Introduction
Cancer stem cells (CSCs), which was first reported
by Dick and his colleagues in acute myeloid leukemia (AML)
(1), have been identified in many
solid tumors. It is extensively accepted the importance of CSCs in
tumorigenesis, tumor progression, metastasis and relapse. CSCs are
capable of preserving cancer heterogeneity through reserving
self-renewal and differentiation abilities. In addition, CSCs
activate the resistance mechanisms, including downregulation of
replication, expression of drug export systems,
epithelial-to-mesenchymal transition (EMT) and enhanced resistance
to hypoxia with induction of angiogenesis and immune escape by
reducing tumor specific antigens, while increasing
anti-inflammatory cytokines and growth factors (2). The positioning of stem cells in normal
organs has been identified, and it has been suggested that the
interaction of microenvironment with stem cells is pivotal for the
maintenance of stemness (3). CSCs may
localize at specific niche like stem cells in normal organs
(4). Due to the significance of tumor
microenvironment, it is a reasonable strategy to regulate the stem
cell niche to inhibit CSC survival. Among the multiple causes of
malignant tumor, infection has been considered as a major navigator
of inflammation-induced tumorigenesis (5). It has been accepted that the
inflammatory microenvironment is an essential component of CSCs
niche. Cancer can be promoted by inflammation, via enhancing
proliferative and survival signaling, induction of invasion and
metastasis. In this review, we summarize the major molecular and
cellular mechanisms that participate in the reciprocal action
between CSCs and inflammatory mediators (Figs. 1 and 2;
Table I). The emerging mechanisms of
inflammatory factors-induced stemness of CSCs are outlined. A
better understanding of the role of the crosstalk between CSCs and
inflammatory microenvironment might prospectively lead to more
efficient therapies against cancer initiation and progression.
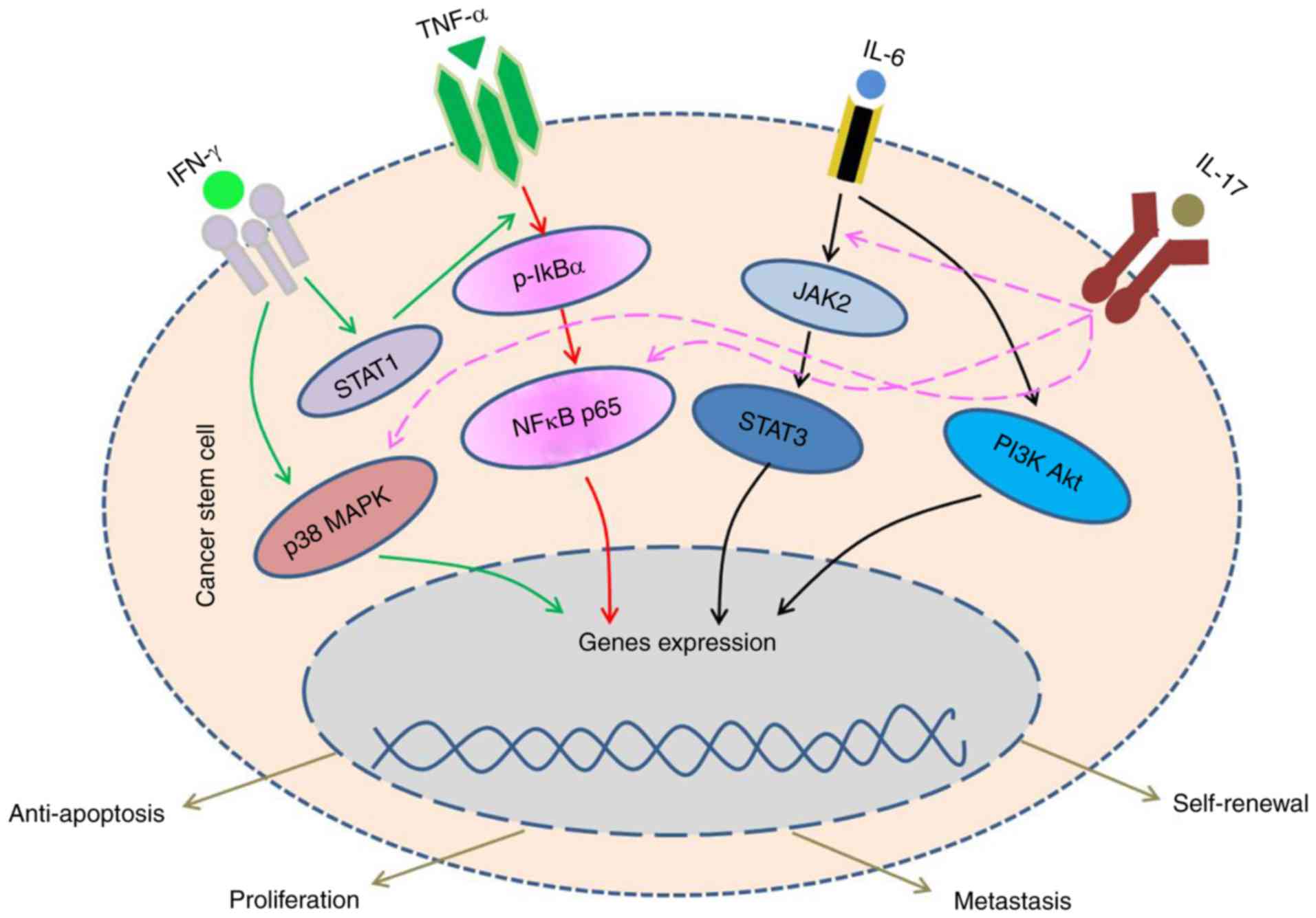 | Figure 1.IFN-γ, by binding to the IFNAR on
CSCs, lead to the activation of NFκB, p38/MAPK and STAT pathways.
After integrated with the TNFR, TNF-α increases the stemness
property and enhance the proliferation of CSCs. IL-6 acts on the
IL-6 receptor expressed by CSCs and activates JAK/STAT, PI3K/Akt
pathways, then to facilitate the self-renewal of stem-like cells
and enhance their tumorigenic potential. The JAK/STAT, NFκB and
p38/MAPK pathways also could be activated by IL-17 and IL-17R.
CSCs, cancer stem cells; IFN, interferon; JAK, Janus kinase; STAT,
signal transducer and activator of transcription; p38, tumor
protein 38; MAPK, mitogen-activated protein kinase; Akt, protein
kinase B; TNF, tumor necrosis factor; TNFR, TNF receptor; NFκB;
nuclear factor κB; JNK, c-Jun N-terminal kinases; PI3K,
phosphatidylinositol 3-kinase; IL, interleukin. |
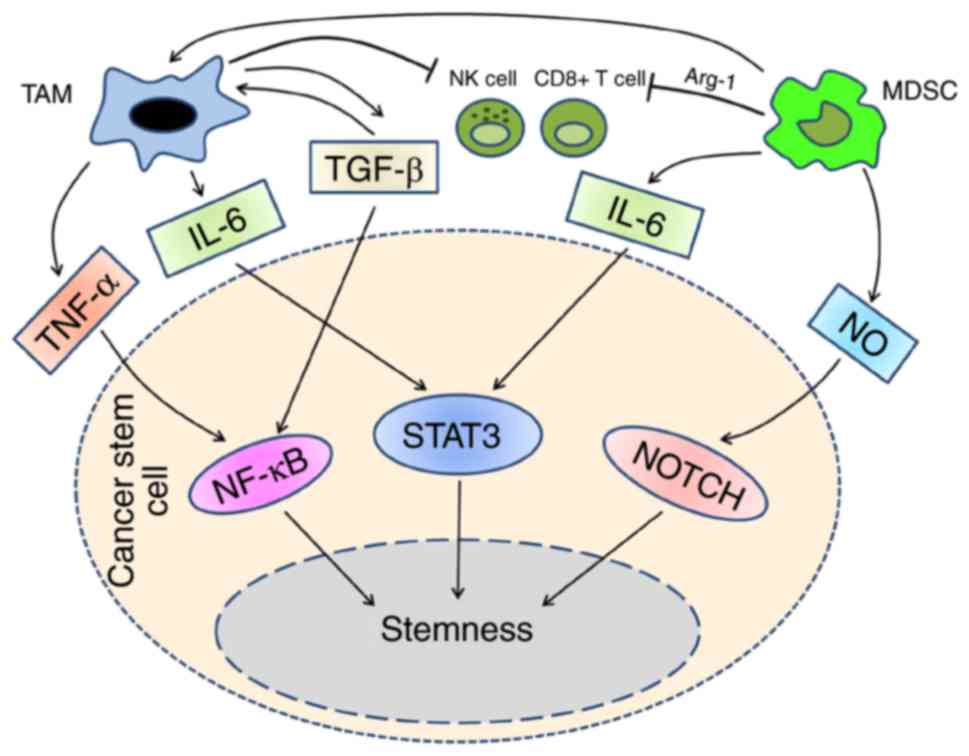 | Figure 2.MDSC population could differentiate
into TAMs, in tumor microenvironment, they suppress NK cells,
CD8+ cells and other immune cells synergistically. The
cytokines exist in tumor microenvironment mediate the interaction
between TAMs and MDSCs with CSCs. TAMs activates NFκB and STAT3
pathways through TNF-α, IL-6 and TGF-β. In addition to IL-6, MDSCs
utilize NO to activate STAT3 and NOTCH pathways. By means of these
signal pathways, TAMs and MDSCs drive CSCs development, enhance the
invasion and migration. CSCs, cancer stem cells; STAT, signal
transducer and activator of transcription; TNF, tumor necrosis
factor; NFκB; nuclear factor κB; IL, interleukin; MDSCs,
myeloid-derived suppressor cells; TAMs, tumor-associated
macrophages; TGF, transforming growth factor. |
 | Table I.A list of some CSC-related
inflammatory cytokines, ligands, origins and their functions. |
Table I.
A list of some CSC-related
inflammatory cytokines, ligands, origins and their functions.
| Item | Origin | Receptors | Signal pathway | Functions (in
tumor) | (Refs.) |
|---|
| IFN | Host cells | IFNGR1,2 | JAK/STAT | Induce EMT | 1,2,7–21 |
|
| (virus-infected
cells) | IFNAR1,2 | p38/MAPK | Downregulate
tumor-antigens |
|
|
| Fibroblasts | IFNLR1 | PI3K/Akt | Facilitate
angiogenesis |
|
|
| Macrophages |
| C3G/Rap1 | Promote CSCs
proliferation |
|
|
| T helper cells |
|
|
|
|
| TNF |
Activated-macrophages | TNFR1 | NFκB/HIF1a | Induce EMT |
7-9,22–27,31–37 |
|
| CD4+
lymphocytes | TNFR2 | JNK/MAPK | Increase stemness
property |
|
|
| NK cells |
|
| Support CSCs
survival |
|
|
| Neutrophils |
|
| Enhance
proliferation |
|
|
| Mast cells |
|
|
|
|
| IL-6 | Macrophages | CD126/IL6RA | JAK/STAT | Downregulate
E-cadherin |
39-42,43,44,47–50 |
|
| Th2 cells | CD130/IR6RB | PI3K/Akt | Moderate CSCs'
differentiation |
|
|
| B cells |
|
| Protect CSCs from
apoptosis |
|
|
| Astrocytes |
|
| Alter DNA
methylation |
|
|
| Endothelium |
|
|
|
|
| IL-17 | Th17 | IL-17RA, B, | p38/MAPK | Drive CSCs'
development | 57-59,61–66 |
|
|
| C, D and E | AP-1 | Promote CSCs'
self-renewal |
|
|
|
|
| JAK/STAT3 | Enhance stemness,
invasion, |
|
|
|
|
| NFκB | and migration |
|
Inflammatory cytokines
Interferons (IFNs)
IFNs are a group of pleiotropic cytokines that
participate in multiple biological activities, such as antivirus
infection, regulation of cell proliferation and immunological
response (6). They are classically
divided among three species: Type I IFNs; Type II IFNs; and Type
III IFNs, and the two main categories in mammals are Type I and II.
Type I contains two primary members, IFN-α and IFN-β, and the IFN-γ
is the lonely member of the type II (7). Indeed, IFNs have been verified in the
therapies of several types of cancer with success, such as chronic
myeloid leukemia, T and B cell lymphomas, melanoma, renal carcinoma
and so on. However, under certain conditions, IFNs was found to
augment the numbers of T regulatory cells (Tregs) and Th17 cells.
The myeloid-derived suppressor cells (MDSCs) that induced by IFNs,
can express as protumorigenesis factors and promote the tumor cells
escape from immunosurveillance (8).
Therefore, IFNs participate in tumor immunology as a ‘double-edged
sword’.
Mesenchymal stem cells (MSCs) are multipotential
stromal cells that can differentiate into kinds of cell types, such
as osteoblasts, chondrocytes, myocytes and adipocytes (9), and with self-renewal and pluripotent
differentiation abilities, possessing the potential to displace
damaged and diseased tissues (10).
There is a closely interaction between the MSCs and the host immune
system, the ability to inhibit proinflammatory cytokines of MSCs
sets up the foundation for clinical applications in treating
autoimmune diseases (11). In recent
years, the MSCs have been proved to accelerate cancer progression
in several types of cancer, and the IFNs have been shown to play
critical roles in it. Wang et al demonstrated that IFN-γ and
TNF-α, as two important representative inflammatory factors, play a
crucial role in synergistically inducing MSCs deficiency and
eventual MSCs tumorigenesis via the NFκB pathway in OVX-induced
osteoporotic mice (12). Their
research indicated that IFN-γ and TNF-α contributes in the
MSCs-based tissue regeneration (13).
It is also reported that IFN-γ and TNF-α in the MSCs enhanced tumor
cells malignancy, induced EMT of breast cancer cells, and papillary
thyroid cancer cells (14,15).
Numerous studies have demonstrated that IFNs are
closely related to the CSCs, in the tumor cells proliferation,
therapy resistance, and metastasis. Schürch, et al gived
evidences of that IFN-γ induce proliferation and differentiation of
chronic myeloid leukemia stem cells (16). In pancreatic carcinoma cells, IFN-α
up-regulates the expression of CSC markers, promotes the metastasis
formation (17). Yamashina et
al revealed that the cancer stem-like cells from
chemo-resistant tumors are able to produce IFN-regulated
transcription factors, which promote macrophage colony-stimulating
factor (M-CSF) production and generate tumorigenic myeloid cells,
then facilitate the tumorigenic and stem cell activities of bulk
tumors (18). In glioma stem cells,
it is also demonstrated that the IFN-regulated factors promote
tumorigenicity, angiogenesis, microglia recruitment and maintain
glioma stem cells properties through induction of interleukin 6,
C-X-C motif chemokine 1 and C-C motif chemokine 2 (19). Ojha R and his colleagues reported that
in bladder cancer cells, JAK-mediated autophagy regulates stemness
and cell survival via IFN-γ (20). In
hepatocellular carcinoma, the researchers demonstrated that the
IFN-γ treatment enriched the CD133+ liver CSCs
population in vitro and in vivo (21).
In addition to the above, the IFNs could promote
tumor progression via downregulating tumor antigens, facilitating
angiogenesis, and maintaining an immunosuppressive tumor
microenvironment (22,23). The roles of IFNs in malignancies maybe
determined by tumor microenvironment, tumor types, and tumor stage
and so on, for the two faces of IFNs in cancer, further studies are
in great request to provide a promising prospect for IFNs-based
treatment.
Tumor necrosis factor (TNF)
TNF superfamily refers to a group of cytokines that
can cause cell death, the two main members of the family are TNF-α
and TNF-β. Given that TNF-α accounts for 70~95% of TNF biological
activities, it can represent the TNF superfamily in general
(24). By virtue of the ability to
cause cytolysis of certain tumor cell lines, TNF-α has been
utilized as a potential anticancer agent for many years (25), but with the development of research,
emerging evidences suggest that TNF-α is significant in promoting
tumor progression, in particular, with CSCs.
TNF-α can induce EMT and increase stemness
properties, that is demonstrated in renal cell carcinoma,
hypopharyngeal cancer and cholangiocarcinoma cells (26,27).
Synergized with IFN-γ, TNF-α stimulates MSCs to enhance malignancy
of cancer cells, tumorigenesis (12–14), and
resistance to chemotherapy (28). Yu
et al validated that TNF-α-activated MSCs promote breast
cancer metastasis via recruiting CXCR2+ neutrophils
(29), a similar result is reported
by Katanov C and his colleague (30).
In recent studies, it is revealed that TNF-α
enhances CSCs phenotype of oral squamous cell carcinoma (OSCC)
cells, such as an increase in tumor sphere-formation ability, stem
cell associated genes expression, chemo-radioresistance, and
tumorigenesis (31). Besides that,
TNF-α upregulates SLUG (a mediator of EMT process) with a
dependency on canonical NFκB/HIF1α signaling, then imparts breast
cancer cells with stem cell-like features (32). In melanoma, it is evidenced that after
treatment of TNF-α, the self-renewing capacity of stem-like cells
is upregulated (33). The
transcription factor Atonal homolog 1 (Atoh1) protein, stabilized
by TNF-α, could enrich colon CSCs, and induce high malignant
potential (34). In myeloid leukemia,
TNF-α secreted by the CSCs could promote NFκB pathway/p65 pathway
and support stem cells survival (35,36).
Similarly, TNF-α induces NFκB pathway activation to protect
colorectal CSCs from death, and induce tumor regression (37).
For the importance of TNF-α in promoting
tumorigenesis and progression, it represents a novel target in
tumor prevention and therapy (28).
However, more studies are needed before ideal clinical outcomes can
be achieved (38).
IL-6
IL-6 is one member of the ILs, which acts as both a
pro-inflammatory cytokine and an anti-inflammatory myokine
(39). IL-6, also referred to as
B-cell stimulatory factor-2 (BSF-2) and IFN β-2, is a cytokine that
regulates the immune response but also plays a role in modulating
cell growth, differentiation, and survival. It is mainly secreted
by T cells, macrophages and fibroblasts. IL-6 belongs to the ‘IL-6
type cytokine’ family that also includes leukemia inhibitory factor
(LIF), IL-11, ciliary neurotrophic factor (CNTF), cardiotrophin-1
(CT-1) and oncostatin M (OSM) (40).
IL-6 mediates its activity by a cell-surface typeIcytokine receptor
complex, which includes the ligand-binding IL-6Rα chain (IL-6R,
CD126), and the signal-transducing component gp130 (CD130)
(41). After interaction with its
receptor, IL-6 triggers the IL-6R and gp130 proteins to form a
complex, which stimulates distinct pathways to perform its
oncogenic consequence, these pathways consist of Janus kinases
(JAK)/signal transducer and activator of transcription (STAT),
mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3
kinase (PI3K)/protein kinase B (Akt) (42).
The varying biological functions of IL-6 make it
critical in the enhancement or suppression of tumor growth and
progress, and it is involved in the proliferation and
differentiation of malignant cells and found to be high in serum
and tumor tissues of a majority of cancers, including prostate
cancer (43), multiple myeloma
(44), renal cell carcinoma (45) and breast cancer (46). Emerging evidences suggest that the
IL-6 levels in the serum are implicated in aggressive tumor growth
and response to therapies in many types of cancer, and also could
be a prognostic marker for assessing disease stage and disease
progression (43).
To be valid in the protumorigenesis, IL-6 frequently
interacts with CSCs, such as moderating CSCs differentiation and
maintaining the stemness of cancer cells. In lung cancer cells,
IL-6 promotes the self-renewal of CD133+ CSC-like cells,
and the IL-6 expressing CD133+ cells have significantly
higher expression of EMT-related molecules (lower E-cadherin,
higher N-cadherin, vimentin, and TWIST), as well as higher
expression of metastasis-related molecules [MMP9, transforming
growth factor (TGF)-β1] than IL-6 knocked down CD133+
cells (47). In subsequent study, it
is reported that IL-6 signaling induces DNA repair while preventing
CD133+ CSC-like cells from apoptotic death after
radiation for lung cancer (44).
Likewise, in cisplatin-resistant lung cancer cells, IL-6 plays an
important role in triggering enhanced stemness during cisplatin
resistance development, through the upregulation of
hypoxia-inducible factors (HIFs) (48). Liu et al (49) reported the IL-6/JAK2/STAT3 pathway
upregulates DNA methyltransferase 1 (DNMT1) and promotes cancer
initiation and lung CSCs proliferation by downregulation of p53 and
p21 resulting from DNA hypermethylation. By the activation of
IL-6/JAK2/STAT3 pathway, MSCs can enhance the capability of tumor
initiation in lung cancer cells (50). Correspondingly, TME and/or tumor cells
can impact the function of IL-6. Lu et al (51) revealed that the adipose-derived stem
cells (ADSCs) enhance the malignant characteristics of Lewis lung
carcinoma cells, including cell growth ability and especially CSC
property. Glioma stem cells (GSCs) initiate microglial IL-6
secretion via TLR4 signaling and that IL-6 induces glioma growth by
supporting GSCs, and glioma-associated microglia/brain macrophages
are the primary source of IL-6 in tumor (52). By secreting high levels of IL-6,
ovarian MSCs (OvMSCs) enhance the proliferation, sphere and colony
formation and tumorigenesis of SKOV3 cells (53). In colorectal cancer (CRC), it is
proposed that CD90+ fibroblasts/myofibroblasts may be
the major source of IL-6 in T2-T3 CRC tumors, then supports the
stemness of tumor cells and mediates the immune adaptive
inflammatory response favoring tumor growth (54). The role of epigenetic control in
cancer onset and development has been extensively accepted in
recent years, e.g., the IL-6/JAK2/STAT3 pathway upregulates DNA
methyltransferase 1 (54), the long
non-coding RNAs (lncRNAs) in liver CSCs (LCSCs) mediate the
crosstalk between TNF-α/NFκB signaling and autocrine IL-6/STAT3
cascade, then suppressed LCSCs expansion by inhibiting IL-6
transcription and STAT3 activation (55). In monoclonal gammopathy of
undetermined significance (MGUS) and multiple myeloma, the IL-6
production in the bone marrow MSC (BMMSC) is driven by the
citrullination of histone H3 (56).
Overall, these evidences suggest that IL-6 is
involved in mechanisms of CSCs stemness, and the significance of
IL-6 in the crosstalk between tumor and its microenvironment.
Therefore, the inhibition of IL-6 may be a useful adjunct to cancer
therapy, with a need of targeting this protein to combat
cancer.
IL-17
IL-17 is a potent proinflammatory cytokine mainly
produced by activated T-helper 17 cells (Th17) and activated by
IL-23. The IL-17, also called IL-17A, is the fundamental member of
a group of cytokines called IL-17 family. Besides of IL-17A, there
are IL-17B, IL-17C, IL-17D, IL-17E (also called IL-25), and IL-17F
constitute this family. The IL-17 receptor group includes five
receptors, IL-17RA, B, C, D and E (57). IL-17 binds IL-17R, and then forms a
complex to mediate a variety of signaling pathway, such as JNK,
Erk1/2, p38, AP-1, JAK/STAT3 and NFκB (58). By virtue of these pathways, IL-17
induces the production of many other cytokines (such as IL-6,
G-CSF, GM-CSF, IL-1β, TGF-β, and TNF-α), chemokines (IL-8, GRO-α,
and MCP-1), and prostaglandins (e.g., PGE2) from many cell
types, then to play an important part in the initiation and
progression of many diseases. As a pivotal factor of tumor
microenvironment, IL-17 could facilitate cancer progression, from
tumorigenesis, migration, invasion, and metastasis, to adapting the
tumor in its ability to confer upon itself both immune evasion, and
chemotherapy resistance (59).
In view of the significance of CSCs in the cancer
progression, the interplay between IL-17 and CSCs has aroused great
interests of the scientists and researchers in recent years. In
CRC, the sphere cells cocultured with
Foxp3+IL-17+ cells could express more CRC
cell markers (CD133, CD44s, CD166, EpCAM, and ALDH1) than the
control sphere cells, and when neutralizing anti-IL-17 antibody was
added to the culture, the expression of CD133 and the rest of the
CRC cell markers was abolished. These data suggest that
Foxp3+IL-17+ cells are capable of driving
cancer-initiating cells (CICs) development (60). In this study, they also demonstrated
that hypoxia induces Foxp3+Tregs to express IL-17. Consistently, it
is revealed that Th17 stemness may be partially controlled by
signaling pathways such as hypoxia inducible factor HIF1α, Notch
and Bcl (61). Lotti et al
(62) reported that IL-17A can
contribute to CICs maintenance through IL-17A receptor in CRC, and
then promote protumorigenic CICs behavior, as well as contribute to
CIC therapeutic resistance. It is also demonstrated that one role
of IL-17 in ovarian CD133+ cancer stem-like cells
(CSLCs) is to promote the self-renewal (63). By IL-17 overexpression, the growth and
sphere formation capacities of ovarian CD133+ CSLCs were
dramaticlly enhanced. The incentive function of IL-17 might be
mediated by the NFκB and p38/MAPK signaling pathway. In human
hepatocellular carcinoma (HCC), IL-17E binding to IL-17RB motivates
NFκB and JAK/STAT3 pathways to facilitate propagation and
self-renewal ability of CSCs, and these beneficial effects could be
prevented by specific inhibitors of JAK and NFκB signals (64). Similarly, by NFκB and STAT3 pathways,
IL-17-IL-17R interaction in glioma stem cells (GSCs) induces an
autocrine and/or paracrine cytokine feedback loop, that may provide
an important signaling pathway for the stemness of GSCs (65). In addition to that, the upregulation
of IL-17 in gastric cancer results in the transformation of
quiescent gastric CSCs into invasive gastric CSCs, from invasion,
migration to tumor formation ability (66).
For the significance of the interaction between
IL-17 and CSCs, targeting IL-17 may emerge as a feasible novel
therapeutic strategy. That clearly demonstrates a need for more
research in this area, and the potential application of anti-IL-17
in the overall management will be hopeful.
Inflammatory cells
MDSCs
MDSCs are one of the major cell populations in
charge of mediating immune responses, and are a heterogeneous
population of cells comprised of macrophages, dendritic cells (DCs)
and granulocytes, which expand during tumor progression, autoimmune
disease, infection and other pathological conditions, and can
efficiently suppress T cell function (67). The immune cells of MDSCs are from the
myeloid lineage (a family of cells that originate from bone marrow
stem cells) and are consist of myeloid-cell progenitors and
precursors of myeloid cells. Under normal condition, the immature
myeloid cells (IMCs) quickly differentiate into mature
granulocytes, macrophages or DCs, but in abnormal conditions such
as cancer, infectious diseases, trauma or some autoimmune
disorders, IMCs differentiate into MDSCs (68). A number of immune suppressive factors
are associated with the immunosuppressive function of MDSCs,
including arginase (encoded by ARG1), inducible nitric oxide
synthase (iNOS, or NOS2), nitric oxide (NO) and reactive
oxygen species (ROS). The signaling pathways activated in MDSCs
induced biological process are mainly STAT6, STAT1, and NFκB.
In addition to the immunological functions,
non-immunological functions of MDSC also should been illustrated,
such as the promotion of tumorigenesis, tumor invasion and
metastasis (69). Given that CSCs are
responsible for cancer initiating, progression, metastasis and
recurrence, it is reasonable to hypothesize that there are mutual
effects between CSCs and MDSCs, and then mounting evidences verify
the existence of the relationship. In an early study, the
CD44+ cancer stem-like cells are shown to not only more
strongly inhibit T-cell proliferation, but also more efficiently
induce regulatory T cells (Treg cells) and MDSCs as compared with
CD44− cells (70). In
glioblastoma, it is shown that MDSCs recruited to the tumor
microenvironment by CSCs, can promote the survival of CSCs, and
also be responsible for the immune-evasive properties. Moreover,
the CSCs conditioned media inhibits MDSCs apoptosis and increases
arginase 1 (Arg1) production of murine bone marrow derived MDSCs
(71). Based on the known evidence
and their experimental findings, Gao et al (72) hypothesize that T cell immunoglobulin
mucin-3 (Tim-3), which specifically expresses on Leukemic stem
cells (LSCs), is beneficial for LSCs survival and AML progression
by promoting expansion of MDSCs and differentiating into
tumor-associated macrophages (TAMs) at the leukemia site. In a
further study, it is demonstrated that the macrophage migration
inhibitory factor (MIF), which was produced at high levels by
glioblastoma stem cells, could increase production of the
immune-suppressive enzyme Arg1 in MDSCs as a CXCR2-dependent manner
(73). In return, MDSCs could enhance
the stemness of CSCs in certain ways. In ovarian carcinoma, MDSCs
trigger the expression of miRNA101 in cancer cells, which
subsequently repressed the corepressor gene C-terminal binding
protein-2 (CtBP2), and CtBP2 directly targets stem cell core genes
leading to increased cancer cell stem cells properties and
increases metastatic and tumorigenic potential (74). Panni et al (75) revealed that, depending on the
activation of the STAT3 pathway, the pancreatic cancer TME
transforms monocytes to monocytic MDSCs (Mo-MDSCs), and these cells
enhance the stemness and mesenchymal properties CSCs. In a recent
study, it is revealed that MDSCs promote tumor initiation by
endowing breast cancer cell stemness as well as repressing T-cell
activation, and these effects are depended on the crosstalk between
STAT3 and NOTCH pathways in cancer cells (76).
As a critical member of the tumor microenvironment,
MDSCs lead to not only the tumor-associated immunosuppression, but
also the CSCs promotion, and could be a prospective target in
anti-tumor treatment, but there are still a lot of efforts needed
to put in, such as how to identify MDSCs, the mechanism of MDSCs
accumulation in TME, and by which means can suppress MDSCs
effectively.
Tumor-associated macrophages
(TAMs)
TAMs are the macrophages infiltrating in tumor
tissue, are the most immune cells in the tumor microenvironment. At
first, it is believed that TAMs are able to recognize and eliminate
tumor cells by a variety of cytokines, but as the development of
research, it is now generally accepted that TAMs play a key role in
tumor growth, invasion and migration (77). TAMs derive from monocytic precursors
circulating in the vessels, when there is a tumor exists, monocytes
could be recruited into TME, via the chemokines and cytokines
secreted by tumor cells, tumor stromal cells, and immune cells,
including chemokine (C-C motif) ligand-2 (CCL2), CCL5, Chemokine
(C-X-C motif) ligand-1 (CXCL1), vascular endothelial growth factor
(VEGF), platelet derived growth factor (PDGF), TGF and macrophage
colony stimulating factor (M-CSF). Affected by the cells and
cytokines, mononuclear phagocytes polarize into different types of
TAM. Typically activated M1 macrophages are induced by IFN-γ alone
or in cooperation with microbial stimuli (i.e., LPS) or
cytokines (i.e., TNF and GM-CSF). On the other hand, IL-4
and IL-13 induce M2 macrophages alternatively (78). M1 macrophages often show a capability
of anti-tumor, such as releasing toxic intermediates and
proinflammatory cytokines, inducing specific immune response, and
enhancing the antigen presenting. In contrast, M2 macrophages
represent as a pro-tumor factor, through suppressing the
inflammatory responses, promoting angiogenesis, inducing the
invasion and metastasis of tumor. It is accepted that TAMs have
functions and phenotype more similar to M2 macrophages, so TAMs are
often considered as promoter of tumor progression (79).
A lot of evidences have clarified that TAMs and CSCs
have close contact with each other, and the interaction between
them play a critical role in tumorigenesis, progression, invasion,
drug resistance and so on (80).
Jinushi et al (81) found that
TAMs produced amount of Milk-fat globule EGF-8 (MFG-E8) in
stimulation with lung CSCs. The MFG-E8 enhanced tumorigenicity and
drug resistance in CSCs, by means of the coordinated activation of
STAT3 and Sonic Hedgehog signals. Furthermore, MFG-E8 and IL-6 from
TAMs could synergistically mediate tumorigenesis and drug
resistance within CSC. In breast cancer, it is reported that after
the cocultivation of M2 macrophages and breast cancer cell lines
MCF-7, hybrids can be isolated from the fusion, and they represent
a more invasive phenotype, including increased migration,
aggression and tumorigenicity, which implicated the role of TAMs in
promoting CSCs (82). Additionally,
by releasing high levels of TGF-β1, TAMs promote the invasion of
glioma stem-like cells (GSLCs) and increase the production of
MMP-9, then induce EMT (83). The
role of TGF-β1/EMT is also revealed in hepatocellular carcinoma
(84). Likewise, the importance of
EGFR/Stat3/Sox-2 and IL-6/STAT3 signaling pathway in regulating
CSCs also shows up in breast cancer and hepatocellular carcinoma
(85–87).
Through the way of interaction, CSCs could recruit
TAMs to promote tumor growth. It is demonstrated that the
CD34− melanoma tumor-initiating cells (TICs) interact
specifically with M2 macrophages, then support their resistance to
chemotherapeutic drugs and accelerate cancer progression by TGF-β
and arginase pathway (88). Zhou
et al (89) and Shi et
al (90) found that in
Glioblastoma, the periostin (POSTN) preferentially secreted by GSCs
could correlates with TAM density in primary GBMs. Silencing POSTN
in GSCs can reduce TAM recruitment, inhibit tumor growth, and
extend survival of mice bearing GSC-derived xenografts.
Furthermore, they revealed that POSTN recruits TAMs via integrin
αvβ3 signaling.
For the significance of the relationship between
TAMs and CSCs, the coexpression of them could be a meaningful
marker to the tumor diagnosis and prognostic, as well as a
promising therapy target (91,92). We
propose that the elimination or re-differentiation of macrophages
within the tumor microenvironment could be a significant prong of
combination therapies designed to treat malignancies.
Conclusion
Currently, cancer is still one of the biggest
threats to human life. Despite of all the efforts have been put in
the studies of tumor treatment in recent decades, the survival rate
has not apparently been improved. Inflammatory TME mainly show up
the promotion of CSCs, but sometimes it also represent as an
inhibitory factor to the tumor. The mechanism of the role
transformation is still not clear, so furthermore experimental and
clinical researches are needed to shed light on the fundamental
mechanisms. Given that Inflammatory TME and CSCs are two major
barriers to valid cancer therapy, targeting them may provide a
promising approach to developing novel treatments.
Acknowledgements
This study was supported by Research Grants
(15411950300) from Science and Technology Commission of Shanghai
Municipality, Science and Technology Commission Foundation of
Shanghai (no. 10DZ1951300) and Shanghai Summit & Plateau
Disciplines (to CPZ), the National Natural Science Foundation of
China (no. 81771046) and Shanghai Summit & Plateau Disciplines
(to LW), Natural Science Foundation of China (no. 81602367 to
XY).
References
|
1
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Albini A, Bruno A, Gallo C, Pajardi G,
Noonan DM and Dallaglio K: Cancer stem cells and the tumor
microenvironment: Interplay in tumor heterogeneity. Connect Tissue
Res. 56:414–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Voog J and Jones DL: Stem cells and the
niche: A dynamic duo. Cell Stem Cell. 6:103–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kise K, Kinugasa-Katayama Y and Takakura
N: Tumor microenvironment for cancer stem cells. Adv Drug Deliv
Rev. 99:197–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin
C and Flavell RA: Inflammation-induced cancer: Crosstalk between
tumours, immune cells and microorganisms. Nat Rev Cancer.
13:759–771. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Corrales L and Gajewski TF: Molecular
pathways: Targeting the stimulator of interferon genes (STING) in
the immunotherapy of cancer. Clin Cancer Res. 21:4774–4779. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trinchieri G: Type I interferon: Friend or
foe? J Exp Med. 207:2053–2063. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zaidi MR and Merlino G: The two faces of
interferon-γ in cancer. Clin Cancer Res. 17:6118–6124. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nardi Beyer N and da Silva Meirelles L:
Mesenchymal stem cells: Isolation, in vitro expansion and
characterization. Handb Exp Pharmacol. 249–282. 2006. View Article : Google Scholar
|
|
10
|
Shi S and Gronthos S: Perivascular niche
of postnatal mesenchymal stem cells in human bone marrow and dental
pulp. J Bone Miner Res. 18:696–704. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharma RR, Pollock K, Hubel A and Mckenna
D: Mesenchymal stem or stromal cells: A review of clinical
applications and manufacturing practices. Transfusion.
54:1418–1437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Zhao Y, Liu Y, Akiyama K, Chen C,
Qu C, Jin Y and Shi S: IFN-γ and TNF-α synergistically induce
mesenchymal stem cell impairment and tumorigenesis via NFκB
signaling. Stem Cells. 31:1383–1395. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Wang L, Kikuiri T, Akiyama K, Chen
C, Xu X, Yang R, Chen W, Wang S and Shi S: Mesenchymal stem
cell-based tissue regeneration is governed by recipient T
lymphocytes via IFN-γ and TNF-α. Nat Med. 17:1594–1601. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trivanović D, Jauković A, Krstić J,
Nikolić S, Okić Djordjević I, Kukolj T, Obradović H, Mojsilović S,
Ilić V, Santibanez JF and Bugarski D: Inflammatory cytokines prime
adipose tissue mesenchymal stem cells to enhance malignancy of
MCF-7 breast cancer cells via transforming growth factor-β1. IUBMB
Life. 68:190–200. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lv N, Gao Y, Guan H, Wu D, Ding S, Teng W
and Shan Z: Inflammatory mediators, tumor necrosis factor-α and
interferon-γ, induce EMT in human PTC cell lines. Oncol Lett.
10:2591–2597. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schürch C, Riether C, Amrein MA and
Ochsenbein AF: Cytotoxic T cells induce proliferation of chronic
myeloid leukemia stem cells by secreting interferon-γ. J Exp Med.
210:605–621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu Y, Karakhanova S, Huang X, Deng SP,
Werner J and Bazhin AV: Influence of interferon-α on the expression
of the cancer stem cell markers in pancreatic carcinoma cells. Exp
Cell Res. 324:146–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamashina T, Baghdadi M, Yoneda A,
Kinoshita I, Suzu S, Dosakaakita H and Jinushi M: Cancer stem-like
cells derived from chemoresistant tumors have a unique capacity to
prime tumorigenic myeloid cells. Cancer Res. 74:2698–2709. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin X, Kim SH, Jeon HM, Beck S, Sohn YW,
Yin J, Kim JK, Lim YC, Lee JH, Kim SH, et al: Interferon regulatory
factor 7 regulates glioma stem cells via interleukin-6 and Notch
signalling. Brain. 135:1055–1069. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ojha R, Singh SK and Bhattacharyya S:
JAK-mediated autophagy regulates stemness and cell survival in
cisplatin resistant bladder cancer cells. Biochim Biophys Acta.
1860:2484–2497. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Chen JN, Zeng TT, He F, Chen SP, Ma
S, Bi J, Zhu XF and Guan XY: CD133+ liver cancer stem cells resist
interferon-gamma-induced autophagy. BMC Cancer. 16:152016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furuta J, Inozume T, Harada K and Shimada
S: CD271 on melanoma cell is an IFN-γ-inducible immunosuppressive
factor that mediates downregulation of melanoma antigens. J Invest
Dermatol. 134:1369–1377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kharma B, Baba T, Matsumura N, Kang HS,
Hamanishi J, Murakami R, McConechy MM, Leung S, Yamaguchi K, Hosoe
Y, et al: STAT1 drives tumor progression in serous papillary
endometrial cancer. Cancer Res. 74:6519–6530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang AM, Creasey AA, Ladner MB, Lin LS,
Strickler J, Van Arsdell JN, Yamamoto R and Mark DF: Molecular
cloning of the complementary DNA for human tumor necrosis factor.
Science. 228:149–154. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roberts NJ, Zhou S, Diaz LA and Matthias
H: Systemic use of tumor necrosis factor alpha as an anticancer
agent. Oncotarget. 2:739–751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Jiao M, Wu K, Li L, Zhu G, Wang
X, He D and Wu D: TNF-α induced epithelial mesenchymal transition
increases stemness properties in renal cell carcinoma cells. Int J
Clin Exp Med. 7:4951–4958. 2014.PubMed/NCBI
|
|
27
|
Techasen A, Namwat N, Loilome W,
Bungkanjana P, Khuntikeo N, Puapairoj A, Jearanaikoon P, Saya H and
Yongvanit P: Tumor necrosis factor-α (TNF-α) stimulates the
epithelial-mesenchymal transition regulator Snail in
cholangiocarcinoma. Med Oncol. 29:3083–3091. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Valizadeh A, Ahmadzadeh A, Saki G,
Khodadadi A and Teimoori A: Role of tumor necrosis factor-producing
mesenchymal stem cells on apoptosis of chronic B-lymphocytic tumor
cells resistant to fludarabine-based chemotherapy. Asian Pac J
Cancer Prev. 16:8533–8539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu PF, Huang Y, Han YY, Lin LY, Sun WH,
Rabson AB, Wang Y and Shi YF: TNFα-activated mesenchymal stromal
cells promote breast cancer metastasis by recruiting CXCR2+
neutrophils. Oncogene. 36:482–490. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Katanov C, Lerrer S, Liubomirski Y,
Leider-Trejo L, Meshel T, Bar J, Feniger-Barish R, Kamer I,
Soria-Artzi G, Kahani H, et al: Regulation of the inflammatory
profile of stromal cells in human breast cancer: Prominent roles
for TNF-α and the NF-κB pathway. Stem Cell Res Ther. 6:872015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee SH, Hong HS, Liu ZX, Kim RH, Kang MK,
Park NH and Shin KH: TNFα enhances cancer stem cell-like phenotype
via Notch-Hes1 activation in oral squamous cell carcinoma cells.
Biochem Biophys Res Commun. 424:58–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Storci G, Sansone P, Mari S, D'Uva G,
Tavolari S, Guarnieri T, Taffurelli M, Ceccarelli C, Santini D,
Chieco P, et al: TNFalpha up-regulates SLUG via the
NF-kappaB/HIF1alpha axis, which imparts breast cancer cells with a
stem cell-like phenotype. J Cell Physiol. 225:682–691. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ostyn P, El Machhour R, Begard S, Kotecki
N, Vandomme J, Flamenco P, Segard P, Masselot B, Formstecher P,
Touil Y and Polakowska R: Transient TNF regulates the self-renewing
capacity of stem-like label-retaining cells in sphere and skin
equivalent models of melanoma. Cell Commun Signal. 12:522014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fukushima K, Tsuchiya K, Kano Y, Horita N,
Hibiya S, Hayashi R, Kitagaki K, Negi M, Itoh E, Akashi T, et al:
Atonal homolog 1 protein stabilized by tumor necrosis factor α
induces high malignant potential in colon cancer cell line. Cancer
Sci. 106:1000–1007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gallipoli P, Pellicano F, Morrison H,
Laidlaw K, Allan EK, Bhatia R, Copland M, Jørgensen HG and Holyoake
TL: Autocrine TNF-α production supports CML stem and progenitor
cell survival and enhances their proliferation. Blood.
122:3335–3339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou X, Zhou S, Li B, Li Q, Gao L, Li D,
Gong Q, Zhu L, Wang J, Wang N, et al: Transmembrane TNF-α
preferentially expressed by leukemia stem cells and blasts is a
potent target for antibody therapy. Blood. 126:1433–1442. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sheng YH, He Y, Hasnain SZ, Wang R, Tong
H, Clarke DT, Lourie R, Oancea I, Wong KY, Lumley JW, et al: MUC13
protects colorectal cancer cells from death by activating the NF-κB
pathway and is a potential therapeutic target. Oncogene.
36:700–713. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nenu I, Tudor D, Filip AG and Baldea I:
Current position of TNF-α in melanomagenesis. Tumor Biol.
36:6589–6602. 2015. View Article : Google Scholar
|
|
39
|
Bromberg J and Wang TC: Inflammation and
cancer: IL-6 and STAT3 complete the link. Cancer Cell. 15:79–80.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Smith PC, Hobisch A, Lin DL, Culig Z and
Keller ET: Interleukin-6 and prostate cancer progression. Cytokine
Growth Factor Rev. 12:33–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yao X, Huang J, Zhong H, Shen N, Faggioni
R, Fung M and Yao Y: Targeting interleukin-6 in inflammatory
autoimmune diseases and cancers. Pharmacol Ther. 141:125–139. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hideshima T, Nakamura N, Chauhan D and
Anderson KC: Biologic sequelae of interleukin-6 induced PI3-K/Akt
signaling in multiple myeloma. Oncogene. 20:5991–6000. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kumari N, Dwarakanath BS, Das A and Bhatt
AN: Role of interleukin-6 in cancer progression and therapeutic
resistance. Tumor Biol. 37:11553–11572. 2016. View Article : Google Scholar
|
|
44
|
Chen Y, Zhang F, Tsai Y, Yang X, Yang L,
Duan S, Wang X, Keng P and Lee SO: IL-6 signaling promotes DNA
repair and prevents apoptosis in CD133+ stem-like cells of lung
cancer after radiation. Radiat Oncol. 10:2272015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Altundag O, Altundag K and Gunduz E:
Interleukin-6 and C-reactive protein in metastatic renal cell
carcinoma. J Clin Oncol. 23:1044–1045. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Knüpfer H and Preiß R: Significance of
interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res
Treat. 102:129–135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ok LS, Yang X, Duan S, Ying T, Strojny LR,
Peter K, et al: IL-6 promotes growth and epithelial-mesenchymal
transition of CD133+ cells of non-small cell lung cancer.
Oncotarget. 7:6626–6638. 2016.PubMed/NCBI
|
|
48
|
Zhang F, Duan S, Ying T, Keng PC and Chen
Y, Lee SO and Chen Y: Cisplatin treatment increases stemness
through upregulation of hypoxia-inducible factors by interleukin-6
in non-small cell lung cancer. Cancer Sci. 107:746–754. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu CC, Lin JH, Hsu TW, Su K, Li AF, Hsu
HS and Hung SC: IL-6 enriched lung cancer stem-like cell population
by inhibition of cell cycle regulators via DNMT1 upregulation. Int
J Cancer. 136:547–559. 2015.PubMed/NCBI
|
|
50
|
Hsu HS, Lin JH, Hsu TW, Su K, Wang CW,
Yang KY, Chiou SH and Hung SC: Mesenchymal stem cells enhance lung
cancer initiation through activation of IL-6/JAK2/STAT3 pathway.
Lung Cancer. 75:167–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lu JH, Wei HJ, Peng BY, Chou HH, Chen WH,
Liu HY and Deng WP: Adipose-derived stem cells enhance cancer stem
cell property and tumor formation capacity in lewis lung carcinoma
cells through an interleukin-6 paracrine circuit. Stem Cells Dev.
25:1833–1842. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
a Dzaye OD, Hu F, Derkow K, Haage V,
Euskirchen P, Harms C, Lehnardt S, Synowitz M, Wolf SA and
Kettenmann H: Glioma stem cells but not bulk glioma cells
upregulate IL-6 secretion in microglia/brain macrophages via
toll-like receptor 4 signaling. J Neuropathol Exp Neurol.
75:429–440. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ding DC, Liu HW and Chu TY: Interleukin-6
from ovarian mesenchymal stem cells promotes proliferation, sphere
and colony formation and tumorigenesis of an ovarian cancer cell
line SKOV3. J Cancer. 7:1815–1823. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Huynh PT, Beswick EJ, Yun AC, Johnson P,
O'Connell MR, Watts T, Singh P, Qiu S, Morris K, Powell DW and
Pinchuk IV: CD90(+) stromal cells are the major source of IL-6,
which supports cancer stem-like cells and inflammation in
colorectal cancer. Int J Cancer. 138:1971–1981. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang X, Sun W, Shen W, Xia M, Chen C,
Xiang D, Ning B, Cui X, Li H, Li X, et al: Long non-coding RNA DILC
regulates liver cancer stem cells via IL-6/STAT3 axis. J Hepatol.
64:1283–1294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mcnee G, Eales KL, Wei W, Williams DS,
Barkhuizen A, Bartlett DB, Essex S, Anandram S, Filer A, Moss PA,
et al: Citrullination of histone H3 drives IL-6 production by bone
marrow mesenchymal stem cells in MGUS and multiple myeloma.
Leukemia. 31:373–381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kolls JK and Lindén A: Interleukin-17
family members and inflammation. Immunity. 21:467–476. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Guéry L and Hugues S: Th17 cell plasticity
and functions in cancer immunity. Biomed Res Int. 2015:3146202015.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yang B, Fung A, Zhao H, Wang T and Ma D:
The Role of Interleukin 17 in Tumour Proliferation, Angiogenesis
and Metastasis. Mediators of Inflammation. 2014:390–392. 2014.
View Article : Google Scholar
|
|
60
|
Yang S, Wang B, Guan C, Wu B, Cai C, Wang
M, Zhang B, Liu T and Yang P: Foxp3+IL-17+ T cells promote
development of cancer-initiating cells in colorectal cancer. J
Leukoc Biol. 89:85–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wei S, Zhao E, Kryczek I and Zou W: Th17
cells have stem cell-like features and promote long-term immunity.
Oncoimmunology. 1:516–519. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lotti F, Jarrar AM, Pai RK, Hitomi M,
Lathia J, Mace A, Gantt GA Jr, Sukhdeo K, DeVecchio J, Vasanji A,
et al: Chemotherapy activates cancer-associated fibroblasts to
maintain colorectal cancer-initiating cells by IL-17A. J Exp Med.
210:2851–2872. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xiang T, Long H, He L, Han X, Lin K, Liang
Z, Zhuo W, Xie R and Zhu B: Interleukin-17 produced by tumor
microenvironment promotes self-renewal of CD133+ cancer stem-like
cells in ovarian cancer. Oncogene. 34:165–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Luo Y, Yang Z, Su L, Shan J, Xu H, Xu Y,
Liu L, Zhu W, Chen X, Liu C, et al: Non-CSCs nourish CSCs through
interleukin-17E-mediated activation of NF-κB and JAK/STAT3
signaling in human hepatocellular carcinoma. Cancer Lett.
375:390–399. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Parajuli P, Anand R, Mandalaparty C,
Suryadevara R, Sriranga PU, Michelhaugh SK, Cazacu S, Finniss S,
Thakur A, Lum LG, et al: Preferential expression of functional
IL-17R in glioma stem cells: Potential role in self-renewal.
Oncotarget. 7:6121–6135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jiang YX, Yang SW, Li PA, Luo X, Li ZY,
Hao YX and Yu PW: The promotion of the transformation of quiescent
gastric cancer stem cells by IL-17 and the underlying mechanisms.
Oncogene. 36:1256–1264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Condamine T and Gabrilovich DI: Molecular
mechanisms regulating myeloid-derived suppressor cell
differentiation and function. Trends Immunol. 32:19–25. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Medina-Echeverz J, Aranda F and Berraondo
P: Myeloid-derived cells are key targets of tumor immunotherapy.
Oncoimmunology. 3:e283982014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chikamatsu K, Okamoto A, Sakakura K,
Hatsushika K, Takahashi G and Masuyama K: P3.14. Immunoregulatory
properties of CD44+ cancer stem-like cells in squamous cell
carcinoma of the head and neck. Oral Oncol Suppl. 3:205–206. 2009.
View Article : Google Scholar
|
|
71
|
Otvos B, Finke J, Vogelbaum M and Lathia
JD: Interrogating the interactions between myeloid derived
suppressor cells and cancer stem cells in glioblastoma. J Immuno
Ther Cancer. 1:2682013. View Article : Google Scholar
|
|
72
|
Gao L, Yu S and Zhang X: Hypothesis:
Tim-3/galectin-9, a new pathway for leukemia stem cells survival by
promoting expansion of myeloid-derived suppressor cells and
differentiating into tumor-associated macrophages. Cell Biochem
Biophys. 70:273–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Otvos B, Silver DJ, Mulkearns-Hubert EE,
Alvarado AG, Turaga SM, Sorensen MD, Rayman P, Flavahan WA, Hale
JS, Stoltz K, et al: Cancer stem cell-secreted macrophage migration
inhibitory factor stimulates myeloid derived suppressor cell
function and facilitates glioblastoma immune evasion. Stem Cells.
34:2026–2039. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cui TX, Kryczek I, Zhao L, Zhao E, Kuick
R, Roh MH, Vatan L, Szeliga W, Mao Y, Thomas DG, et al:
Myeloid-derived suppressor cells enhance stemness of cancer cells
by inducing microRNA101 and suppressing the corepressor CtBP2.
Immunity. 39:611–621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Panni RZ, Sanford DE, Belt BA, Mitchem JB,
Worley LA, Goetz BD, Mukherjee P, Wang-Gillam A, Link DC, Denardo
DG, et al: Tumor-induced STAT3 activation in monocytic
myeloid-derived suppressor cells enhances stemness and mesenchymal
properties in human pancreatic cancer. Cancer Immunol Immunother.
63:513–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Peng D, Tanikawa T, Li W, Zhao L, Vatan L,
Szeliga W, Wan S, Wei S, Wang Y, Liu Y, et al: Myeloid-derived
suppressor cells endow stem-like qualities to breast cancer cells
through IL6/STAT3 and NO/NOTCH cross-talk signaling. Cancer Res.
76:3156–3165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Shih JY, Yuan A, Chen JW and Yang PC:
Tumor-associated macrophage: Its role in cancer invasion and
metastasis. J Cancer Mol. 2:101–106. 2006.
|
|
78
|
Allavena P, Sica A, Solinas G, Porta C and
Mantovani A: The inflammatory micro-environment in tumor
progression: The role of tumor-associated macrophages. Crit Rev
Oncol Hematol. 66:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Franklin RA, Liao W, Sarkar A, Kim MV,
Bivona MR, Liu K, Pamer EG and Li MO: The cellular and molecular
origin of tumor-associated macrophages. Science. 344:921–925. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Raggi C, Mousa HS, Correnti M, Sica A and
Invernizzi P: Cancer stem cells and tumor-associated macrophages: A
roadmap for multitargeting strategies. Oncogene. 35:671–682. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jinushi M, Chiba S, Yoshiyama H, Masutomi
K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A and Tahara H:
Tumor-associated macrophages regulate tumorigenicity and anticancer
drug responses of cancer stem/initiating cells. Proc Natl Acad Sci
USA. 108:12425–12430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ding J, Jin W, Chen C, Shao Z and Wu J:
Tumor associated macrophage × cancer cell hybrids may acquire
cancer stem cell properties in breast cancer. PLoS One.
7:e419422012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen
L, Xiao HL, Wang B, Yi L, Wang QL, et al: Tumor-associated
microglia/macrophages enhance the invasion of glioma stem-like
cells via TGF-β1 signaling pathway. J Immunol. 189:444–453. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Fan QM, Jing YY, Yu GF, Kou XR, Ye F, Gao
L, Li R, Zhao QD, Yang Y, Lu ZH and Wei LX: Tumor-associated
macrophages promote cancer stem cell-like properties via
transforming growth factor-beta1-induced epithelial-mesenchymal
transition in hepatocellular carcinoma. Cancer Lett. 352:160–168.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yang J, Liao D, Chen C, Liu Y, Chuang TH,
Xiang R, Markowitz D, Reisfeld RA and Luo Y: Tumor-associated
macrophages regulate murine breast cancer stem cells through a
novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells.
31:248–258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhou N, Zhang Y, Zhang X, Lei Z, Hu R, Li
H, Mao Y, Wang X, Irwin DM, Niu G and Tan H: Exposure of
tumor-associated macrophages to apoptotic MCF-7 cells promotes
breast cancer growth and metastasis. Int J Mol Sci. 16:11966–11982.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wan S, Zhao E, Kryczek I, Vatan L,
Sadovskaya A, Ludema G, Simeone DM, Zou W and Welling TH:
Tumor-associated macrophages produce interleukin 6 and signal via
STAT3 to promote expansion of human hepatocellular carcinoma stem
cells. Gastroenterology. 147:1393–1404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Tham M, Tan KW, Keeble J, Wang X, Hubert
S, Barron L, Tan NS, Kato M, Prevost-Blondel A, Angeli V and
Abastado JP: Melanoma-initiating cells exploit M2 macrophage TGFβ
and arginase pathway for survival and proliferation. Oncotarget.
5:12027–12042. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhou W, Ke SQ, Huang Z, Flavahan W, Fang
X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, et al: Periostin
secreted by glioblastoma stem cells recruits M2 tumour-associated
macrophages and promotes malignant growth. Nat Cell Biol.
17:170–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Shi Y, Ping Y, Zhang X and Bian XW:
Hostile takeover: Glioma stem cells recruit TAMs to support tumor
progression. Cell Stem Cell. 16:219–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
He KF, Zhang L, Huang CF, Ma SR, Wang YF,
Wang WM, Zhao ZL, Liu B, Zhao YF, Zhang WF and Sun ZJ: CD163+
tumor-associated macrophages correlated with poor prognosis and
cancer stem cells in oral squamous cell carcinoma. Biomed Res Int.
2014:8386322014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hou YC, Chao YJ, Tung HL, Wang HC and Shan
YS: Coexpression of CD44-positive/CD133-positive cancer stem cells
and CD204-positive tumor-associated macrophages is a predictor of
survival in pancreatic ductal adenocarcinoma. Cancer.
120:2766–2777. 2014. View Article : Google Scholar : PubMed/NCBI
|
















