Introduction
Prostate cancer is one of the most common types of
cancer in men and the mortality rate was 9.37/100,000 in 2012,
which has increased yearly in China (1). An increasing amount of evidence has
indicated that age, ethnicity and family history of prostate cancer
are factors associated with morbidity of prostate cancer (2). The occurrence and development of
prostate cancer may be the result of the interaction between
genetic predisposition and environmental factors, while genetic
variation determines the susceptibility of individuals to suffer
from prostate cancer (3). At present,
the involvement of whole-genome analysis of different ethnic groups
worldwide has revealed that there are >30 susceptible sites on
the genome associated with the risks of prostate cancer occurrence
(4). The study of a genetic
predisposition to prostate cancer, has demonstrated notable results
(4,5).
The apoptosis signaling pathway is stimulated by
exogenous and endogenous stresses (6). These specific stresses are: Unfolded
protein accumulation in the endoplasmic reticulum; disordered
ingestion and release of endoplasmic reticulum calcium; and
incorrect processing of specific proteins, either accompanied or
unaccompanied, in the endoplasmic reticulum (6). Sustained endoplasmic reticulum stress
results in apoptosis (7). Caspase-3
is a member of the caspase protein family, and is located in the
endoplasmic reticulum (8). The
specificity of caspase-3 may be activated by endoplasmic reticulum
stress.
Cofilin is a type of actin binding protein in
eukaryons with a low molecular weight (9). The cofilin-1 gene is positioned
at chromosome 11q13 and is expressed in non-muscular tissues
(9). F-actin regulates the
reconstruction of the actin framework and moves cells forward by
reconstructing the schistose pseudopodia and lamellar structures of
the front histiocyte (10). Highly
activated cofilin-1 has been demonstrated in glioma, Lymphocytoma
cutis, colon cancer, hepatoma carcinoma, renal carcinoma,
esophageal squamous cancer and prostate cancer cells (11).
Paxillin is a phosphoric acid protein, with a
molecular mass of 68×103, and its main role is in the
process of focal adhesion (12). It
functions to combine vinculin and actin (12). The human paxillin gene is positioned
at chromosome 12q24, and there are 11 expressed regions (13). The paxillin molecule contains multiple
structural domains and a combination of a series of signal proteins
and structural proteins to mediate cell signaling transduction
(13). It serves an important role in
cell adhesion and transport processes, and has a close association
with localized cancer cell movement (13).
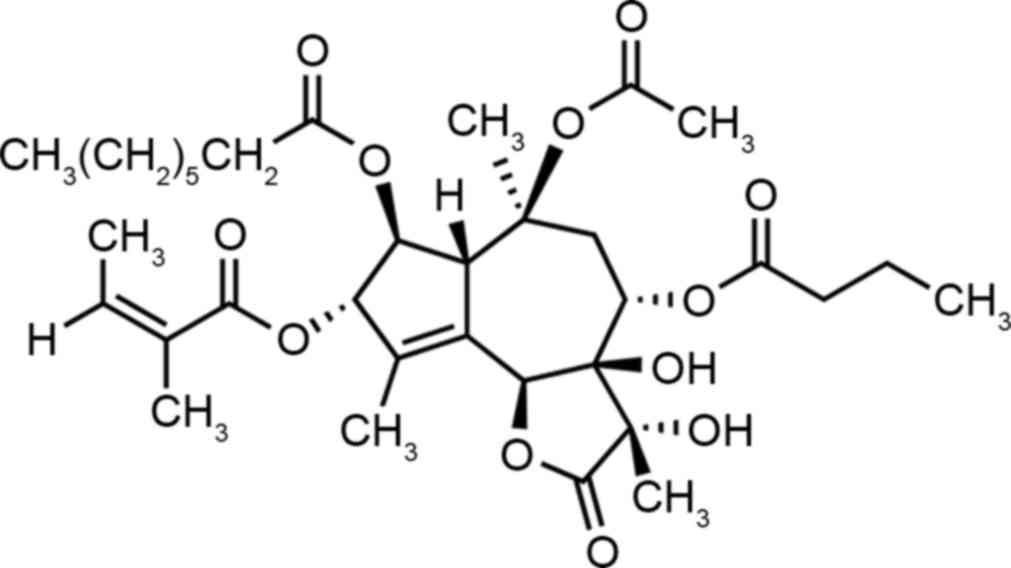
It is widely hypothesized that thapsigargin
(Fig. 1) induces endoplasmic
reticulum stress, and may induce multiple cells to undergo
endoplasmic reticulum stress and apoptosis (14). It has been demonstrated that
thapsigargin may inhibit A549 cell growth, the source of which type
II alveolar epithelial cells, and induce apoptosis (14). Concurrently, thapsigargin may induce
leukemic K562 cells to undergo apoptosis in response to endoplasmic
reticulum stress (15). The action of
thapsigargin is to suppress activity of the endoplasmic reticulum
membrane Ca2+-adenosine 5′-triphosphate enzyme, induce
the increase in concentration of intracytoplasmic Ca2
and reduce the level of stored calcium in the endoplasmic
reticulum, so as to cause endoplasmic reticulum stress and induce
apoptosis (16). The present study
explored whether thapsigargin induced apoptosis in prostate cancer
cells, and explored its possible mechanism.
Materials and methods
Cell culture
The human prostate cancer PC3 cell line was
purchased from the Shanghai Cell Bank of The Chinese Academy of
Sciences (Shanghai, China) and maintained in RPMI-1640 media
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) containing
10% fetal bovine serum (Hyclone; GE Healthcare Life Sciences) and
100 µg/ml penicillin-streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C and 5%
CO2.
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8, Beyotime Institute
of Biotechnology, Haimen, China) was selected to determine the
effect of thapsigargin (0, 1, 10 and 100 nM) on PC3 cell
proliferation. The PC3 cells were seeded in 96-well plates at a
density of 1×104 cells/well and incubated with
thapsigargin (0, 1, 10 and 100 nM) for 12, 24 and 48 h at 37°C.
Then, 10 µl CCK-8 reagent was added and incubated for 4 h at 37°C.
Cell proliferation was detected at 450 nm using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Flow cytometric analysis for cell
apoptosis
The PC3 cells were seeded in 6-well plates at a
density of 1×106 cells/well and incubated with
thapsigargin (0, 1, 10 and 100 nM) for 24 h at 37°C. The PC3 cells
were washed with cold PBS twice, and re-suspended with 500 µl
binding buffer (BD Biosciences, Franklin Lakes, NJ, USA). Then, 5
µl Annexin V-fluorescein isothiocyanate (BD Biosciences) was added
to the cells and incubated for 30 min at 4°C in the dark.
FACSCalibur flow cytometry (BD Biosciences) was performed following
the addition of 10 µl propidium iodide for 15 min in the dark at
room temperature and Flowjo software (version 7.6.1; (FlowJo LLC,
Ashland, OR, USA) was used to analyze apoptosis rate.
Caspase-3 and caspase-9 activity
analysis
The PC3 cells were seeded in 96-well plates at
1×104 cells/well and incubated with thapsigargin (0, 1,
10 and 100 nM) for 24 h at 37°C. A total of 100 µl caspase-3 or
caspase-9 reagent (C1136 or C1158, Beyotime Institute of
Biotechnology) was added and incubated at room temperature for an
additional 2 h. Caspase-3 and caspase-9 activity was detected at
490 nm using a microplate reader (Bio-Rad Laboratories, Inc.).
Western blot analysis
The PC3 cells were seeded in 6-well plates at
1×106 cells/well and incubated with thapsigargin (0, 1,
10 and 100 nM) for 24 h at 37°C. Then, the PC3 cells were washed
with cold PBS twice and prepared using a ProteoJET cytoplasmic
protein extraction kit (Fermentas; Thermo Fisher Scientific, Inc.).
Protein concentrations were measured using a BCA Protein Assay kit
(Thermo Fisher Scientific Inc.). Protein (30 µg) was separated by
10–12% SDS-PAGE and transferred electrophoretically using a PVDF
membrane by standard procedures. The PVDF membrane was blocked for
2 h with 5% non-fat milk in TBST (TBS + 0.1% Tween-20) at 37°C and
probed overnight using the following primary antibodies: Anti-RAC-α
serine threonine-protein kinase (Akt, sc-135829; 1:1,000; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA); anti-phosphorylated
(p)-Akt (sc-7985-R; 1:500; Santa Cruz Biotechnology, Inc.);
anti-p-mechanistic target of rapamycin (p-mTOR; sc-101738; 1:500;
Santa Cruz Biotechnology, Inc.); anti-F-actin (ab205; 1:1,000;
Santa Cruz Biotechnology, Inc.); anti-cofilin-1 (sc-376476;
1:1,000; Santa Cruz Biotechnology, Inc.); anti-paxillin (sc-390738;
1:1,000; Santa Cruz Biotechnology, Inc.); and anti-β-actin
(sc-1616; 1:2,000; Santa Cruz Biotechnology, Inc.), in PBST at 4°C.
Then, the membrane was incubated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:2,000
dilution; sc-2004 or sc-2005, Santa Cruz Biotechnology, Inc.) for 2
h at room temperature and detected by Super Signal enhanced
chemiluminescence development (ECL) reagent (Pierce; Thermo Fisher
Scientific, Inc.) and analyzed using sodium Image Lab software
(version 3.0; Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are expressed as mean ± standard deviation and
were analyzed using the SPSS version 17.0 software (SPSS, Inc.,
Chicago, IL, USA). A one-way analysis of variance and Tukey's
post-hoc test was used to compare data between groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Thapsigargin inhibits cell
proliferation in prostate cancer cells
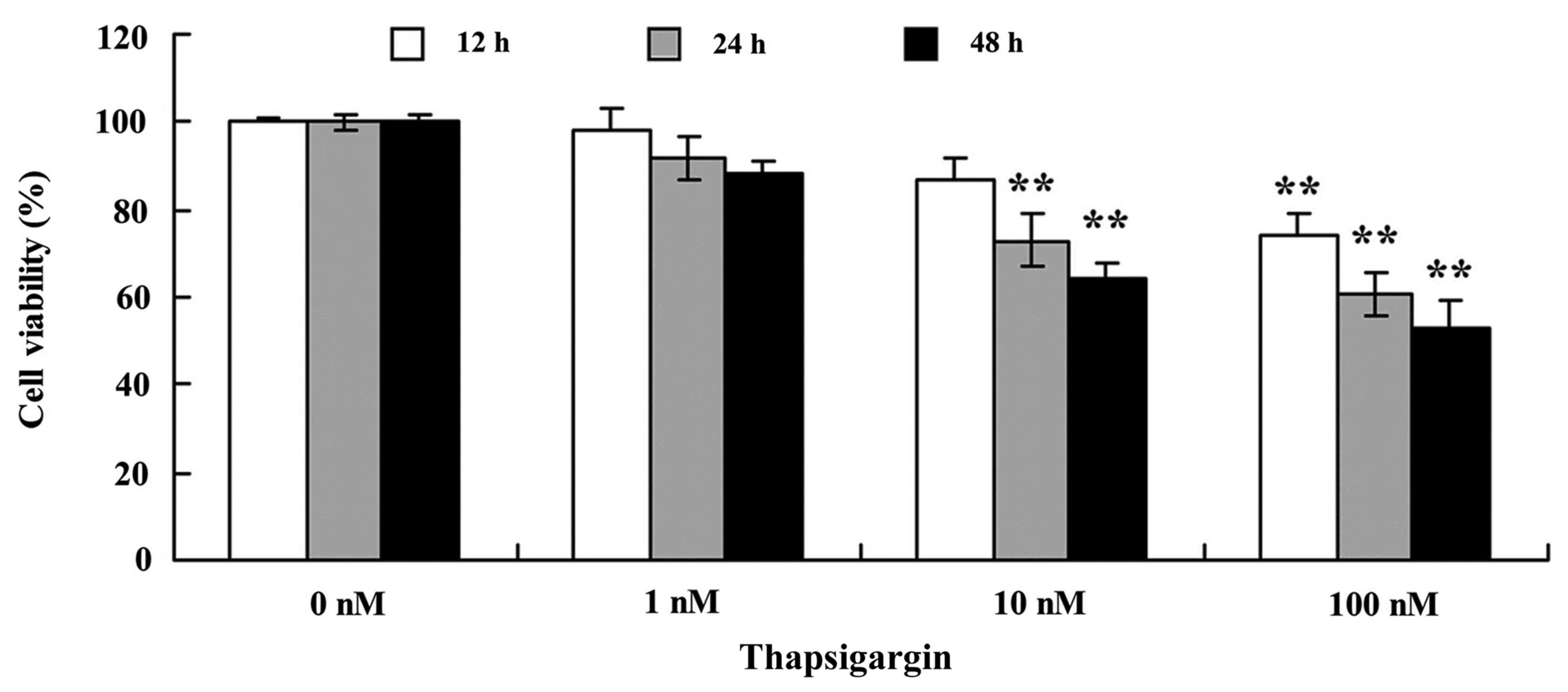
The present study demonstrated that thapsigargin may
suppress cell proliferation of prostate cancer PC3 cells in a dose-
and time-dependent manner (Fig. 2).
Treatment with 10 and 100 nM thapsigargin at 24 or 48 h or 1, 10
and 100 nM thapsigargin at 12 h significantly suppressed cell
proliferation of PC3 cells, compared with 0 nM of thapsigargin
(Fig. 2).
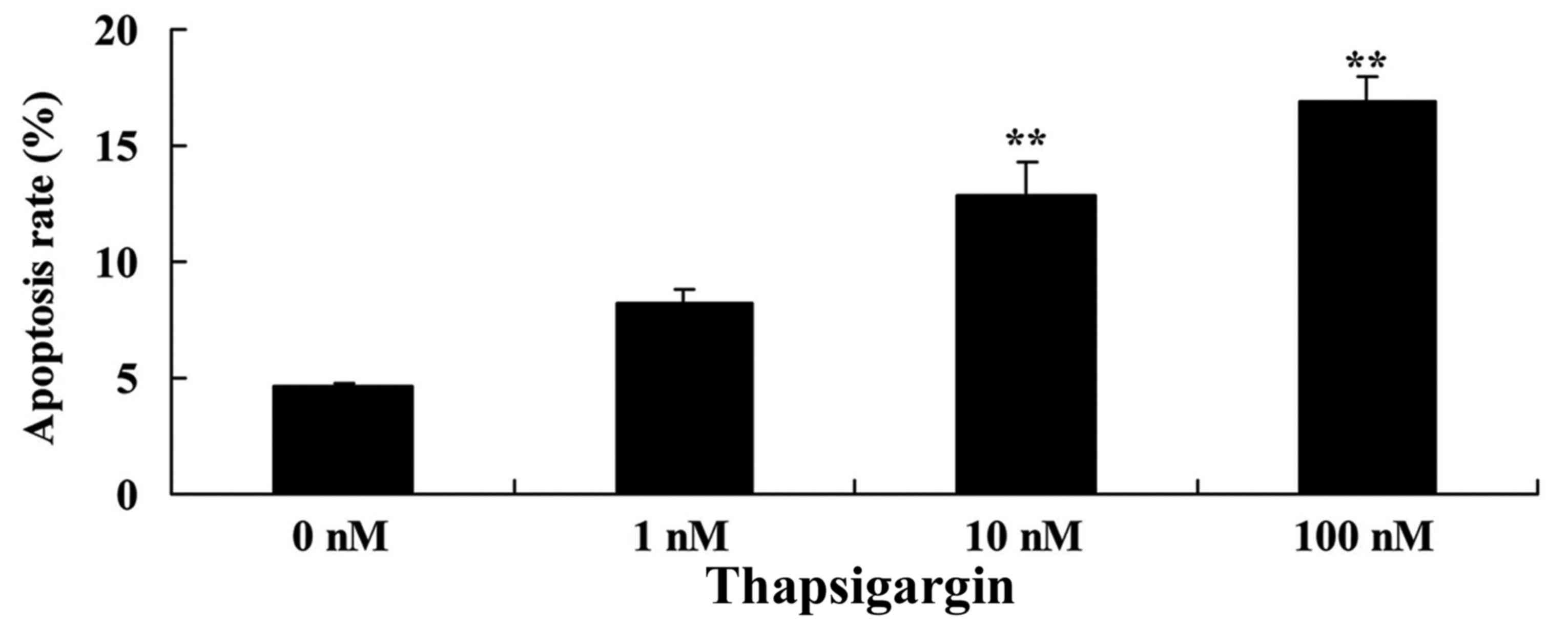
Thapsigargin increases the apoptosis
rate in prostate cancer cells
Consistent with the aforementioned data, 10 and 100
nM thapsigargin significantly increased the apoptosis rate of
prostate cancer PC3 cells in a dose-dependent manner (Fig. 3). These data suggest that thapsigargin
may suppress cell proliferation and increase the apoptosis rate of
PC3 cells as a potential treatment for prostate cancer.
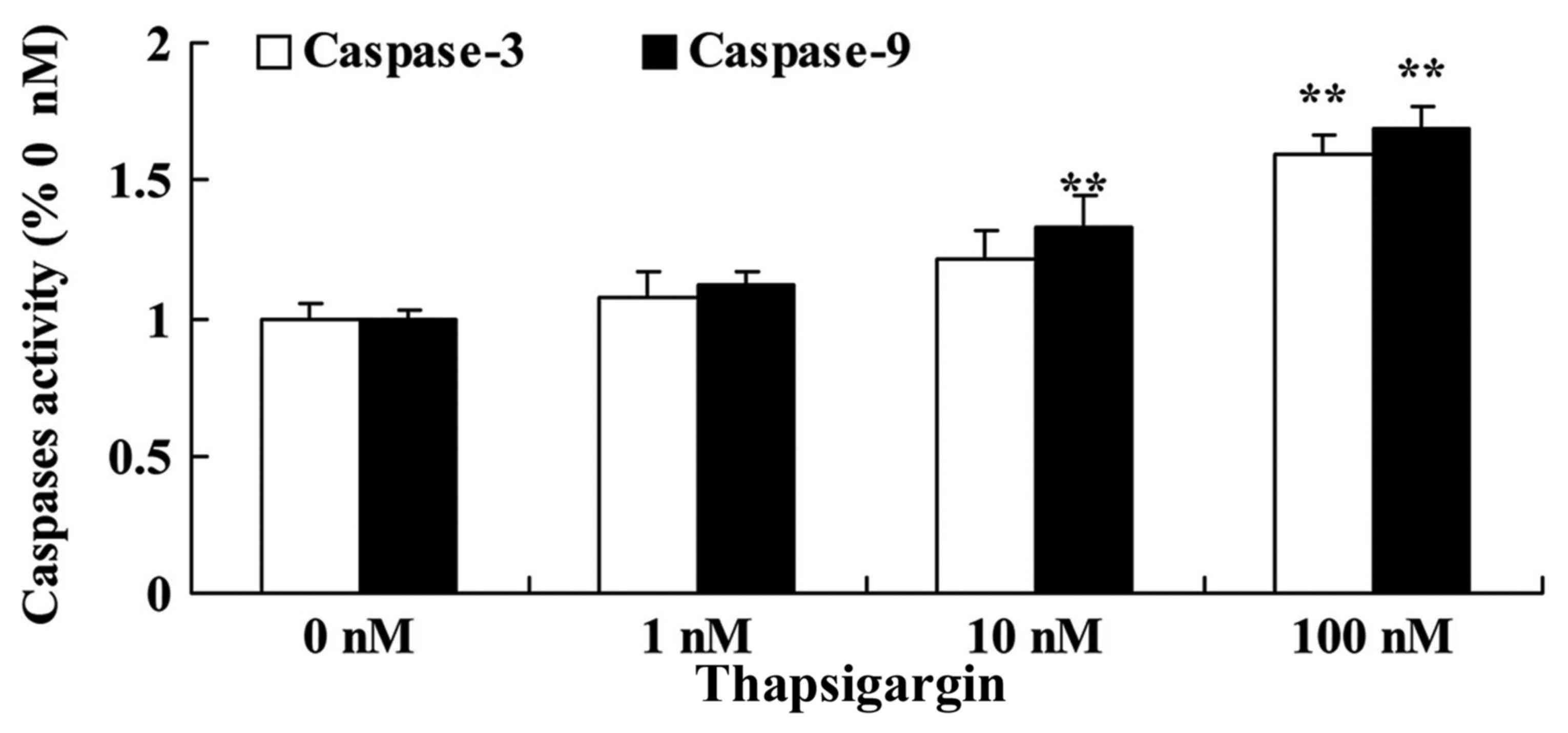
Thapsigargin induces caspase-3/9
activities in prostate cancer cells
To explore the anticancer effects of thapsigargin on
cell apoptosis, caspase-3/9 activities in PC3 cells were then
examined. Treatment with 10 and 100 nM thapsigargin significantly
increased caspase-9 activities, and treatment with 100 nM
thapsigargin significantly increased caspase-3 activities in PC3
cells compared with the 0 µM thapsigargin group (Fig. 4).
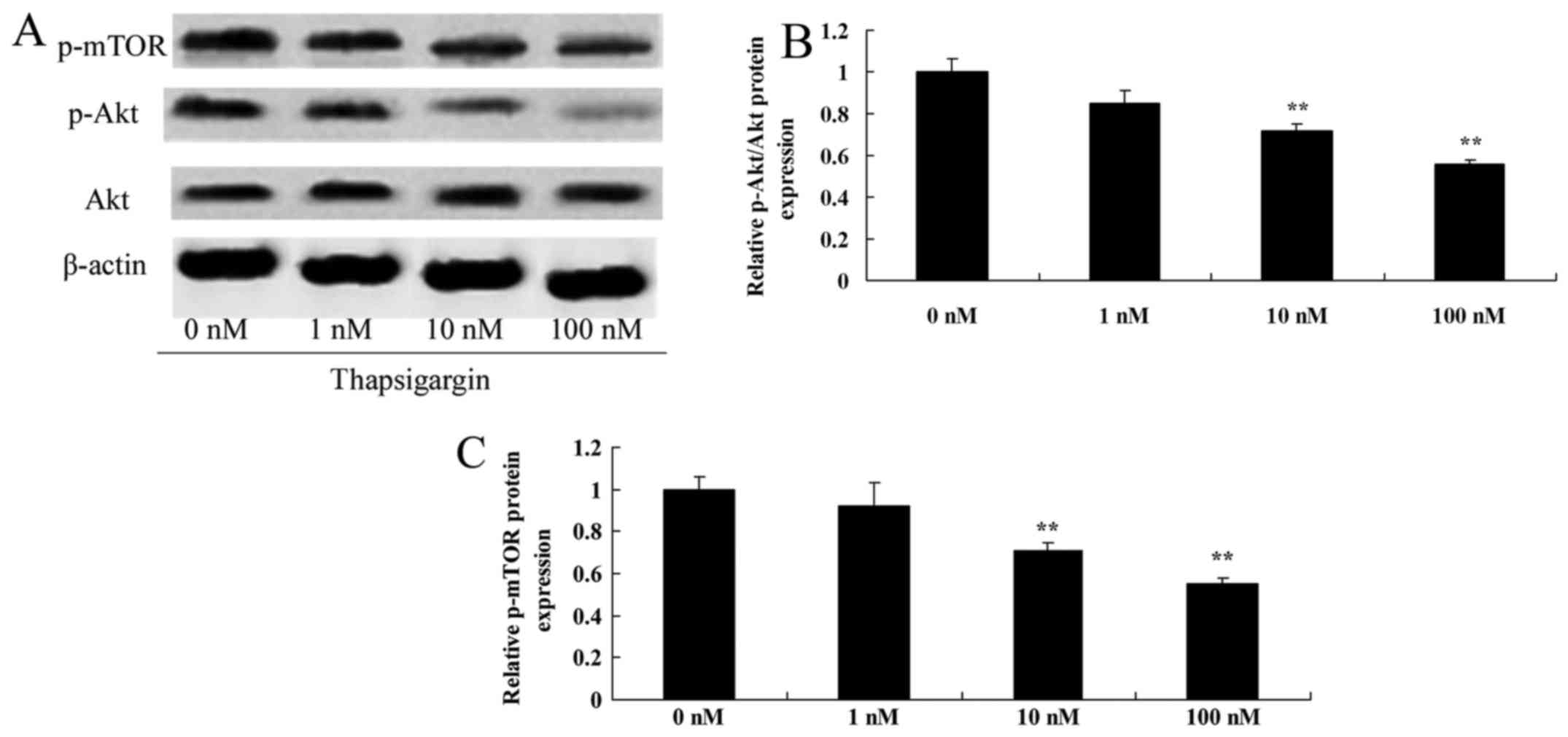
Thapsigargin inhibits Akt in prostate
cancer cells
To confirm the potential mechanism of thapsigargin
action, p-Akt and Akt protein expression levels were investigated
by western blot analysis. As demonstrated in Fig. 5A and B, p-Akt protein expression was
significantly decreased in PC3 cells by treatment with 10 and 100
nM thapsigargin compared with the 0 nM treatment group.
Thapsigargin inhibits mTOR expression
in prostate cancer cells
Next, the p-mTOR expression level in prostate cancer
cells was examined by treatment with thapsigargin in PC3 cells.
Treatment with 10 and 100 nM thapsigargin significantly decreased
p-mTOR protein expression in PC3 cells compared with the 0 µM
thapsigargin group (Fig. 5C).
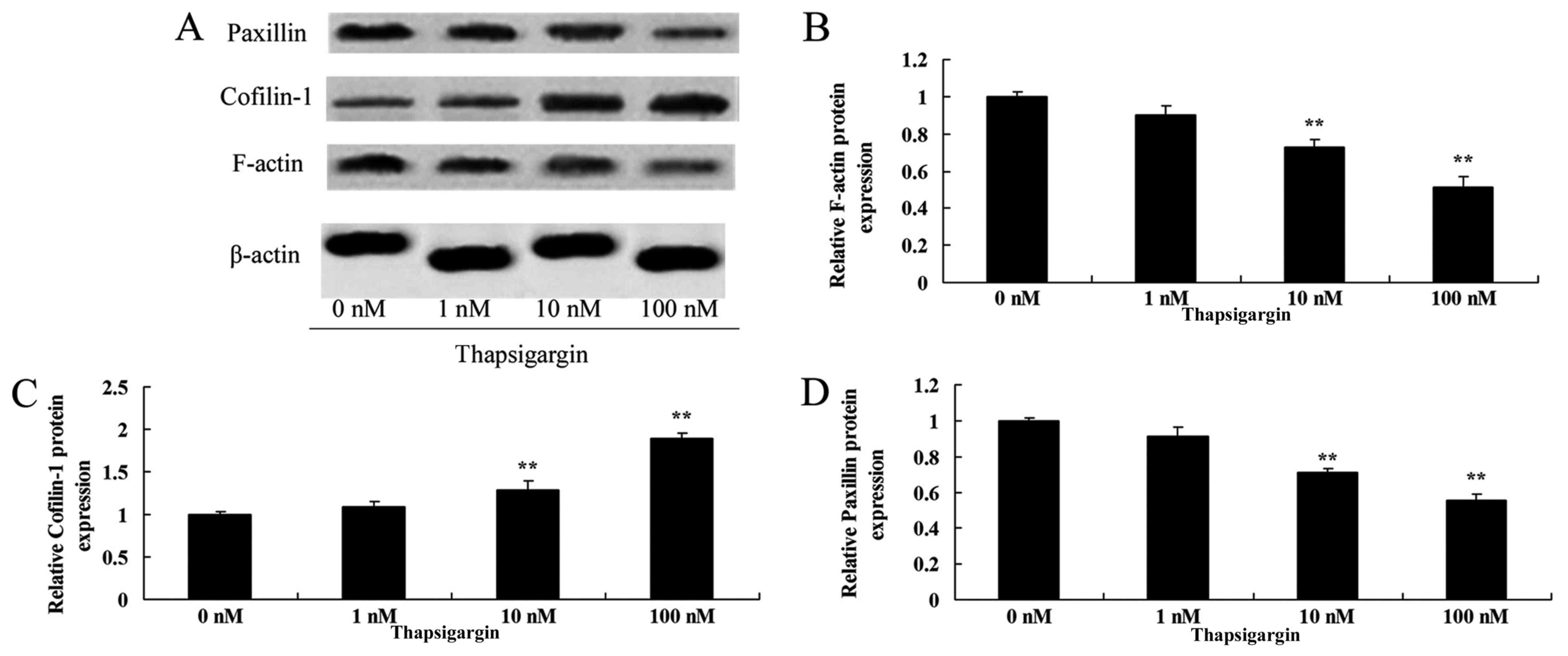
Thapsigargin inhibits F-actin
expression in prostate cancer cells
To determine the functional significance of F-actin
in the regulation of the effect of thapsigargin on prostate cancer
cells, F-actin protein expression was analyzed using western blot
analysis. The western blot analysis data from the present study
indicated that F-actin protein expression was significantly
decreased by treatment with 100 nM thapsigargin in PC3 cells
compared with the 0 µM thapsigargin group (Fig. 6A and B).
Thapsigargin induces cofilin-1
expression in prostate cancer cells
Furthermore, the effect of thapsigargin on cofilin-1
expression in prostate cancer cells was determined using western
blot analysis. As indicated in Fig. 6A
and C, treatment with 100 nM thapsigargin significantly
increased cofilin-1 protein expression in prostate cancer PC3 cells
compared with the 0 µM thapsigargin group (Fig. 6A and C).
Thapsigargin inhibits paxillin
expression in prostate cancer cells
To additionally confirm the inhibitory effect of
thapsigargin on paxillin expression in prostate cancer cells, the
protein expression of paxillin was measured using western blot
analysis. Treatment with 10 and 100 nM thapsigargin significantly
decreased paxillin protein expression in prostate cancer PC3 cells
compared with the 0 µM thapsigargin group (Fig. 6D).
Discussion
Prostate cancer is one of the most common types of
malignant tumors. Its mortality rate ranks sixth globally (17). There are ~903,500 incident cases
globally every year, including 258,400 mortalities (17). Despite the improvement of diagnostic
technology and effective development of screening processes, the
morbidity of prostate cancer in Asian and European countries
including China in recent years has increased (18). However, during the progression of
treatment, it is inevitable for patients with prostate cancer to
develop resistance to hormone therapy within several years, namely
castrate-resistant prostate cancer (17). For hormone-independent prostate
cancer, which is insensitive to endocrinotherapy, there is no
consistent ideal therapeutic method; therefore, it has become an
increasingly difficult issue (4). The
activation of caspases associated with endoplasmic reticulum stress
occurs during the early phase of apoptosis, when cells suffer from
a stress reaction, resulting in increases in mitochondrial outer
membrane permeability (19).
Cytochrome c is released into the cytoplasm, which then
promotes the formation of the apoptosis complex, activates effector
caspases and causes apoptosis (8).
The present study indicated that thapsigargin significantly
decreased cell proliferation, and increased the apoptosis rate and
caspase-9/3 activities in PC3 cells.
The mTOR signal transduction pathway primarily
participates in the synthesis of proteins (20). A previous study concerning the
suspected associations between single nucleotide polymorphisms in
the signal transduction pathway gene and prostate cancer conducted
worldwide (21). The mTOR signaling
pathway is an important therapeutic target of prostate cancer
(21). mTOR is a highly conserved
serine/threonine kinase, belonging to the phosphoinositide 3-kinase
(PI3K) family, and is also the downstream effector of the PI3K/Akt
signaling pathway (21). mTOR is
widely expressed in cells and regulates multiple cellular functions
in different cells, including survival and proliferation (21). On the one hand, the mammalian target
of rapamycin complex 1 regulates translation, including 5′terminal
oligopyrimidine tract mRNAs (20).
Conversely, mTORC1 serves as the central pivot of the cascade
signal channel that regulates RNA translation. The present study
identified that thapsigargin inhibits p-Akt and p-mTOR protein
expression in prostate cancer cells. Chiu et al (22) demonstrated that thapsigargin induces
pro-death autophagy through Akt-mTOR-Ribosomal protein S6 kinase
β-1 pathway inhibition in multidrug-resistant lung cancer cells.
These data suggest the role of thapsigargin-mediated inhibition of
the Akt-mTOR pathway in prostate cancer cells.
The cytoskeleton is a network structure consisting
of cellular internal proteins, including canaliculi, microfilaments
and intermediate filaments (23).
Microfilaments are the smallest of the three skeleton structures,
are composed of actin and exist in the form of free and globular
actin G-actin or F-actin (23).
Previous data indicate that the changes in actin
polymerization/depolymerization, namely actin skeleton
reconstruction, serve important regulatory roles in phenotypes of
malignant cells (24). It has been
suggested that intervention in cellular skeleton microfilament
actin reconstruction may be a functional target of anticancer
drugs, and may be regarded as a basis for developing novel
antineoplastic drugs (25). Yip et
al (26) suggested that
thapsigargin modulates osteoclastogenesis through the regulation of
F-actin and reactive oxygen species production (27). The present study indicated that
thapsigargin inhibits F-actin expression in prostate cancer
cells.
The highly localized activities of cofilin-1
generate schistose pseudopodia and determine cellular motor
direction; cofilin-1 serves an important role in cell migration
(9). Concomitantly, there have been
studies demonstrating that cofilin-1 is an important regulatory
factor of cancer cell metastasis and invasion (9). The overexpression of cofilin-1 protein
levels increases the migratory rate of cancer cells, but inhibiting
its expression may markedly reduce cancer cell growth (9). An overexpression of cofilin-1 at a mRNA
level has been revealed in breast cancer cell subsets (11). A previous study identified that
cofilin-1 also exhibited overexpression in a number of types of
cancer cells (11). The present study
suggested that thapsigargin induces cofilin-1 expression in
prostate cancer cells. Wang et al (27) demonstrated that thapsigargin induces
apoptosis of human lung adenocarcinoma cells through cofilin-1 and
paxillin.
Protein-tyrosine kinase 6 (Brk) promotes the
migration and infiltration of prostate cancer, and is activated by
paxillin phosphorylation (10).
Paxillin is the binding protein and acting substrate of Brk
(10). Then, the adaptor molecule
CrkII isoform activates guanosine triphosphatase Ras-related C3
botulinum toxin substrate 1, to cause cell migration and
infiltration (10). In addition,
paxillin may also combine with multiple oncogenic proteins,
disordering or even dysregulating normal adhesion, and control the
growth factor signaling passageway required for cell proliferation,
so as to participate in tumor metastasis (23). The abnormal expression of paxillin is
associated with the occurrence, invasion and metastasis of prostate
cancer (23). The present study
identified that thapsigargin significantly inhibited paxillin
protein expression in prostate cancer PC3 cells. Wang et al
(27) revealed that thapsigargin
induces apoptosis in human lung adenocarcinoma cells through
cofilin-1 and paxillin. These results of the present study suggest
that curcumin inhibits the tumor growth of prostate cancer cells by
modulating the F-actin/cofilin-1/paxillin pathway.
In summary, the present study suggests that
thapsigargin significantly decreased cell proliferation, and
increased the apoptosis rate and caspase-9/3 activities in PC3
cells. Additionally, inhibition of Akt-mTOR pathway and modulation
of the F-actin/cofilin-1/paxillin pathway by thapsigargin may
decreased cell growth in prostate cancer cells. These data suggest
that thapsigargin may be a novel drug that suppresses the growth of
prostate cancer cells through the Akt-mTOR and
F-actin/cofilin-1/paxillin pathways.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
XW designed the experiment. FH and PW performed the
experiments. XW and FH analyzed the data. XW wrote the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
D'Amico AV, Chen MH, Renshaw A, Loffredo M
and Kantoff PW: Long-term follow-up of a randomized trial of
radiation with or without androgen deprivation therapy for
localized prostate cancer. JAMA. 314:1291–1293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fedorov A, Fluckiger J, Ayers GD, Li X,
Gupta SN, Tempany C, Mulkern R, Yankeelov TE and Fennessy FM: A
comparison of two methods for estimating DCE-MRI parameters via
individual and cohort based AIFs in prostate cancer: A step towards
practical implementation. Magn Reson Imaging. 32:321–329. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu EY, Massard C, Gross ME, Carducci MA,
Culine S, Hudes G, Posadas EM, Sternberg CN, Wilding G, Trudel GC,
et al: Once-daily dasatinib: Expansion of phase II study evaluating
safety and efficacy of dasatinib in patients with metastatic
castration-resistant prostate cancer. Urology. 77:1166–1171. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Houédé N, Pulido M, Mourey L, Joly F,
Ferrero JM, Bellera C, Priou F, Lalet C, Laroche-Clary A, Raffin
MC, et al: A phase II trial evaluating the efficacy and safety of
efavirenz in metastatic castration-resistant prostate cancer.
Oncologist. 19:1227–1228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thomas R, Williams M, Sharma H, Chaudry A
and Bellamy P: A double-blind, placebo-controlled randomised trial
evaluating the effect of a polyphenol-rich whole food supplement on
PSA progression in men with prostate cancer-the U.K. NCRN Pomi-T
study. Prostate Cancer Prostatic Dis. 17:180–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Obakan P, Arisan ED, Coker-Gurkan A and
Palavan-Unsal N: Epibrassinolide-induced apoptosis regardless of
p53 expression via activating polyamine catabolic machinery, a
common target for androgen sensitive and insensitive prostate
cancer cells. Prostate. 74:1622–1633. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reshma RS, Sreelatha KH, Somasundaram V,
Satheesh Kumar S, Nadhan R, Nair RS and Srinivas P: Plumbagin, a
naphthaquinone derivative induces apoptosis in BRCA 1/2 defective
castrate resistant prostate cancer cells as well as prostate cancer
stem-like cells. Pharmacol Res. 105:134–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kwegyir-Afful AK, Ramalingam S,
Purushottamachar P, Ramamurthy VP and Njar VC: Galeterone and
VNPT55 induce proteasomal degradation of AR/AR-V7, induce
significant apoptosis via cytochrome c release and suppress growth
of castration resistant prostate cancer xenografts in vivo.
Oncotarget. 6:27440–27460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu B, Fukada K, Zhu H and Kyprianou N:
Prohibitin and cofilin are intracellular effectors of transforming
growth factor beta signaling in human prostate cancer cells. Cancer
Res. 66:8640–8647. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu LI, Fu NI, Luo XU, Li XY and Li XP:
Overexpression of cofilin 1 in prostate cancer and the
corresponding clinical implications. Oncol Lett. 9:2757–2761. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sundram V, Chauhan SC, Ebeling M and Jaggi
M: Curcumin attenuates β-catenin signaling in prostate cancer cells
through activation of protein kinase D1. PLoS One. 7:e353682012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hammes SR, Miedlich SU and Sen A: Paxillin
and steroid signaling: From frog to human. Methods Mol Biol.
1204:95–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bokobza SM, Ye L, Kynaston HG and Jiang
WG: Growth and differentiation factor-9 promotes adhesive and
motile capacity of prostate cancer cells by up-regulating FAK and
Paxillin via Smad dependent pathway. Oncol Rep. 24:1653–1659. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Janyou A, Changtam C, Suksamrarn A,
Tocharus C and Tocharus J: Suppression effects of
O-demethyldemethoxycurcumin on thapsigargin triggered on
endoplasmic reticulum stress in SK-N-SH cells. Neurotoxicology.
50:92–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Drexler HC: Synergistic apoptosis
induction in leukemic cells by the phosphatase inhibitor salubrinal
and proteasome inhibitors. PLoS One. 4:e41612009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muramatsu Y, Maemoto T, Iwashita A and
Matsuoka N: Novel neuroprotective compound SCH-20148 rescues
thymocytes and SH-SY5Y cells from thapsigargin-induced
mitochondrial membrane potential reduction and cell death. Eur J
Pharmacol. 563:40–48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolpin BM, O'Reilly EM, Ko YJ, Blaszkowsky
LS, Rarick M, Rocha-Lima CM, Ritch P, Chan E, Spratlin J, Macarulla
T, et al: Global, multicenter, randomized, phase II trial of
gemcitabine and gemcitabine plus AGS-1C4D4 in patients with
previously untreated, metastatic pancreatic cancer. Ann Oncol.
24:1792–1801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsumoto K, Hagiwara M, Tanaka N,
Hayakawa N, Ishida M, Ninomiya A, Nakajima Y and Nakamura S:
Survival following primary androgen deprivation therapy for
localized intermediate- or high-risk prostate cancer: Comparison
with the life expectancy of the age-matched normal population. Med
Oncol. 31:9792014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cella D, Ivanescu C, Holmstrom S, Bui CN,
Spalding J and Fizazi K: Impact of enzalutamide on quality of life
in men with metastatic castration-resistant prostate cancer after
chemotherapy: Additional analyses from the AFFIRM randomized
clinical trial. Ann Oncol. 26:179–185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kato M, Banuelos CA, Imamura Y, Leung JK,
Caley DP, Wang J, Mawji NR and Sadar MD: Cotargeting androgen
receptor splice variants and mTOR signaling pathway for the
treatment of castration-resistant prostate cancer. Clin Cancer Res.
22:2744–2754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang F and Wang L, Zhang S, Fang Q, Hao F,
Sun Y, Zhao L, Chen S, Liao H and Wang L: CD147 modulates autophagy
through the PI3K/Akt/mTOR pathway in human prostate cancer PC-3
cells. Oncol Lett. 9:1439–1443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chiu LY, Hu ME, Yang TY, Hsin IL, Ko JL,
Tsai KJ and Sheu GT: Immunomodulatory protein from ganoderma
microsporum induces pro-death autophagy through Akt-mTOR-p70S6K
pathway inhibition in multidrug resistant lung cancer cells. PLoS
One. 10:e01257742015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Asahara S, Shibutani Y, Teruyama K, Inoue
HY, Kawada Y, Etoh H, Matsuda T, Kimura-Koyanagi M, Hashimoto N,
Sakahara M, et al: Ras-related C3 botulinum toxin substrate 1
(RAC1) regulates glucose-stimulated insulin secretion via
modulation of F-actin. Diabetologia. 56:1088–1097. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu Y, Yang O, Fazli L, Rennie PS, Gleave
ME and Dong X: Progesterone receptor expression during prostate
cancer progression suggests a role of this receptor in stromal cell
differentiation. Prostate. 75:1043–1050. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo Y, Cui X, Zhao J, Han Y, Li M, Lin Y,
Jiang Y and Lan L: Cells susceptible to epithelial-mesenchymal
transition are enriched in stem-like side population cells from
prostate cancer. Oncol Rep. 31:874–884. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yip KH, Zheng MH, Steer JH, Giardina TM,
Han R, Lo SZ, Bakker AJ, Cassady AI, Joyce DA and Xu J:
Thapsigargin modulates osteoclastogenesis through the regulation of
RANKL-induced signaling pathways and reactive oxygen species
production. J Bone Miner Res. 20:1462–1471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang F, Liu DZ, Xu H, Li Y, Wang W, Liu BL
and Zhang LY: Thapsigargin induces apoptosis by impairing
cytoskeleton dynamics in human lung adenocarcinoma cells.
ScientificWorldJournal. 2014:6190502014.PubMed/NCBI
|