Introduction
Though considered rare, anal cancer incidence has
increased in the last 20 years in the USA (1), with an estimated 8,080 new cases and
1,080 associated mortalities in 2016 (2). The most frequent histological type is
squamous cell carcinoma (SCC), responsible for ~95% of anal tumors
(3). The risk factors for anal cancer
include human papillomavirus (HPV) infection, immunodeficiency,
immunosuppression and tobacco smoking; however being
HPV+ has been demonstrated to be the most important
factor (4,5). Among the distinct HPV types, HPV16 is
the most frequently identified in anal SCC (6) and may cause infected cells to progress
from intraepithelial neoplasia to high-grade dysplasia, and finally
to invasive cancer (5,7). Additionally, an increased prevalence of
infection with high-risk HPV (HR-HPV) has been identified in the
anal canals of women with HPV-associated pathologies, including
cancer of the vulva, vagina and cervix, compared with in women
without these HPV-associated pathologies (8).
It has been revealed that HR-HPV infections,
particularly with concomitantly elevated expression of the viral
gene products E6 and E7, are involved in cell cycle entry and cell
proliferation (9,10). E7 protein binds retinoblastoma
protein, inducing its degradation and aberrant cell cycle
progression by upregulating p21 and p16 (11). E6 protein is involved in several
oncogenic events, leading to p53 degradation, nuclear factor
(NF)-κB pathway activation under hypoxic conditions, and the
upregulation of human telomerase reverse transcriptase, which
promotes cell immortalization (11).
A previous study identified NF-κB as an independent predictor of
disease-free survival (DFS) in patients treated with chemotherapy
(12).
Raf kinase inhibitor protein (RKIP) is described as
an NF-κB suppressor (13), an
inhibitor of the Raf/mitogen-activated protein kinase/extracellular
signal-regulated kinase (ERK) kinase (MEK)/ERK signaling pathway
(14), and a regulator of G
protein-coupled receptors (15). RKIP
is expressed in several normal human tissues (16), and previous studies have demonstrated
the prognostic value of the loss of RKIP expression in various
gastrointestinal tumors, including colorectal cancer,
gastrointestinal stromal tumors and gastric cancer (17–19). To
date, only a limited number of molecular biomarkers have been
identified that are able to predict treatment outcomes or response
in anal cancer, including HPV infection, p16 protein expression
(20,21), and the overexpression of multidrug
resistance-associated protein 1, excision repair
cross-complementation group 1 and thymidylate synthase (22). KRAS proto-oncogene (KRAS)
mutations, which are predictors of patient outcomes (23) in colorectal tumors, were identified at
a low frequency in tumors of the anal canal (24). Therefore, the aim of the present study
was to evaluate the clinical implications of RKIP expression using
immunohistochemistry in a series of invasive lesions (SCCs and
adenocarcinomas) and in situ lesions [high-grade squamous
intra-epithelial lesions (HSILs)] of the anal canal.
Materials and methods
Patients and samples
The resected tumors of 48 patients with anal cancer
(27 female, 21 male), diagnosed at Barretos Cancer Hospital
(Barretos, SP, Brazil) between June 2000 and August 2010, were
evaluated and the clinical data are summarized in Table I. The mean age of the patients was
55.9 years (range, 27–86 years). KRAS and BRAF
mutation status data were retrieved from our previous study
(24), while HPV16 and HPV18 status,
and β-globin, p16, Ki-67, minichromosome maintenance protein
complex (MCM) and topoisomerase II α (TOP2A) expression
immunohistochemical analyses were retrieved from another previous
study (25) and are summarized in
Table II. All patients included in
the present study provided written informed consent, and the study
was approved by the Barretos Cancer Hospital Ethical Committee
(Barretos, SP, Brazil) approval under the protocol number 310/2010.
The data of all patients alive by December 2012 were censored.
 | Table I.Clinicopathological characteristics of
the patients and association with RKIP expression. |
Table I.
Clinicopathological characteristics of
the patients and association with RKIP expression.
| Parameter | n | Low RKIP, n
(%) | High RKIP, n
(%) | P-value |
|---|
| Sex |
|
|
| 0.999 |
|
Female | 27 | 24 (88.9) | 3 (11.1) |
|
|
Male | 21 | 18 (85.7) | 3 (14.3) |
|
| Ethnicity |
|
|
| 0.019a |
|
Caucasian | 40 | 37 (92.5) | 3 (7.5) |
|
|
Non-caucasian | 8 | 5 (62.5) | 3 (37.5) |
|
| Age, years |
|
|
| 0.047a |
|
≤46 | 12 | 8 (66.7) | 4 (33.3) |
|
|
47–66 | 24 | 22 (91.7) | 2 (8.3) |
|
|
≥67 | 12 | 12 (100) | 0 (0.0) |
|
| Tobacco
consumption |
|
|
| 0.665 |
| No | 23 | 19 (82.6) | 4 (17.4) |
|
| Yes
(current or past) | 22 | 20 (90.9) | 2 (9.1) |
|
| Clinical grade |
|
|
| 0.038a |
| Stage
0 | 6 | 3 (50) | 3 (50) |
|
| Stages
I and II | 24 | 22 (91.7) | 2 (8.3) |
|
| Stages
III and IV | 15 | 14 (93.3) | 1 (6.7) |
|
| Histological
type |
|
|
| 0.037a |
|
SCC | 26 | 23 (88.5) | 3 (11.5) |
|
|
Adenocarcinoma | 14 | 14 (100) | 0 |
|
|
HSIL | 8 | 5 (62.5) | 3 (37.5) |
|
| Clinical
response |
|
|
| 0.847 |
| No
evidence | 24 | 20 (83.3) | 4 (16.7) |
|
|
Complete | 12 | 11 (91.7) | 1 (8.3) |
|
|
Progression | 7 | 6 (85.7) | 1 (14.3) |
|
| Status |
|
|
| 0.743 |
|
Deceased (by cancer) | 13 | 12 (92.3) | 1 (7.7) |
|
|
Deceased (not by cancer) | 4 | 4 (100.0) | 0 (0.0) |
|
| Alive
without disease | 26 | 21 (80.8) | 5 (19.2) |
|
| Alive
with disease | 5 | 5 (100.0) | 0 (0.0) |
|
 | Table II.Pathological characteristics of the
patients and association with RKIP expression. |
Table II.
Pathological characteristics of the
patients and association with RKIP expression.
| Parameter | n | Low RKIP labeling,
n (%) | High RKIP labeling,
n (%) | P-value |
|---|
| HPV16 |
|
|
| 0.013b |
|
Negative | 10 | 6
(60.0) | 4
(40.0) |
|
|
Positive | 38 | 36 (94.7) | 2 (5.3) |
|
|
β-globina |
|
|
| 0.999 |
|
Weak | 3 | 3
(100.0) | 0 (0.0) |
|
|
Strong | 45 | 39 (86.7) | 6
(13.3) |
|
| p16a |
|
|
| 0.999 |
|
Negative | 13 | 11 (84.6) | 2 (15.4) |
|
|
Positive | 34 | 30 (88.2) | 4 (11.8) |
|
| NA | 1 | NA | NA |
|
| Ki-67a |
|
|
| 0.637 |
|
1+2+3+ | 12 | 10 (83.3) | 2 (16.7) |
|
| 4+ | 35 | 31 (88.6) | 4 (11.4) |
|
| NA | 1 | NA | NA |
|
| MCMa |
|
|
| 0.999 |
|
Negative | 9 | 8
(88.9) | 1 (11.1) |
|
|
Positive | 39 | 34 (87.2) | 5 (12.8) |
|
| TOP2Aa |
|
|
| 0.329 |
|
1+2+ | 32 | 27 (90.0) | 3 (10.0) |
|
| 3+ | 12 | 9 (75.0) | 3 (25.0) |
|
| NA | 4 | NA | NA |
|
Samples, fixed for 24 h at room temperature in 10%
buffered formalin and embedded in paraffin blocks, were retrieved
from the Department of Pathology of Barretos Cancer Hospital.
Subsequently, 5-µm-thick sections were placed on histological
slides, and hematoxylin and eosin staining (1 min in hematoxylin
and 30 sec in eosin at room temperature) was performed to confirm
the initial diagnosis using a light microscope (magnification,
×400).
Immunohistochemistry
Sections (5-µm thick) were subjected to
immunohistochemical staining according to the streptavidin-biotin
peroxidase method using an UltraVision Large Volume Detection
system Anti-Polyvalent, HRP kit (cat. no. TP-125-HL; LabVision;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Briefly, slides
were xylene-deparaffinized and rehydrated in a descending alcohol
series (100, 90, 70 and 50%, followed by water), prior to
antigen-retrieval for 20 min at 98°C in 10 mM citrate buffer
(pH=6.0; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Endogenous
peroxidase blocking was performed by incubation with hydrogen
peroxide (3% in methanol v/v; Sigma-Aldrich; Merck KGaA) for 10 min
at room temperature. Protein blocking was performed for 5 min with
UV Block Plus (LabVision; Thermo Fisher Scientific, Inc.).
Subsequently, the slides were incubated with a polyclonal primary
anti-RKIP antibody (dilution, 1:1,000; cat. no. 07–137; Upstate
Biotechnology, Inc., Lake Placid, NY, USA) for 60 min at room
temperature, followed by a secondary biotinylated goat
anti-polyvalent antibody (part of the aforementioned kit) for 5 min
at room temperature, after which they were incubated with the
streptavidin-peroxidase complex for 5 min (LabVision; Thermo Fisher
Scientific, Inc.) at room temperature. Chromogen color development
was accomplished with 3,3′-diaminobenzidine (5 min at room
temperature) and a Gill-2 hematoxylin counterstain (30 sec at room
temperature).
The slides were scored in a blinded manner by one
pathologist and one histologist using semi-quantitative criteria,
as previously described (18,26) using a light microscope (magnification,
×400). The labeling score was determined as the sum of the
percentage of positive cells (0, negative; 1, <5% immunoreactive
cells; 2, 5–50% immunoreactive cells; 3, >51% immunoreactive
cells) and the labeling intensity (0, negative; 1, weak; 2,
moderate; 3, strong) in the tumor tissue. RKIP expression was
classified as low (scores between 0 and 4) or high (scores 5 and
6). This scoring system was used to compare strongly positive RKIP
samples vs. samples with weak/moderate expression. Normal stomach
sections expressing RKIP were used as a positive control (19).
Statistical analysis
Analysis of descriptive characteristics was
performed to characterize the study population using SPSS
statistical software (version 21; IBM Corp., Armonk, NY, USA).
Comparisons between groups were performed using Fisher's exact
tests and the Kaplan-Meier method was applied to assess survival
rates, using a log-rank test to compare the curves. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics and survival
analysis
Follow-up was performed every 6 months or as
necessary (until the point of mortality), with a mean follow-up
time of 57.2 months (range, 2.1–158.4 months). From the 48 cases, 8
were diagnosed with HSIL, 14 were diagnosed with adenocarcinomas
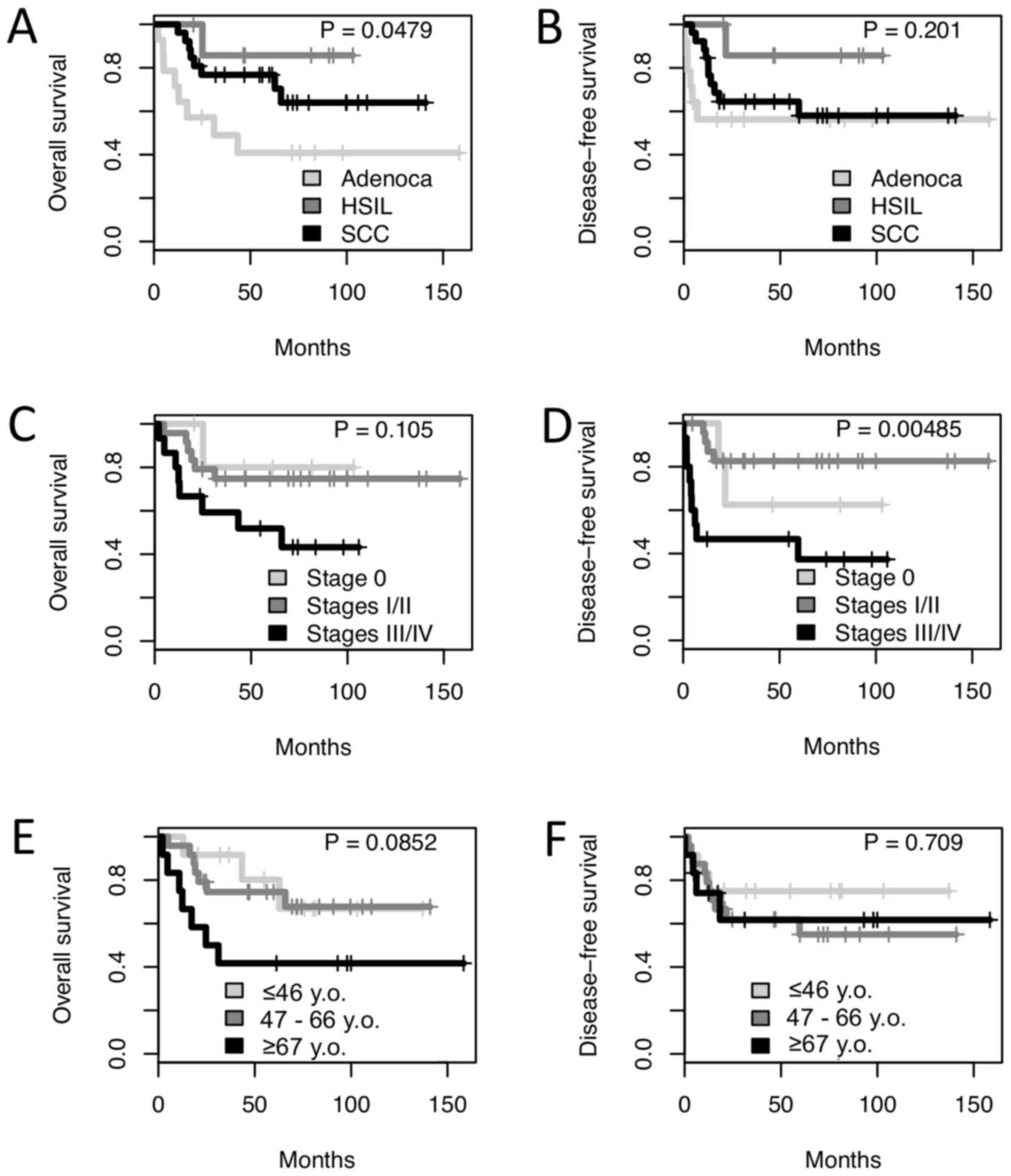
and 26 were diagnosed with SCC. Log-rank analysis revealed a lower
5-year survival rate for patients with adenocarcinoma (40.8%),
compared with for those with SCC and HSIL (76.7 and 80%,
respectively; P=0.0479; Fig. 1A). No
significant difference was observed in the disease-free survival
rates between the three tumor types (P=0.201; Fig. 1B). There was no significant difference
in the overall survival of patients based on clinical stage
(Fig. 1C), but clinical stages III/IV
were associated with a poorer disease-free survival rate (a 5-year
disease-free survival rate of 37.3% vs. 62.5 and 82.6% for clinical
stages 0 and I/II, respectively; P<0.01; Fig. 1D). Additionally, older patients (≥67
years old) were revealed to experience a poorer 5-year survival
rate [41.7 vs. 80.2 and 74.5% for young-(≤46 years old) and
medium-age patients (47–66 years old), respectively; P=0.085,
Fig. 1E], although there was no
significant difference in disease-free survival (Fig. 1F).
Immunohistochemistry findings
Table I presents the
clinicopathological features of the patients as associated with
RKIP expression. High RKIP expression was associated with
non-Caucasian patients (37.5 vs. 7.5% in Caucasians; P=0.019) and
younger (≤46 years old) patients (33.3 vs. 0.0% of older patients;
P=0.047). Additionally, high RKIP expression was associated with
lower tumor clinical stages (50% in stage 0 vs. 6.7% in stages
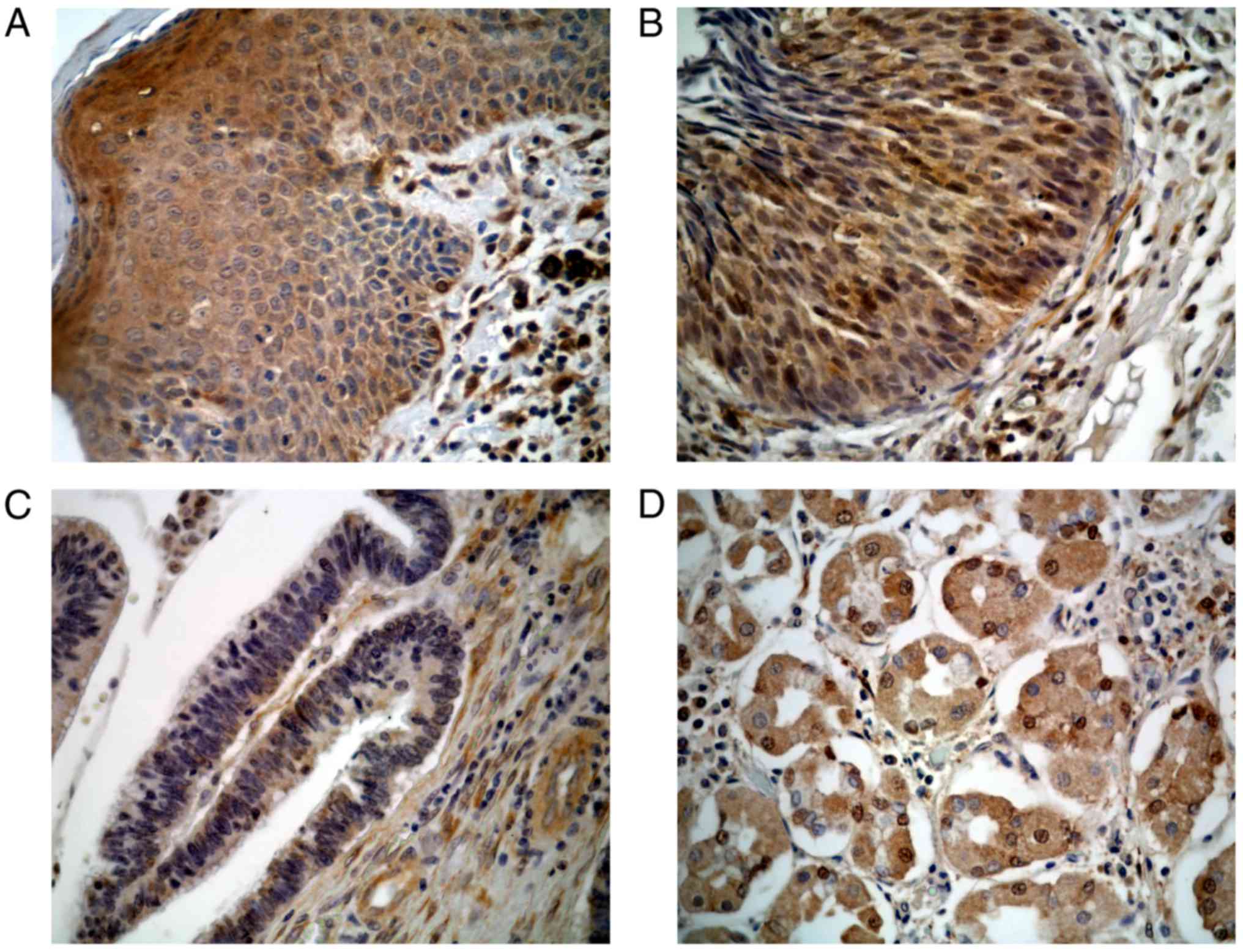
III/IV; P=0.038). Finally, anal HSILs (37.5%) exhibited the
greatest percentage of high RKIP expression, followed by anal SCCs
(11.5%) and adenocarcinomas (0.0%) (P=0.037; Table I, Fig.
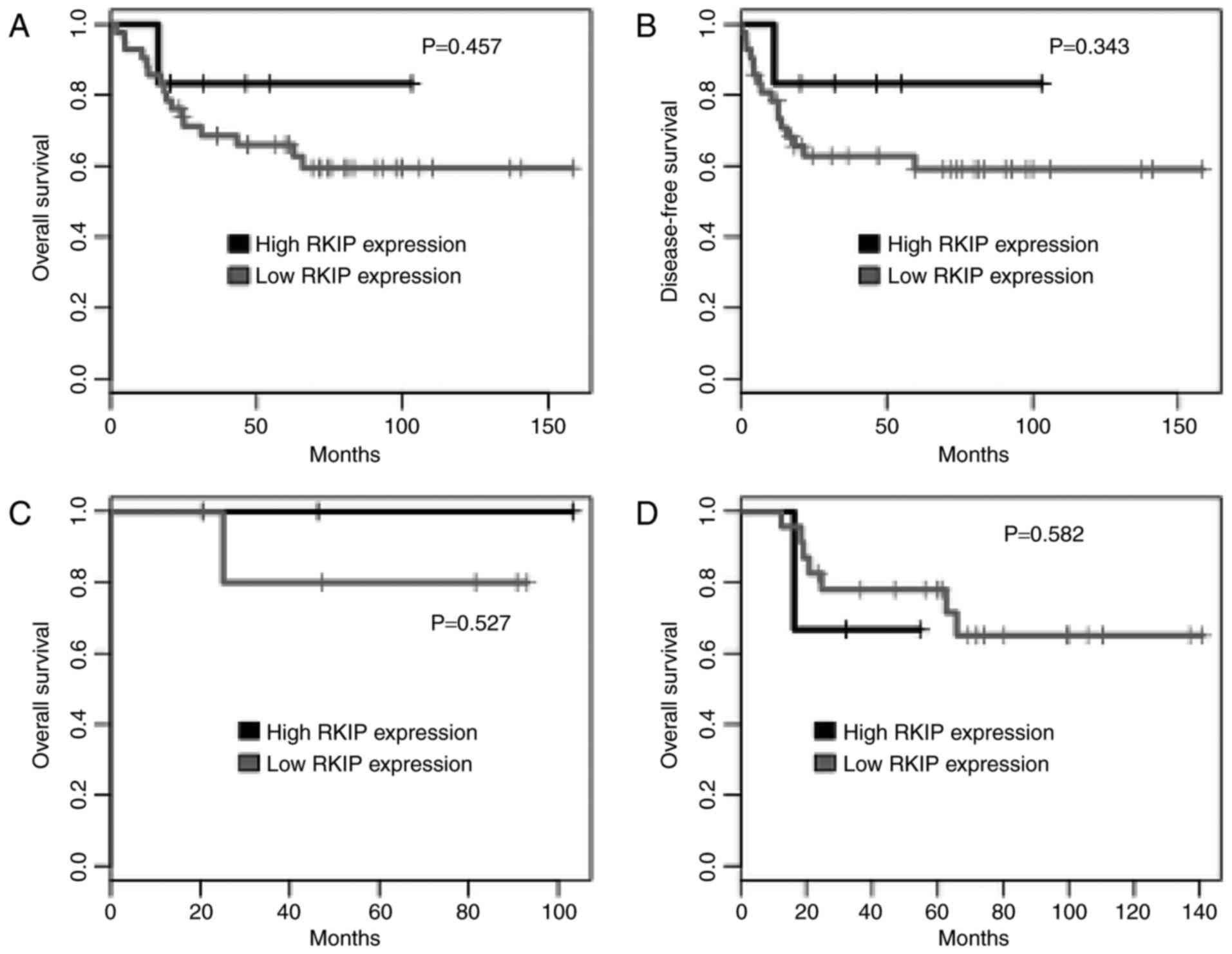
2). There was no significant difference in overall- or
disease-free survival between RKIP-high and RKIP-low expressing
patients (Fig. 3A and B). Similarly,
following the stratification of patients by tumor type (HSIL and
SCC), there was no significant difference in survival based on RKIP
expression (Fig. 3C and D). All the
patients with adenocarcinoma demonstrated low tumor RKIP
expression.
RKIP expression was correlated with HPV16 infection,
KRAS and BRAF mutation status, and the expression of
β-globin, p16, Ki-67, MCM and TOP2A, as detected by
immunohistochemistry. There were no positive cases of HPV18. All 30
cases that were analyzed for KRAS mutation were wild type,
and only 1 case of the 35 analyzed was positive for a BRAF
V600E mutation. A decreased percentage of HPV16+ cases
were identified with high expression of RKIP, compared with the
HPV16− cases (5.3 vs. 40%; P=0.013; Table II), indicating a possible correlation
between HPV16 infection and RKIP expression.
Discussion
A number of previous studies have demonstrated that
RKIP expression has prognostic value in several tumor types,
including gastric, colorectal and gastrointestinal stromal tumors
(17–19). However, other studies have identified
a number of molecular distinctions between anal canal tumors and
their colorectal counterparts (24,27).
Therefore, the present study aimed to evaluate RKIP expression in
invasive and in situ tumors of the anal canal, and to
correlate findings with the clinicopathological data.
The results of the present study revealed a poorer
overall survival rate in patients diagnosed with adenocarcinoma,
compared with in those diagnosed with SCC or HSIL. An improved
prognosis was expected in patients with HSIL, considering that
these in situ lesions are associated with <30% recurrence
following treatment (5,28). Additionally, the results of the
present study demonstrated an improved survival rate in patients
with SCC, compared with in patients with adenocarcinoma. This
supports the findings of a previous study that compared the overall
survival and prognosis of patients with anal adenocarcinoma, SCC
and rectal adenocarcinoma, in which the authors demonstrated a
poorer overall survival rate and prognosis for patients with anal
adenocarcinoma (28). This same trend
was described by our group in a previous study (24). Furthermore, when considering patient
age, the present study identified poorer survival rates in patients
≥67 years old compared with in younger patients (≤47 years old),
supporting the results presented by a prior study that revealed
younger patients experienced improved overall- and disease-free
survival rates compared with older patients with anal cancer
(29). In addition, the present study
did not identify high RKIP expression in the tumors of any older
patients.
RKIP is considered a tumor suppressor gene, and its
expression may prevent RAF/MEK/ERK signal transduction (30), thus serving a function in the
inhibition of cancer development. The present study identified an
increased percentage of high RKIP expression in patients with in
situ lesions (HSIL) than in patients with invasive lesions (SCC
and adenocarcinoma). Notably, an increased percentage of patients
at clinical stage 0 exhibited high RKIP expression, compared with
patients with higher clinical stages (I/II or III/IV). Considering
the patient stratification was dependent on RKIP expression, the
lack of receiver operating characteristic analysis may be a
limitation of the present study; however, an RKIP scoring system
was used according to our previously published studies (26), a method that is widely used for the
assessment of immunohistochemistry staining (31), and allowed for inter-study
comparisons.
Infection with high-risk HPV (HR-HPV16 and HR-HPV18)
is considered the major risk factor for anal cancer development
(4). Additionally, a previous study
demonstrated that HPV−/p16− patients
experienced poorer outcomes compared with
HPV+/p16+ patients (20). Another study revealed the relevance of
p16 expression, reporting an association with improved overall- and
disease-free survival rates (6);
however, the results of the present study did not identify any
correlation between the expression of RKIP and of p16, which may
explain the lack of correlation between RKIP expression and
survival. In the cohort of the present study, a decreased number
proportion of HPV16+ patients exhibited high RKIP
protein expression compared with HPV16− patients. Hu
et al (32) analyzed RKIP
expression via immunohistochemical analysis in normal and cancerous
cervical tissues and revealed high RKIP expression in normal
tissue, low expression in primary cancer and the lowest (or absent)
levels of expression in metastatic disease. Considering that HR-HPV
infection is a fundamental step for cervical cancer development
(9,10), there may be an inverse association
between HR-HPV infection and RKIP expression, as was observed in
the present study cohort. However, more studies are required in
order to elucidate the molecular basis of this finding.
In conclusion, to the best of our knowledge, this is
the first study to investigate RKIP expression levels in a set of
anal tumors. The results of the present study demonstrated that
high RKIP expression is present in lesions with clinical features
that are generally associated with a good prognosis, including
lower age at diagnosis, in situ lesions and lesions of a
lower clinical grade, and that HPV16 infection may affect RKIP
expression; a finding that requires further investigation.
Acknowledgements
The present study was supported by the São Paulo
Research Foundation (grant nos. 2010/16795-4 and 2011/08523-7) and
Ministry of Science, Technology, Innovation and Communication
(grant no. MCT/FINEP/CT-INFRA-PROINFRA 01/2011).
References
|
1
|
Nelson RA, Levine AM, Bernstein L, Smith
DD and Lai LL: Changing patterns of anal canal carcinoma in the
United States. J Clin Oncol. 31:1569–1575. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deans GT, McAleer JJ and Spence RA:
Malignant anal tumours. Br J Surg. 81:500–508. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bosman FT: World Health Organization and
International Agency for Research on Cancer: WHO classification of
tumours of the digestive system. IARC Press; Lyon; 2010
|
|
5
|
Leonard D, Beddy D and Dozois EJ:
Neoplasms of anal canal and perianal skin. Clin Colon Rectal Surg.
24:54–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Serup-Hansen E, Linnemann D,
Skovrider-Ruminski W, Høgdall E, Geertsen PF and Havsteen H: Human
papillomavirus genotyping and p16 expression as prognostic factors
for patients with American joint committee on cancer stages I to
III carcinoma of the anal canal. J Clin Oncol. 32:1812–1817. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burgos J, Curran A, Tallada N, Guelar A,
Navarro J, Landolfi S, Villar J, Crespo M, Ribera E and Falcó V:
Risk of progression to high-grade anal intraepithelial neoplasia in
HIV-infected MSM. AIDS. 29:695–702. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stier EA, Sebring MC, Mendez AE, Ba FS,
Trimble DD and Chiao EY: Prevalence of anal human papillomavirus
infection and anal HPV-related disorders in women: A systematic
review. Am J Obstet Gynecol. 213:278–309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doorbar J: Molecular biology of human
papillomavirus infection and cervical cancer. Clin Sci (Lond).
110:525–541. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Doorbar J: Model systems of human
papillomavirus-associated disease. J Pathol. 238:166–179. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ajiro M and Zheng ZM: Oncogenes and RNA
splicing of human tumor viruses. Emerg Microbes Infect. 3:e632014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ajani JA, Wang X, Izzo JG, Crane CH, Eng
C, Skibber JM, Das P and Rashid A: Molecular biomarkers correlate
with disease-free survival in patients with anal canal carcinoma
treated with chemoradiation. Dig Dis Sci. 55:1098–1105. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yeung KC, Rose DW, Dhillon AS, Yaros D,
Gustafsson M, Chatterjee D, McFerran B, Wyche J, Kolch W and Sedivy
JM: Raf kinase inhibitor protein interacts with NF-kappaB-inducing
kinase and TAK1 and inhibits NF-kappaB activation. Mol Cell Biol.
21:7207–7217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yeung K, Seitz T, Li S, Janosch P,
McFerran B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H, et
al: Suppression of Raf-1 kinase activity and MAP kinase signalling
by RKIP. Nature. 401:173–177. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lorenz K, Lohse MJ and Quitterer U:
Protein kinase C switches the Raf kinase inhibitor from Raf-1 to
GRK-2. Nature. 426:574–579. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Odabaei G, Chatterjee D, Jazirehi AR,
Goodglick L, Yeung K and Bonavida B: Raf-1 kinase inhibitor
protein: Structure, function, regulation of cell signaling, and
pivotal role in apoptosis. Adv Cancer Res. 91:169–200. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Wang LY, Feng F, Zhao Y, Huang MY,
Shao Q, Chen C, Sheng H, Chen DL, Zeng ZL, et al: Effect of Raf
kinase inhibitor protein expression on malignant biological
behavior and progression of colorectal cancer. Oncol Rep.
34:2106–2114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martinho O, Gouveia A, Silva P, Pimenta A,
Reis RM and Lopes JM: Loss of RKIP expression is associated with
poor survival in GISTs. Virchows Arch. 455:277–284. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martinho O, Simões K, Longatto-Filho A,
Jacob CE, Zilberstein B, Bresciani C, Gama-Rodrigues J, Cecconello
I, Alves V and Reis RM: Absence of RKIP expression is an
independent prognostic biomarker for gastric cancer patients. Oncol
Rep. 29:690–696. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mai S, Welzel G, Ottstadt M, Lohr F,
Severa S, Prigge ES, Wentzensen N, Trunk MJ, Wenz F, von
Knebel-Doeberitz M and Reuschenbach M: Prognostic relevance of HPV
infection and p16 overexpression in squamous cell anal cancer. Int
J Radiat Oncol Biol Phys. 93:819–827. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meulendijks D, Tomasoa NB, Dewit L, Smits
PH, Bakker R, van Velthuysen ML, Rosenberg EH, Beijnen JH,
Schellens JH and Cats A: HPV-negative squamous cell carcinoma of
the anal canal is unresponsive to standard treatment and frequently
carries disruptive mutations in TP53. Br J Cancer. 112:1358–1366.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smaglo BG, Tesfaye A, Halfdanarson TR,
Meyer JE, Wang J, Gatalica Z, Reddy S, Arguello D and Boland PM:
Comprehensive multiplatform biomarker analysis of 199 anal squamous
cell carcinomas. Oncotarget. 6:43594–43604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Roock W, Claes B, Bernasconi D, de
Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V,
Papamichael D, Laurent-Puig P, et al: Effects of KRAS, BRAF, NRAS,
and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy
in chemotherapy-refractory metastatic colorectal cancer: A
retrospective consortium analysis. Lancet Oncol. 11:753–762. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bidinotto LT, Véo CA, Loaiza EA, de França
AP, Lorenzi AT, Rosa LA, de Oliveira CM, Levi JE, Scapulatempo-Neto
C, Longatto-Filho A and Reis RM: Low mutation percentage of KRAS
and BRAF genes in Brazilian anal tumors. Mol Med Rep. 14:3791–3797.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scapulatempo-Neto C, Veo C, Fregnani JHTG,
Lorenzi A, Mafra A, Melani AGF, Loaiza EAA, Rosa LAR, de Oliveira
CM, Levi JE and Longatto-Filho A: Characterization of topoisomerase
II α and minichromosome maintenance protein 2 expression in anal
carcinoma. Oncol Lett. 13:1891–1898. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martinho O, Campos M, Ribeiro G, Penna V,
Curcelli EC, Olivieri MV, Morini S, Scapulatempo C, Abrahão-Machado
LF and Reis RM: Raf kinase inhibitor protein expression and
prognostic value in soft tissue sarcomas. Pathobiology. 83:41–46.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matalon SA, Mamon HJ, Fuchs CS, Doyle LA,
Tirumani SH, Ramaiya NH and Rosenthal MH: Anorectal cancer:
Critical anatomic and staging distinctions that affect use of
radiation therapy. Radiographics. 35:2090–2107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Franklin RA, Giri S, Valasareddy P, Lands
LT and Martin MG: Comparative survival of patients with anal
adenocarcinoma, squamous cell carcinoma of the anus, and rectal
adenocarcinoma. Clin Colorectal Cancer. 15:47–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bilimoria KY, Bentrem DJ, Rock CE, Stewart
AK, Ko CY and Halverson A: Outcomes and prognostic factors for
squamous-cell carcinoma of the anal canal: Analysis of patients
from the national cancer data base. Dis Colon Rectum. 52:624–631.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vandamme D, Herrero A, Al-Mulla F and
Kolch W: Regulation of the MAPK pathway by raf kinase inhibitory
protein. Crit Rev Oncog. 19:405–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fedchenko N and Reifenrath J: Different
approaches for interpretation and reporting of immunohistochemistry
analysis results in the bone tissue-a review. Diagn Pathol.
9:2212014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu CJ, Zhou L, Zhang J, Huang C and Zhang
GM: Immunohistochemical detection of Raf kinase inhibitor protein
in normal cervical tissue and cervical cancer tissue. J Int Med
Res. 39:229–237. 2011. View Article : Google Scholar : PubMed/NCBI
|