Introduction
Endometrial cancer (EC) is one of the most malignant
forms of cancer of the female reproductive system. Chemotherapy
combined with surgery and radiotherapy is the most important method
of contemporary EC treatment. Previously, studies demonstrated that
YKL-40 is one of the candidate biomarkers of EC; the YKL-40 gene
may serve an important role in the proliferation (1), angiogenesis (2), and anti-apoptosis (3) of cancer cells. However, the function of
YKL-40 requires further investigation. A previous study (4) reported that the YKL-40 protein is highly
expressed in a variety of tissues and in the serum of patients with
EC, compared with patients with uterine myoma and healthy
individuals.
In the present study, a unique small interfering
(si)RNA sequence was used to prepare a recombinant lentivirus which
was transduced into tumor cells and was effective in inhibiting the
expression of the YKL-40 gene. MTT, migration, invasion and flow
cytometry (FCM) assays were performed to identify the effects of
si-YKL-40 on the proliferative, migratory and invasive abilities,
and apoptotic rate, of EC HEC-1A cells. FCM analysis of the average
cellular apoptotic rate following treatment with cisplatin, pre-
and post-si-YKL-40 transduction, was used to understand the effects
of si-YKL-40 on the sensitivity of EC HEC-1A cells to
cisplatin-based chemotherapy. Additionally, further investigation
is required to understand the underlying mechanism of EC
chemoresistance. Combining YKL-40 siRNA with other chemotherapies
may provide efficacious therapy for the treatment of cancer.
Targeting the YLK-40 gene may control the occurrence of EC
development in the future.
Materials and methods
Cell lines and cell culture
HEC-1A cells were obtained from the Affiliated Tumor
Hospital of Guangxi Medical University (Nanning, China). The cells
were maintained at 37°C in a humidified incubator under 5%
CO2 and cultured in Dulbecco's modified Eagle's medium
(DMEM)/F12 culture medium (GE Healthcare, Chicago, IL, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin, and 100
µg/ml streptomycin. The cells were subcultured every 2 days. Cells
in the logarithmic growth phase were used for the experiments.
YKL-40 siRNA sequences
A DNA oligomer specifically targeting the sequence
of YKL-40 5′-GACTCTCTTTCTGTCGGA-3′ (si-YKL-40; Hanbio Biotechnology
Co., Ltd., Shanghai, China) was selected and subcloned into a
retroviral pSUPER-puro-vector (Hanbio Biotechnology Co., Ltd.).
Subsequently, 293T retroviral packaging cells (American Type
Culture Collection, Manassas, VA, USA) were transfected with the
si-YKL-40-pSUPER puro-vector (1×108/ml; Hanbio
Biotechnology Co., Ltd.). After 48 h post-transfection, the
supernatant which was centrifuged at 72,000 × g/min was harvested
and filtered in 4°C for 120 min through a 0.45 µm pore-size filter.
All of the transfected reagents were purchased from Hanbio
Biotechnology Co., Ltd.
Cell transfection and knockdown of
YKL-40 within HEC-1A cells
HEC-1A cells were divided into three groups: The
experimental group was transfected with siRNA, the mock-treatment
group was treated with transfection reagent only, and the blank
control group was left untransfected. HEC-1A (5×104
cells) were transferred into 6-well plates, then transfected when
cells reached 50% confluence. The results of a previous study
(5) indicated that the optimal
multiplicity of infection value was 20; therefore, this number was
selected to transfect the cells (si-YKL-40; Hanbio Biotechnology
Co., Ltd.). Cells were transfected with si-YKL-40 or nontargeting
green fluorescence protein (GFP; Hanbio Biotechnology Co., Ltd.) in
the presence of Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
cells that were successfully transfected exhibited GFP. Following
transfection, a lentivirus was used to transduce HEC-1A cells.
Selection with 1 µg/ml puromycin was performed for 2 weeks
following transduction; puromycin-resistant cells were used for
subsequent experiments.
Detection of YKL-40 expression by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), according to the
manufacturer's protocol. The optical density (OD) 260/OD280 ratio
for determining the purity of RNA was reported to be between
1.8-2.0. A total of 1 µg of RNA was reverse transcribed using the
first cDNA strand (cDNA synthesis kit; Takara Bio, Inc., Otsu,
Japan). RT-qPCR was performed using an ABI StepOne Plus (Applied
Biosystems; Thermo Fisher Scientific, Inc.) system with SYBR Green
PCR Master Mix (Takara Bio, Inc.). RT-qPCR reactions were performed
according to the manufacturers' protocols. RT-qPCR assays were
performed in triplicate, and the mean values were used to calculate
mRNA expression. Gene expression was normalized to β-actin mRNA.
The results were calculated and presented as 2−∆∆Cq
(6). The primer sequences were as
follows: YKL-40, 5′-ATCACCAAGGAGCCAAACATC-3′ (sense) and
5′-GGGGAAGTAGGATAGGGGACA-3′ (antisense); β-actin,
5′-ACACTGTGCCCATCTACG-3′ (sense) and 5′-TGTCACGCACGATTTCC-3′
(antisense).
Cell proliferation assays
For the proliferation assays, cells
(5×103) were seeded onto 96-well plates with 100 µl
DMEM/F12 medium and incubated overnight at 37°C. Five replicates
were prepared for each cell type. Cells from each of the groups
were cultured for 24, 48, 72, 96 and 108 h. To measure
proliferation, MTT reagent [20 µl 5 mg/ml thiazolyl blue
tetrazolium bromide in PBS (Sigma-Aldrich; Merck KGaA)] was added
into each well and incubated for 4 h. The reaction was stopped by
adding 150 µl dimethyl sulfoxide to each well. The OD value of blue
emission was analyzed with a microplate reader (Thermo Fisher
Scientific, Inc.) at a 570 nm wavelength. The cell growth curve was
determined using OD values as the ordinate and duration as the
abscissa. All the experiments were conducted in triplicate.
Cell migration assays and Matrigel
invasion assays
Cells (1×104) were seeded onto Costar
Transwells (polycarbonate membrane, 24-well format, 0.8 mm pore
size; Corning Incorporated, Corning, NY, USA) over the upper
chamber coated with 60 µl Matrigel (1:7; BD Biosciences, San Jose,
CA, USA) which was diluted with serum-free DMEM/F12. This was
followed by the addition of 500 µl DMEM/F12 containing 10% FBS at
the bottom. The cells were incubated for 48 h at 37°C. The cells in
the upper chamber were wiped with wet wipes, while the cells in the
bottom were washed with PBS. Subsequently, the cells were fixed
with 4% paraformaldehyde for 20 min at room temperature and stained
with 0.1% crystal violet for 30 min at room temperature. Average
cell numbers were calculated from five different fields
(magnification, ×20) in each sample by an light inverted microscope
(CKX41SF; Olympus Corporation, Tokyo, Japan). In addition, cells
required incubation for only 24 h in the cell migration assay. The
experiments were repeated three times.
Effect of cisplatin on the expression
of YKL-40 mRNA within HEC-1A cells
HEC-1A cells which were not transduced with
si-YKL-40 were divided into two groups constituting untreated cells
(group A) and those treated with 25 mmol/l cisplatin for 48 h
(group B). RT-qPCR analysis was performed to investigate the mRNA
levels of YKL-40 within HEC-1A cells. The experiment was performed
in accordance with the aforementioned protocols. The experiments
were conducted in triplicate.
MTT assays and apoptotic assays
Apoptotic assays of the transduced HEC-1A cells
treated with cisplatin were performed by detecting 7-actinomycin D
(7AAD) and Annexin V-phycoerythrin (PE) in these cells. The cells
were treated with 25 µmol/l cisplatin for 48 h. Cells treated with
normal saline were used as the control. Cisplatin was purchased
from Shandong Qilu King-Phar Pharmaceutical Co., Ltd. (Jinan,
China). Preparation of the drug was performed according to the
manufacturer's protocols; a concentration gradient of cisplatin (0,
50, 25, 12.5, 6.25, 3.125, and 100 µmol/l) was produced. The dosage
of the cisplatin was administered according to the results of the
MTT assay; as the proliferative ability of the experimental group
cells was significantly inhibited compared with the blank control
group cells and the mock-treatment group cells when treated with 25
µmol/l cisplatin. At this concentration, the chemosensitivity of
HCE-1A cells to cisplatin was increased following silencing of the
YKL-40 gene. The cells (1×106/ml) were incubated with
7-AAD and PE-conjugated Annexin V (BD Biosciences) for 15 min at
37°C, respectively, according to the manufacturer's protocol, and
were analyzed by FCM (FACSCalibur and CellQuest software version
5.2.1; BD Biosciences).
Statistical analysis
Statistical analysis was performed using SPSS
Statistics for Windows, version 17.0 (SPSS Inc., Chicago, USA).
Data are presented as the mean ± standard deviation, and
significance was determined by one-way analysis of variance (ANOVA)
and two independent sample t-test. The Student-Newman-Keuls and
Least Significant Difference analysis were used as post-hoc tests
following ANOVA to determine the difference between specific
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
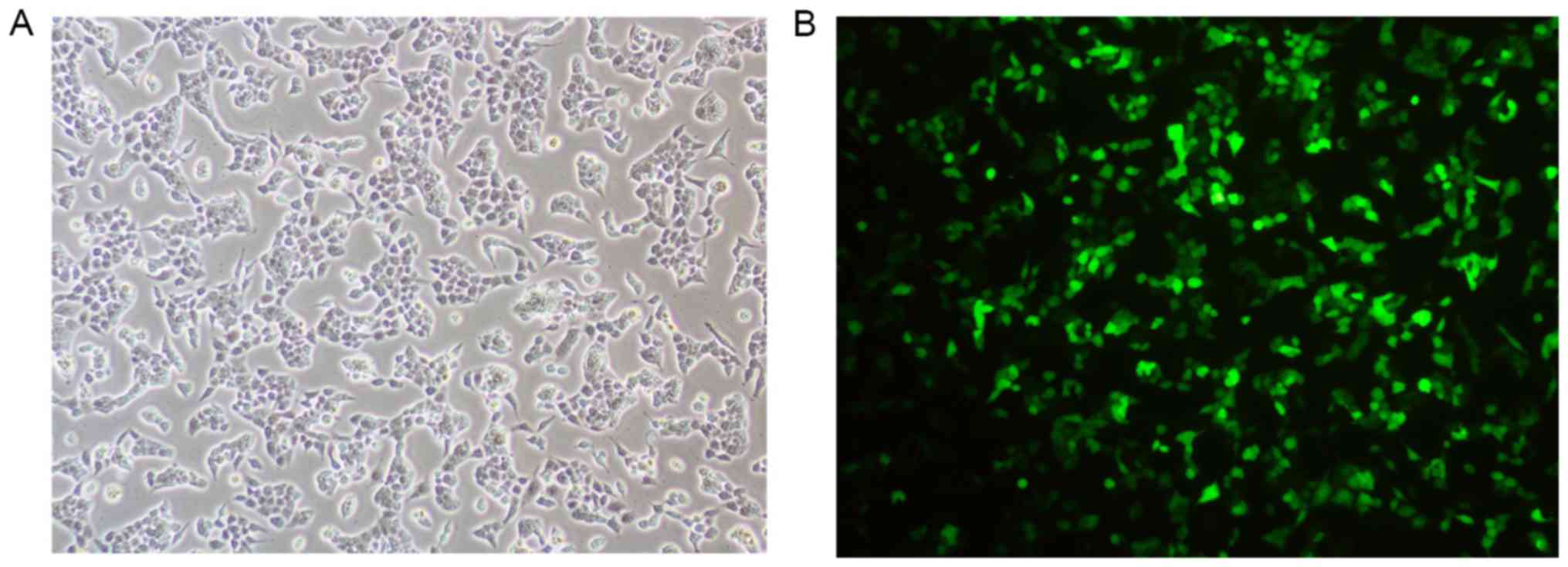
Detection of fluorescence in
transfected cells
Treated cells were observed under a microscope.
HEC-1A cells that were successfully transfected with siRNA
exhibited green fluorescence (Fig.
1).
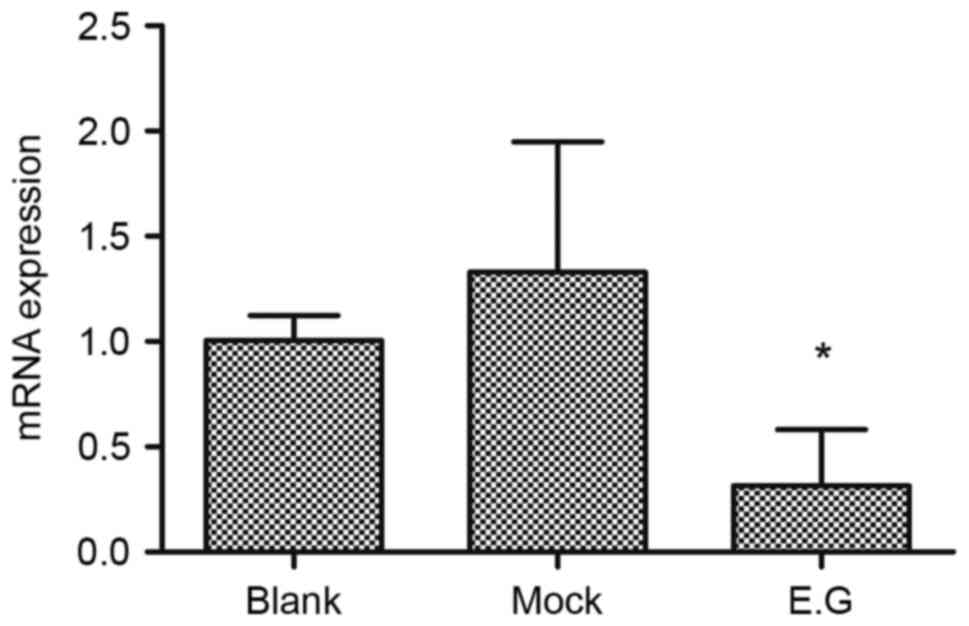
Interference effects of YKL-40
siRNA
Following lentivirus-mediated transduction, the RNA
transcription levels of YKL-40 within HEC-1A cells were lower
compared with in the blank control and mock-treatment groups
(F=6.875; P=0.015). No significant difference was observed between
the blank control group and mock-treatment group (P<0.05;
Fig. 2).
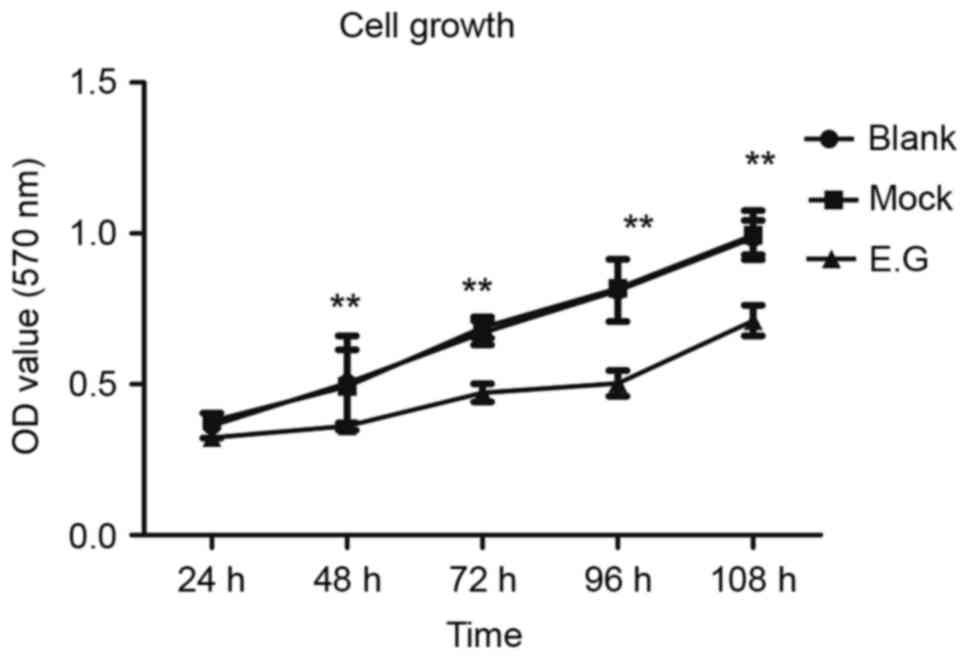
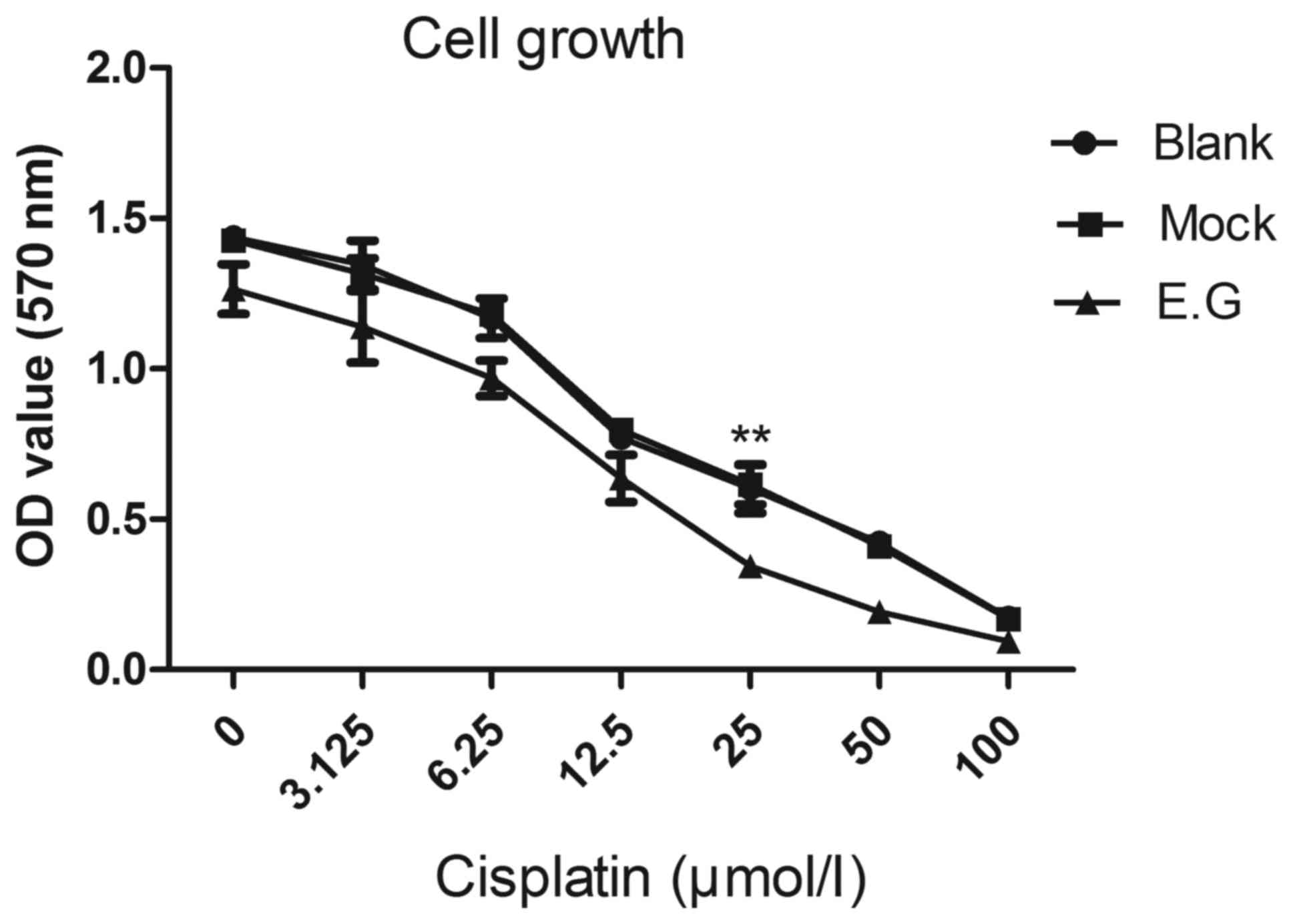
YKL-40 gene controls the proliferation
potential of HEC-1A cells
MTT assays indicated that si-YKL-40 inhibited HEC-1A
cell proliferation compared with the blank control and
mock-treatment groups (P<0.05; Fig.
3).
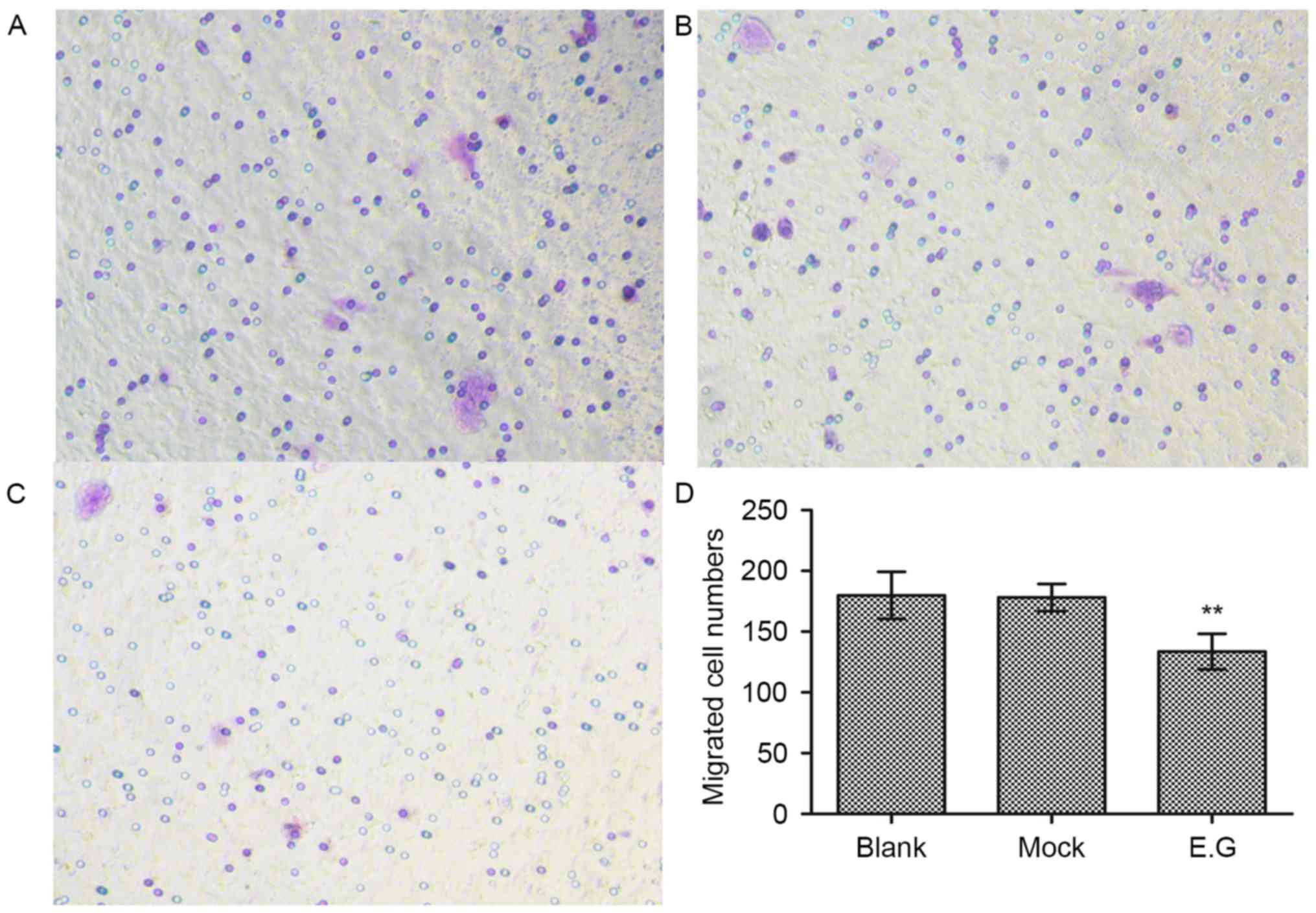
YKL-40 gene promotes the migratory
potential of HEC-1A cells
Cell migration assays demonstrated that si-YKL-40
(133±14 migrated cells) inhibited the HEC-1A cell migration
abilities compared with in the blank control (179±19 migrated
cells) and mock-treatment groups (178±11 migrated cells; F=14.494;
P<0.05; Fig. 4).
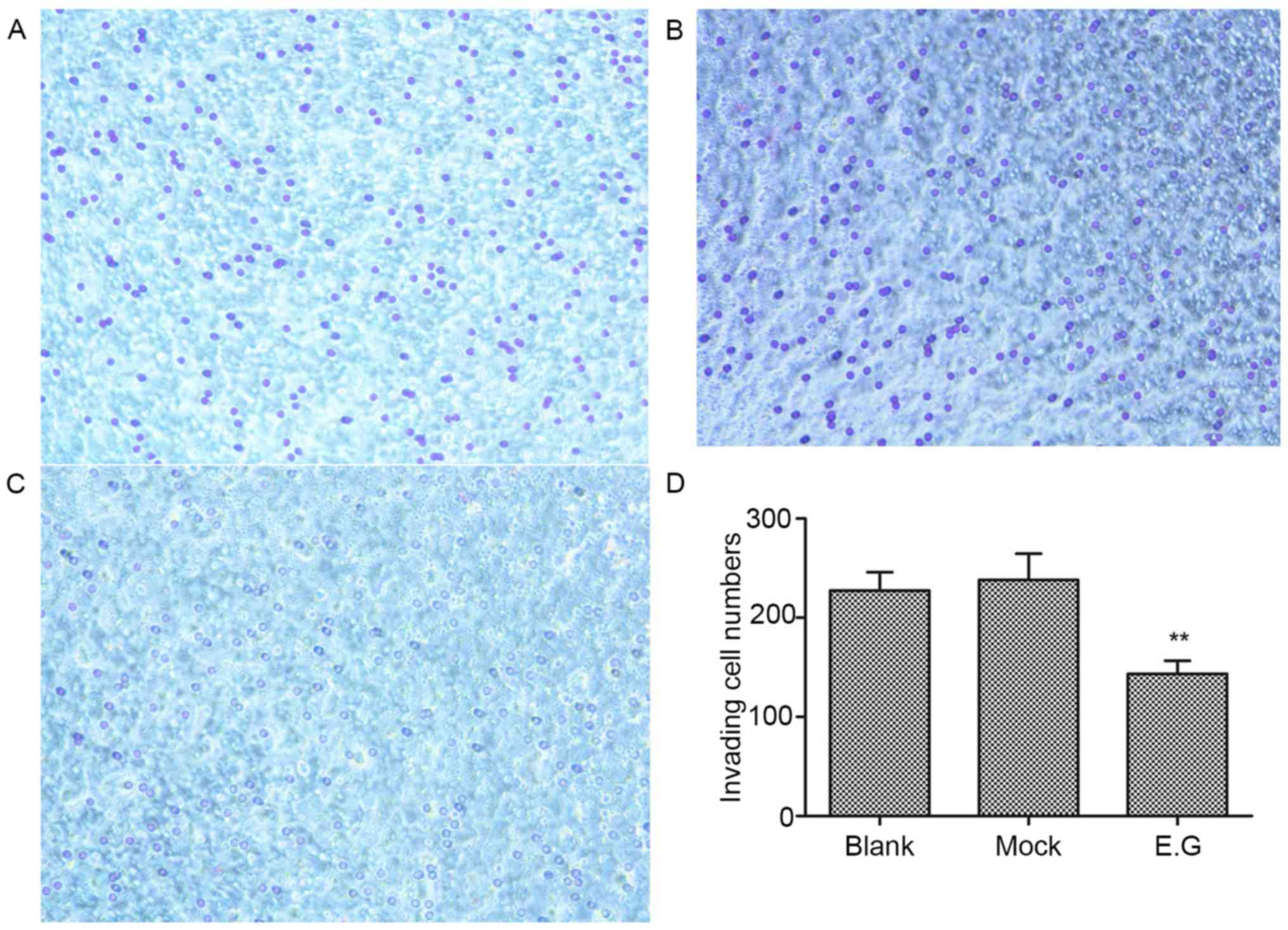
YKL-40 gene regulates the invasive
ability of HEC-1A cells
Matrigel invasion assays revealed that the invasive
ability of HEC-1A cells within the experimental group was
significantly inhibited; the invasion number (143±13) was lower
compared with the blank control (227±18) and mock-treatment groups
(238±26; F=33.476; P<0.05; Fig.
5).
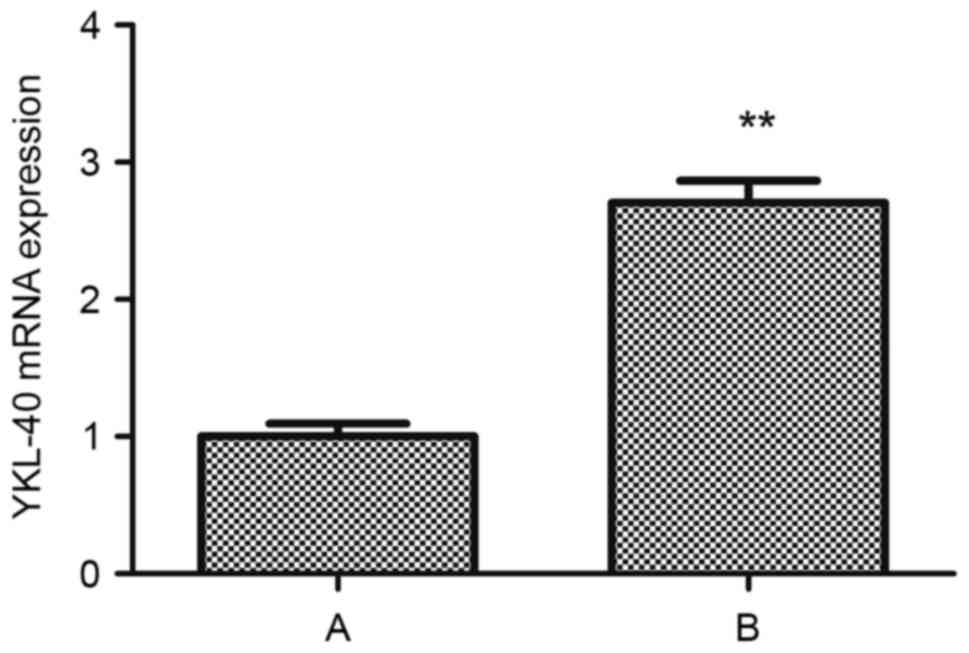
Cisplatin upregulates the expression
of YKL-40 mRNA in human HEC-1A cells
Following treatment with cisplatin, the RNA
transcription levels of YKL-40 within HEC-1A cells of group B was
upregulated compared with in group A (t=−15.986, P<0.05;
Fig. 6).
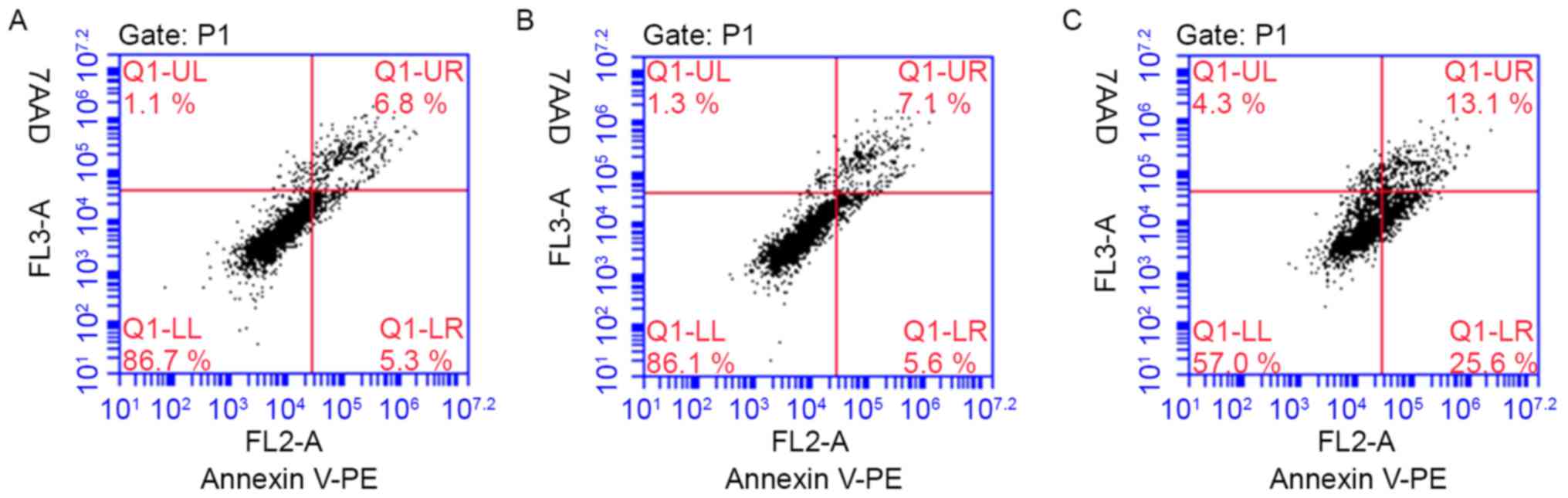
YKL-40 gene inhibits the apoptosis of
HEC-1A cells following treatment with cisplatin
YKL-40 inhibited the apoptosis of human EC cells
treated with a cytotoxic drug. To investigate the possible
biological effects of the YKL-40 gene on EC cells,
YKL-40-transfected HEC-1A cells were generated for in vitro
apoptosis assays. The HEC-1A cell apoptosis rate increased
following the inhibition of YKL-40 expression (experimental group,
38.07±4.88; blank group, 13.3±1.01; mock group, 12.5±0.17), when
treated with cisplatin for 48 h (Fig.
7). Following the downregulation of YKL-40 expression levels
via YKL-40 siRNA, cisplatin chemotherapeutic sensitivity and the
apoptosis of EC cells increased. However, there was no significant
difference observed between the blank control and mock-treatment
groups. The representative figures of the FCM analysis for the
detection of Annexin V-positive and 7AAD-positive cells in
transduced HEC-1A cells treated with cisplatin are presented in
Fig. 8.
Discussion
EC is now the most common gynecologic malignancy,
which more commonly occurs in postmenopausal women. EC consists of
a group of endometrial epithelial malignant tumors with a number of
pathological types, including endometrial adenocarcinoma (7). Studies have revealed that the
pathogenesis of EC is associated with numerous factors (8). Clinical characterization of the type of
disease is an essential step for correct diagnosis and treatment.
However, the cause of EC requires further investigation. Type I EC
develops in an environment of high levels of estrogen and
frequently develops from endometrial hyperplasia, whereas type II
cancer is not an estrogen-associated cancer, occurring
predominantly in postmenopausal women. An excessive increase in
estrogen may promote the accumulation of body fat, and
obesity-associated proteins may promote the proliferation of
endometrial carcinoma (9).
YKL-40, additionally termed CHI3L1, is produced by a
variety of cells (10), including
macrophages, neutrophils, eosinophils, vascular smooth muscle
cells, endothelial cells and cancer cells. Elevated levels of serum
YKL-40 protein have been reported in a number of other types of
cancer, including glioma (11)
cholangiocarcinoma (12), colorectal
cancer (13), non-small cell lung
cancer (14), renal cell cancer
(15) and osteosarcoma (16). YKL-40 may also be expressed within
local inflammatory cells in inflamed tissues (17,18) and it
is considered to be a biomarker in inflammatory disease. The YKL-40
gene can regulate the extent of inflammation, and angiogenesis, and
the process of inflammation resolution. In gynecologic malignant
tumors, including ovarian cancer (19) and EC (20), YKL-40 serum levels were observed to be
higher compared with healthy individuals. One previous study has
have demonstrated that plasma YKL-40 protein levels are elevated in
patients with EC and are associated with disease severity and
prognosis (4). Previous studies also
demonstrated that the expression of YKL-40 protein in EC tissue was
associated with histology, stage and reduced survival time via
immunohistochemistry (20,21). YKL-40 gene and protein expression
levels in cancerous tissue may determine the concentration in the
blood. The serum YKL-40 level as a biomarker may have values in the
diagnosis and prognosis of cancer.
YKL-40 mRNA may serve an important role in the
proliferation, angiogenesis, and anti-apoptosis of cancer cells. A
previous study reported potential biological functions and
mechanisms of the YKL-40 gene within cancer cells. For example, in
cholangiocarcinoma cells, YKL-40 mRNA may promote cell
proliferation and migration by regulating the RAC-α
serine/threonine-protein kinase/extracellular signal-regulated
kinase (ERK) pathway (12). The
YKL-40 protein has additionally been reported to promote tumor
angiogenesis by inducing the ERK1/2 pathway (22). Research has demonstrated that the
YKL-40 protein regulates the release of inflammatory cytokines and
the activation of the mitogen-activated protein kinase signaling
pathway in colorectal carcinoma (23). A research has revealed that YKL-40
mRNA may inhibit ovarian cancer cell apoptosis via the MCL-1 BCL2
family apoptosis regulator gene (6).
The YKL-40 protein may be induced by interleukin-8 and tumor
necrosis factor-α in colitis, and it promotes the development of
colitis to tumor by regulating the nuclear factor-κB pathway
(24). In addition, YKL-40 mRNA also
promotes macrophage recruitment and angiogenesis in colorectal
cancer (23). A previous study
reported that YKL-40 may regulate the sensitivity of a glioblastoma
cell line to chemotherapy treatment via the signal transducer and
activator of transcription 3 signaling pathway (25). These previous findings indicate that
YKL-40 mRNA may have a role in cancer cells as an inflammatory
factor. However, further investigation into the underlying
mechanism is required.
RNA interference is a powerful tool for gene
silencing. In astrocytoma, YKL-40 has been reported to induce
angiogenesis and metastasis (2). In
the present study, the expression of the YKL-40 gene within HEC-1A
cells was significantly knocked down following transduction with a
lentivirus. Additionally, YKL-40 gene activity was successfully
inhibited by si-YKL-40; however, the same effect was not observed
in the mock-treatment group. In cell proliferation assays, the
proliferative ability of HEC-1A cells was inhibited by YKL-40
siRNA. In addition, the silencing of the YKL-40 gene within HEC-1A
cells was associated with a reduction in the number of migrating
and invading cells during the migration and Matrigel invasion
assays. The number of migratory cells in the experimental group was
significantly reduced compared with in the blank and the
mock-treatment groups. These assays revealed that YKL-40 may
control the migratory and invasive potential of HEC-1A cells.
Postoperative chemotherapy for residual tumor cells
is an important and a primary treatment for advanced EC. At
present, platinum drugs are widely used in a variety of first-line
chemotherapy treatments; however, resistance to chemotherapy is a
major limiting factor in the treatment of cancer, and EC is no
exception. It is important to understand the underlying mechanism
of EC chemotherapy drug resistance.
In the present study, the effects of cisplatin on
the YKL-40 mRNA in human HEC-1A cells were investigated. The
expression levels of YKL-40 mRNA within human HEC-1A cells were
elevated following treatment with cisplatin for 48 h. The results
were consistent with those of van Linde et al (26); an ELISA revealed that serum YKL-40
levels were increased following chemotherapy in glioblastoma.
YKL-40 is associated with chemotherapy sensitivity in
drug-resistant tumor cell lines, and a reduction in the expression
of YKL-40 in these cancer cell lines may increase the sensitivity
of tumor chemotherapy drugs (25).
However, a study of non-small cell lung cancer demonstrated that
serum YKL-40 levels were downregulated following chemotherapy.
Additionally, YKL-40 may be associated with the proliferation of EC
and exerted an anti-apoptotic function when the HEC-1A cells were
treated with chemotherapy drugs YKL-40 may serve an important role
in the sensitivity to chemotherapy in certain cancer cells. In the
present study, the effects of si-YKL-40 on the cisplatin
sensitivity of EC HEC-1A cells 48 h post-treatment with cisplatin
were determined via an MTT assay. The proliferative ability of the
experimental group cells was significantly inhibited compared with
the blank and mock-treatment groups when treated with 25 µmol/l
cisplatin. This dosage of cisplatin was associated with the
greatest sensitivity to chemotherapy exhibited by HEC-1A cells. FCM
revealed that the average cellular apoptosis rate increased
following the inhibition of YKL-40 gene expression within EC HEC-1A
cells under the same cisplatin concentration (25 µmol/l). The
proportion of apoptotic cells was significantly increased within
the blank and mock-treatment groups compared with the experimental
group. These assays demonstrated the association between the YKL-40
gene and cisplatin-based chemotherapy in HEC-1A cells. The YKL-40
gene may increase the sensitivity of cisplatin-based chemotherapy,
while undertaking a mechanism of EC chemoresistance. Research into
ovarian cancer has demonstrated that YKL-40 may act as an
evaluation index to aid the prognosis of cancer chemotherapy
(27). Cell migration assays revealed
that si-YKL-40 inhibited the HEC-1A cell migration abilities, as
well as chemoresistance in gliomas (28). These findings provide support for the
hypothesis that the YKL-40 gene may serve an important role in the
chemoresistant, proliferative and apoptotic abilities of EC.
A wealth of tumorigenic evidence from human cancer
and animal tumor models indicates that elevated levels of
angiogenic factors in cancer tissue correlate with tumor
angiogenesis. Studies have investigated the inhibitory effects of
YKL-40 gene on tumor growth and angiogenesis induced by YKL-40
siRNA (2). Other studies have
demonstrated that the migratory and invasive abilities of ovarian
cancer cells were inhibited by the silencing of YKL-40 gene,
whereas YKL-40 overexpression may be associated with angiogenesis
(6). In other malignancies (29–31), the
proliferative, migratory and invasive abilities of cancer cells
were inhibited by si-YKL-40 in vitro; tumor formation in the
experimental group was reduced compared with the controlled group
in vivo (2). In the present
study, si-YKL-40 increased the sensitivity to cisplatin-based
chemotherapy in EC HEC-1A cells. Biological behaviors, including
proliferative, migration, invasive and anti-apoptotic abilities of
HEC-1A cells were inhibited by YKL-40 gene RNA interference. In
addition, the overexpression of YKL-40 may induce the
proliferative, migratory and invasive abilities of EC cells, as in
other tumors. Further investigation into the effects of YLK-40 and
the underlying mechanism are required.
In conclusion, the results of the present study
indicated that the expression of YKL-40 was effectively suppressed
by si-YKL-40, which was associated with the inhibition of the
biological behaviors of HEC-1A cells. YKL-40 siRNA increased the
sensitivity of cisplatin-based chemotherapy in HEC-1A cells.
Combining YKL-40 siRNA with other postoperative chemotherapies may
provide more efficacious therapies for patients with EC. The YKL-40
gene may serve as a molecular target for the diagnosis and
treatment of EC in the future. However, further clinical trials are
required to understand the effects of YLK-40 in the management of
EC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Scientific Foundation of China (grant no. 81360388) and the
Natural Scientific Foundation of Guangxi Zhuang Autonomous Region,
China (grant no. 2016GXNSFAA380258).
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
JF conceived the study and analyzed the data. LL and
DL analyzed data. LL, YL, PS, CZ, XX and XH performed the
experiments. LL wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
YKL-40
|
chitinase-3-like protein 1
|
|
siRNA
|
small interfering RNA
|
|
FCM
|
flow cytometry
|
|
EC
|
endometrial cancer
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
OD
|
optical density
|
|
7AAD
|
7-actinomycin D
|
|
PE
|
phycoerythrin
|
|
ERK
|
extracellular signal-regulated
kinase
|
References
|
1
|
Brøchner CB, Johansen JS, Larsen LA, Bak
M, Mikkelsen HB, Byskov AG, Andersen CY and Møllgård K: YKL-40 is
differentially expressed in human embryonic stem cells and in cell
progeny of the three germ layers. J Histochem Cytochem. 60:188–204.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Francescone RA, Scully S, Faibish M,
Taylor SL, Oh D, Moral L, Yan W, Bentley B and Shao R: Role of
YKL-40 in the angiogenesis, radioresistance, and progression of
glioblastoma. J Biol Chem. 286:15332–15343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee CG, Hartl D, Lee GR, Koller B,
Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, et
al: Role of breast regression protein 39 (BRP-39)/chitinase
3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J
Exp Med. 206:1149–1166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan JT, Si XH, Liao Y and Shen P: The
diagnostic and prognostic value of serum YKL-40 in endometrial
cancer. Arch Gynecol Obstet. 287:111–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li LL, Fan JT, Li DH and Liu Y: Effects of
a small interfering RNA targeting YKL-40 gene on the proliferation
and invasion of endometrial cancer HEC-1A cells. Int J Gynecol
Cancer. 26:1190–1195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chiang YC, Lin HW, Chang CF, Chang MC, Fu
CF, Chen TC, Hsieh SF, Chen CA and Cheng WF: Overexpression of
CHI3L1 is associated with chemoresistance and poor outcome of
epithelial ovarian carcinoma. Oncotarget. 6:39740–39755. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lax SF: Pathology of endometrial
carcinoma. Adv Exp Med Biol. 943:75–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dossus L, Allen N, Kaaks R, Bakken K, Lund
E, Tjonneland A, Olsen A, Overvad K, Clavel-Chapelon F, Fournier A,
et al: Reproductive risk factors and endometrial cancer: The
European prospective investigation into cancer and nutrition. Int J
Cancer. 127:442–451. 2010.PubMed/NCBI
|
|
9
|
Zhu Y, Shen J, Gao L and Feng Y: Estrogen
promotes fat mass and obesity-associated protein nuclear
localization and enhances endometrial cancer cell proliferation via
the mTOR signaling pathway. Oncol Rep. 35:2391–2397. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roslind A and Johansen JS: YKL-40: A novel
marker shared by chronic inflammation and oncogenic transformation.
Methods Mol Biol. 511:159–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steponaitis G, Skiriutė D, Kazlauskas A,
Golubickaitė I, Stakaitis R, Tamašauskas A and Vaitkienė P: High
CHI3L1 expression is associated with glioma patient survival. Diagn
Pathol. 11:422016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thongsom S, Chaocharoen W, Silsirivanit A,
Wongkham S, Sripa B, Choe H, Suginta W and Talabnin C:
YKL-40/chitinase-3-like protein 1 is associated with poor prognosis
and promotes cell growth and migration of cholangiocarcinoma.
Tumour Biol. 37:9451–9463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Johansen JS, Christensen IJ, Jørgensen LN,
Olsen J, Rahr HB, Nielsen KT, Laurberg S, Brünner N and Nielsen HJ:
Serum YKL-40 in risk assessment for colorectal cancer: A
prospective study of 4,496 subjects at risk of colorectal cancer.
Cancer Epidemiol Biomarkers Prev. 24:621–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang XW, Cai CL, Xu JM, Jin H and Xu ZY:
Increased expression of chitinase 3-like 1 is a prognosis marker
for non-small cell lung cancer correlated with tumor angiogenesis.
Tumour Biol. 36:901–907. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vom Dorp F, Tschirdewahn S, Niedworok C,
Reis H, Krause H, Kempkensteffen C, Busch J, Kramer G, Shariat SF,
Nyirady P, et al: Circulating and tissue expression levels of
YKL-40 in renal cell cancer. J Urol. 195:1120–1125. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thorn AP, Daugaard S, Christensen LH,
Christensen IJ and Petersen MM: YKL-40 protein in osteosarcoma
tumor tissue. APMIS. 124:453–461. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lai T, Chen M, Deng Z, L Y, Wu D, Li D and
Wu B: YKL-40 is correlated with FEV1 and the asthma control test
(ACT) in asthmatic patients: Influence of treatment. BMC Pulm Med.
15:12015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gudmundsdottir S, Lieder R, Sigurjonsson
OE and Petersen PH: Chitosan leads to downregulation of YKL-40 and
inflammasome activation in human macrophages. J Biomed Mater Res A.
103:2778–2785. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zou L, He X and Zhang JW: The efficacy of
YKL-40 and CA125 as biomarkers for epithelial ovarian cancer. Braz
J Med Biol Res. 43:1232–1238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan JT, Li MJ, Shen P, Xu H, Li DH and Yan
HQ: Serum and tissue level of YKL-40 in endometrial cancer. Eur J
Gynaecol Oncol. 35:304–308. 2014.PubMed/NCBI
|
|
21
|
Kemik P, Saatli B, Yıldırım N, Kemik VD,
Deveci B, Terek MC, Koçtürk S, Koyuncuoğlu M and Saygılı U:
Diagnostic and prognostic values of preoperative serum levels of
YKL-40, HE-4 and DKK-3 in endometrial cancer. Gynecol Oncol.
140:64–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang W, Kawanishi M, Miyake K, Kagawa M,
Kawai N, Murao K, Nishiyama A, Fei Z, Zhang X and Tamiya T:
Association between YKL-40 and adult primary astrocytoma. Cancer.
116:2688–2697. 2010.PubMed/NCBI
|
|
23
|
Kawada M, Seno H, Kanda K, Nakanishi Y,
Akitake R, Komekado H, Kawada K, Sakai Y, Mizoguchi E and Chiba T:
Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis
in colorectal cancer. Oncogene. 31:3111–3123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen CC, Pekow J, Llado V, Kanneganti M,
Lau CW, Mizoguchi A, Mino-Kenudson M, Bissonnette M and Mizoguchi
E: Chitinase 3-like-1 expression in colonic epithelial cells as a
potentially novel marker for colitis-associated neoplasia. Am J
Pathol. 179:1494–1503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Akiyama Y, Ashizawa T, Komiyama M, Miyata
H, Oshita C, Omiya M, Iizuka A, Kume A, Sugino T, Hayashi N, et al:
YKL-40 downregulation is a key factor to overcome temozolomide
resistance in a glioblastoma cell line. Oncol Rep. 32:159–166.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Linde ME, van der Mijn JC, Pham TV,
Knol JC, Wedekind LE, Hovinga KE, Aliaga ES, Buter J, Jimenez CR,
Reijneveld JC and Verheul HM: Evaluation of potential circulating
biomarkers for prediction of response to chemoradiation in patients
with glioblastoma. J Neurooncol. 129:221–230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boisen MK, Madsen CV, Dehlendorff C,
Jakobsen A, Johansen JS and Steffensen KD: The prognostic value of
plasma YKL-40 in patients with chemotherapy-resistant ovarian
cancer treated with bevacizumab. Int J Gynecol Cancer.
26:1390–1398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ku BM, Lee YK, Ryu J, Jeong JY, Choi J,
Eun KM, Shin HY, Kim DG, Hwang EM, Yoo JC, et al: CHI3L1 (YKL-40)
is expressed in human gliomas and regulates the invasion, growth
and survival of glioma cells. Int J Cancer. 128:1316–1326. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jefri M, Huang YN, Huang WC, Tai CS and
Chen WL: YKL-40 regulated epithelial-mesenchymal transition and
migration/invasion enhancement in non-small cell lung cancer. BMC
Cancer. 15:5902015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mylin AK, Abildgaard N, Johansen JS,
Heickendorff L, Kreiner S, Waage A, Turesson I and Gimsing P:
Nordic Myeloma Study Group; Serum YKL-40: A new independent
prognostic marker for skeletal complications in patients with
multiple myeloma. Leuk Lymphoma. 56:2650–2659. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jeet V, Tevz G, Lehman M, Hollier B and
Nelson C: Elevated YKL40 is associated with advanced prostate
cancer (PCa) and positively regulates invasion and migration of PCa
cells. Endocr Relat Cancer. 21:723–737. 2014. View Article : Google Scholar : PubMed/NCBI
|