Introduction
Recent advances in laparoscopic gastrectomy for
gastric cancer, including reduced invasiveness and development of
standardized procedures, have been beneficial for patients
(1). For example, laparoscopic
surgical procedures for lymph node (LN) dissection, a
well-recognized crucial step in curative gastrectomy for gastric
cancer, have been standardized during the past two decades.
Classically, complete LN dissection around arterial vessels in
cases of advanced gastric cancer, particularly when performed with
curative intent, involved exposure of the tunica adventitia
(2). However, more recent
laparoscopic approaches have enabled surgeons to visualize and
recognize the exact borders between the LNs and perivascular
tissues, including the neuronal fibers, lymphatic vessels, and
fatty tissues that surround arteries. Kanaya et al (3), described this visible layer as the
‘outermost layer’ and insisted that during lymphadenectomy, it
would be beneficial to preserve the perivascular connective tissues
surrounding various arteries, including the hepatic and splenic
arteries (4). Dissection of
suprapancreatic LNs (e.g., 8a and 11) is considered a critical
point during lymphadenectomy because damage to the pancreas can
lead to major post-gastrectomy complications. Lymphadenectomy
procedures that preserve the periarterial connective tissue are
therefore considered superior to suprapancreatic LN dissection in
terms of preventing pancreatic damage while removing the LN.
Majority of the surgeons in Japan and other countries, therefore
adhere to the former procedures, especially for prophylactic LN
dissection in cases with early gastric cancer.
During a gastrectomy, the main gastric arteries
designated for resection are often bundled and cut along with the
periarterial connective tissue, which includes thick nerve fibers.
Recently, however, an LN dissection procedure that aims to preserve
these periarterial connective tissues has been applied to patients
with both early and advanced gastric cancer (4,5). The
prevalence and severity of neural invasion from the primary tumor
have been shown to correlate significantly with reduced survival
among patients with gastrointestinal malignancies such as
adenocarcinoma of the esophagogastric junction-II/III and gastric
cancer (6). Neural invasion from the
primary tumor has also been identified as a significant prognostic
factor in cases of diffuse invasive gastric cancer (7) as well as gastric cancer after curative
resection (8). Currently, the
incidence of cancer cell invasion into this periarterial connective
tissue, which also comprises of lymphatic vessels and fat tissue,
is thought to be almost zero in cases with early gastric cancer.
Also, neural invasion into these periarterial tissues is thought to
occur infrequently in cases with gastric cancer, compared to other
cancers such as hepatobiliary or pancreatic cancer (9). However, no study has investigated the
presence of cancer cells in periarterial tissues from patients with
gastric cancer.
We conducted the present study to evaluate the
safety of lymphadenectomy with periarterial connective tissue
preservation for treatment of gastric cancer. Proximal roots of the
left gastric artery (LGA) and right gastroepiploic arteries (RGEA)
obtained from surgical specimens following gastrectomy were
microscopically examined for the presence of cancer cells,
including neural invasion in the periarterial tissues.
Materials and methods
From April 2014 to November 2015, 23 roots of the
RGEA and 26 roots of the LGA were collected from 28 consecutive
gastric cancer patients and processed for histopathological
examination. All patients underwent D1+ or D2 gastrectomy with
preservation of the perivascular tissues surrounding the hepatic
and splenic artery at the Shiga University of Medical Science
Hospital. Case data were collected from our hospital database and
clinical records.
We pathologically examined the proximal roots of the
LGA and RGEA to detect cancer cells in the periarterial tissues. We
sampled the periarterial connective tissues around these arteries
which we considered as the distal vascular lymphadenectomy margin.
Resected specimens were stained with hematoxylin and eosin
according to standard procedures prior to evaluation.
Histopathological analysis was performed by 2 independent
pathologists (M.I. and R.K.). Histological diagnoses and responses
to neoadjuvant chemotherapy were evaluated based on the Japanese
guidelines for gastric cancer surgery and pathology (10). The ethics committee in Shiga
University of Medical Science approved this retrospective
observational study, including the patient's passive consent method
and it conformed to the 1995 provisions of the Declaration of
Helsinki (as revised in Brazil 2013). Passive consent, commonly
termed opt-out consent, assumes agreement to study participation
unless consent is deliberately withdrawn. For this type of study,
formal consent is not required (11,12).
Results
The patient characteristics are shown in Table I. The tumor stages of I, II, III and
IV were seen in 8, 8, 9 and 3 patients, respectively. We detected
cancer cells in the periarterial tissues from 3 (15%) of the 20
advanced gastric cancer cases. These 3 cases were classified as
pathological stages IIB, IIIC, and IV, and 2 of them had received
neoadjuvant chemotherapy. Cancer cells were detected in the thick
fibrous tissue with neural invasion in one case, in fat tissues in
the second case, and in fat tissues with neural invasion in the
third case. While 2 cases were obtained from specimens surrounding
the LGA, one case was from the RGEA. In contrast, cancer cells were
not detected in the periarterial tissues from the 8 patients with
early gastric cancer.
 | Table I.Patient characteristics (n=28
patients). |
Table I.
Patient characteristics (n=28
patients).
| Variables | no. |
|---|
| Mean age (range) | 67.3 (35–90) |
| Sex |
|
| Male | 20 |
|
Female | 8 |
| Location in
stomach |
|
| Upper
third of stomach | 6 |
| Middle
third of stomach | 15 |
| Lower
third of stomach | 7 |
| Final gastric cancer
stage stage |
|
| I | 8 |
| II | 8 |
| III | 9 |
| IV | 3 |
| pT |
|
| T1 | 9 |
| T2 | 3 |
| T3 | 8 |
| T4 | 8 |
| pN |
|
| N0 | 9 |
| N1 | 4 |
| N2 | 8 |
| N3 | 7 |
| Lymphatic invasion
grade |
|
| 0 | 7 |
| 1 | 10 |
| 2 | 6 |
| 3 | 5 |
| Vascular invasion
grade |
|
| 0 | 12 |
| 1 | 11 |
| 2 | 4 |
| 3 | 1 |
| Neoadjuvant
chemotherapy |
|
| Yes | 4 |
| No | 24 |
Neural invasion was observed in the first case of a
61-year-old male patient with esophagogastric junction carcinoma
(Siewert type 3). This patient underwent total gastrectomy with D2
LN dissection with exposure of the arterial tunica adventitia and
received additional hyperthermic intraperitoneal chemotherapy
following a diagnosis of T4 advanced gastric cancer with serosal
invasion and LN metastasis (SE, N3b, H0, P0, CY0). Following a
macroscopic and histopathological examination, the tumor was
diagnosed as a carcinoma at the esophagogastric junction that
comprised of a well to moderately differentiated tubular
adenocarcinoma with serosal invasion (T4a, ly2, v2) and two
perigastric LNs (no. 2; 1/1, no. 3a; 6/10). Cancer cells and
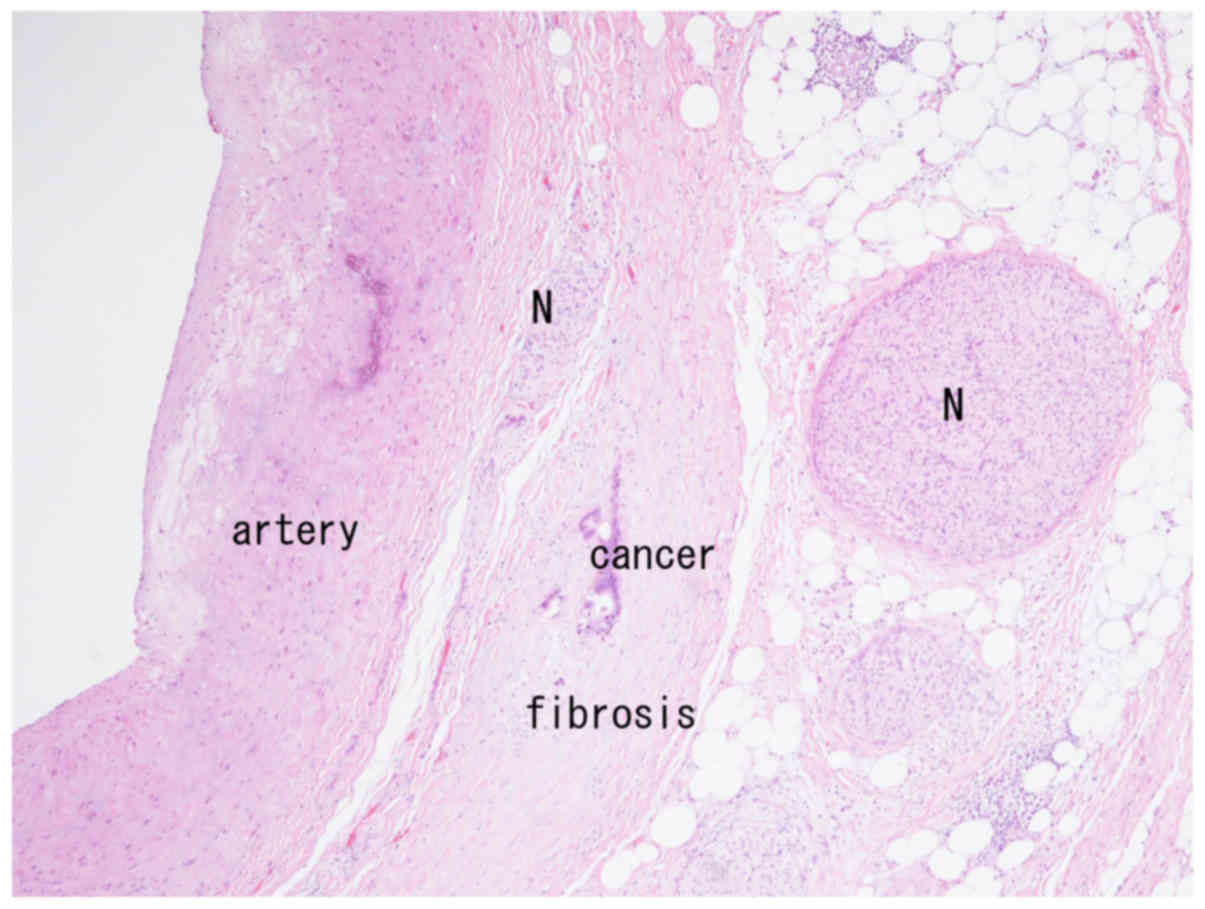
accompanying fibrosis were observed in very close proximity to the
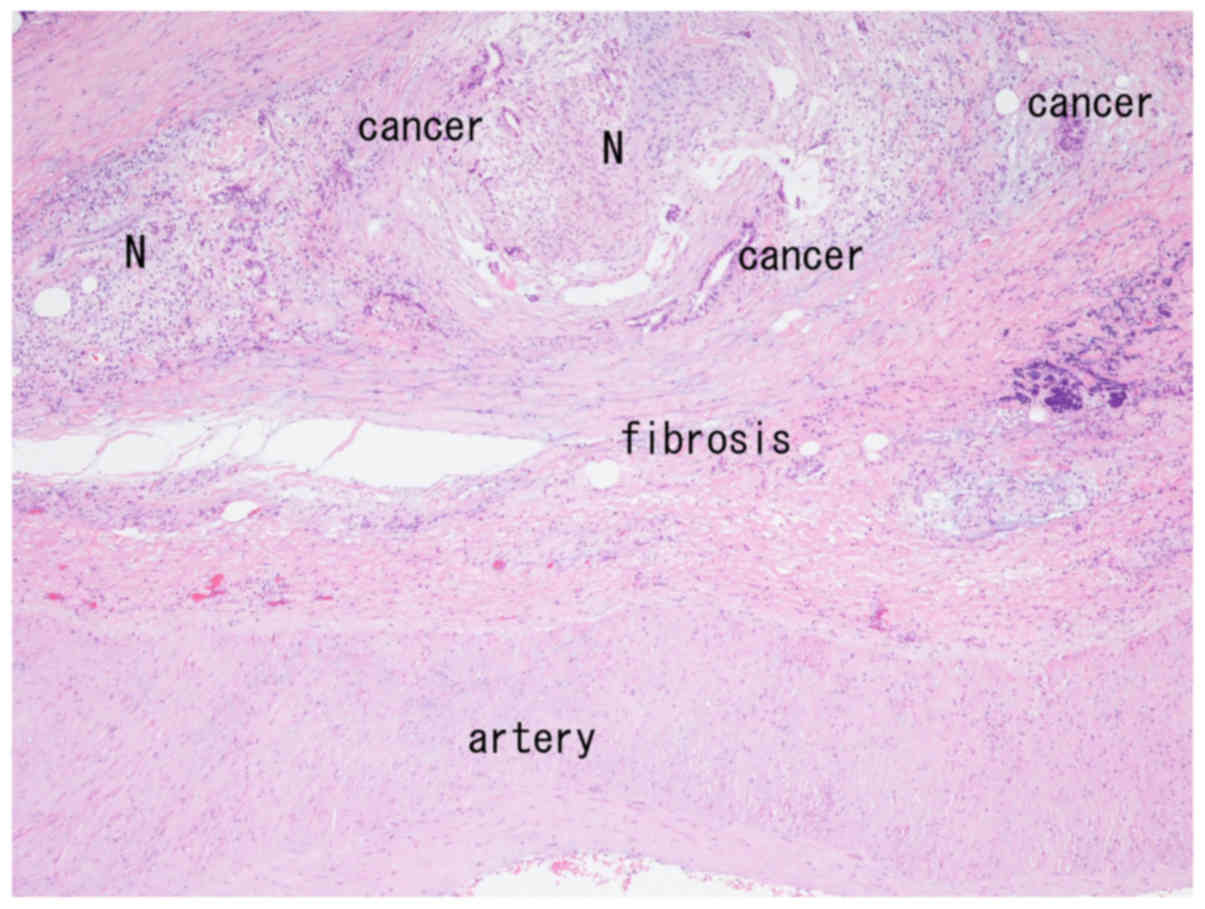
arterial wall (Fig. 1). Neural fibers
were observed both within and outside the fibrous tissue (Fig. 1), and neural invasion was seen in the
periarterial tissue (Fig. 2). The
patient received adjuvant chemotherapy with S-1. Metastatic
descending colon cancer was detected 27 months after surgery, and
he underwent colectomy. Another 10 months later, peritoneal
metastasis was detected, and he underwent resection of the small
intestine for an obstructive ileus due to peritoneal metastasis.
Forty months after surgery, he received best supportive care.
The second case involved a 71-year-old female
patient with a T4 advanced gastric cancer located in the middle of
the greater curvature of the stomach, with liver invasion (T4b, N0,
H0, P0). She underwent total gastrectomy, splenectomy, and D2 LN
dissection with exposure of the arterial tunica adventitia after 2
courses of neoadjuvant S-1/CDDP chemotherapy, as well as partial
resection of the lateral segment of the liver. The pathological
diagnosis was mucinous adenocarcinoma of the stomach, whereas no
carcinoma invasion to the liver was observed. Metastasis was
confirmed histologically in 12/26 LNs (no. 1: 2/2, no. 3a: 1/1, no.
3b: 2/2, no. 4d: 1/1, no. 6: 1/2, no. 7: 1/1, no. 9: 2/2, no. 11d:
2/2). The final histological diagnosis was gastric carcinoma (UML,
type 4, T4a(SE), med, INFc, ly2, v1, P0, H0, M0, N3, CY1, stage
IV), and the patient exhibited a grade 1a pathological response to
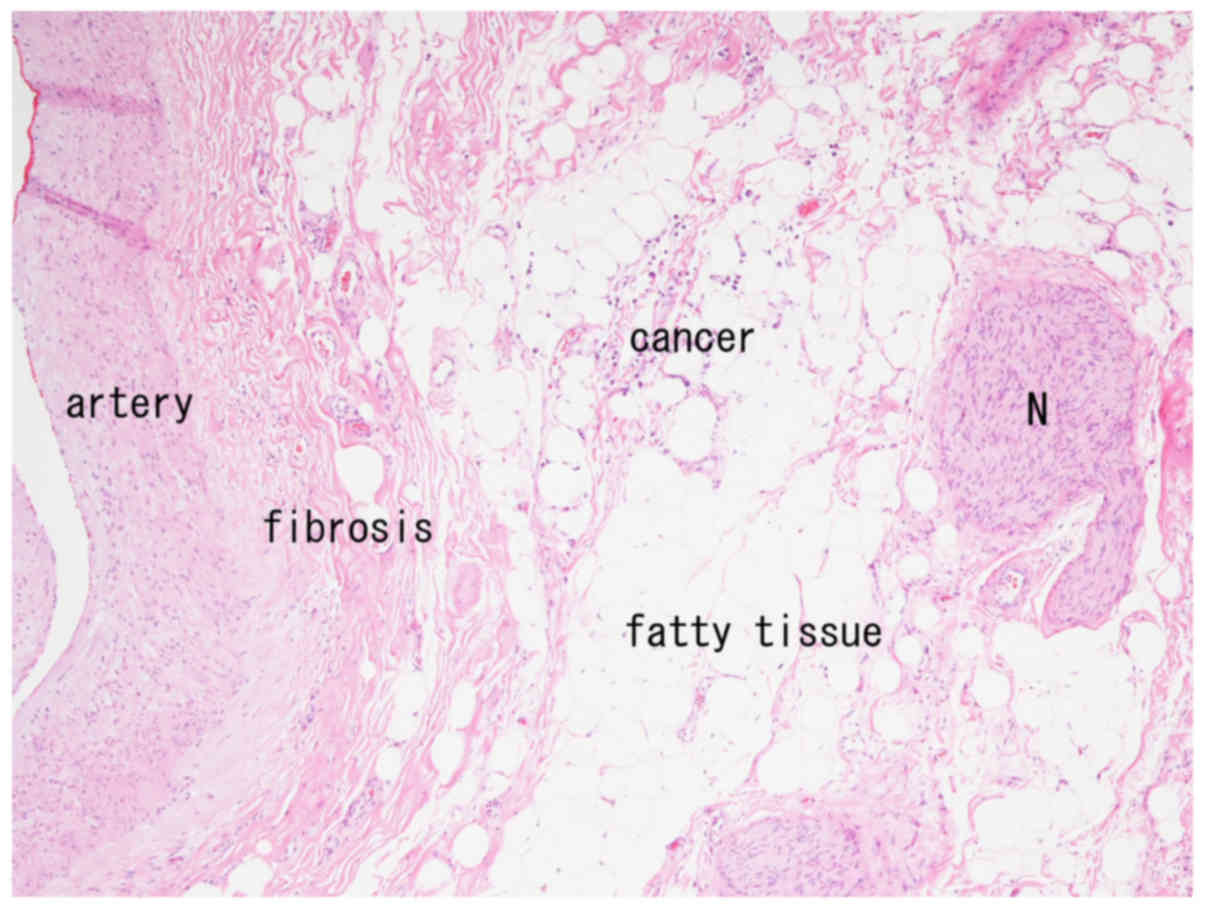
chemotherapy. A specimen from the root of the LGA contained cancer
cells in the periarterial fat tissue, with no neural invasion
(Fig. 3). Although she received
adjuvant chemotherapy with 8 courses of S-1 and oxaliplatin,
pleural effusion and ascites were detected which turned out to be
peritoneal carcinomatosis. Nine months after surgery, she received
a second line of chemotherapy with ramucirumab and paclitaxel.
However, eighteen months after surgery she died of peritoneal
carcinomatosis.
The third case involved a 79-year-old female patient
with gastric cancer located in the lower greater curvature of the
stomach. The tumor had invaded the pancreas body and affected the
LNs (T4b, N2, H0, P0). After 2 courses of neoadjuvant S-1/CDDP
chemotherapy, she underwent total gastrectomy, splenectomy, and D2
LN dissection with exposure of the arterial tunica adventitia,
leading to a histological tumor diagnosis of poorly differentiated
adenocarcinoma of the stomach. Histologically, 8/20 LN metastases
were confirmed (no. 1: 1/5, no. 4d: 3/6, no. 6: 3/5, no. 12a: 1/1).
The pathological diagnosis was gastric carcinoma (L, type 3, T2
(MP), sci, INFc, ly1, v0, P0, H0, M0, N2, CY1, stage IV), and the
primary tumor exhibited a grade 2 histological response after
preoperative chemotherapy. Cancer cells were detected in the
periarterial fat tissue, and neural fibers were found in a specimen
from the root of the RGEA. She received chemotherapy with 4 courses
of S-1 and cisplatin followed by S-1 for 15 months. Twenty-six
months after surgery, she is currently in remission.
Discussion
In our study of specimens resected during
gastrectomy, we observed cancer cells in the perivascular tissues
surrounding the LGA and RGEA of patients with advanced gastric
cancer. Although previously perivascular tissue preservation was
usually performed in patients undergoing curative gastrectomy with
lymphadenectomy for early gastric cancer, extended lymphadenectomy
with D2 LN dissection has become popular for patients with advanced
gastric cancer (13). However, in
Japan, the last 2 decades have seen changes in the actual
lymphadenectomy procedure used around the roots of gastric arteries
intended for resection and along arterial vessels, including the
hepatic and splenic arteries.
In Japan, prior to the popularization of
laparoscopic gastrectomy, lymphadenectomy for gastric cancer
(particularly advanced gastric cancer) had involved exposure of the
arterial tunica adventitia (2). The
recent laparoscopic approaches have introduced new surgical anatomy
with particular reference to the membrane structures, leading to
the establishment of sophisticated lymphadenectomy procedures that
preserve the arterial connective tissues of patients with gastric
cancer. The designated ‘outermost layer’ appears to be a reasonable
anatomical landmark during lymphadenectomy in patients with early
gastric cancer. Indeed, a recent multicenter phase II trial
involving patients who underwent laparoscopy-assisted distal
gastrectomy with suprapancreatic nodal dissection for clinical
stage I gastric cancer found that only 1.7% of the patients (a low
rate) developed an anastomotic leakage or a pancreatic fistula
(14). However, we became concerned
about the safety of this lymphadenectomy procedure in patients with
advanced gastric cancer. Although the main gastric arteries are
usually resected at their roots during radical LN dissection for
gastric cancers, it was unclear whether the periarterial tissues
surrounding the roots of arteries should be totally removed. In the
current study, we detected cancer cells in the periarterial tissues
surrounding the LG and RGEA roots in 15% of the patients with
advanced gastric cancer and LN metastasis. To the best of our
knowledge our study is the first to have employed this approach.
This finding suggests that in advanced cases, periarterial tissues
should be removed completely under arterial tunica adventitia
exposure in order to eliminate any residual cancer cells at the
roots of major gastric arteries.
In addition, although we did not present direct
evidence, cases of advanced gastric cancer with LN metastasis may
also involve periarterial tissues around the hepatic and splenic
arteries. A recent paper that described the standardization of D2
lymphadenectomy and surgical quality control used the term
‘exposure of vessels’ to describe suprapancreatic lymphadenectomy
(15). However, previous reports have
not distinguished between the preservation and removal of
periarterial tissues. We propose that there are two types of
curative lymphadenectomy for patients with gastric cancer. One is
prophylactic, and the other is therapeutic lymphadenectomy. While
the prophylactic lymphadenectomy involves dissection of potentially
metastasized LNs, therapeutic lymphadenectomy involves dissection
of obviously metastasized LNs. In cases of therapeutic
lymphadenectomy, especially those involving advanced gastric
cancers, there is no consensus regarding whether periarterial
tissues should be preserved or resected. Therefore, future
evaluations should address the issues of periarterial cancer
invasion and adequate removal of periarterial tissue around the
major arteries with the intent to establish radical regional
lymphadenectomy along with an oncologically adequate layer,
especially for advanced gastric cancers with LN metastases.
Importantly, surgeons who perform almost all gastrectomies by the
laparoscopic approach should not select the outermost layer for LN
dissection of far advanced tumors, especially those with bulky LNs.
Several reports on laparoscopic surgery for advanced gastric cancer
have emphasized on lymphadenectomy (4,6,16).
Neural invasion by hepatobiliary and pancreatic
cancers is a popular research topic with demonstrated prognostic
relevance (9). By contrast, neural
invasion by gastric cancer was previously thought to be rare but
has recently been identified as a critical prognostic factor (6–8).
However, neural invasion in the context of gastric cancer was
defined within the primary tumor and not in the periarterial
tissue, as discussed in the present study. Because the advanced
gastric cancers in this study clearly demonstrated the occurrence
of neural invasion in the periarterial tissues, future studies will
be needed to distinguish the clinical behaviors of neural-invasive
cancer cells in periarterial tissues from those within the main
tumor.
Pathologically, the effectiveness of gastric surgery
has not yet been evaluated based on the residual cancer cells at
the lymphadenectomy margins. Our study suggests that periarterial
connective tissues around major gastric arteries should be sampled
and considered as a distal vascular lymphadenectomy margin in order
to pathologically confirm the curability of surgical specimens from
advanced gastric cancers. Failure to perform such an evaluation
could have potentially led to an underestimation of tumor stage in
3 positive cases in this study. Furthermore, the presence of cancer
cells around the vascular margin suggests the potential need for
intensive postoperative chemotherapy. However, further studies will
be needed to clarify the clinical significance of the residual
cancer cells in the vascular margin with periarterial connective
tissue invasion. Immunohistochemical staining for claudin 4 has
been shown to be useful for detecting a small number of cancer
cells (17). However, in the present
study, as there were a sufficient number of cancer cells, we were
able to detect them via standard hematoxylin and eosin staining
without requiring to additionally stain for claudin 4.
We must note a particular limitation of this study,
namely the small number of examined cases. A larger number of
patients and additional arterial specimens would have provided more
information about cancer cell infiltration around the periarterial
connective tissues associated with gastric cancer, particularly in
terms of the resection margins of main gastric arteries, the
adequate periarterial layer for lymphadenectomy, and pathological
distal lymphadenectomy margins.
In conclusion, the present study microscopically
demonstrated the presence of cancer cells in periarterial tissues
from patients with advanced gastric cancer. Surgeons must,
therefore, consider the possibility of cancer cell invasion within
preserved periarterial tissues, although the clinical relevance of
these cells remains to be determined. Further studies to elucidate
the clinical effects of perivascular cancer cells on patient
prognosis are warranted.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HY contributed to the study conception and design,
HY, SM, SK, TY and MI were involved in the acquisition of data. HY,
MI, MT and RK were involved in the analysis and interpretation of
data. HY and SM drafted the manuscript and RK and MT critically
revised the manuscript.
Ethics approval and consent to
participate
The protocol for this research project was approved
by the Ethics Committee of the Shiga University of Medical Science,
and it conformed to the 1995 provisions of the Declaration of
Helsinki (as revised in Brazil 2013).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
Koeda K, Nishizuka S and Wakabayashi G:
Minimally invasive surgery for gastric cancer: The future standard
of care. World J Surg. 35:1469–1477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Funabiki T: Extended radical subtotal
gastrectomy with R3 lymphnodal dissection as well as saccate
bursctomy. Jpn J Gastroenterol Surg. 24:162–166. 1991. View Article : Google Scholar
|
|
3
|
Kanaya S, Haruta S, Kawamura Y, Yoshimura
F, Inaba K, Hiramatsu Y, Ishida Y, Taniguchi K, Isogaki J and Uyama
I: Video: Laparoscopy distinctive technique for suprapancreatic
lymph node dissection: Medial approach for laparoscopic gastric
cancer surgery. Surg Endosc. 25:3928–3929. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uyama I, Suda K and Satoh S: Laparoscopic
surgery for advanced gastric cancer: Current status and future
perspectives. J Gastric Cancer. 13:19–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu B and Lu KY: Neural invasion in
pancreatic carcinoma. Hepatobiliary Pancreat Dis Int. 1:469–476.
2002.PubMed/NCBI
|
|
6
|
Shinohara T, Satoh S, Kanaya S, Ishida Y,
Taniguchi K, Isogaki J, Inaba K, Yanaga K and Uyama I: Laparoscopic
versus open D2 gastrectomy for advanced gastric cancer: A
retrospective cohort study. Surg Endosc. 27:286–294. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liebl F, Demir IE, Mayer K, Schuster T,
D'Haese JG, Becker K, Langer R, Bergmann F, Wang K, Rosenberg R, et
al: The impact of neural invasion severity in gastrointestinal
malignancies: A clinicopathological study. Ann Surg. 260:900–907.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka A, Yoshikawa H, Okuno K, Koh K,
Watatani M, Matsumura E and Yasutomi M: The importance of neural
invasion (NI) as a prognostic factor in diffuse invasive gastric
cancer. Surg Today. 27:692–695. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bilici A, Seker M, Ustaalioglu BB, Kefeli
U, Yildirim E, Yavuzer D, Aydin FM, Salepci T, Oncel M and Gumus M:
Prognostic significance of perineural invasion in patients with
gastric cancer who underwent curative resection. Ann Surg Oncol.
17:2037–2044. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Japanese Gastric Cancer Association:
Japanese Classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Littenberg B and MacLean CD: Passive
consent for clinical research in the age of HIPAA. J Gen Intern
Med. 21:207–211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vellinga A, Cormican M, Hanahoe B, Bennett
K and Murphy AW: Opt-out as an acceptable method of obtaining
consent in medical research: A short report. BMC Med Res Methodol.
11:402011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sakuramoto S, Sasako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: Adjuvant chemotherapy for gastric cancer with
S-1, an oral fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katai H, Sasako M, Fukuda H, Nakamura K,
Hiki N, Saka M, Yamaue H, Yoshikawa T and Kojima K: JCOG Gastric
Cancer Surgical Study Group: Safety and feasibility of
laparoscopy-assisted distal gastrectomy with suprapancreatic nodal
dissection for clinical stage I gastric cancer: A multicenter phase
II trial (JCOG 0703). Gastric Cancer. 13:238–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim HI, Hur H, Kim YN, Lee HJ, Kim MC, Han
SU and Hyung WJ: Standardization of D2 lymphadenectomy and surgical
quality control (KLASS-02-QC): A prospective, observational,
multicenter study [NCT01283893]. BMC Cancer. 14:2092014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fukunaga T, Hiki N, Kubota T, Nunobe S,
Tokunaga M, Nohara K, Sano T and Yamaguchi T: Oncologic outcomes of
laparoscopy-assisted distal gastrectomy for gastric cancer. Ann
Surg Oncol. 20:2676–2682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Facchetti F, Lonardi S, Gentili F, Bercich
L, Falchetti M, Tardanico R, Baronchelli C, Lucini L, Santin A and
Murer B: Claudin 4 identifies a wide spectrum of epithelial
neoplasms and represents a very useful marker for carcinoma versus
mesothelioma diagnosis in pleural and peritoneal biopsies and
effusions. Virchows Arch. 451:669–680. 2007. View Article : Google Scholar : PubMed/NCBI
|