Introduction
As the most common malignancy of head and neck, oral
squamous cell carcinoma (OSCC) ranks the top six of malignant
tumors worldwide with unsatisfactory prognosis (1). Tongue squamous cell carcinoma (TSCC) is
one of the leading subtypes of OSCC, which frequently results in
the malfunction of mastication, speech and deglutition, with the
characteristics of high degree of malignancy, high rate of tumor
metastasis and high recurrence (2,3). Despite
of surgery combined with chemoradiotherapy as the primary treatment
for TSCC, postoperative recurrence and the rate of distant
metastasis remain high and the total 5-year survival rate is only
~50~60%. This critically affects influencing the quality of life of
patients (4–6). With the broad development of tumor
marker and molecular targeted therapy (7), a more detailed understanding of the
mechanisms contributing to the carcinogenesis of TSCC would be of
value to improve the therapeutic effect of TSCC at the molecular
level.
microRNA (miRNA/miR), a type of highly conserved
non-coding small molecules, serves an important role in numerous
biological activities by regulating the expression of the target
mRNAs at the post-transcriptional level, giving rise to mRNA
degradation or translational suppression (8–10).
miRNA-409-3p, located on chromosome 14q32.31, has been demonstrated
to regulate several cellular processes, including cell
proliferation, apoptosis and metastasis (11). Notably, miR-409-3p was credited as a
promising tumor suppressor to inhibit cell proliferation, invasion
and migration by suppressing the target gene c-Met in lung
adenocarcinoma (12) and bladder
cancer (13). Similarly in gastric
cancer, downregulation of miR-409-3p was apparent. The
overexpression of miR-409-3p in vitro and in vivo may
inhibit the target gene PHD finger protein 10 to restrict cell
proliferation and accelerate apoptosis (14). However, Josson et al (15) identified elevated miR-409-3p/-5p
levels in prostate cancer, thereby indicating that it may
facilitate tumorigenesis, epithelial-to-mesenchymal transition, as
well as bone metastasis of prostate cancer. This suggests that
miR-409-3p may serve important functions in different tumor
progression, including TSCC.
Radixin (RDX) is a tumor-associated factor that
belongs to the ezrin-radixin-moesin (ERM) family (16), which is involved in the regulation of
diverse cellular functions, including cell morphogenesis and
polarization, as well as adhesion and migration (17,18).
Previous studies reported a close association of RDX with
invasion and migration of tumor cells. As reported by Tsai et
al (19), miR-196a/-196b may
enhance the invasion and migration of gastric cancer cells by
inhibiting the mRNA and protein expression of RDX. Additionally,
with the assistance of target gene prediction website, it was
demonstrated that RDX may be a potential target gene of
miR-409-3p, but little evidence is available regarding the
association between miR-409-3p and RDX in TSCC (20). Therefore, the aim of the present study
was to investigate the effects of miR-409-3p on RDX in the
development of TSCC and potential underlying molecular mechanisms,
thereby providing the potential strategy to improve the diagnosis,
intervention and treatment of TSCC.
Materials and methods
Ethics statement
The present study conformed to the criteria issued
by the Declaration of Helsinki (21).
All patients were informed, agreed to the experiment and signed
informed consent forms, and the present study was granted
permission from the Clinical Ethics Committee of Jingzhou Central
Hospital (Hubei, China). All animal procedures were performed
according to the Guide for the Care and Use of Laboratory Animals
published by the US National Institutes of Health (22).
Study objects
A total of 68 patients, (38 males and 30 females;
mean age, 57.18±9.77 years) treated with surgery and diagnosed as
primary TSCC at the Department of Oral and Maxillofacial Surgery at
Jingzhou Central Hospital from December 2014 to December 2016, were
collected as case group. Inclusion criteria were as follows;
histological diagnosis of TSCC confirmed on hematoxylin and
eosin-stained sections. Exclusion criteria were as follows;
immunosuppressed patients, patients who had previously been
diagnosed with cancer (of any type and location), or patients who
had previously undergone radiation therapy. Among the 68 patients,
42 cases were poorly and moderately differentiated and 26 cases
were well differentiated. Lymph node metastasis was absent in a
total of 48 cases and present in 20 cases. Of these patients, 49
cases were aged ≥55 years, and 19 cases were aged <55 years.
According to the tumor-node metastasis (TNM) staging of World
Health Organization (23), 43 cases
were in stage I–II and 25 cases were in stage III–IV. Based on
tumor diameter, 44 cases were ≥2 cm and 24 cases were <2 cm.
Additionally, samples from an additional 25 cases
were obtained from the Department of Oral and Maxillofacial
Surgery, Jingzhou Central Hospital (Jingzhou, China) consisting of
13 males and 12 females (mean age, 56.45±8.69 years) taken from
non-neoplastic surgery of the tongue and confirmed as normal tongue
mucosa tissues by routine pathological examination were selected as
the normal control group. There were no statistical differences in
terms of gender and age between the two groups of patients (both
P>0.05). All specimens were immediately stored in liquid
nitrogen.
Selection of cells and culture
The human oral keratinocytes (HOK) and human TSCC
cell lines, including Tca8113, SCC9, SCC25, and Ca127, were
purchased from the Type Culture Collection of the Chinese Academy
of Sciences. Dulbecco's modified Eagle medium (DMEM, Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
bovine serum (FBS) (Invitrogen; Thermo Fisher Scientific, Inc.) and
1% penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.)
were used for culture with 5% CO2 at 37°C under a
humidified atmosphere. The culture medium was replaced every 2 days
or following the thawing of cells. When the cells were at a
confluence of ~80~90% cell passaging was carried out. The cells in
logarithmic phase were seeded in 6-well plate at a density of
5×103.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cells using
Trizol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
optical density (OD) of RNA at 260 and 280 nm was determined by
ultraviolet spectrophotometer, and RNA concentration was
calculated. The RNA samples were stored at −80°C. On the basis of
gene sequences published in Genbank, primers (Table I) were designed using Primer software
(version 5.0; Applied Biosystems; Thermo Fisher Scientific, Inc.)
and synthesized by Shanghai Biological Engineering Co., Ltd.
(Shanghai, China) Total RNA reverse transcription PCR was performed
according to the manufacturer's protocol (Takara Biotechnology
Ltd., Dalian, China). PCR conditions consisted of pre-denaturation
at 95°C for 15 min and 40 cycles of denaturation at 94°C for 15 sec
and annealing/extension at 60°C for 30 sec. U6 was used as the
internal reference control for miR-409-3p, and β-actin was employed
as the internal reference control for RDX, 2−ΔΔCq
(24) was used to calculate the
relative expression of the target gene (25). Each experiment was repeated three
times.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequences
(5′-3′) |
|---|
| miR-409-3p |
|
|
Forward |
GAATGTTGCTCGGTGA |
|
Reverse |
GTGCAGGGTCCGAGGT |
| U6 |
|
|
Forward |
GCGCGTCGTGAAGCGTTC |
|
Reverse |
GTGCAGGGTCCGAGGT |
| RDX |
|
|
Forward |
GAATCAGGAGCAGCTAGCAGCAGAACTT |
|
Reverse |
TTGGTCTTTTCCAAGTCTTCCTGGGCTGCA |
| β-actin |
|
|
Forward |
CAAACTGAAGCTCGCACTCTC |
|
Reverse |
GCTGCAGATTCTTGGGTTGTG |
Dual-luciferase reporter gene
assay
TargetScan (www.targetscan.org) was used to predict the binding
site of miR-409-3p and RDX 3′untranslated region (UTR).
Then, wild-type (WT) RDX 3′UTR plasmid (named as RDX
3′UTR-WT) and mutated (MUT) RDX 3′UTR (named as RDX
3′UTR-MUT) were constructed. The target sequences were as follows;
WT-RDX, 3′UTR is 5′-UUAAGGGAGCUCUUCAACAUUA-3′ and MUT
RDX, 5′-UUAAGGGAGCUCUUGTCGGAAA-3′. For the transfection
experiments, the following were transfected separately into the
Tca8113 cells: miR-409-3p mimics + RDX-WT, miR-409-3p mimics +
RDX-MUT, miR-409-3p NC + RDX-WT and miR-409-3p NC + RDX-MUT.
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for transfection. The luciferase
activity was detected by dual-luciferase reporter gene assay kit
(Promega Corporation, Madison, WI, USA) following the
manufacturer's protocol. The results were expressed as relative
luciferase activity (firefly luciferase/Renilla luciferase).
Each experiment was repeated three times.
Cell grouping and transfection
Tca8113 cells at the logarithmic phase were divided
into six groups, including blank (without any transfection),
negative control (NC; 5′-ACTACTGAGTGACAGTAGA-3′) (transfected with
NC sequence), miR-409-3p mimic (transfected with miR-409-3p mimic
sequence), miR-409-3p inhibitor (transfected with miR-409-3p
inhibitor sequence), small-interfering (si)-RDX group (transfected
with si-RDX) and miR-409-3p inhibitor + si-RDX group (transfected
with miR-409-3p inhibitor and si-RDX) groups. miR-409-3p
mimic/inhibitor was purchased from Applied Biosystems (Thermo
Fisher Scientific, Inc.), while si-RDX was obtained from Guangzhou
RiboBio Co., Ltd (Guangzhou, China). Cell transfection in each
group was performed using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). Each experiment was
repeated three times.
Western blotting
Total protein was extracted from each group, and
protein concentration was determined using bicinchoninic acid assay
kit (Beyotime Institute of Biotechnology, Haimen, China). With the
addition of loading buffer, the extracted protein was heated at
95°C for 10 min and 30 µg protein was loaded on 10% polyacrylamide
gel. Following the transfer of the proteins to a polyvinylidene
(PVDF) membrane, the PVDF membrane was blocked with 5% bovine serum
albumin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 1 h at
room temperature, and then was incubated with primary antibody
against RDX (1:500; cat. no. ab52495; Epitomics; Abcam, Cambridge,
UK) and β-actin (1:1,000; cat. no. A1978; Sigma-Aldrich; Merck
KGaA) at 4°C overnight. Subsequently, PVDF membrane was washed by
Tris-buffered saline with Tween-20 three times at room temperature
(5 min per wash), followed by 1-h incubation with the corresponding
horseradish peroxidase (HRP)-conjugated secondary antibody
(1:1,000; cat. no. ab131368; Epitomics; Abcam, Cambridge, UK) at
room temperature. Following an additional washing step, PVDF
membrane was developed using chemiluminescence reagent (GE
Healthcare, Chicago, IL, USA) and analyzed by Bio-Rad Gel Doc EZ
imager (Bio-Rad Laboratories, Inc., Hercules, CA, USA) with β-actin
as an internal reference. Each band was analyzed using the Image
Pro Plus 6.0 (Olympus, Tokyo, Japan). Each experiment was repeated
three times.
CCK-8 assay
The cells at the logarithmic phase were collected
from each group, made into cell suspension and added into a 96-well
plate at 100 µl/well. Each group was provided with 3 parallel
control wells. The cells were cultured at 37°C with 5%
CO2 for 24, 48 and 72 h respectively. An additional 1-h
incubation in each well was performed with the addition of 10 µl
Cell Counting Kit-8 (CCK-8) (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). The microplate reader (Thermo Fisher Scientific,
Inc.) was used to determine the OD at 450 nm. Every experiment was
repeated three times using the mean OD value.
Scratch wound-healing assay
Tca8113 cells were seeded in a 6-well plate at a
density of 5×104. After reaching from 70–80% confluence,
a cell scraper (width, 2 mm) was used to the scratch cells. The
cells were observed at 0 and 48 h and imaged using an IX71 inverted
microscope (Olympus), and Image-Pro Plus 6.0 software was used to
measure the distance between the scratches. The cell migration
distance was calculated using the following equation: Cell
migration distance (mm)=(scratch distance at 0 h)-(scratch distance
at 48 h). Each experiment was repeated three times.
Transwell invasion assay
Matrigel basement membrane matrix (BD Biosciences,
Franklin Lakes, NJ or San Jose, CA, USA) was thawed at 4°C.
Briefly, 5×104 cells in serum-free media were placed
into the upper chamber. Subsequently, Matrigel was diluted as 1:3
with serum-free DMEM medium, and added to the upper Transwell
chamber (EMD Millipore, Billerica, MA, USA) and dried at room
temperature. Following digestion with trypsin, the cells in each
group were made into single cell suspensions using serum-free DMEM
and starved for 24 h. Subsequently, cell suspension was added in
the upper chamber with 200 and 500 µl DMEM medium (containing 10%
FBS) were added to the 24-well plate. Subsequently, the chamber was
put into each well and cultured for 48 h at 37°C. Subsequently, the
chamber was taken out and washed using phosphate-buffered saline.
The culture solution in the upper chamber was emptied. The residual
Matrigel and cells on the micro-porous film of the chamber were
wiped. The cells were fixed with 95% ethyl alcohol for 15 min and
stained with 0.1% crystal violet at 37°C for 30 min and observed
under an inverted microscope to record the mean number of cells
passing through the basement membrane. Each experiment was repeated
three times.
Tumor xenograft model of nude
mice
The experimental animals used were 24 female BALB/C
mice (4–6 weeks; mean body weight, 18±2 g), which were bought from
the Institute of Laboratory Animal Sciences, Chinese Academy of
Medical Sciences and Peking Union Medical College. The mice were
fed at the Experimental Animal Center of Jingzhou Central Hospital
and bred under specified pathogen-free conditions (26°C, 70%
relative humidity and a 12-h light/12-h dark cycle) in a germ-free
environment with free access to food and water. The nude mice were
divided into three groups with eight mice in each group, including
blank group (inoculated with non-transfected Tca8113 cells), NC
group (inoculated with Tca8113 cells transfected with NC sequence)
and miR-409-3p mimic group (inoculated with Tca8113 cells
transfected with miR-409-3p mimic). Subsequently, the cell
concentration was adjusted to 5×106/ml, and this was
subcutaneously injected into the nude mice in each group at the
right lingual margin with 0.05 ml/mouse. At the end of the fourth
week, the mice were sacrificed by cervical dislocation, and tumor
volume was calculated using the formula:
Volume=(length/width2)/2. Subsequently, the tumor tissue
was separated, transplanted and weighted. Each axillary lymph node
was fixed in 4% formaldehyde at 4°C for 12 h, embedded in paraffin
and cut in 4 µm sections, examined and judged by two pathologists
to determine whether lymph node metastasis was present. H&E
staining and pathological examination were performed. Sections were
stained for 3 min in 1:1 hematoxylin, and for 1 min in 0.5% eosin
at room temperature. For immunohistochemistry, endogenous
peroxidase was blocked by 3% H2O2 for 5 min
at 37°C. The slide was blocked in 5% goat antiserum for 10 min at
37°C and incubated with lymphatic vessel endothelial hyaluronic
acid receptor 1 (LYVE1; monoclonal anti-LYVE1 antibody; cat. no.
sc-65647; Santa Cruz Biotechnology Inc., Dallas, TX, USA) at a
dilution of 1:100 at 4°C overnight. The sections were then
incubated with biotinylated secondary antibody (1:2,000) in a
two-step detection reagent PV-6004 (OriGene Technologies, Inc.,
Beijing, China). Lymphatic microvessel density (LMVD) was
determined as previously described by Weidner et al
(26).
Statistical analysis
All data was analyzed using SPSS software (version
21.0; IBM Corp., Armonk, NY, USA) and expressed as the mean ±
standard deviation. The comparison of normally distributed data
between two groups were analyzed using unpaired t-tests, and the
comparison among multiple groups was analyzed one-way analysis of
variance followed by Tukey post hoc tests for multiple comparisons.
The data are expressed as percentages and ratios. Discrete data
were analyzed using a chi-square test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of miR-409-3p in TSCC
tissues and cell lines
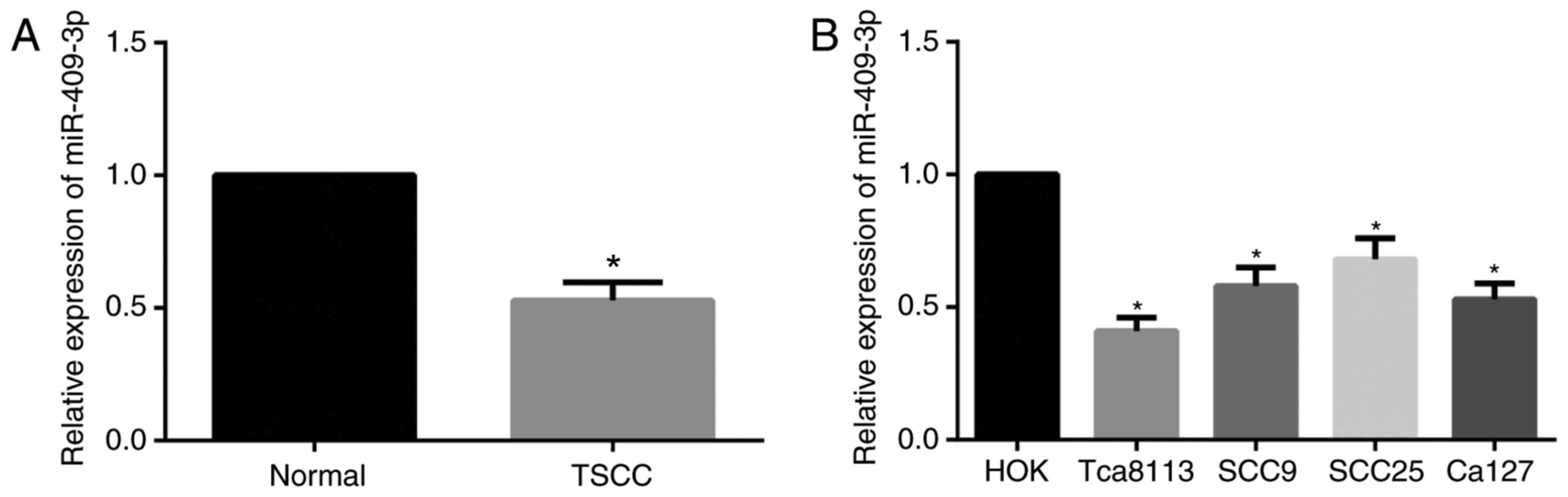
RT-qPCR was carried out to detect miR-409-3p
expression in TSCC tissues and cell lines. As demonstrated in
Fig. 1, significant downregulation of
miR-409-3p expression was demonstrated in tissues from patients
with TSCC when compared with normal tongue mucosa tissues
(P<0.05). Furthermore compared with the HOK cells, the
expression of miR-409-3p was significantly decreased in TSCC cell
lines (Tca8113, SCC9, SCC25 and Ca127; all P<0.05), and the most
significant decrease in miR-409-3p expression was observed in
Tca8113 cells (P<0.01), and therefore the subsequent
vitro experiments were performed using Tca8113 cells.
Association between miR-409-3p
expression and clinicopathological features of patients with
TSCC
A significant association between miR-409-3p
expression and lymph node metastasis and TNM stage was presented in
Table II (all P<0.05). miR-409-3p
expression was lower in patients with TSCC and lymph node
metastasis and advanced TNM stages compared with those with no
lymph node metastasis and with low TNM stages (Table II). However, no statistically
significant difference was observed between miR-409-3p expression
with age, sex, tumor diameter and differentiation of patients with
TSCC (all P>0.05; Table II).
 | Table II.Association between miR-409-3p
expression and clinicopathological features of patients with tongue
squamous cell carcinoma. |
Table II.
Association between miR-409-3p
expression and clinicopathological features of patients with tongue
squamous cell carcinoma.
| Clinicopathological
features | n | miR-409-3p
expression | P-value |
|---|
| Sex |
|
| 0.710 |
|
Male | 38 | 0.534±0.068 |
|
|
Female | 30 | 0.527±0.070 |
|
| Age |
|
| 0.979 |
|
≥55 | 49 | 0.527±0.068 |
|
|
<55 | 19 | 0.528±0.069 |
|
| Tumor diameter |
|
| 0.869 |
| ≥2
cm | 44 | 0.528±0.069 |
|
| <2
cm | 24 | 0.531±0.069 |
|
|
Differentiation |
|
| 0.330 |
|
Moderately/poorly
differentiated | 42 | 0.521±0.075 |
|
| Well
differentiated | 26 | 0.538±0.057 |
|
| TNM stage |
|
| 0.003 |
|
I–II | 43 | 0.547±0.060 |
|
|
III–IV | 25 | 0.495±0.072 |
|
| Lymph node
metastasis |
|
| <0.001 |
|
With | 20 | 0.471±0.051 |
|
|
Without | 48 | 0.551±0.062 |
|
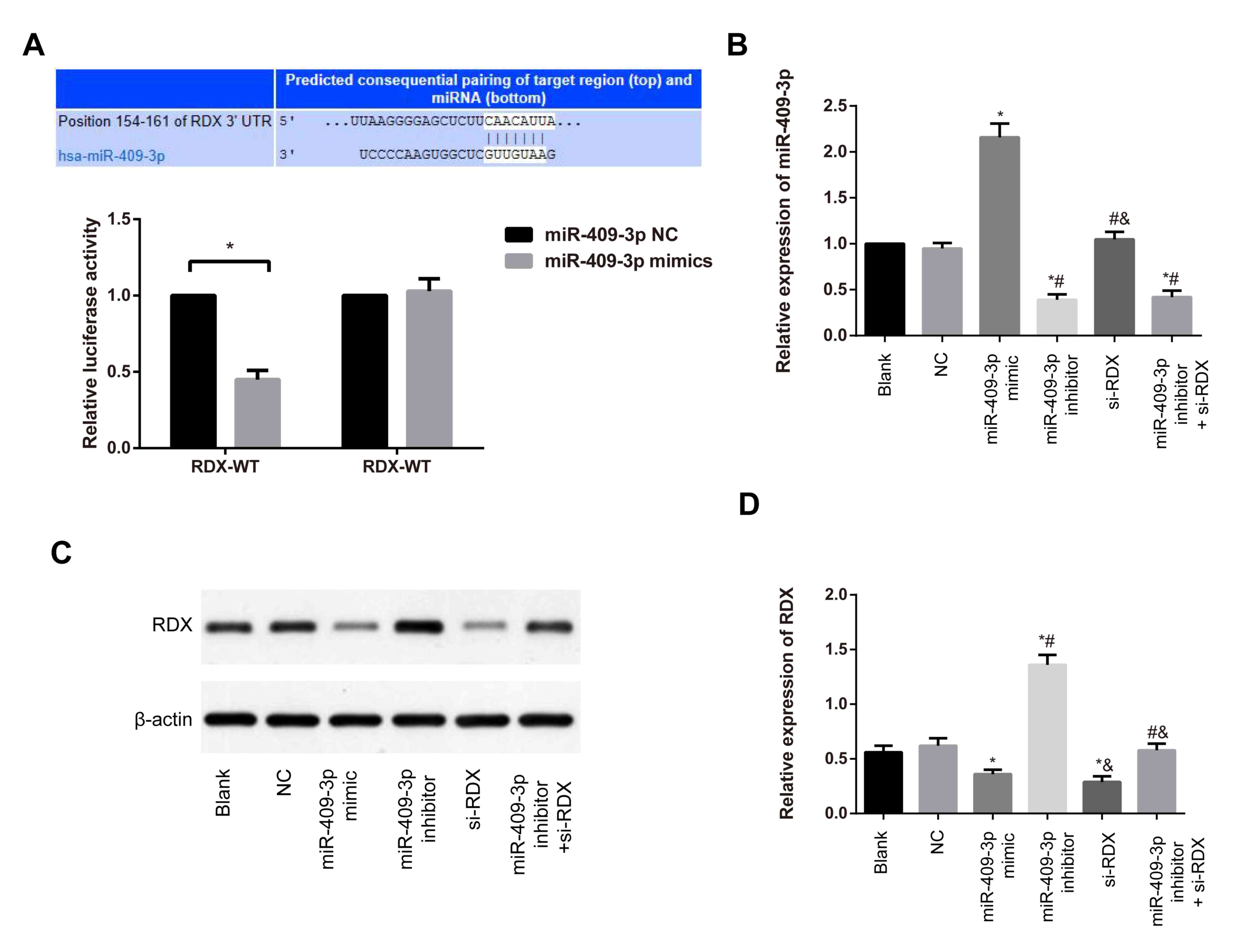
RDX is the target gene of
miR-409-3p
Targetscan (www.targetscan.org/) was used to predict the target
gene of miR-409-3p, and the miR-409-3p binding site within the
3′-UTR of RDX was identified (Fig.
2A). Furthermore, results from the dual-luciferase reporter
gene assay revealed that following co-transfection with miR-409-3p
mimic and RDX 3′UTR-WT, a decrease in the relative
luciferase activity was present compared with the luciferase
activity in cells that were co-transfected with miR-409-3p NC and
RDX 3′UTR-WT (P<0.05). The luciferase activity in cells
co-transfected with RDX 3′UTR-MUT + miR-409-3p NC and
RDX 3′UTR-MUT + miR-409-3p mimic was not significantly
different (P>0.05, Fig. 2A),
suggesting that RDX may be a target gene of miR-409-3p.
Expression of miR-409-3p and RDX in
various transfection groups
RT-qPCR and western blotting were conducted to
determine the expression of miR-409-3p and RDX. In comparison with
the blank group, no significant differences in miR-409-3p
expression were exhibited in NC and si-RDX groups (both P>0.05;
Fig. 2B). By contrast, an increase
was demonstrated in miR-409-3p mimic group compared with the blank
group. Furthermore, a marked decrease was detected in the
miR-409-3p inhibitor and miR-409-3p inhibitor + si-RDX groups
compared with the blank group (all P<0.05; Fig. 2B). As presented in Fig. 2C and D, the protein expression of RDX
was significantly decreased in the miR-409-3p mimic and si-RDX
groups compared with the blank group (both P<0.05; Fig. 2B). By contrast, an increase in RDX
expression was detected in the miR-409-3p inhibitor group, compared
with the blank group (P<0.05). When compared with the miR-409-3p
inhibitor group, RDX protein expression was reduced in the
miR-409-3p inhibitor + si-RDX group (P<0.05; Fig. 2B).
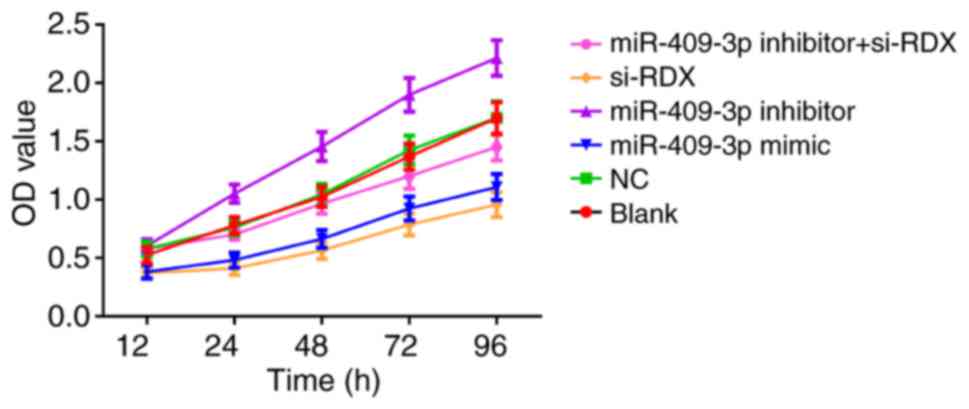
Proliferation of Tca8113 cells in each
transfection group
Cell proliferation in each transfected group was
detected using CCK-8 assay. No significant difference in cell
proliferation was detected between the blank and NC groups
(P>0.05; Fig. 3). However,
compared with the blank group, cell proliferation was decreased in
the miR-409-3p mimic and si-RDX groups respectively, and was
increased in the miR-409-3p inhibitor group (all P<0.05;
Fig. 3). Compared with the miR-409-3p
inhibitor group, cell proliferation was decreased in the miR-409-3p
inhibitor + si-RDX group (P<0.05; Fig.
3).
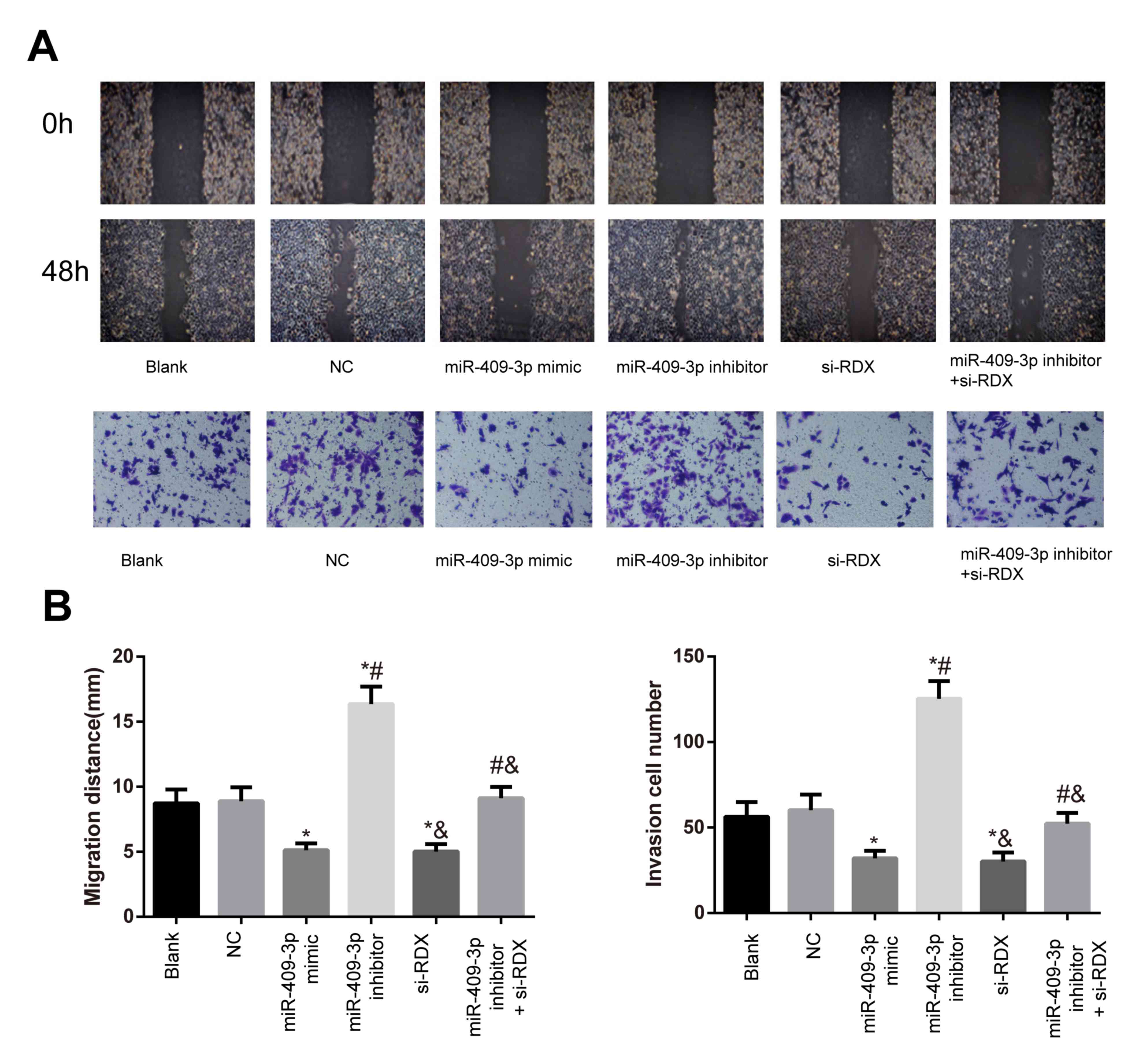
Migratory and invasive abilities of
Tca8113 cells in each transfection group
Compared with the blank group, the migratory and
invasive abilities of the cells in the NC group were not
significant (both P>0.05; Fig. 4).
There was a reduction in the miR-409-3p mimic and si-RDX groups and
an increase in the miR-409-3p inhibitor group with respect to the
migration and invasion abilities compared with the blank group (all
P<0.05). Furthermore, a significant decrease in migratory and
invasive abilities was detected in the miR-409-3p inhibitor +
si-RDX group compared with the miR-409-3p inhibitor group
(P<0.05, Fig. 4A and B).
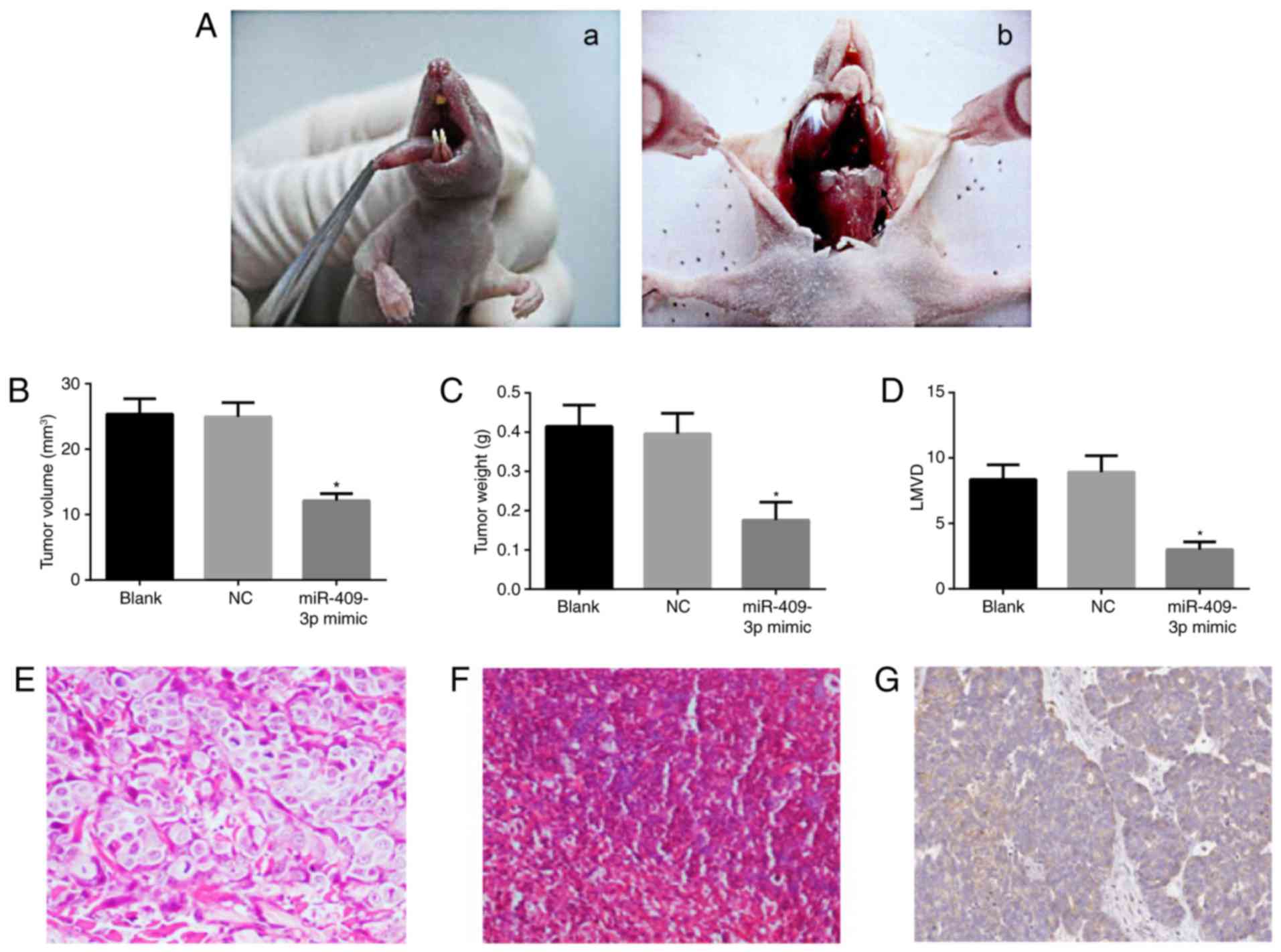
Effects of miR-409-3p on tumor growth
and lymphatic metastasis in nude mice
In order to further investigate the role of
miR-409-3p in vivo, a tumor xenograft model in nude mice
with Tca8113 cells was generated. The tumor formation rates of the
nude mice in three groups were 100%. Following tumor inoculation,
the tongue tumor presented outward growth with a marked increase in
the number of blood vessels (Fig.
5Aa), and neck dissection demonstrated that the nude mice
exhibited enlarged lymph nodes of the neck (Fig. 5Ab). Subsequently, the tumor volume and
weight were measured at the end of the experiment. Compared with
the blank group, tumor volume and weight were significantly reduced
in the miR-409-3p mimic group (both P<0.05; Fig. 5B and C). There were no significances
between the blank group and the NC group in terms of tumor volume
and weight (both P>0.05; Fig. 5B and
C).
Lymphatic metastasis in miR-409-3p mimic group was
0%, which was lower compared with the blank (6/8, 75%) and NC
groups (7/8, 87.5%), data not shown. In addition, LMVD in each
group was as follows: 8.36±1.12 for blank group, 8.92±1.26 for NC
group, and 3.01±0.58 for the miR-409-3p mimic group (Fig. 5D). The LMVD in the miR-409-3p mimic
group was significantly decreased compared with the blank group and
the NC group (both P<0.05; Fig.
5D), suggesting that miR-409-3p mimic exerts inhibitory effects
on lymphangiogenesis. H&E staining of tumor tissues is
presented in Fig. 5E. The tumor cells
appeared round or arranged in nest or sheet. In Fig. 5F, H&E staining results of a lymph
node illustrated that tumor nest were present in lymph nodes, and
the cell nuclei were darkly stained in unequal size. In Fig. 5G, the immunohistochemical staining
indicated a strong brown positive staining of LYVE-1 in cytoplasm
or cytomembrane.
Discussion
Recently, miR-409-3p was reported to be
downregulated in various types of tumors. For instance, Cao et
al (27) and Tang et al
(28) reported that miR-409-3p may
act as a promising prognostic indicator that is pronouncedly
decreased in breast cancer and osteosarcoma respectively.
miR-409-3p was also significantly associated with advanced TNM
stage and metastasis, representing a poor prognosis. Consistently,
in the present study, a significantly reduced expression of
miR-409-3p was detected in TSCC tissues and cell lines compared
with normal controls. miR-409-3p expression was lower in TSCC
patients with lymph node metastasis and higher TNM stage compared
with those without lymph node metastasis and lower TNM stage,
suggesting miR-409-3p may act as a tumor suppressor in the
progression of TSCC. Notably, Zhang et al (29) reported an association between
miR-409-3p and the suppression of breast cancer growth in
vitro and in vivo partially via targeting Akt1.
Additionally, E74 like ETS transcription factor 2 (ELF2) was
demonstrated to be a novel direct target of miR-409-3p in
osteosarcoma cells, where the overexpression of ELF2 was
able to rescue the suppressive function of miR-409-3p in cell
proliferation and tumor growth (30).
Consistent with the aforementioned findings,
RDX was identified as a target gene of miR-409-3p via
dual-luciferase reporter gene assay in the present study.
RDX is a type of cytoskeletal protein, which belongs to the
ERM family (31). Notably, previous
studies support that RDX serves an important role in the
proliferation, migration, infiltration of tumor cells and the
destruction of vascular endothelial barrier function (32,33),
indicating that miR-409-3p might exert a tumor suppressor function
during the development of TSCC via targeting RDX.
In the present study an in vitro experiment
was performed using Tca8113 cells, and a decrease in RDX
expression, cell proliferation, the number of invaded cells and
migration distance following the transfection of miR-409-3p mimic
or RDX siRNA were observed, suggesting that overexpression of
miR-409-3p and silencing of RDX are able to decrease the
migratory and invasive abilities of TSCC cells to inhibit the
growth of tumor cells.
Furthermore, it was also demonstrated that si-RDX is
able to reverse the effect of miR-409-3p inhibitor in TSCC.
Notably, a similar expression pattern of miR-409-3p and RDX
was reported in gastric cancer, where miR-409-3p was able to
suppress RDX expression via directly binding to its 3′-UTR region,
thereby decreasing cell invasion and metastasis (20). Furthermore, the silencing of
RDX in gastric cancer may inhibit the migration and invasion
of SGC-7901 cells by upregulating E-cadherin expression, as
indicated by Zhu et al (34),
and the nuclear factor (NF)-κB/snail signaling pathway may also be
involved. In addition, it was documented in a previous study that
RDX is able to activate the Rac1-extracellular signal
regulated kinase signaling pathway, and increase the secretion of
matrix metallopeptidase (MMP)-7, thereby decreasing the invasion
and migration of colon cancer cells (35). Therefore, miR-409-3p is able to
regulate the transduction of associated downstream signaling
pathways via downregulating the expression of RDX and
suppress the proliferative, invasive and migratory abilities of
TSCC.
Due to the close association of the migratory and
invasive abilities of tumor cells with their metastatic features
(36), a tumor xenograft model of
TSCC in nude mice was established with Tca8113 cells to investigate
the role of miR-409-3p on tumor growth and lymphatic metastasis. It
was demonstrated that a significant decrease in tumor volume,
weight, lymphatic metastasis rate and LMVD was observed in the SCC
nude mice that were transfected with miR-409-3p mimics compared
with the blank group. The increase in LMVD was closely associated
with lymphatic metastasis (37).
Current literature suggests that an increased LMVD
indicates an increased risk for malignancies and a relatively poor
prognosis (38). The present findings
indicated that the overexpression of miR-409-3p exerted inhibitory
effects on tumor growth and metastasis, which further confirms
results of the in vitro experiments. In addition, it was
previously reported that the overexpression of miR-409-3p in HT1080
cells was able to downregulate the expression of the target gene
angiogenin so as to inhibit the growth of the transplantation
tumor, angiogenesis and metastasis in nude mice (39). In pancreatic cancer cell line PANC-1,
the inhibition of RDX via small hairpin (sh)RNA was able to
inhibit the proliferation and migration of cancer cells, and the
tumor microvessel density was decreased so as to significantly
inhibit the tumor growth following the implantation of RDX
shRNA-transfected cells in nude mice (40). These findings suggest that the
overexpression of miR-409-3p in nude mice may downregulate the
expression of RDX and inhibit the metastasis of tumor
cell.
In conclusion, the present findings indicated that
miR-409-3p expression was decreased in TSCC tissues and cells
compared with normal tongue mucosa tissues. miR-409-3p exerts a
tumor inhibitory function, which delays cell proliferation by
targeting RDX. This leads to the inhibition of migratory and
invasive abilities of TSCC cells, providing a potential strategy
for the treatment of TSCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HC designed the study and performed experiments; JD
analyzed the data and made the figures; HC and JD drafted and
revised the paper; all authors approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Clinical
Ethics Committee of Jingzhou Central Hospital (Hubei, China). All
animal procedures were performed according to the Guide for the
Care and Use of Laboratory Animals published by the US National
Institutes of Health (22). All
patients provided written informed consent to participate.
Consent for publication
All patients provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khan Sannam R, Khurshid Z, Akhbar S and
Moin Faraz S: Advances of salivary proteomics in oral squamous cell
carcinoma (OSCC) detection: An update. Proteomes. 4:2016.
|
|
2
|
Kidani K, Osaki M, Tamura T, Yamaga K,
Shomori K, Ryoke K and Ito H: High expression of EZH2 is associated
with tumor proliferation and prognosis in human oral squamous cell
carcinomas. Oral Oncol. 45:39–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao W, Feng Z, Cui Z, Zhang C, Sun Z, Mao
L and Chen W: Up-regulation of enhancer of zeste homolog 2 is
associated positively with cyclin D1 overexpression and poor
clinical outcome in head and neck squamous cell carcinoma. Cancer.
118:2858–2871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mücke T, Kanatas A, Ritschl LM, Koerdt S,
Tannapfel A, Wolff KD, Loeffelbein D and Kesting M: Tumor thickness
and risk of lymph node metastasis in patients with squamous cell
carcinoma of the tongue. Oral Oncol. 53:80–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaboodkhani R, Karimi E, Ashtiani
Khorsandi MT, Kowkabi S, Firouzifar MR, Yazdani F and Yazdani N:
Evaluation of the correlation between CD44, tumor prognosis and the
5-Year survival rate in patients with oral tongue SCC. Iran J
Otorhinolaryngol. 28:407–411. 2016.PubMed/NCBI
|
|
6
|
Taghavi N and Yazdi I: Prognostic factors
of survival rate in oral squamous cell carcinoma: Clinical,
histologic, genetic and molecular concepts. Arch Iran Med.
18:314–319. 2015.PubMed/NCBI
|
|
7
|
McIntire M and Redston M: Targeted
therapies and predictive markers in epithelial malignancies of the
gastrointestinal tract. Arch Pathol Lab Med. 136:496–503. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Djuranovic S, Nahvi A and Green R: A
parsimonious model for gene regulation by miRNAs. Science.
331:550–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
94:776–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
11
|
Bai R, Weng C, Dong H, Li S, Chen G and Xu
Z: MicroRNA-409-3p suppresses colorectal cancer invasion and
metastasis partly by targeting GAB1 expression. Int J Cancer.
137:2310–2322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wan L, Zhu L, Xu J, Lu B, Yang Y, Liu F
and Wang Z: MicroRNA-409-3p functions as a tumor suppressor in
human lung adenocarcinoma by targeting c-Met. Cell Physiol Biochem.
34:1273–1290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu X, Chen H, Lin Y, Hu Z, Mao Y, Wu J, Xu
X, Zhu Y, Li S, Zheng X and Xie L: MicroRNA-409-3p inhibits
migration and invasion of bladder cancer cells via targeting c-Met.
Mol Cells. 36:62–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li C, Nie H, Wang M, Su L, Li J, Yu B, Wei
M, Ju J, Yu Y, Yan M, et al: MicroRNA-409-3p regulates cell
proliferation and apoptosis by targeting PHF10 in gastric cancer.
Cancer Lett. 320:189–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Josson S, Gururajan M, Hu P, Shao C, Chu
GY, Zhau HE, Liu C, Lao K, Lu CL, Lu YT, et al: miR-409-3p/-5p
promotes tumorigenesis, epithelial-to-mesenchymal transition, and
bone metastasis of human prostate cancer. Clin Cancer Res.
20:4636–4646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Valastyan S, Chang A, Benaich N, Reinhardt
F and Weinberg RA: Concurrent suppression of integrin alpha5,
radixin, and RhoA phenocopies the effects of miR-31 on metastasis.
Cancer Res. 70:5147–5154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu G and Voyno-Yasenetskaya TA: Radixin
stimulates Rac1 and Ca2+/calmodulin-dependent kinase, CaMKII:
Cross-talk with Galpha13 signaling. J Biol Chem. 280:39042–39049.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arpin M, Chirivino D, Naba A and
Zwaenepoel I: Emerging role for ERM proteins in cell adhesion and
migration. Cell Adh Migr. 5:199–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsai MM, Wang CS, Tsai CY, Chen CY, Chi
HC, Tseng YH, Chung PJ, Lin YH, Chung IH, Chen CY and Lin KH:
MicroRNA-196a/-196b promote cell metastasis via negative regulation
of radixin in human gastric cancer. Cancer Lett. 351:222–231. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng B, Liang L, Huang S, Zha R, Liu L,
Jia D, Tian Q, Wang Q, Wang C, Long Z, et al: MicroRNA-409
suppresses tumour cell invasion and metastasis by directly
targeting radixin in gastric cancers. Oncogene. 31:4509–4516. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
The Helsinki Declaration of the World
Medical Association (WMA). Ethical principles of medical research
involving human subjects. polski merkuriusz lekarski: Organ
Polskiego Towarzystwa Lekarskiego. 36:298–301. 2014.PubMed/NCBI
|
|
22
|
Bayne K: Revised guide for the care and
use of laboratory animals available. American physiological
society. Physiologist. 39(199): 208–111. 1996.
|
|
23
|
Okuyemi OT, Piccirillo JF and Spitznagel
E: TNM staging compared with a new clinicopathological model in
predicting oral tongue squamous cell carcinoma survival. Head Neck.
36:1481–1489. 2014.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma Z, Li Y, Xu J, Ren Q, Yao J and Tian X:
MicroRNA-409-3p regulates cell invasion and metastasis by targeting
ZEB1 in breast cancer. IUBMB Life. 68:394–402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weidner N, Carroll PR, Flax J, Blumenfeld
W and Folkman J: Tumor angiogenesis correlates with metastasis in
invasive prostate carcinoma. Am J Pathol. 143:401–409.
1993.PubMed/NCBI
|
|
27
|
Cao GH, Sun XL, Wu F, Chen WF, Li JQ and
Hu WC: Low expression of miR-409-3p is a prognostic marker for
breast cancer. Eur Rev Med Pharmacol Sci. 20:3825–3829.
2016.PubMed/NCBI
|
|
28
|
Tang B, Liu C, Zhang QM and Ni M:
Decreased expression of miR-490-3p in osteosarcoma and its clinical
significance. Eur Rev Med Pharmacol Sci. 21:246–251.
2017.PubMed/NCBI
|
|
29
|
Zhang G, Liu Z, Xu H and Yang Q:
miR-409-3p suppresses breast cancer cell growth and invasion by
targeting Akt1. Biochem Biophys Res Commun. 469:189–195. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Hou W, Jia J, Zhao Y and Zhao B:
MiR-409-3p regulates cell proliferation and tumor growth by
targeting E74-like factor 2 in osteosarcoma. FEBS Open Bio.
7:348–357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Neisch AL and Fehon RG: Ezrin, radixin and
moesin: Key regulators of membrane-cortex interactions and
signaling. Curr Opin Cell Biol. 23:377–382. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Valderrama F, Thevapala S and Ridley AJ:
Radixin regulates cell migration and cell-cell adhesion through
Rac1. J Cell Sci. 125:3310–3319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yogesha SD, Sharff AJ, Giovannini M,
Bricogne G and Izard T: Unfurling of the band 4.1, ezrin, radixin,
moesin (FERM) domain of the merlin tumor suppressor. Protein Sci.
20:2113–2120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu YW, Yan JK, Li JJ, Ou YM and Yang Q:
Knockdown of radixin suppresses gastric cancer metastasis in vitro
by up-regulation of e-cadherin via NF-κB/snail pathway. Cell
Physiol Biochem. 39:2509–2521. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang QH, Wang AX and Chen Y: Radixin
enhances colon cancer cell invasion by increasing MMP-7 production
via Rac1-ERK pathway. ScientificWorldJournal. 2014:3402712014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Courtneidge SA: Cell migration and
invasion in human disease: The Tks adaptor proteins. Biochem Soc
Trans. 40:129–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eloy C, Santos J, Soares P and
Sobrinho-Simões M: Intratumoural lymph vessel density is related to
presence of lymph node metastases and separates encapsulated from
infiltrative papillary thyroid carcinoma. Virchows Arch.
459:595–605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jardim JF, Francisco AL, Gondak R,
Damascena A and Kowalski LP: Prognostic impact of perineural
invasion and lymphovascular invasion in advanced stage oral
squamous cell carcinoma. Int J Oral Maxillofac Surg. 44:23–28.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Weng C, Dong H, Chen G, Zhai Y, Bai R, Hu
H, Lu L and Xu Z: miR-409-3p inhibits HT1080 cell proliferation,
vascularization and metastasis by targeting angiogenin. Cancer
Lett. 323:171–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen SD, Song MM, Zhong ZQ, Li N, Wang PL,
Cheng S, Bai RX and Yuan HS: Knockdown of radixin by RNA
interference suppresses the growth of human pancreatic cancer cells
in vitro and in vivo. Asian Pac J Cancer Prev. 13:753–759. 2012.
View Article : Google Scholar : PubMed/NCBI
|