Introduction
Multiple myeloma (MM) is the second most common
hematological malignancy resulting from the growth of plasma cells
within the bone marrow (BM) and is typically accompanied by the
secretion of monoclonal immunoglobulins (1). Despite recent progress in treatment
modalities, MM is considered to be incurable, but treatable
(2). Affecting plasma cells, MM is a
hematopoietic malignancy characterized by a peculiar dependency on
the bone microenvironment. The interaction of MM with its
microenvironment, including BM stromal cells, osteoblasts,
endothelial cells, and cells of the innate and adaptive immune
system, including regulatory T cells, serves a crucial function in
the progression, dissemination and drug resistance of MM cells
(3). MM cells may reside in the
osteoblastic niche for protection from apoptotic stimuli, where
they also suppress the formation and function of osteoblasts,
leading to impairment of bone formation and the development of
osteolytic lesions (4). Improvement
in understanding MM and its microenvironment may result in novel
targets for treatment.
Integrin-linked kinase (ILK) was first described as
a serine/threonine protein kinase that binds directly to, and
transduces signals from, β1 integrin subunits, which appeared to
require the interaction of integrin cytoplasmic domains with
cellular proteins (5). ILK is
associated with multiple cellular functions including cell
migration, cell proliferation, cell adhesions and signal
transduction (6). ILK overexpression
was investigated in primary samples and MM cell lines, and the
molecular and physiological consequences of small interfering RNA
(siRNA)-mediated ILK ablation were compared with treatment with the
small-molecule inhibitor QLT0267 (7).
ILK was investigated for its potential as a nodal signal integrator
for microenvironmental cues in survival pathway activation
(7). A number of mechanisms are
involved in the crosstalk between stem cells and BM stromal cells,
including soluble cytokines, adhesion molecules and certain
signaling pathways, including protein kinase B, mammalian target of
rapamycin/FK506-binding protein-and rapamycin-associated protein,
the Wnt, Notch and parathyroid hormone signaling pathways (7–10).
In a previous study, we investigated the
participation of MSCs in inducing the angiogenic response in MM
(11). In the present study, the
effects of the ILK signaling pathway on MSC-mediated angiogenesis
were investigated. It was identified that MSCs induced by MM cells
differentiate into smooth muscle cells (SMCs), which depends on the
upregulation of ILK expression. Using a co-culture system, an
increase in secreted angiogenic factors was identified, which may
be involved in MSC differentiation.
Materials and methods
Cell culture
MM cell lines investigated included RPMI8226 and
U266 (American Type Culture Collection, Manassas, VA, USA). Ficoll
gradients were centrifuged for 40 min at 400 × g without pause. The
bone marrow mononuclear cell layer was then collected, washed in
PBS counted and prepared for culture (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). BM cells were cultured at 37°C
at an initial density of 5×104 cells/cm2 in
minimum essential medium-α (Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 10% defined fetal bovine serum (DFBS;
HyClone; GE Healthcare, Logan, UT, USA), L-glutamine (2 mM;
Invitrogen; Thermo Fisher Scientific, Inc.), bFGF (1 ng/ml; R&D
Systems, Inc., Minneapolis, MN, USA) and antibiotic/antimycotic
(amphotericin B, penicillin and streptomycin) (Invitrogen; Thermo
Fisher Scientific, Inc. #15240062).
After 1 or 2 days, non-adherent cells were removed
and fresh medium replaced the existing culture medium. Medium was
changed every 2 or 3 days until reaching confluence. For the
co-culture system, 5×104 MSCs were cultured in the
conditioned culture medium for 3 days. The supernatant was
harvested and added to the MM cultures, which were cultured for a
further 1 day. Adherent cells were removed by trypsinization,
harvested and cultured by seeding at 1×103
cells/cm2.
Immunophenotyping of MSCs
Following culture of MSCs for 3 days without MM
cells until reaching confluence, MSCs were stained with antibodies
against cluster of differentiation (CD)11a (#555380), CD11b
(#553310), CD14 (#557831), CD29 (#556047), CD31 (#560984), CD34
(#560940), CD44 (#560977), CD45 (#560777), CD105 (#562408), CD106
(#744313), human leukocyte antigen D-related (HLA-DR; #562805),
CD73 (#562430), CD90 (#550402) or matched isotype control (#562521)
(BD Biosciences, Franklin Lakes, NJ, USA), and with anti-CD138
antibody (#561703, BD Biosciences) to assess potential
contamination with residual plasma cells. Immunofluorescence
analysis was performed using fluorescence-activated cell sorting
(FACS) on a five-parameter flow cytometer (FACS Calibur; BD
Biosciences). The FACS protocol was performed as described
previously (12).
Preparation of conditioned medium
(CM)
MSCs were grown in Dulbecco's modified Eagle's
medium (DMEM), supplemented with 10% DFBS and 2 mM L-glutamine.
When the cultures were between 80 and 90% confluent, the medium was
exchanged for DMEM without FBS, and CM was collected following 2
days of incubation. CM was filtered through a 0.22-µM filter, and
used for experiments immediately following preparation.
SMC differentiation
MM cells were fixed directly in a 6-well plate with
4% formaldehyde for 10 min prior to being incubated with primary
antibodies against α-smooth muscle actin (α-SMA; 1:200; cat no.
ab21027, Abcam, Cambridge, MA, USA) at 4°C overnight. Following
incubation with a fluorescein isothiocyanate-conjugated
immunoglobulin G secondary mouse IgG2b antibody or control
phycoerythrin for 30 min at room temperature, (1:150; kit: cat no.
62-6511; Thermo Fisher Scientific, Inc.), washing and resuspension
in PBS, flow cytometry analyses were performed with a FACS Aria
instrument (BD Biosciences). Analyses were conducted using the
CellQuest software (version 5.2.1, BD CellQuest™ Pro; BD
Biosciences) and graphical outputs were obtained using FlowJo
software (FlowJo version 10.0; FlowJo LLC, Ashland, OR, USA).
siRNA transfection
MSCs were transfected with ILK siRNA (#AM16708,
Invitrogen; Thermo Fisher Scientific, Inc) (10 pmol) and irrelevant
scrambled [non-targeting (NT)] control siRNA (#AM4611, Invitrogen;
Thermo Fisher Scientific, Inc) using Lipofectamine™ 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. MSCs at 50% confluence were treated with
siRNA for 1 day in medium lacking serum and antibiotics. Inhibition
of ILK protein expression was observed within 1 day, and this
knockdown was maintained for at least 3 days following removal of
the medium containing the siRNA.
Western blotting
Total protein was extracted using standard protocols
using RIPA lysis buffer (#sc-24948, Santa Cruz Biotechnology).
Lysates from whole cell extracts or membrane pellets containing 20
µg of proteins were subjected to gel electrophoresis. Proteins were
then transferred to polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). The blots were blocked in 4% BSA in
tris-buffered Tween-20 solution for 30 min at room temperature and
were then incubated at 4°C overnight with the primary antibody. The
proteins were probed by ILK (1:1,000, #sc-20019, Santa Cruz
Biotechnology), VEGF receptor 2 (1:1,000, #ab9530, Abcam), α-SMA
(1:1,000, #ab21027; Abcam) and β-actin. After incubation with goat
anti-rabbit IgG horseradish peroxidase-conjugated secondary
antibodies (1:2,000, #12-348, Millipore) at room temperature for
1h, the blot was visualized by ChemiDoc™ XRS+ System device
(Bio-Rad Laboratories, Hercules, CA, USA). Images were analyzed by
Image J software (version 1.40 g; National Institutes of Health,
Bethesda, MD, USA)
Quantification of angiogenic factors
using ELISA
Recombinant vascular endothelial growth factor
(VEGF) (#900-K10), hepatocyte growth factor (HGF) (#100-39) and
basic fibroblast growth factor (bFGF) (#900-K08) ELISA Development
kits (PeproTech, Inc., Rocky Hill, NJ, USA) was used to quantify
the angiogenic factors VEGF, HGF and bFGF that were released into
the CM of MSCs, according to the manufacturer's protocol.
Formation of tube-like structures
Formation of tube-like structures of MSCs were
monitored by light microscopic observation at ×100 magnification
over six different fields for each well, when MSCs were cultured in
CM for 6 days. Custom image recognition and analysis software was
implemented in the C++ programming language with the use of the
dlib library (http://dlib.net/; date of access,
01/03/2017) for image processing and graphical user interface,
Anti-Grain Geometry library (http://www.antigrain.com/; date of access, 01/03/2017)
as a vector graphics engine and FFTW library (http://www.fftw.org/; date of access, 01/03/2017) for
calculating tube-like structures. Images of the cells were captured
for measurement of the length of tube-like structures, as described
previously (11,12).
Statistical analysis
All experiments were performed in triplicate.
Results are expressed as the mean ± standard deviation. Multiple
groups were compared using two-way analysis of variance followed by
a Bonferroni's post-test. All statistical analysis was performed by
using SPSS (version 11.0; SPSS Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
FACS analysis
The MSC immunophenotype was analyzed using FACS.
MSCs expressed CD44, CD105, CD106, CD90, CD73 and CD29, but did not
express CD11a, CD11b, CD14, CD31, CD34, CD45 or HLA-DR (Table I).
 | Table I.Immunophenotypes of cultured MSCs. |
Table I.
Immunophenotypes of cultured MSCs.
| Antigen | Proportion of MSCs
positive for the antigen, % (range) |
|---|
| CD11a | 2.1 (0.6–3.4) |
| CD11b | 1.3 (0.8–2.5) |
| CD14 | 3.2 (1.1–4.7) |
| CD29 | 95.4 (91.2–99.8) |
| CD31 | 3.1 (1.2–4.1) |
| CD34 | 2.5 (1.1–4.3) |
| CD44 | 97.5 (93.1–98.5) |
| CD45 | 1.9 (1.1–3.8) |
| CD105 | 97.6 (94.4–98.6) |
| CD106 | 94.5 (91.6–99.1) |
| HLA-DR | 3.1 (1.3–5.1) |
| CD73 | 96.4 (91.5–98.2) |
| CD90 | 94.1 (91.2–99.3) |
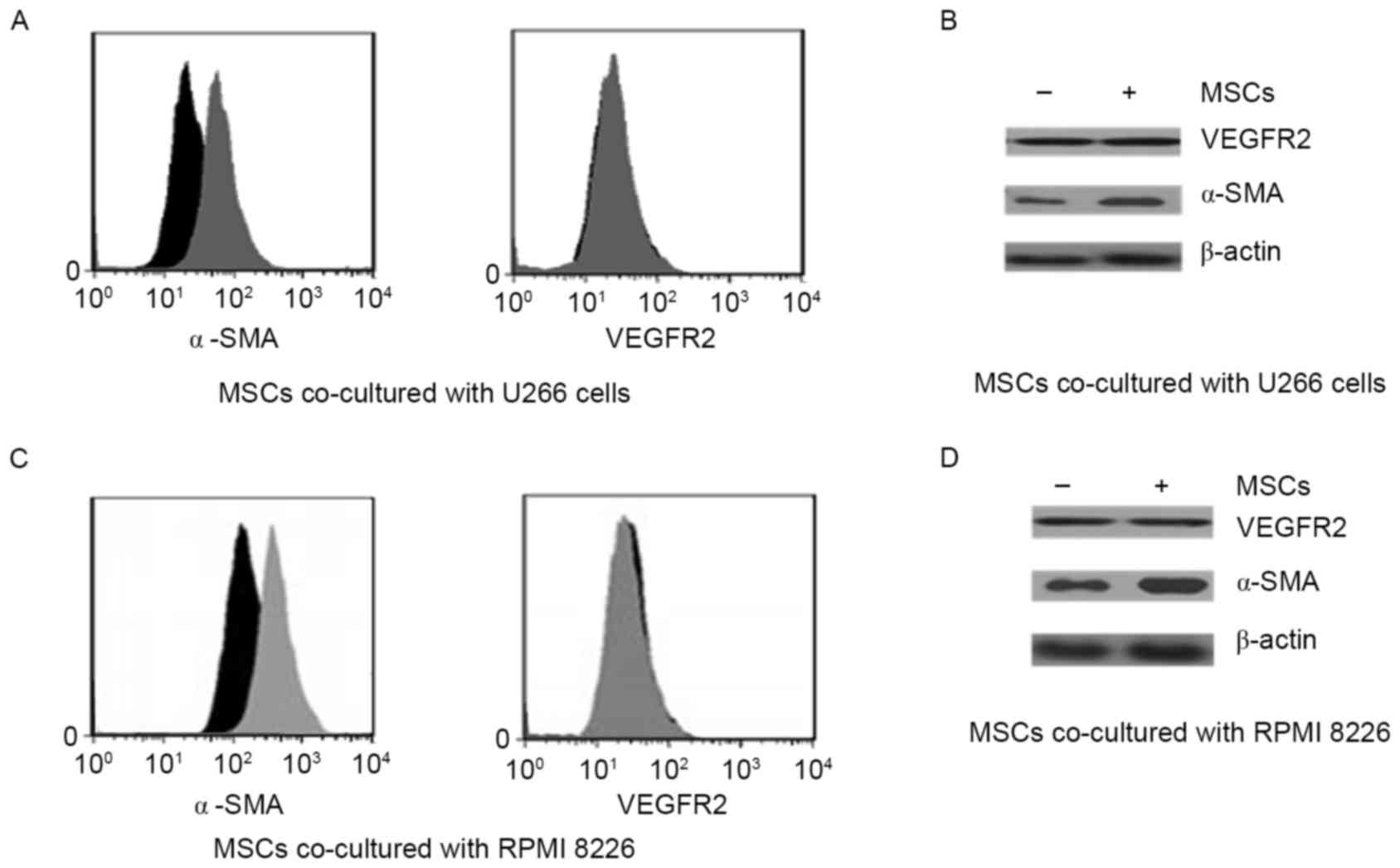
Angiogenic factors were upregulated in
MSCs that were co-cultured with MM cell lines
The classical angiogenic factors VEGF receptor 2
(VEGFR2) and α-SMA were identified using FACS (Fig. 1A and B). Importantly, α-SMA was
strongly upregulated by co-culture conditions, whereas VEGFR2 was
not significantly increased by co-culture with MM cell lines.
Co-culture with U266 and PMI8226 myeloma cells appeared to elicit
the same results. Angiogenic factors were also detected using
western blotting in the two co-culture systems (Fig. 1C and D). In agreement with the FACS
results, co-culture of MSCs with the MM cell lines increased α-SMA
protein expression, but not VEGFR2 expression.
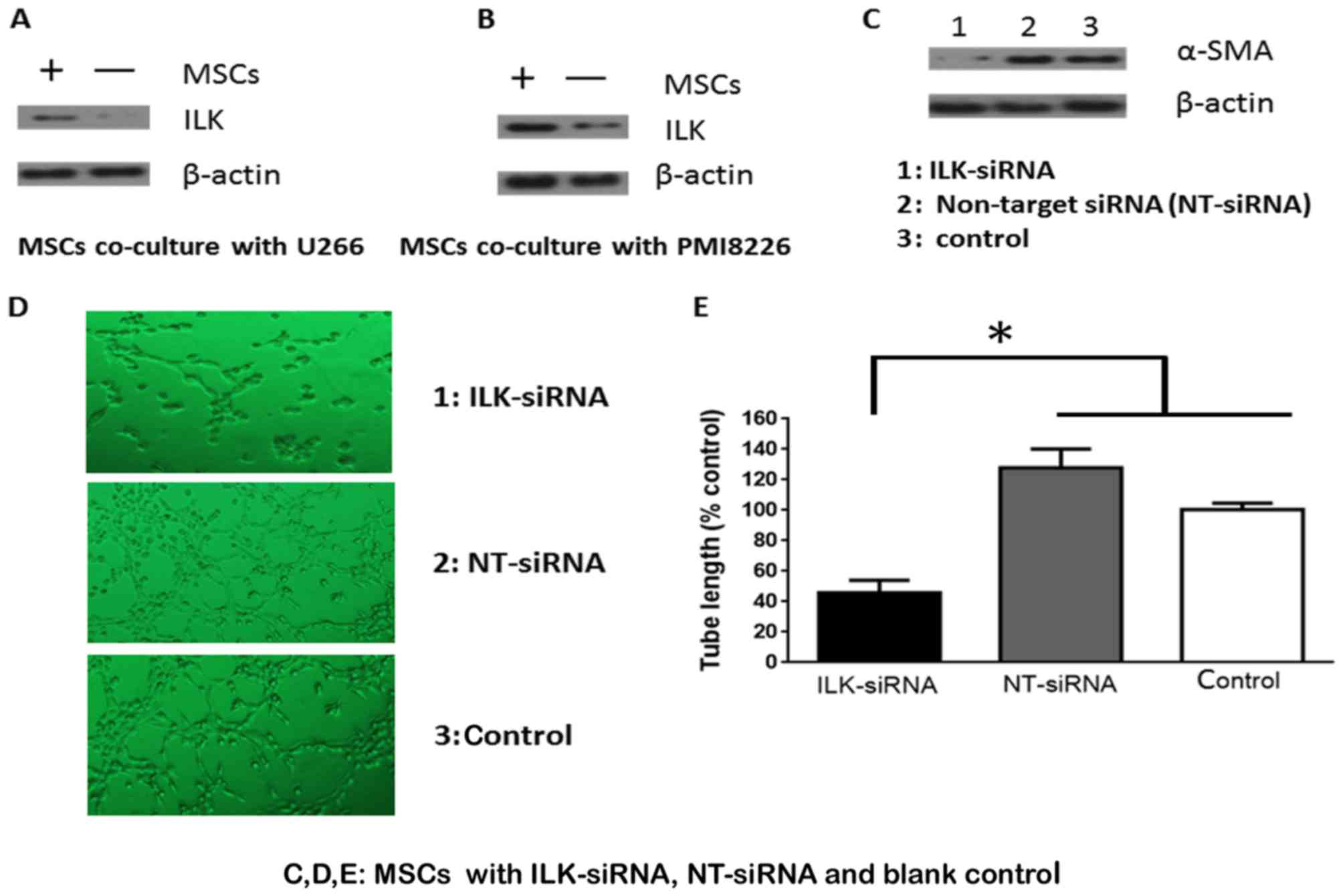
ILK promotes MSC differentiation into
SMCs
ILK protein expression was increased in MSC cells
co-cultured with U266 and PMI8226 MM cells (Fig. 2A and B). To elucidate whether ILK
mediated the effects of MSC differentiation, ILK protein expression
in MSCs was knocked down using siRNA. ILK expression was
successfully knocked down and α-SMA expression appeared to be
dependent on ILK (Fig. 2C). These
results were investigated further by evaluating the formation of
tube-like structures. There was a 5–10% difference in tube
formation ability between knockdown cells compared with controls,
while the number of the intersecting branches of assembled cells
networks was observed at a magnification of ×200 (Fig. 2D). MSCs transfected with ILK siRNA
significantly decreased the formation of capillary-like structures
in vitro, compared with cells transfected with control and
NT siRNA (P<0.05; Fig. 2E).
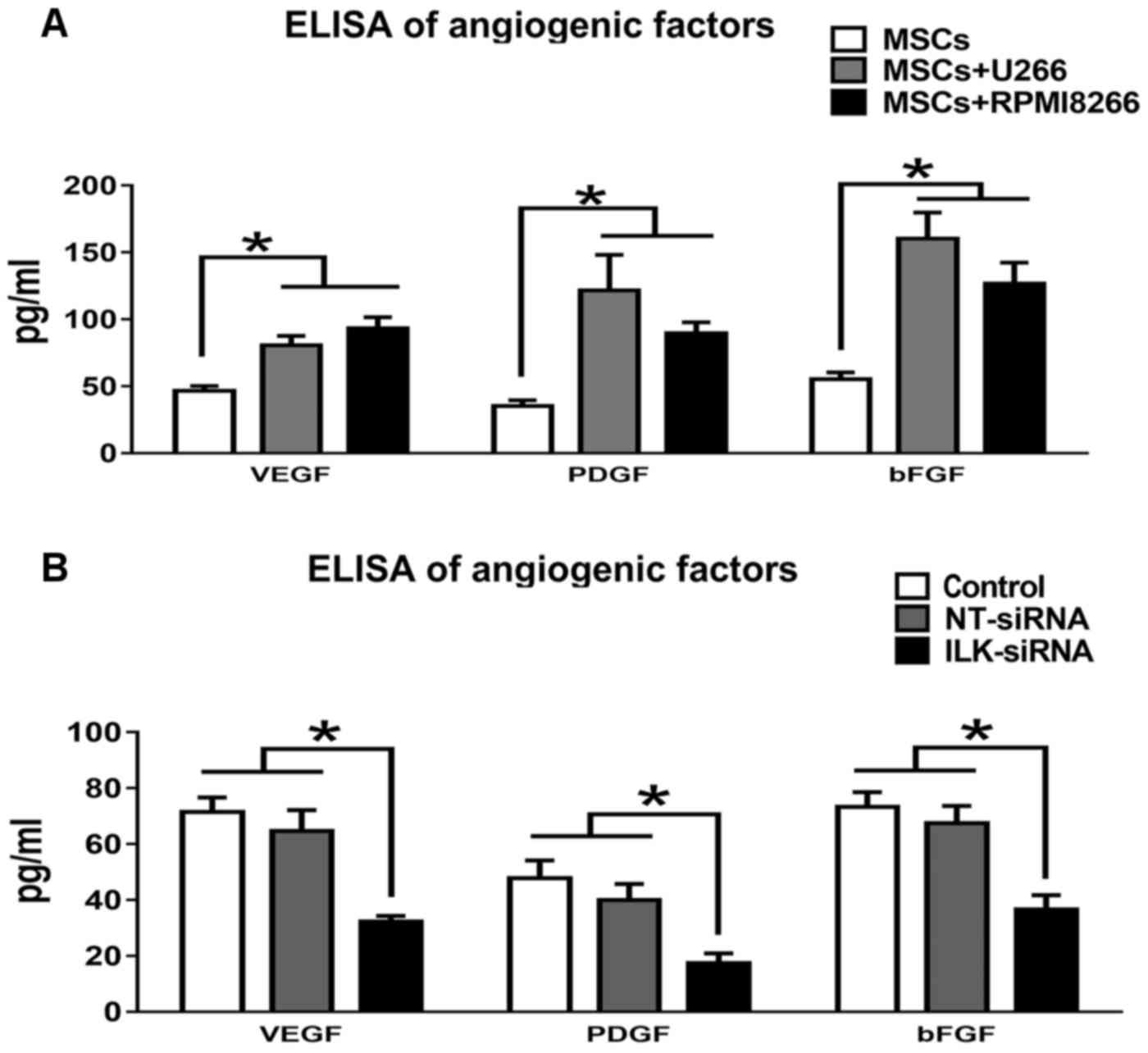
Quantification of angiogenic factors
using ELISA
The levels of the angiogenic factors including bFGF,
HGF and VEGF, which were released into the CM of MSCs cultured
alone or co-cultured with U266 or RPMI8266, were determined using
ELISA (Fig. 3A). All angiogenic
factors were significantly increased under co-culture conditions
(P<0.05; Table II). The levels of
the angiogenic factors were also determined in MSCs following
knockdown with ILK siRNA (Fig. 3B).
In Table III, bFGF, VEGF and PDGF
levels in ILK-knockdown MSCs were significantly decreased compared
with NT siRNA-transfected and control MSCs.
 | Table II.ELISA for determination of the
concentration of angiogenic factors (bFGF, VEGF and PDGF) in
conditioned medium of MSCs in co-culture or cultured alone. |
Table II.
ELISA for determination of the
concentration of angiogenic factors (bFGF, VEGF and PDGF) in
conditioned medium of MSCs in co-culture or cultured alone.
| Culture | VEGF, pg/ml | PDGF, pg/ml | bFGF, pg/ml |
|---|
| MSCs+U266 |
80.41±7.13a |
121.56±26.46a |
160.15±19.53a |
| MSCs+RPMI8266 |
93.25±8.34a |
89.53±8.26a |
126.64±15.67a |
| MSCs | 46.62±3.41 | 35.13±4.23 | 55.09±5.13 |
 | Table III.ELISA for determination of the
concentration of angiogenic factors (bFGF, VEGF and PDGF) in
conditioned medium of MSCs following siRNA treatment or blank
control. |
Table III.
ELISA for determination of the
concentration of angiogenic factors (bFGF, VEGF and PDGF) in
conditioned medium of MSCs following siRNA treatment or blank
control.
| Treatment | VEGF, pg/ml | PDGF, pg/ml | bFGF, pg/ml |
|---|
| ILK siRNA |
32.23±2.09a |
17.34±3.52a |
36.53±5.21a |
| NT siRNA | 64.56±7.53 | 39.96±5.76 | 67.45±6.17 |
| Control | 71.35±5.31 | 47.78±6.35 | 73.23±5.35 |
Discussion
Tumor development and progression, which occur in
the tumor microenvironment and are characterized by various
angiogenic factors, have been demonstrated to be supported by
angiogenesis. The BM microenvironment in MM is characterized by an
increased microvessel density (13,14). There
is growing evidence that integrins serve an essential function in
the recruitment of inflammatory cells to tumor sites, and
inhibition of integrin function has been suggested as a very
promising therapeutic approach for inflammatory diseases and tumors
(8,15,16). The
pathophysiology of MM-induced angiogenesis is complex, and involves
direct production of angiogenic cytokines by plasma cells and their
induction within the microenvironment. It has been widely reported
that tumor cells produce numerous angiogenic factors, including
VEGF, bFGF, epidermal growth factor and matrix metalloproteinases.
Previously, the levels of the angiogenic factors bFGF, HGF and VEGF
in the conditioned medium of MSCs and the capillary-formation
ability of MSCs were determined in vitro (11).
The efficient tumor seeding in a number of, if not
all, instances of MM is likely to be supported by the
epithelial-mesenchymal transition (EMT) program. Indeed, the
induction of EMT suffices to confer on cancer cells an increased
tumor-initiating potential and a number of other attributes of MM,
including enhanced resistance to chemotherapeutic agents and a
lower rate of proliferation (17).
Gupta et al (18) reported
activation of the ILK/β-parvin/cofilin signaling axis is critical
to the metastatic colony-forming ability of multiple aggressive
cancer cell types.
In the present study, it was demonstrated that ILK
may be involved in MSC differentiation into SMCs. MM may induce the
secretion of angiogenic factors dependent on the ILK signaling
pathway. MM may affect MSCs by direct contact and secreting
angiogenic factors. The stromal cells and extracellular matrix have
been well-documented to promote the survival of myeloma cells;
however, less is known about the influence of MSCs on the
angiogenesis of myeloma tumors. In the present study, α-SMA was
markedly upregulated under co-culture conditions, whereas VEGFR2
was not significantly increased by co-culture with MSCs. The
repeated western blot results indicated that α-SMA was upregulated,
whereas further study is required to explain why VEGFR2 is not.
α-SMA is commonly used as a marker of myofibroblast formation. The
presence of pericytes and SMCs surrounding blood vessels has been
described as a structural parameter indicative of vascular
maturity. Differentiating fibroblasts express α-SMA, indicating the
acquisition of a secretory, myofibroblast phenotype, a transition
that is associated with increased secretion of profibrotic
molecules including collagen and fibronectin (19–21).
VEGFR2 appears to be a principal receptor that mediates the
mitogenic and chemotactic effects of VEGF on endothelial cells, but
it is not clear how. ILK may be associated with α-SMA expression.
Determination of the underlying molecular mechanism and regulation
of ILK requires further study, investigate the efficacy of
treatment strategies that directly target the bone
microenvironment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81272562 and
81472183).
Availability of data and materials
The datasets generated during and analyzed during
this study included in the published article are available from the
corresponding author on reasonable request.
Authors' contributions
WZ and XZ were responsible for data collection and
interpretation. LZ also participated in data collection. PZ
analysed the data and YC performed the statistical analysis. XW was
responsible for application of funding, designed the present study,
and prepared and revised the manuscript.
Ethics approval and consent to
participate
The Institutional Ethics Committee of Tianjin
Medical University Cancer Institute and Hospital (Tianjin, China)
approved the present study.
Consent for publication
Written informed consents for the publication of
related figures had been obtained from the study participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rollig C, Knop S and Bornhäuser M:
Multiple myeloma. Lancet. 385:2197–2208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quarona V, Ferri V, Chillemi A, Bolzoni M,
Mancini C, Zaccarello G, Roato I, Morandi F, Marimpietri D, Faccani
G, et al: Unraveling the contribution of ectoenzymes to myeloma
life and survival in the bone marrow niche. Ann N Y Acad Sci.
1335:10–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kikuchi J and Furukawa Y: The mechanisms
of drug resistance via the interaction of myeloma cells with
stromal cells. Nihon Rinsho. 73:57–61. 2015.(In Japanese).
PubMed/NCBI
|
|
4
|
Toscani D, Bolzoni M, Accardi F, Aversa F
and Giuliani N: The osteoblastic niche in the context of multiple
myeloma. Ann N Y Acad Sci. 1335:45–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hannigan GE, McDonald PC, Walsh MP and
Dedhar S: Integrin-linked kinase: Not so ‘pseudo’ after all.
Oncogene. 30:4375–4385. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cabodi S, del Pilar Camacho-Leal M, Di
Stefano P and Defilippi P: Integrin signalling adaptors: Not only
figurants in the cancer story. Nat Rev Cancer. 10:858–870. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Steinbrunn T, Siegmund D, Andrulis M,
Grella E, Kortüm M, Einsele H, Wajant H, Bargou RC and Stühmer T:
Integrin-linked kinase is dispensable for multiple myeloma cell
survival. Leuk Res. 36:1165–1171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shibue T, Brooks MW and Weinberg RA: An
integrin-linked machinery of cytoskeletal regulation that enables
experimental tumor initiation and metastatic colonization. Cancer
Cell. 24:481–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song SW, Chang W, Song BW, Song H, Lim S,
Kim HJ, Cha MJ, Choi E, Im SH, Chang BC, et al: Integrin-linked
kinase is required in hypoxic mesenchymal stem cells for
strengthening cell adhesion to ischemic myocardium. Stem Cells.
27:1358–1365. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goessler UR, Bugert P, Bieback K,
Stern-Straeter J, Bran G, Hörmann K and Riedel F: Integrin
expression in stem cells from bone marrow and adipose tissue during
chondrogenic differentiation. Int J Mol Med. 21:271–279.
2008.PubMed/NCBI
|
|
11
|
Wang X, Zhang Z and Yao C: Angiogenic
activity of mesenchymal stem cells in multiple myeloma. Cancer
Invest. 29:37–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Portalska Janeczek K, Leferink A, Groen N,
Fernandes H, Moroni L, van Blitterswijk C and de Boer J:
Endothelial differentiation of mesenchymal stromal cells. PLoS One.
7:e468422012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Zhang Z and Yao C: Bortezomib
inhibits the angiogenesis mediated by mesenchymal stem cells.
Cancer Invest. 30:657–662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ribatti D, Basile A, Ruggieri S and Vacca
A: Bone marrow vascular niche and the control of angiogenesis in
multiple myeloma. Front Biosci (Landmark Ed). 19:304–311. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vacca A, Ria R, Reale A and Ribatti D:
Angiogenesis in multiple myeloma. Chem Immunol Allergy. 99:180–196.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mao Q, Lin CX, Liang XL, Gao JS and Xu B:
Mesenchymal stem cells overexpressing integrin-linked kinase
attenuate cardiac fibroblast proliferation and collagen synthesis
through paracrine actions. Mol Med Rep. 7:1617–1623. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malinin NL, Pluskota E and Byzova TV:
Integrin signaling in vascular function. Curr Opin Hematol.
19:206–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shibue T, Brooks MW, Inan MF, Reinhardt F
and Weinberg RA: The outgrowth of micrometastases is enabled by the
formation of filopodium-like protrusions. Cancer Discov. 2:706–721.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Elzamly S, Agina HA, Elbalshy AE,
Abuhashim M, Saad E and Elmageed Abd ZY: Integration of VEGF and
α-SMA expression improves the prediction accuracy of fibrosis in
chronic hepatitis C liver biopsy. Appl Immunohistochem Mol Morphol.
25:261–270. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tonino P and Abreu C: Microvessel density
is associated with vegf and alpha-sma expression in different
regions of human gastrointestinal carcinomas. Cancers (Basel).
3:3405–3418. 2011. View Article : Google Scholar : PubMed/NCBI
|