Introduction
Granular cell tumors (GCTs) are rare and were first
described by Abrikossof in 1926 (1).
These tumors are generally benign and only 1–2% of cases were
reported malignant (2,3). The malignant form has a reported 3-year
mortality rate of 60% (4). GCTs have
been reported in soft tissue, including skin, tongue, subcutaneous
tissue and skeletal muscle. Multiple lesions in the skin occur in
~16% of all cases (2); however,
gastrointestinal involvement, particularly the colon, is extremely
rare (5,6). Colonic GCTs are often asymptomatic and
may be detected incidentally during colonoscopy screening or
through examination performed for non-specific gastrointestinal
symptoms.
To date, the majority of what is known regarding
GCTs is limited to case reports. For example, Hong and Lim
(7) reported a case of GCT in the
cecum, which was removed via laparoscopic approach. Cha et
al (8) described a case of GCT in
the descending colon, which was treated by endoscopic mucosal
resection. Due to its rarity, arguments remain regarding diagnosis
and treatment. The development of endoscopic technology has
provided a new perspective on diagnosis and treatment for colonic
GCTs. The present study describes 11 cases of colonic GCTs,
including clinical manifestations, endoscopic and endoscopic
ultrasonography (EUS) features, pathology, immunohistochemistry,
treatment, and morbidity. Special attention is given to the safety
and feasibility of endoscopic treatment.
Materials and methods
Preoperative evaluation
Patients who received a diagnosis of GCT between
March 2010 and April 2015 at Gastrointestinal Endoscopy Center of
Fujian Provincial Hospital (Fuzhou, China) were included in the
present study. The following data were registered: Patient
demographics, clinical manifestations, endoscopic characteristics,
EUS appearance, pathology, immunohistochemistry, treatment and
associated morbidity.
All patients underwent blood tests, including
hematic biometry and clotting times, prior to the endoscopic
procedure. Colonoscopy, EUS, chest X-ray and abdominal computerized
tomography were performed to determine the feasibility of
endoscopic treatment. All patients provided signed informed consent
to accept the endoscopic procedure after receiving information
regarding the treatment, risks, and benefits. Written informed
consent was received from all patients for publication of the data
in the present study.
Under EUS, the following were recorded: Tumor
location, color, maximum diameter, superficial appearance and the
involved layer within the colonic wall. Lesions ≤2 cm in maximum
diameter and limited to the mucosa or submucosal layer were
selected for endoscopic submucosal dissection (ESD). Lesions in the
submucosal layer or >2 cm in diameter were selected for
endoscopic submucosal excavation (ESE).
Endoscopic resection of GCTs
A single-accessory-channel endoscope with a
water-jet (PCF-Q260AZI; Olympus Corporation, Tokyo, Japan) was
used. A short transparent cap (ND-201-11802; Olympus Corporation)
was used to ensure a clear endoscopic view and apply tension to the
connective tissue during the dissection. CO2
insufflation was employed to alleviate abdominal discomfort during
the ESD procedure. Several types of electrosurgical knives were
applied depending on necessity, including the dual, hook or
insulated-tip (KD-650Q, KD-260R and KD611, respectively; Olympus
Corporation). The HybridKnife system (ERBE, Tübingen, Germany) was
used as the electrosurgical generator. Injection needles (NM4L1),
argon plasma coagulation unit (APC300, ERBE), snares, hot biopsy
forceps and clips (SD230U20, FD410LR and HX610-135, respectively;
Olympus Corporation) were used during the operation.
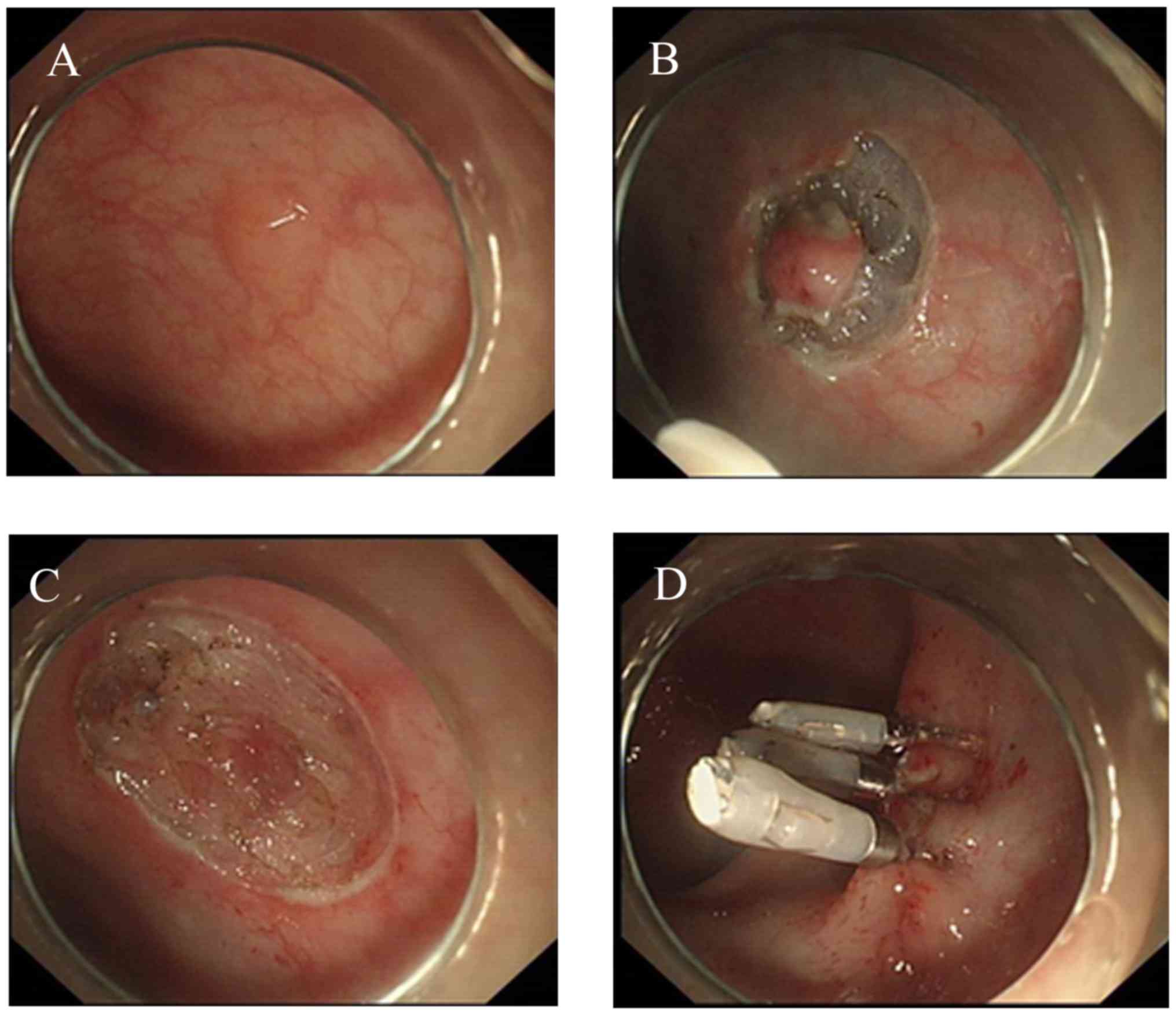
The standard ESD procedure has been previously
described by numerous authors (9)
(Fig. 1). Herein, the specific
protocol used in the submucosal injection step was highlighted. Due
to the duration required for ESD, a solution consisting of 10%
sodium hyaluronate, 5% lidocaine and 0.5% indicarmine dye was
injected into the submucosa around the lesion, helping to lift the
mucosa, and reduce the risk of perforation.
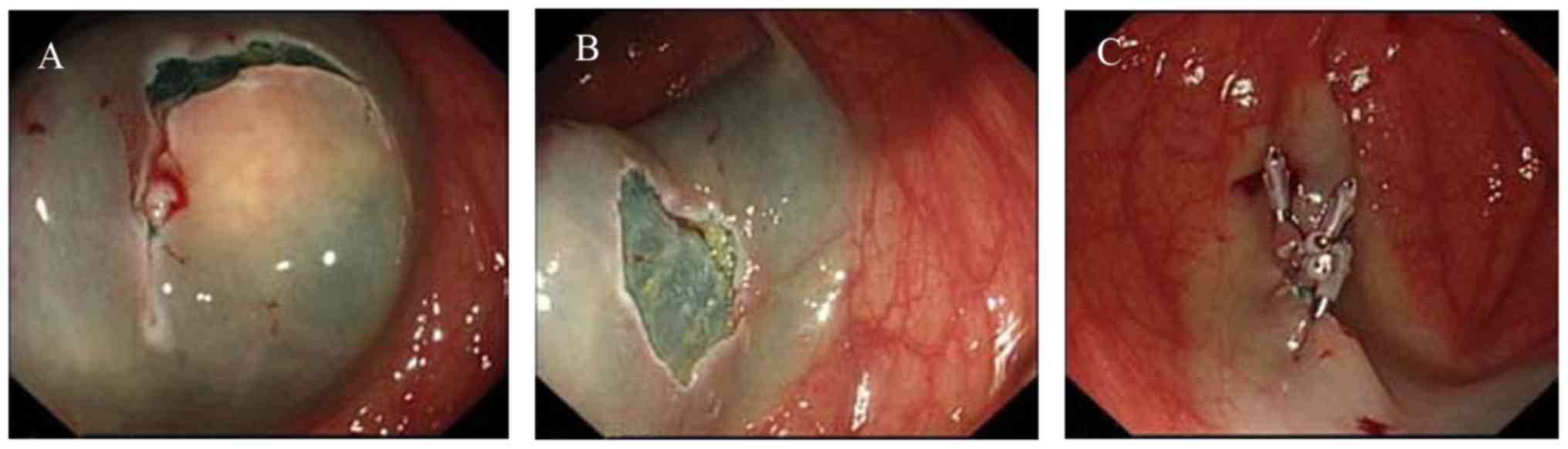
One patient with a tumor 3 cm in diameter received
ESE treatment (Fig. 2), as follows.
To lift the tumor, a submucosal injection of several milliliters of
the mixed solution aforementioned around the lesion was performed
using a 23-gauge disposable needle. A mucosectomy was performed in
which the superficial mucosa was incised using a dual knife. The
tumor was carefully resected from the connecting tissue using a
proper knife (dual or insulation-tipped) to achieve an en
bloc resection. For closure, exposed vessels on the surface or
at the edge of the wound were coagulated with hot biopsy forceps to
prevent delayed bleeding and metallic clips were used to close the
wound. The duration between marking foci and complete resection of
the tumor was noted.
Pathological diagnosis
All resected specimens were fixed in 10% formalin
solution for 6–8 h at room temperature. Thereafter, they were
embedded in paraffin and cut into 5-µm sections. Hematoxylin-eosin
staining was performed in accordance with a previous study
(10). Immunohistochemical
examination was conducted for suspected GCTs. For
immunohistochemical analysis, paraffin-embedded sections were
deparaffinized in xylene for 5 min and hydrated in graded ethanol
(100% ethanol for 5 min, 95% ethanol for 3 min, 85% ethanol for 3
min, 80% ethanol for 3 min and 75% ethanol for 3 min, respectively)
to prior to immunostaining. Sections were soaked in 10 mM citrate
buffer (pH 6.0) and treated at 125°C for 5 min using a pressure
boiler (Decloaking chamber, Biocare Medical LCC, Pacheco, CA, USA)
to perform heat-induced antigen retrieval. Sections were left in
the pressure boiler to cool following complete boiling. Specimens
were then immersed in 5% bovine serum albumin solution at 37°C for
40 mins to block non-specific interaction sites. Sections were
incubated with rabbit anti-human S100 (1:6,000; cat. no. PL032696R;
Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) overnight
at 4°C, and then with goat anti-rabbit biotin-conjugated secondary
antibody (1:2,000; cat. no. A21 220; Abcam, Cambridge, UK) at 37°C
for 30 min, and subsequently washed three times with PBS. Staining
was performed at room temperature for 30 min, using the
avidin-biotin-peroxidase complex method (Vectastain; Vector
Laboratories, Inc., Burlingame, CA, USA). Sections were
counterstained with hematoxylin-eosin for 1 min at room
temperature. Following staining, sections were dehydrated, mounted
and viewed via light microscopy (original magnification, ×200).
Follow-up protocol
Patients with lesions <1 cm and benign tumors
determined by pathology were followed-up annually. Patients with
tumors >1 cm or considered histopathologically atypical were
supervised every 6 months for the first 2 years and annually
thereafter. All patients were monitored for recurrence using
thoracic computed tomography scans, endoscopy and EUS.
Results
Eleven patients (7 males and 4 females) with a mean
age of 49.91 years (range, 40–67 years) were diagnosed with GCT
(Table I). Eight patients were
asymptomatic and the tumors were identified via colonoscopy
screening. Three patients were referred to the Gastrointestinal
Endoscopy Center of Fujian Provincial Hospital complaining of
hematochezia (1 patient) and abdominal pain (2 patients). Eight
lesions were located in the ascending colon and 3 in the cecum; all
of them were solitary.
 | Table I.Patient clinical characteristics. |
Table I.
Patient clinical characteristics.
| Patient | Age, years | Sex | Presentation | Tumor location | Invading layer | Tumor size,
cma | Excision |
|---|
| 1 | 60 | Male | Asymptomatic | Ascending colon | Submucosa | 0.6 | ESD |
| 2 | 67 | Female | Asymptomatic | Cecum | Submucosa | 0.5 | ESD |
| 3 | 42 | Male | Hematochezia | Ascending colon | Submucosa | 3.0 | ESE |
| 4 | 50 | Male | Asymptomatic | Ascending colon | Muscularis
mucosa | 0.4 | ESD |
| 5 | 40 | Female | Abdominal pain | Cecum | Submucosa | 1.2 | ESD |
| 6 | 52 | Male | Asymptomatic | Ascending colon | Muscularis
mucosa | 0.8 | ESD |
| 7 | 47 | Female | Asymptomatic | Ascending colon | Submucosa | 1.5 | ESD |
| 8 | 45 | Male | Abdominal pain | Cecum | Submucosa | 1.0 | ESD |
| 9 | 57 | Female | Asymptomatic | Ascending colon | Muscularis
mucosa | 0.8 | ESD |
| 10 | 45 | Male | Asymptomatic | Ascending colon | Submucosa | 1.5 | ESD |
| 11 | 44 | Male | Asymptomatic | Ascending colon | Submucosa | 0.8 | ESD |
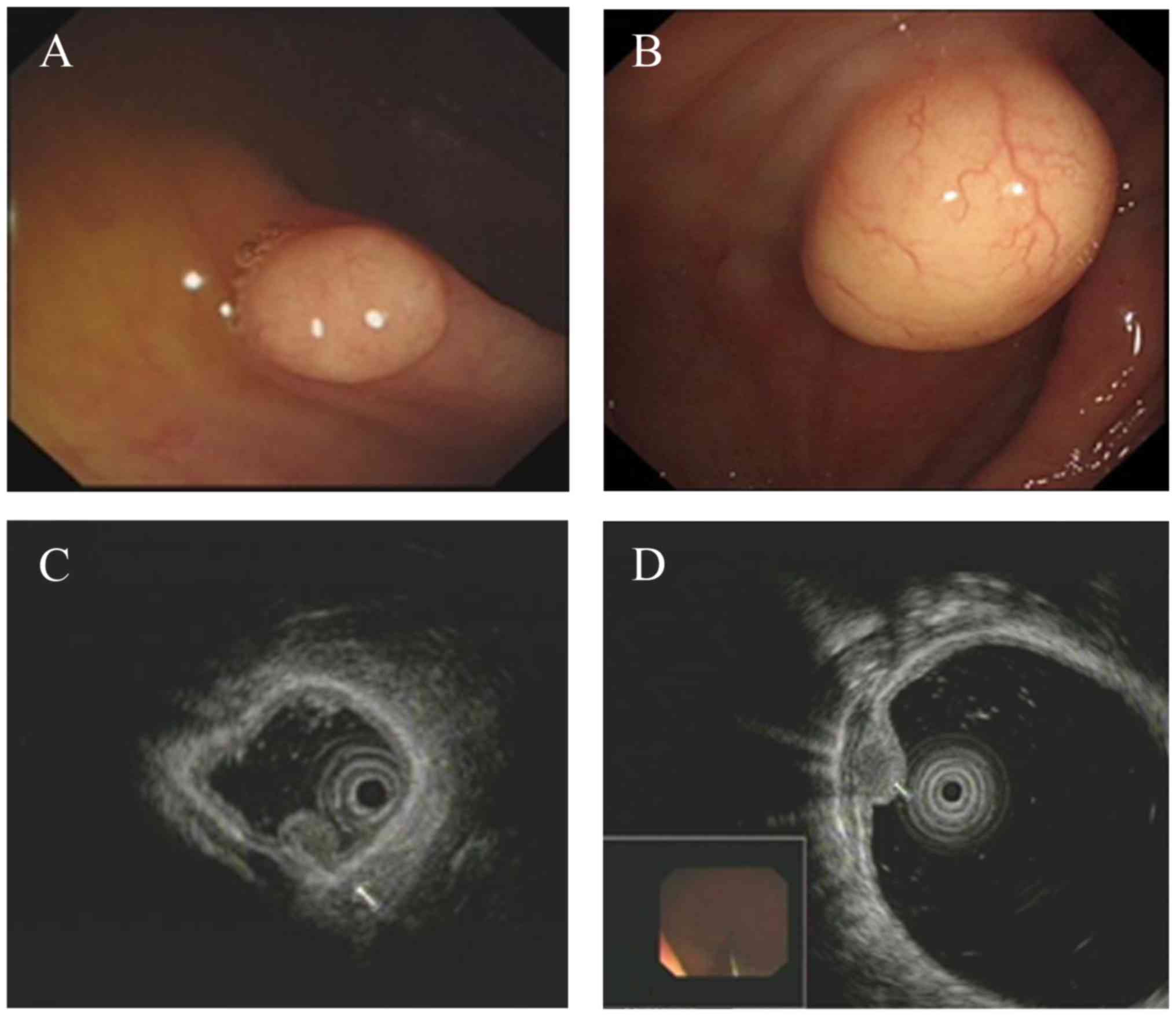
White-light colonoscopy revealed a yellowish or
white, solid and well-circumscribed tumor covered by smooth
superficial mucosa (Fig. 3A and B).
On EUS, these colonic GCTs appeared as homogenous originating from
the muscularis mucosa (3 cases; Fig.
3C) or a granular-type heterogeneous hypoechoic solid tumor
arising from the submucosal layer (8 cases; Fig. 3D). The tumors were well circumscribed
with no compression of neighboring organs and no lymph node was
affected. The tumor diameters ranged between 0.3 and 3.0 cm, with a
mean diameter of 1.1 cm.
All patients underwent endoscopic resection, based
on the tumor characteristics. Ten patients with tumor size ≤2 cm
and located within the muscularis mucosa or submucosal layer
underwent standardized ESD to insure complete resection of the
tumor. Another patient with a GCT 3 cm in diameter and originating
from the submucosal layer accepted ESE for treatment. Tumors were
removed en bloc without complications. The mean operative
duration was 38 min (range, 31 to 50 min).
Pathological diagnosis of the tumor was performed on
all patients. No GCT tissue was detected at the bottom or on the
edge of the specimens, indicating complete resection.
Histologically, all GCT lesions showed a well-circumscribed growth
pattern within the submucosal layer. The gross specimen was a
solitary and peanut-like nodule with a yellowish cross-section.
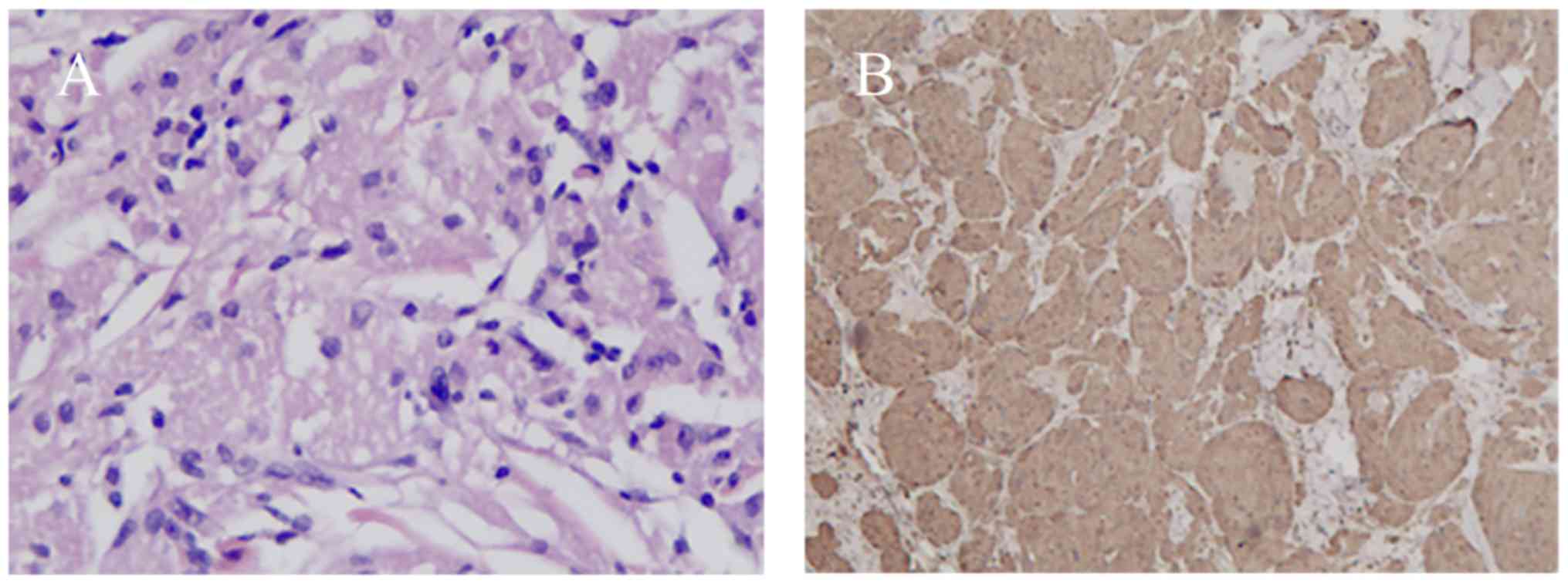
Microscopically, the neoplastic cells were characterized by small,
uniform nuclei surrounded by abundant granular eosinophilic
cytoplasm (Fig. 4A). Typical
histopathological features confirmed the diagnosis of GCT. Further
immunohistochemical analysis revealed positive staining for S-100
in the nucleus and cytoplasm for all cases (Fig. 4B). None of the lesions met the
criteria for malignant or atypical GCT (3). There, all were determined to be
histologically benign.
All patients completed follow-ups of 11 months to 5
years. None of the patients complained of any discomfort or
experienced massive weight loss during their follow-up period.
Colonoscopy and EUS were performed in all patients and none
exhibited recurrence or metastasis.
Discussion
GCTs are a rare type of soft tissue tumors that may
be detected anywhere throughout the body, most commonly in the oral
cavity, skin, skeletal muscle and subcutaneous tissue, but also in
the nervous system, respiratory tract, and female genital organs
(11,12). GCTs are relatively uncommon in the
gastrointestinal track, accounting for 5–11% of all GCTs (5,6,13). To date, ~130 cases of colonic GCTs
have been reported in the English literature (14). Among the relatively rare locations,
the esophagus is involved most frequently, then the duodenum, anus
and stomach. Least common is the colon and rectum, where primarily
the ascending colon, cecum, appendix, and rectum are affected
(8). In the present report, the
tumors were identified in the ascending colon (8 cases) and cecum
(3 cases).
The sex and age distribution of GCT remains
controversial in the literature, partially due to cases that were
insufficiently described. Singhi et al (14) reported an equal sex distribution, with
ages ranging between 31 and 60 years. An et al (15) observed a male predominance, with a
wider age distribution between 21 and 75 years. In the present
study, the male-to-female ratio was 7:4, with ages ranging between
40 and 67 years.
Colonic GCTs tend to be asymptomatic and usually
follow a benign course. The lesions are usually identified during
colonoscopy screening or examinations performed for other reasons.
Patients who had discomfort may exhibit larger lesions. However,
this association is not strong regarding colonic GCTs. Common
symptoms are non-specific, including abdominal discomfort,
hematochezia and changes in bowel habits. In the present study, 8
patients were asymptomatic and 3 complained of hematochezia (1
patient) and abdominal pain (2 patients). An association between
the symptoms of the 3 patients and colonic GCT of the mucosal layer
is unlikely. Multiple GCTs in children may be indicative of
neurofibromatosis (16), Noonan
syndrome (17), growth retardation or
Hodgkin's disease in remission (18).
However, the statistical power is not sufficient due to the
scarcity of cases.
In the present cases, the white-light colonoscopy
showed a yellowish or white, well-circumscribed tumor covered by
normal mucosa. A few cases may show rough mucosa or ulceration on
the top of the lesion, due to an inflammatory reaction to the
mucosa. The lesion is usually solitary and <2 cm in diameter.
However, multiple lesions within the gastrointestinal track or
involved extra-gastrointestinal sites have also been reported in
10–20% of all GCTs (19), and the
largest tumor has been 4 cm in diameter (20).
EUS is essential to determine the invasion depth and
nature of GCTs. Typical colonic GCTs are characterized by
homogeneous or mild heterogeneous hypoechoic nodules with a growth
pattern within the mucosa or submucosa. In the current study, 8
tumors were detected in the submucosal layer with a hypoechoic but
granular-type heterogeneous ultrasonography image. This particular
feature may facilitate the diagnosis of colonic GCTs. However,
colonoscopy examination or EUS features are not reliable enough to
confirm a diagnosis of GCT.
The final diagnosis of GCT is based on
histopathology findings. The typical pathological characteristics
of GCT and positive staining for S-100 protein support an accurate
diagnosis; the key is to get precise GCT tissue. GCTs located at
the mucosa may benefit from biopsy. However, for those restricted
to the submucosal area an endoscopic forceps biopsy is not
recommended, because it may not pick up the actual lesion, but
destroy the tumor integrity with subsequent bleeding.
EUS-guided fine needle aspiration is valuable for
pathological determination. However, the extracted biopsy tissue is
of limited size and the malignant location may be missed, leading
to a false diagnosis. Complete resection is the optimal strategy to
obtain specimens of submucosal tumors. None of the cases in the
present report underwent biopsy, considering that all lesions were
submucosal tumors.
GCTs are generally considered benign with malignant
potential (1–2%) (19,21). For benign cases, endoscopic mucosal
resection or ESD is the best strategy for tumors <2 cm in
diameter (22,23). Previously, partial colectomy was
advised for tumors >2 cm in diameter (24). However, in our experience, lesions
measuring 3–5 cm and limited to the submucosa may be completely
resected by ESE, without bleeding or perforation. This requires a
thorough hemostatic procedure at the edge and bottom of the wound,
and excavation should be performed close to the submucosal layer
while incising the bottom of the tumor.
At present, perforation is the greatest concern of
endoscopists (25). The development
of endoscopic techniques provides several closure approaches during
ESD to deal with perforation, including the following: Clipping;
clipping and then strengthening with an endoloop; purse string
suture with metallic clips and endoloop; and interrupted suture
with endoloop and metallic clip (26). The advent of endoscopic closure
techniques has expanded the indications for ESD for colonic GCTs
located within the submucosal layer, avoiding invasive
procedures.
The first malignant GCT was described by Ravich in
1945 (27). Notably, this particular
clinically malignant tumor may be histologically malignant or
benign. The histological features of malignant GCTs have been
described previously (3,11). Herein, the clinical features that may
indicate a GCT with malignant potential, all of which are high-risk
signals that call for clinicians' special attention are summarized
as follows: Advanced patient age, tumor size >5 cm, rapid recent
growth, and an infiltrative growth pattern. It is recommended that
patients with suspected GCTs undergo a preoperative examination
that includes EUS and abdominal computerized tomography, to
determine the possibility of endoscopic treatment. Traditional
surgery is indicated for a GCT measuring 5 cm in maximum diameter,
an infiltrative growth pattern invading the muscular layer and
lympho-vascular invasion, or further metastasis. The role of
chemotherapy and radiotherapy remains indefinite, as there are only
a few cases of malignant GCTs that responded to chemotherapy
(28,29).
In summary, colonic GCTs are rare, primarily occur
in the right colon and typically follow a benign course. The
initial clinical manifestation and endoscopic appearance is
non-specific, and therefore a correct diagnosis is difficult to
achieve. The advancement of endoscopic technique has partially
replaced partial colectomy for the treatment of benign GCTs.
However, traditional surgery remains the optimal strategy for
malignant cases.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YYC conceived and designed the study. XC and LC
collected all of the data. YHC and WL analyzed the data, drafted
the article and made critical revisions to the article. All of the
authors gave final approval of the version to be published.
Ethics approval and consent to
participate
All patients provided written informed consent to
accept the endoscopic procedure, after receiving information
regarding the treatment, risks and benefits.
Consent for publication
Written informed consent was received from all
patients for publication of the data in the present study.
Competing interests
The authors declare no competing interests.
References
|
1
|
Abrikossoff AI: Weitere untersuchungen
über myoblastenmyome. Virchows Archiv Für Pathologische Anatomie
Und Physiologie Und Für Klinische Medicin. 280:723–740. 1931.
|
|
2
|
Khansur T, Balducci L and Tavassoli M:
Granular cell tumor. Clinical spectrum of the benign and malignant
entity. Cancer. 60:220–222. 1987.
|
|
3
|
Fanburg-Smith JC, Meis-Kindblom JM, Fante
R and Kindblom LG: Malignant granular cell tumor of soft tissue:
Diagnostic criteria and clinicopathologic correlation. Am J Surg
Pathol. 22:779–794. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akahane K, Kato K, Ogiso S, Sakaguchi K,
Hashimoto M, Ishikawa A, Kato T, Fuwa Y, Takahashi A and Kobayashi
K: Malignant granular cell tumor of the breast: Case report and
literature review. Breast Cancer. 22:317–323. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lack EE, Worsham GF, Callihan MD, Crawford
BE, Klappenbach S, Rowden G and Chun B: Granular cell tumor: A
clinicopathologic study of 110 patients. J Surg Oncol. 13:301–316.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnston J and Helwig EB: Granular cell
tumors of the gastrointestinal tract and perianal region: A study
of 74 cases. Dig Dis Sci. 26:807–816. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hong R and Lim SC: Granular cell tumor of
the cecum with extensive hyalinization and calcification: A case
report. World J Gastroenterol. 15:3315–3318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cha JM, Lee JI, Joo KR, Choe JW, Jung SW,
Shin HP and Lim SJ: Granular cell tumor of the descending colon
treated by endoscopic mucosal resection: A case report and review
of the literature. J Korean Med Sci. 24:337–341. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Take I, Shi Q, Qi ZP, Cai SL, Yao LQ, Zhou
PH and Zhong YS: Endoscopic resection of colorectal granular cell
tumors. World J Gastroenterol. 21:13542–13547. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feldman AT and Wolfe D: Tissue processing
and hematoxylin and eosin staining. Methods Mol Biol. 1180:31–43.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ensari A: Granular cell tumor: What's new
in diagnosis and treatment? Turk J Gastroenterol. 18:135–138.
2007.PubMed/NCBI
|
|
12
|
Haikal F, Maceira J, Dias E and
Ramos-E-Silva M: Histogenesis of Abrikossoff tumour of the oral
cavity. Int J Dent Hyg. 8:53–62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Parfitt JR, McLean CA, Joseph MG,
Streutker CJ, Al-Haddad S and Driman DK: Granular cell tumours of
the gastrointestinal tract: Expression of nestin and
clinicopathological evaluation of 11 patients. Histopathology.
48:424–430. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singhi AD and Montgomery EA: Colorectal
granular cell tumor: A clinicopathologic study of 26 cases. Am J
Surg Pathol. 34:1186–1192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
An S, Jang J, Min K, Kim MS, Park H, Park
YS, Kim J, Lee JH, Song HJ, Kim KJ, et al: Granular cell tumor of
the gastrointestinal tract: Histologic and immunohistochemical
analysis of 98 cases. Hum Pathol. 46:813–819. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sahn EE, Dunlavey ES and Parsons JL:
Multiple cutaneous granular cell tumors in a child with possible
neurofibromatosis. J Am Acad Dermatol. 36:327–330. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moos D, Droitcourt C, Rancherevince D,
Marec Berard P and Skowron F: Atypical granular cell tumor
occurring in an individual with Noonan syndrome treated with growth
hormone. Pediatr Dermatol. 29:665–666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Raeve L, Roseeuw D and Otten J:
Multiple cutaneous granular cell tumors in a child in remission for
Hodgkin's disease. J Am Acad Dermatol. 47(Suppl 2): S180–S182.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saleh H, El-Fakharany M and Frankle M:
Multiple synchronous granular cell tumors involving the colon,
appendix and mesentery: A case report and review of the literature.
J Gastrointestin Liver Dis. 18:475–478. 2009.PubMed/NCBI
|
|
20
|
Choi SM, Hong SG, Kang SM, Chae BG, Kim
SJ, Park PK and Park HS: A case of malignant granular cell tumor in
the sigmoid colon. Clin Endosc. 47:197–200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thacker MM, Humble SD, Mounasamy V, Temple
HT and Scully SP: Case report. Granular cell tumors of extremities:
Comparison of benign and malignant variants. Clin Orthop Relat Res.
455:267–273. 2007.
|
|
22
|
Hoteya S, Yahagi N, Iizuka T, Kikuchi D,
Kawano K, Noguchi T, Mizuno H and Hashimoto M: Endoscopic resection
for early gastric cancers by EMR/ESD. Gan To Kagaku Ryoho.
34:16–20. 2007.(In Japanese). PubMed/NCBI
|
|
23
|
Goto O, Uraoka T, Horii J and Yahagi N:
Expanding indications for ESD: Submucosal disease (SMT/carcinoid
tumors). Gastrointest Endosc Clin N Am. 24:169–181. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen J, Wang L, Xu J, Pan T, Shen J, Hu W
and Yuan X: Malignant granular cell tumor with breast metastasis: A
case report and review of the literature. Oncol Lett. 4:63–66.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma MX and Bourke MJ: Complications of
endoscopic polypectomy, endoscopic mucosal resection and endoscopic
submucosal dissection in the colon. Best Pract Res Clin
Gastroenterol. 30:749–767. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi Q, Chen T, Zhong YS, Zhou PH, Ren Z,
Xu MD and Yao LQ: Complete closure of large gastric defects after
endoscopic full-thickness resection, using endoloop and metallic
clip interrupted suture. Endoscopy. 45:329–334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ravich A, Stout AP and Ravich RA:
Malignant granular cell myoblastoma involving the urinary bladder.
Ann Surg. 121:361–372. 1945. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiang MJ, Fang TJ, Li HY, Chen IH and Lee
KF: Malignant granular cell tumor in larynx mimicking laryngeal
carcinoma. Am J Otolaryngol. 25:270–273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morita S, Hiramatsu M, Sugishita M,
Gyawali B, Shibata T, Shimokata T, Urakawa H, Mitsuma A, Moritani
S, Kubota T, et al: Pazopanib monotherapy in a patient with a
malignant granular cell tumor originating from the right orbit: A
case report. Oncol Lett. 10:972–974. 2015. View Article : Google Scholar : PubMed/NCBI
|