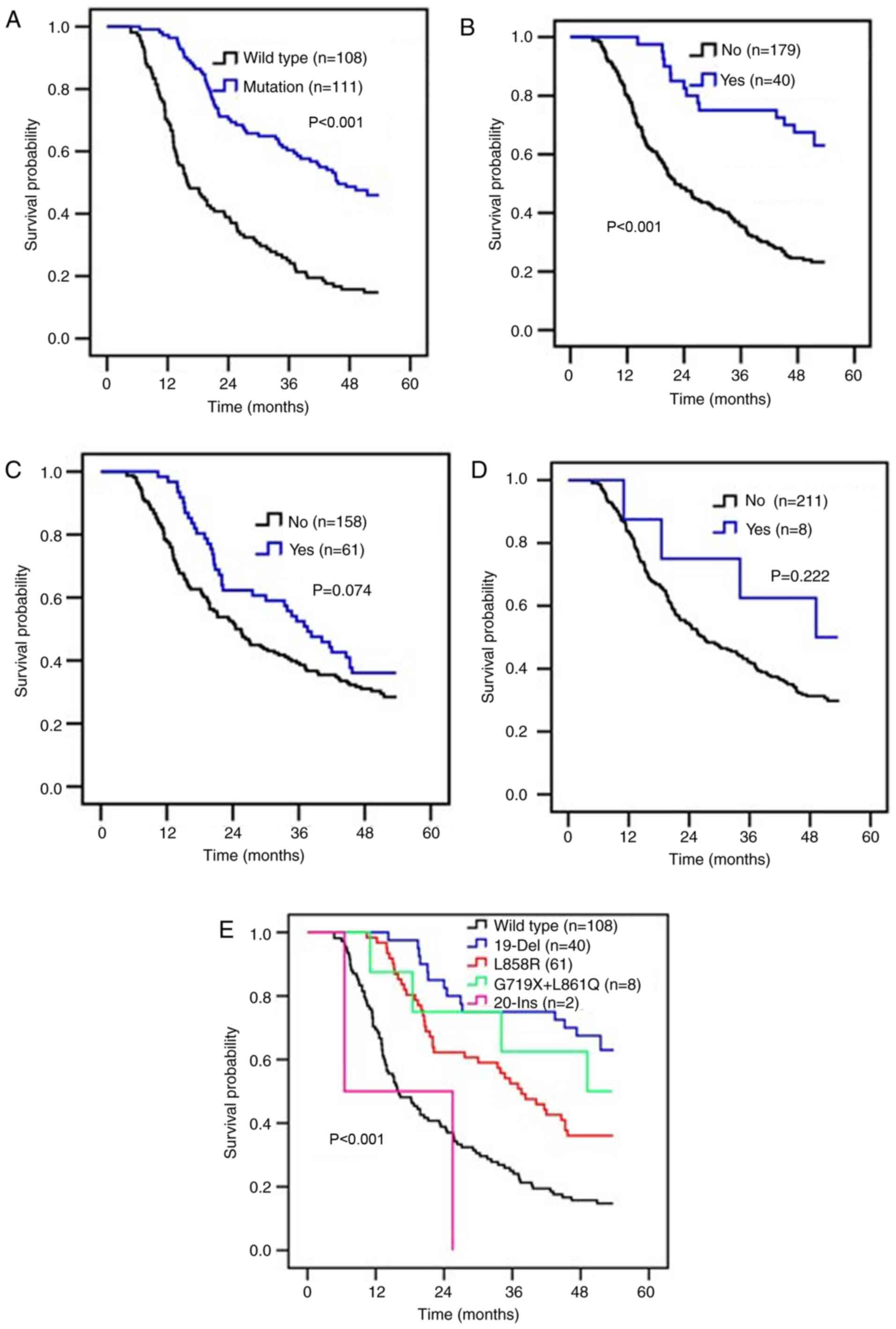
was markedly increased in patients with EGFR mutations or 19-Del
compared with patients without EGFR mutations or 19-Del (Fig. 2A and B). There was no significant
difference in OS time between patients with or without the L858
mutation (P=0.074; Fig. 2C), or with
or without other mutations of EGFR (P=0.222; Fig. 2D).
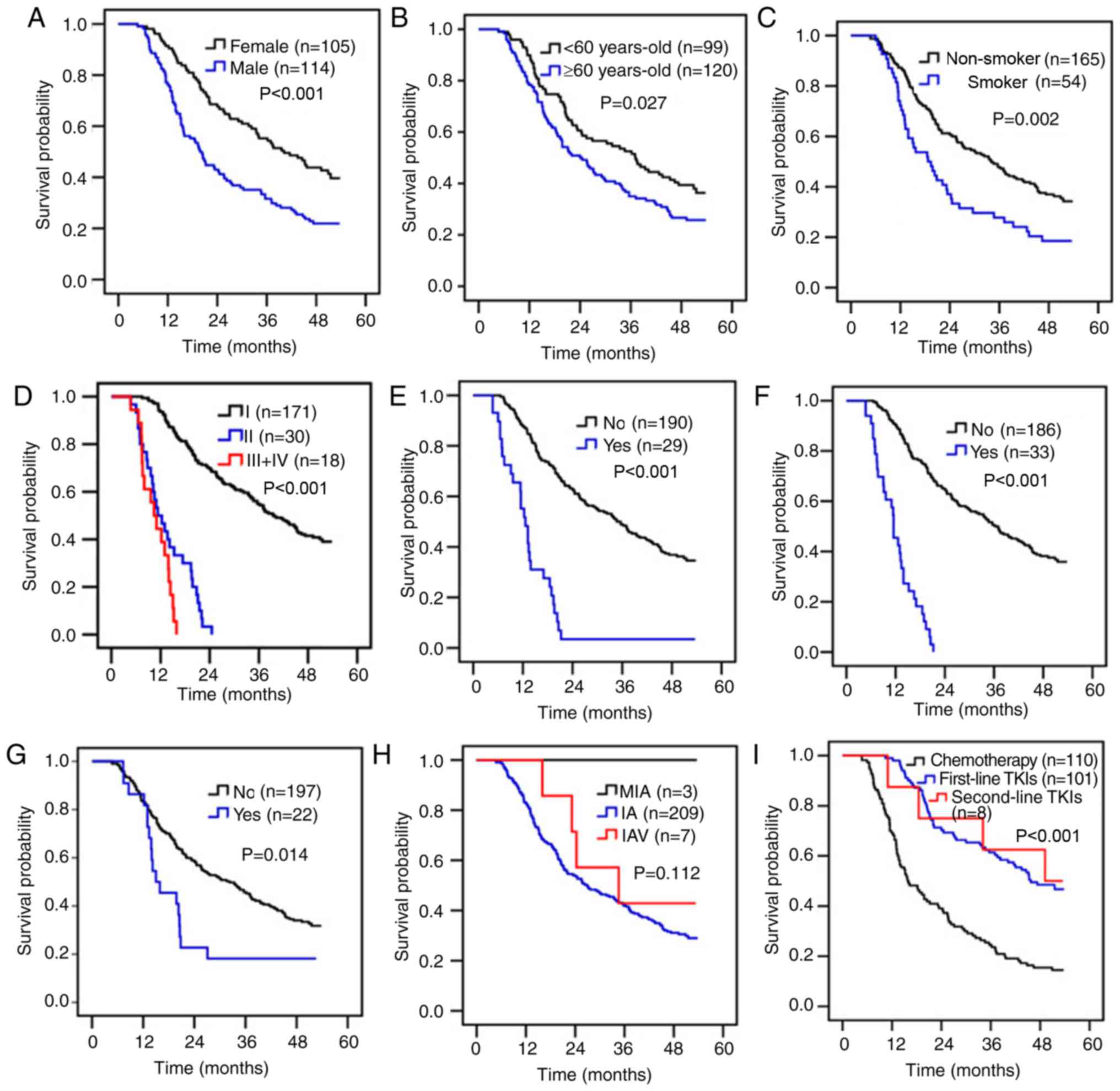 | Figure 1.Survival analysis of 219 patients
with lung adenocarcinoma. Analysis of the association of OS time
and (A) sex, (B) age, (C) smoking history (D) pTNM stage, (E) nerve
invasion status, (F) vascular invasion status, (G) clinical
recurrence, (H) adenocarcinoma subtypes, and (I) post-surgical
therapeutic regimen. OS, overall survival; pTNM pathological
Tumor-Node-Metastasis; TKI, tyrosine kinase inhibitor; n,
number. |
A total of 110 patients received cisplatin-based
chemotherapy, while 109 patients received TKI targeted therapies
(Table II). The present study
suggested that TKI targeted therapies could prolong survival time
compared with cisplatin-based chemotherapy (47.3 months vs. 15.8
months, P<0.001). However, there was no significant difference
in terms of survival time with first- or second-line TKI treatment
(45.7 months vs. 49.2 months; Fig.
1I). Cox's multiple regression analysis was used to analyze the
association of various clinical characteristics and patient
prognosis. Multivariate analysis revealed that tumor pTNM stage,
nerve invasion, vascular invasion and EGFR mutation types were
independent predictors for patient prognosis. The results also
suggested that patients with the 19-Del mutation were associated
with a relatively good prognosis, while patients with an L858R
mutation were not (Table III).
 | Table III.Univariate and multivariate analysis
of clinical characteristics and the overall survival time of
patients with lung adenocarcinoma. |
Table III.
Univariate and multivariate analysis
of clinical characteristics and the overall survival time of
patients with lung adenocarcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | Number, (MST,
months) | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
Female | 105 (40.2) | 29.976–50.424 | <0.001 |
|
|
|
|
Male | 114 (19.8) | 16.487–23.113 |
|
|
|
|
| Age, years |
|
|
|
|
|
|
|
<60 | 99 (37.1) | 28.325–45.875 | 0.027 |
|
|
|
|
≥60 | 120 (24.0) | 18.249–29.751 |
|
|
|
|
| Smoking status |
|
|
|
|
|
|
|
Non-smoker | 165 (34.1) | 26.695–41.505 | 0.002 |
|
|
|
|
Smoker | 54 (18.7) | 12.579–24.821 |
|
|
|
|
| pTNM stage |
|
|
|
|
|
|
| I | 171 (39.6) | 32.872–46.328 | <0.001 | 1 |
|
|
| II | 30 (11.3) | 7.945–14.655 |
| 8.904 | 6.175–12.840 |
<0.001c |
|
III+IV | 18 (10.4) | 7.490–13.310 |
|
|
|
|
| Nerve invasion |
|
|
|
|
|
|
| No | 190 (34.7) | 28.081–41.319 | <0.001 | 1 |
|
|
|
Yes | 29 (12.6) | 10.358–14.842 |
| 6.692 | 3.591–12.470 |
<0.001c |
| Vascular
invasion |
|
|
|
|
|
|
| No | 186 (36.2) | 29.517–42.883 | <0.001 | 1 |
|
|
|
Yes | 33 (11.5) | 9.706–13.294 |
| 3.579 | 1.961–6.533 |
<0.001c |
| Recurrence |
|
|
|
|
|
|
| No | 197 (32.3) | 24.899–39.701 | 0.014 |
|
|
|
|
Yes | 22 (15.0) | 8.335–21.664 |
|
|
|
|
| Histologic
subtypes |
|
|
|
|
|
|
|
MIA | 3 (NR) | – | 0.112 |
|
|
|
| IA | 209 (26.9) | – |
|
|
|
|
|
IAV | 7 (34.6) | – |
|
|
|
|
| EGFR mutation |
|
|
|
|
|
|
| Wild
type | 108 (15.8) | 11.472–20.128 |
<0.001a | 1 |
|
|
|
Mutation | 111 (45.7) | 36.326–57.290 |
|
|
|
|
|
19-Del | 40 (NR) | – |
<0.001b | 0.432 | 0.101–1.855 | 0.259c |
|
L858R | 61 (37.6) |
29.182–46.018b |
| 0.051 | 0.011–0.244 |
<0.001c |
|
G719X+L861Q | 8 (49.2) |
28.500–54.146b |
| 0.110 | 0.025–0.493 | 0.004c |
|
20-Ins | 2 (6.5) | 6.500-b |
| 0.066 | 0.011–0.390 | 0.003c |
| EGFR 19-Del
mutation |
|
|
|
|
|
|
| No | 179 (22.2) | 17.853–26.547 | <0.001 | 1 |
|
|
|
Yes | 40 (NR) | – |
| 0.463 | 0.241–0.889 | 0.021d |
| EGFR L858R
mutation |
|
|
|
|
|
|
| No | 158 (24.5) | 19.804–29.196 | 0.074 |
|
|
|
|
Yes | 61 (37.6) | 29.182–46.018 |
|
|
|
|
| EGFR rare
mutatione |
|
|
|
|
|
|
| No | 211 (26.9) | 19.906–33.894 | 0.222 |
|
|
|
|
Yes | 8 (49.2) | 29.501–52.199 |
|
|
|
|
| Treatment |
|
|
|
|
|
|
|
Chemotherapy | 110 (15.8) | 11.432–20.168 | <0.001 |
|
|
|
|
TKI | 109 (47.3) | 21.500–1.711 |
|
|
|
|
|
First-line | 101 (45.7) | 21.500–1.678 |
|
|
|
|
|
Second-line | 8 (49.2) | 18.500–14.146 |
|
|
|
|
Discussion
In the present study, EGFR mutations were detected
in 111/219 patients with lung adenocarcinoma (50.7%), and the most
common mutations were L858R (54.9%) and 19-Del (36%), accounting
for 90.9% of all EGFR mutations. Sex, age, smoking status, pTNM
stage, nerve invasion, vascular invasion, EGFR mutation status,
recurrence and therapeutic regimen were all associated with OS
time. Multivariate analysis revealed that pTNM stage, nerve
invasion, vascular invasion and EGFR mutations were independent
predictors for patient prognosis.
The detection of EGFR mutations in lung
adenocarcinoma patients has been widely performed worldwide
(5–7,10,21). A number of methods for detecting EGFR
mutations now, exist, with direct sequencing and F-PCR being the
most common clinically used methods (22–24).
Direct sequencing can detect unknown gene mutations and is the
‘gold standard’ for detecting gene mutations. However, the low
sensitivity, the requirement for large specimen size, the
complexity and duration of the protocol, the high cost and
difficult interpretation of results are disadvantages of direct
sequencing (22,23). The F-PCR method combines specific
primers with a double loop probe technique. The amplified products
are detected by double ring probes, and the mutation status of
sample DNA are observed using a PCR platform, specific reaction
procedures and highly specific Taq DNA polymerases (22,24). This
method has the advantages of high specificity and sensitivity for
detecting rare mutations, a simple and rapid protocol, simple data
interpretation, and suitability for large-scale screening in
clinical laboratories (22,24). In the present study, EGFR mutations in
lung adenocarcinoma patients were detected using the F-PCR
method.
The EGFR gene is located on the short arm of human
chromosome 7, composed of 188,307 bases and 28 exons, and its
tyrosine kinase functional domain is encoded by exons 18–24
(5). Previous studies have
demonstrated that mutations in exons 18–21 in patients with lung
cancer were associated with patient-responsiveness to EGFR-TKIs.
This is likely due to changes in the structure of the EGFR ATP
binding area, enhancing the combining capacity of EGFR-TKIs
(5,7,21). To
date, >30 mutations of EGFR have been reported, including 19-Del
(~45% all EGFR mutations), L858R (~40–45%), G719X (~5%), 20-Ins
(~1%) (5,12,21).
Studies have suggested that EGFR mutation rates in patients with
lung adenocarcinoma differ among countries and ethnicities, between
sexes, and with smoking status (25–27). The
overall mutation rate of EGFR in Chinese patients with lung
adenocarcinoma in the present study was 50.7%, which is consistent
with previous reports (6,25–27). The
mutation rate in female patients was significantly higher than that
of male patients (P<0.001) while the mutation rate in smokers
was low compared with non-smokers (P=0.003), which was also
consistent with previous reports (25,26,28). It
was also demonstrated that EGFR mutations were more common in
patients with T1 stage tumors or without vascular invasion compared
with T2 stage patients, or those with vascular invasion (P=0.027
and P=0.031, respectively; Table
I).
Previous studies have revealed that EGFR mutations
are predictors of TKI treatment response and prognosis of patients
with lung adenocarcinoma (10,20,27,29).
However, clinical studies indicated that patients with different
EGFR mutations were associated with different outcomes (10,29).
Patients with 19-Del or L858R have been demonstrated to be
associated with a relatively good prognosis following TKI treatment
compared with other mutations (5). A
number of studies have suggested that 19-Del or L858R mutations do
not have different effects on prognosis (21,30), while
others have indicated that patients with 19-Del survived
significantly longer than patients with L858R (31,32). In
the present study, the survival time of patients with EGFR
mutations was significantly higher than patients without EGFR
mutations (P<0.001; Fig. 2A), and
multivariate analyses demonstrated that the presence of an EGFR
mutation was a predictor of favorable prognosis for patients with
lung adenocarcinoma (P<0.001; Table
III). These results were consistent with previous reports
(10,29). Patients with 19-Del were associated
with an improved prognosis compared with those without (Fig. 2B; P<0.001), and 19-Del was
demonstrated to be a predictor of good outcome (HR, 0.463; 95% CI,
0.241–0.889; P=0.021). L858R was not demonstrated to be an
independent predictor of lung adenocarcinoma prognosis, although
the survival time of patients with L858R was longer than those
without (37.6 months vs. 24.5 months; P=0.074; Fig. 2C). This may be associated with the
differences in the sequences and structures of exons 19 and 21, and
the differences in TKI activity in patients with different
mutations (33,34).
A number of rare EGFR mutations were also detected,
including 3 cases of G719X (2.7%), 5 cases of L861Q (4.5%) and 2
cases of 20-Ins (1.8%), a rate which was consistent with previous
reports (35,36). Patients with G719 or L861Q had an MST
of 49.2 months following second-line TKI treatment, which was
longer than that of patients without G719 or L861Q mutations (49.2
months vs. 26.9 months; P=0.222; Fig.
2D). In the present study, only 10 cases of rare mutations were
detected (9.0%), and only 8 of these patients were treated with
second-line TKIs. Further larger scale studies are required.
It has been reported that ~5% EGFR mutations occur
in exon 20, and patients with these mutations are often insensitive
to TKI treatment (37). Clinical
studies have confirmed that patients with NSCLC acquired resistance
to TKIs following a period of treatment. A mutation in T790M in
exon 20 was detected in ~50% patients with acquired resistance
(37,38). Other studies have demonstrated that
T790M mutations existed in a minority of untreated tumor cells
prior to treatment, and that the mutation rates increased
significantly following treatment (21,38). No
T790M mutation was detected in exon 20, which may be due to all
specimens being collected from surgical patients, or because no
patients were treated with chemotherapeutic drugs prior to surgery.
Alternatively, the number of tumor cells exhibiting the T790M
mutation may have been too low for detection. In following studies,
it is necessary to perform analyze T790M in patients with acquired
drug-resistant lung adenocarcinoma.
Previous studies have indicated that IASLC/ATS/ERS
classification of lung adenocarcinoma may be an independent
prognostic factor (13,15,20). In
the present study, it was demonstrated that EGFR mutations were
more common in MIA (100%) than in IA (51.7%) and IAV (0%). The
survival time of patients with MIA was longer than that of patients
with IA and IAV, however, this was not statistically significant
(26.9 months vs. 34.6 months, P=0.112; Fig. 1I). The present study has a number of
limitations: ~95.4% specimens were collected from patients with IA.
Furthermore, the majority of cases of lung adenocarcinoma were at
an early stage. All patients who participated in the present study
were treated in a single area (Yantai, China), which may cause
selection bias. Furthermore, the histological categories of lung
adenocarcinoma, including MIA, IA and IAV, were considered in the
present study, but the histological subtypes of lung
adenocarcinoma, including lepidic, acinar, papillary,
micropapillary, solid and mucinous predominant subtypes, were not
considered. A further study with a more even ratio of all lung
adenocarcinoma subtypes and stages is required.
Sumiyoshi et al (39) indicated that nerve invasion and
clinical recurrence could serve as independent predictors for
patients with lung adenocarcinoma, and Matsumura et al
(12) suggested that vascular
invasion could also function as an independent predictor (40,41). In
the present study, multivariate analysis demonstrated that nerve
invasion, vascular invasion and clinical recurrence were
independent predictors for patients with lung adenocarcinoma
(Fig. 1E-G). Previous studies have
suggested that high pTNM stage is associated with poor prognosis
(41–43). In the present study, high pTNM stage
was associated with a relatively short survival time, and pTNM
stage was demonstrated to be a predictor of prognosis (Fig. 1D).
To conclude, the present study suggests that
prognosis of patients with lung adenocarcinoma is associated with
pTNM staging, nerve invasion, vascular invasion and EGFR mutation
status. Patients exhibiting 19-Del were associated with a good
prognosis compared with those exhibiting L858R following TKI
targeted therapy. Overall, the present study demonstrated that EGFR
mutation detection is conducive for selecting a favorable
therapeutic regimen for patients with lung adenocarcinoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Program of Shandong Province (grant no. J15LK02) and the
Scientific Research Project of Yantai (grant no. 2016ZH081).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XZ, LC and JL were major contributors toward data
collection, data analysis and manuscript writing. XH, YZ and HZ
performed the histological examination of lung adenocarcinoma. BW,
BL and PG, were responsible for manuscript preparation, study
design, data analysis and article finalization. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval was granted by the Medical Ethics
Committee of Binzhou Medical University (reference no. 2012-37).
Written informed consent was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EGFR
|
epidermal growth factor receptor
|
|
ARMS
|
amplification refractory mutation
system
|
|
CT
|
computed tomography
|
|
F-PCR
|
fluorescence-polymerase chain
reaction
|
|
G719X
|
point mutations in exon 18
|
|
19-Del
|
deletion mutations in exon 19
|
|
20-Ins
|
insertion mutations in exon 20
|
|
L858R and L861Q
|
two base-pair substitution mutations
in exon 21
|
|
MIA
|
minimally invasive adenocarcinoma
|
|
IA
|
invasive adenocarcinoma
|
|
IAV
|
invasive adenocarcinoma variant
|
|
IASLC/ATS/ERS
|
International Association for the
Study of Lung Cancer, American Thoracic Society and European
Respiratory Society
|
|
MST
|
median survival time
|
|
NSCLC
|
non-small cell lung cancer
|
|
OS
|
overall survival
|
|
pTNM
|
pathological tumor-node-metastasis
|
|
TKIs
|
tyrosine kinase inhibitors
|
References
|
1
|
Jia Y, Li F, Liu YF, Zhao JP, Leng MM and
Chen L: Depression and cancer risk: A systematic review and
meta-analysis. Public Health. 149:138–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Devarakonda S, Morgensztern D and Govindan
R: Genomic alterations in lung adenocarcinoma. Lancet Oncol.
16:e342–e351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pao W and Miller VA: Epidermal growth
factor receptor mutations, small-molecule kinase inhibitors, and
non-small-cell lung cancer: Current knowledge and future
directions. J Clin Oncol. 23:2556–2568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho J, Choi SM, Lee J, Lee CH, Lee SM, Yim
JJ, Chung DH, Yoo CG, Kim YW, Han SK and Park YS: The association
of EGFR mutations with stage at diagnosis in lung adenocarcinomas.
PLoS One. 11:e01668212016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin JH, Lin D, Xu L, Wang Q, Hu HH, Xu HP
and He ZY: The association between clinical prognostic factors and
epidermal growth factor receptor-tyrosine kinase inhibitor
(EGFR-TKI) efficacy in advanced non-small-cell lung cancer
patients: A retrospective assessment of 94 cases with EGFR
mutations. Oncotarget. 8:3412–3421. 2017.PubMed/NCBI
|
|
8
|
Na II, Rho JK, Choi YJ, Kim CH, Park JH,
Koh JS, Ryoo BY, Yang SH and Lee JC: The survival outcomes of
patients with resected non-small cell lung cancer differ according
to EGFR mutations and the P21 expression. Lung Cancer. 57:96–102.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eichler AF, Kahle KT, Wang DL, Joshi VA,
Willers H, Engelman JA, Lynch TJ and Sequist LV: EGFR mutation
status and survival after diagnosis of brain metastasis in nonsmall
cell lung cancer. Neuro Oncol. 12:1193–1199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Isaka T, Nakayama H, Yokose T, Ito H,
Miyagi Y, Matsuzaki T, Nagata M, Furumoto H, Nishii T, Katayama K,
et al: Epidermal growth factor receptor mutations and prognosis in
pathologic N1-N2 pulmonary adenocarcinoma. Ann Thorac Surg.
102:1821–1828. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dancey JE: Epidermal growth factor
receptor inhibitors in non-small cell lung cancer. Drugs.
67:1125–1138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumura Y, Owada Y, Yamaura T, Muto S,
Osugi J, Hoshino M, Higuchi M, Ohira T, Suzuki H and Gotoh M:
Epidermal growth factor receptor gene mutation as risk factor for
recurrence in patients with surgically resected lung
adenocarcinoma: A matched-pair analysis. Interact Cardiovasc Thorac
Surg. 23:216–222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshizawa A, Sumiyoshi S, Sonobe M,
Kobayashi M, Fujimoto M, Kawakami F, Tsuruyama T, Travis WD, Date H
and Haga H: Validation of the IASLC/ATS/ERS lung adenocarcinoma
classification for prognosis and association with EGFR and KRAS
gene mutations: Analysis of 440 Japanese patients. J Thorac Oncol.
8:52–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Memon AA, Zhang H, Gu Y, Luo Q, Shi J,
Deng Z, Ma J and Ma W: EGFR with TKI-sensitive mutations in exon 19
is highly expressed and frequently detected in Chinese patients
with lung squamous carcinoma. Onco Targets Ther. 10:4607–4613.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Russell PA, Barnett SA, Walkiewicz M,
Wainer Z, Conron M, Wright GM, Gooi J, Knight S, Wynne R, Liew D
and John T: Correlation of mutation status and survival with
predominant histologic subtype according to the new IASLC/ATS/ERS
lung adenocarcinoma classification in stage III (N2) patients. J
Thorac Oncol. 8:461–468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Y, Hou L, Yang Y, Xie H, Yang Y, Li Z,
Zhao H, Gao W and Su B: Two-gene signature improves the
discriminatory power of IASLC/ATS/ERS classification to predict the
survival of patients with early-stage lung adenocarcinoma. Onco
Targets Ther. 9:4583–4591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim D, Kim HK, Kim SH, Lee HY, Cho JH,
Choi YS, Kim K, Kim J, Zo JI and Shim YM: Prognostic significance
of histologic classification and tumor disappearance rate by
computed tomography in lung cancer. J Thorac Dis. 10:388–397. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abdollah F, Sun M, Suardi N, Gallina A,
Capitanio U, Bianchi M, Tutolo M, Passoni N, Karakiewicz PI,
Rigatti P, et al: National Comprehensive Cancer Network practice
guidelines 2011: Need for more accurate recommendations for pelvic
lymph node dissection in prostate cancer. J Urol. 188:423–428.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger K, Yatabe Y, Powell CA, Beer D, Riely G,
Garg K, et al: International association for the study of lung
cancer/american thoracic society/european respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Warth A, Muley T, Meister M, Stenzinger A,
Thomas M, Schirmacher P, Schnabel PA, Budczies J, Hoffmann H and
Weichert W: The novel histologic International Association for the
Study of Lung Cancer/American Thoracic Society/European Respiratory
Society classification system of lung adenocarcinoma is a
stage-independent predictor of survival. J Clin Oncol.
30:1438–1446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Angulo B, Conde E, Suárez-Gauthier A,
Plaza C, Martínez R, Redondo P, Izquierdo E, Rubio-Viqueira B,
Paz-Ares L, Hidalgo M, et al: A comparison of EGFR mutation testing
methods in lung carcinoma: Direct sequencing, real-time PCR and
immunohistochemistry. PLoS One. 7:e438422012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Cai Y, Dong Y, Nong J, Zhou L, Liu
G, Su D, Li X, Wu S, Chen X, et al: Clinical characteristics and
outcomes of patients with primary lung adenocarcinoma harboring ALK
rearrangements detected by FISH, IHC, and RT-PCR. PLoS One.
9:e1015512014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim EK, Kim KA, Lee CY and Shim HS: The
frequency and clinical impact of HER2 alterations in lung
adenocarcinoma. PLoS One. 12:e01712802017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi Y, Au JS, Thongprasert S, Srinivasan
S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G and Yang PC: A
prospective, molecular epidemiology study of EGFR mutations in
Asian patients with advanced non-small-cell lung cancer of
adenocarcinoma histology (PIONEER). J Thorac Oncol. 9:154–162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Midha A, Dearden S and McCormack R: EGFR
mutation incidence in non-small-cell lung cancer of adenocarcinoma
histology: A systematic review and global map by ethnicity
(mutMapII). Am J Cancer Res. 5:2892–2911. 2015.PubMed/NCBI
|
|
27
|
Martin P, Shiau CJ, Pasic M, Tsao M,
Kamel-Reid S, Lin S, Tudor R, Cheng S, Higgins B, Burkes R, et al:
Clinical impact of mutation fraction in epidermal growth factor
receptor mutation positive NSCLC patients. Br J Cancer.
114:616–622. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei WE, Mao NQ, Ning SF, Li JL, Liu HZ,
Xie T, Zhong JH, Feng Y, Wei CH and Zhang LT: An analysis of EGFR
mutations among 1506 cases of non-small cell lung cancer patients
in Guangxi, China. PLoS One. 11:e01687952016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Won YW, Han JY, Lee GK, Park SY, Lim KY,
Yoon KA, Yun T, Kim HT and Lee JS: Comparison of clinical outcome
of patients with non-small-cell lung cancer harbouring epidermal
growth factor receptor exon 19 or exon 21 mutations. J Clin Pathol.
64:947–952. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lai Y, Zhang Z, Li J, Sun D, Zhou Y, Jiang
T, Han Y, Huang L, Zhu Y, Li X and Yan X: EGFR mutations in
surgically resected fresh specimens from 697 consecutive Chinese
patients with non-small cell lung cancer and their relationships
with clinical features. Int J Mol Sci. 14:24549–24559. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Riely GJ, Pao W, Pham D, Li AR, Rizvi N,
Venkatraman ES, Zakowski MF, Kris MG, Ladanyi M and Miller VA:
Clinical course of patients with non-small cell lung cancer and
epidermal growth factor receptor exon 19 and exon 21 mutations
treated with gefitinib or erlotinib. Clin Cancer Res. 12:839–844.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee VH, Tin VP, Choy TS, Lam KO, Choi CW,
Chung LP, Tsang JW, Ho PP, Leung DK, Ma ES, et al: Association of
exon 19 and 21 EGFR mutation patterns with treatment outcome after
first-line tyrosine kinase inhibitor in metastatic non-small-cell
lung cancer. J Thorac Oncol. 8:1148–1155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gazdar AF and Minna JD: Inhibition of EGFR
signaling: All mutations are not created equal. PLoS Med.
2:e3772005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carey KD, Garton AJ, Romero MS, Kahler J,
Thomson S, Ross S, Park F, Haley JD, Gibson N and Sliwkowski MX:
Kinetic analysis of epidermal growth factor receptor somatic mutant
proteins shows increased sensitivity to the epidermal growth factor
receptor tyrosine kinase inhibitor, erlotinib. Cancer Res.
66:8163–8171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Beau-Faller M, Prim N, Ruppert AM,
Nanni-Metéllus I, Lacave R, Lacroix L, Escande F, Lizard S, Pretet
JL, Rouquette I, et al: Rare EGFR exon 18 and exon 20 mutations in
non-small-cell lung cancer on 10 117 patients: A multicentre
observational study by the French ERMETIC-IFCT network. Ann Oncol.
25:126–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Watanabe S, Minegishi Y, Yoshizawa H,
Maemondo M, Inoue A, Sugawara S, Isobe H, Harada M, Ishii Y, Gemma
A, et al: Effectiveness of gefitinib against non-small-cell lung
cancer with the uncommon EGFR mutations G719X and L861Q. J Thorac
Oncol. 9:189–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Q, Ke E, Niu F, Deng W, Chen Z, Xu
C, Zhang X, Zhao N, Su J, Yang J, et al: The role of T790M mutation
in EGFR-TKI re-challenge for patients with EGFR-mutant advanced
lung adenocarcinoma. Oncotarget. 8:4994–5002. 2017.PubMed/NCBI
|
|
38
|
Su KY, Chen HY, Li KC, Kuo ML, Yang JC,
Chan WK, Ho BC, Chang GC, Shih JY, Yu SL and Yang PC: Pretreatment
epidermal growth factor receptor (EGFR) T790M mutation predicts
shorter EGFR tyrosine kinase inhibitor response duration in
patients with non-small-cell lung cancer. J Clin Oncol. 30:433–440.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sumiyoshi S, Yoshizawa A, Sonobe M,
Kobayashi M, Fujimoto M, Tsuruyama T, Date H and Haga H: Pulmonary
adenocarcinomas with micropapillary component significantly
correlate with recurrence, but can be well controlled with EGFR
tyrosine kinase inhibitors in the early stages. Lung Cancer-J
Iaslc. 81:53–59. 2013. View Article : Google Scholar
|
|
40
|
Ohtaki Y, Shimizu K, Kakegawa S, Nagashima
T, Nakano T, Atsumi J, Enokida Y, Igai H, Ibe T, Sugano M, et al:
Postrecurrence survival of surgically resected pulmonary
adenocarcinoma patients according to EGFR and KRAS mutation status.
Mol Clin Oncol. 2:187–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li J, Yang X, Xia T, Guan Y and Zhong N:
Stage I synchronous multiple primary non-small cell lung cancer: CT
findings and the effect of TNM staging with the 7th and 8th
editions on prognosis. J Thorac Dis. 9:5335–5344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ramlau R, Krawczyk P, Dziadziuszko R,
Chmielewska I, Milanowski J, Olszewski W, Stencel K, Ramlau-Piątek
K, Segiet A, Skroński M, et al: Predictors of EGFR mutation and
factors associated with clinical tumor stage at diagnosis:
Experience of the INSIGHT study in Poland. Oncol Lett.
14:5611–5618. 2017.PubMed/NCBI
|
|
43
|
Saji H, Tsuboi M, Shimada Y, Kato Y,
Yoshida K, Nomura M, Matsubayashi J, Nagao T, Kakihana M, Usuda J,
et al: A proposal for combination of total number and anatomical
location of involved lymph nodes for nodal classification in
non-small cell lung cancer. Chest. 143:1618–1625. 2013. View Article : Google Scholar : PubMed/NCBI
|