Introduction
Gliomas are the most common primary intra-axial
brain tumor in adults. According to the WHO classification of
tumors in the central nervous system (1), glioma is classified into four grades
based on histopathological features. Median overall survival
remains about 5–10 years for grade II, 2–3 years for grade III, and
12–15 months for grade IV gliomas (2). In particular, grade IV glioma has an
extremely poor prognosis due to its strong tendency to infiltrate
adjacent brain tissue and low response to chemoradiotherapy. Grade
IV tumors include glioblastomas with mesenchymal-like features,
which originate from progenitor or stem cells in the astrocytic
lineage (1). In addition, when
gliomas with non-mesenchymal features recur, their cellular
characteristics are altered to exhibit typical mesenchymal
features. A shift towards the mesenchymal subtype appears to be a
common pattern in disease progression of various cancers, analogous
to cancer cells undergoing the epithelial-to-mesenchymal transition
(EMT).
The EMT is involved in differentiation of immature
embryos, wound healing, and tumor metastasis (3,4). In most
cases, the EMT is regulated by several transcription factors,
including the δEF1 family of two-handed zinc-finger factors
(ZEB1/δEF1 and ZEB2/SIP1). Several extracellular signaling
molecules, including fibroblast growth factor (FGF)-2 and tumor
necrosis factor (TNF)-α, as well as intracellular signaling
molecules such as Ras, cooperate with TGF-β to upregulate ZEBs and
promote the EMT (5,6). The EMT induced by TGF-β and FGF-2
specifically causes upregulation of integrin α3, which is
positively correlated with aggressiveness of breast cancer cells
(7). Previously, we reported that
integrin α3 and laminin are highly expressed in and promote
invasiveness of glioma cells, in which basal levels of ERK1/2
phosphorylation status are extremely high. Importantly, when ERK1/2
in glioma cells are inactivated by the inhibitor U0126, expression
of integrin α3 and δEF1 family proteins (ZEB1 and ZEB2) is
downregulated (7). Moreover, a
neutralizing antibody targeting integrin α3 inhibits motile
properties (8). Thus, aggressiveness
in glioma appears to be correlated with EMT phenotypes.
In this study, we found that ZEB1 and ZEB2 were
expressed at high levels in glioma cells. Although single knockdown
of either ZEB1 or ZEB2 alone moderately inhibited invasion and
anchorage-independent growth, double knockdown of both proteins had
more pronounced effects. Glioma cells in which both ZEB1 and ZEB2
were silenced, formed smaller tumors in mice. Immunohistochemical
(IHC) analyses using human surgical specimens revealed that ZEB1
and ZEB2 were more highly expressed in specimens of grade IV than
grade II glioma. Importantly, ZEB-positive cells were more abundant
in specimens from patients with recurrent glioma, suggesting that
both ZEB1 and ZEB2 are overexpressed in glioma with more aggressive
phenotypes. Thus, δEF1 family proteins represent promising
prognostic markers for glioma.
Materials and methods
Cell culture and antibodies
MCF-7, MDA-MB-231, and A172 cells were from the
American Type Culture Collection (Manassas, VA, USA). KG-1-C, U251,
and T98G cells were from Japanese Collection of Research
Bioresources Cell Bank (Tokyo, Japan). GL261 cells was from Cell
Resource Center for Biomedical Research, Tohoku University (Sendai,
Japan). All cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Nacalai Tesque, Kyoto, Japan) supplemented with 10%
fetal bovine serum (FBS), 100 U/ml penicillin (Nacalai Tesque), and
100 µg/ml streptomycin (Nacalai Tesque). Mouse monoclonal
anti-α-tubulin antibody was purchased from Sigma-Aldrich. Rabbit
polyclonal anti-ZEB1 and -ZEB2 antibodies were from Novus
Biologicals (Littleton, CO, USA).
RNA interference, RNA isolation, and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) analysis
Transfection of siRNA was performed according to the
protocol recommended for RNAiMAX (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). siRNAs and shRNAs against ZEB1
and ZEB2 were also described previously (6,7). Total RNA
was purified using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA)
and used for RT-qPCR analyses. RT-qPCR analysis was performed using
the Power SYBR Green Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.). mRNA levels were normalized against the
corresponding levels of glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) mRNA. The primer sequences were described previously
(6).
Immunoblotting
The procedures used for immunoblotting were
previously described (6).
Immunodetection was performed with the ECL blotting system
(Amersham Bioscience, Piscataway, NJ, USA) on a Luminescent Image
Analyzer (LAS4000; Fujifilm, Tokyo, Japan). α-tubulin was used as a
loading control.
Cell invasion, proliferation and
colony formation assays
Procedures were as previously described (6,7,9). For the invasion assay, cells were
photographed and visually counted, and the cell counts were
subjected to statistical analyses. For proliferation assays, 24 h
after infection or transfection, cells were trypsinized, counted,
and re-seeded in triplicate in 96-well plates. Four days later,
Cell Count Reagent SF (Nacalai Tesque) was used according to the
recommended protocol, and the results were evaluated by statistical
analyses. For colony formation assays, cells in 0.3% agar (Nacalai
Tesque) were covered with culture media for 3 weeks. Cell viability
was measured using Cell Count Reagent SF, which was added to the
media and incubated for 60 min. An aliquot was taken and
colorimetrically quantified at 450–650 nm.
Allo/Xenograft glioma implants in
mouse brain
All experimental animals were reared in accordance
with the animal experiment protocols approved by University of
Yamanashi's Administrative Panel on Laboratory Animal Models.
Five-week-old male mice were used for intracranial model of tumor
cells implantation. Intraperitoneally anesthetized mice were set in
a stereotactic frame (NARISHIGE Type SR5N), the scalp was linearly
incised, and a burr hole 1 mm in diameter was introduced 3 mm
lateral and 1 mm anterior from bregma. Tumor cells (1.0×105) were
stereotactically transplanted at a depth of 3.5 mm under cranial
bone using a Hamilton syringe. The syringe was pulled out slowly
over the course of 1 min to avoid a rapid change in intracranial
pressure. The burr hole was covered with sterile wax, followed by
scalp suture (10). All the animal
experiments were conducted in compliance with the protocol which
was reviewed by the Institutional Animal Care and Use Committee and
approved by the president of Yamanashi University (A24-70).
IHC analyses using human glioma
specimens
Thirty-two surgical specimens were prepared from 25
glioma patients treated at Yamanashi University Hospital from 1985
to 2016. Informed consent was obtained from all subjects. Of the 32
specimens, 19 were from patients with only primary tumor, 10 were
from patients who had undergone two surgical operations due to
tumor recurrence, and three specimens were from a patient who had
undergone three surgeries. The specimens were chosen according to
the following criteria: i) no preoperative chemotherapy or
irradiation before recurrence of glioma, and ii) definitive
diagnosis (grade II, III, or IV) by pathologists. Intensity of
ZEB1- and ZEB2-positive cells was scored: 0 for negative (fewer
than 1% positive cells in a field); 1 for weak (1 to 24%); 2 for
intermediate (25 to 49%); and 3 for strongly positive (more than
50%). All studies were conducted using the protocol approved by the
Ethics Committee of the University of Yamanashi (1642).
Statistical analysis
Survival analyses of implanted mice were performed
using Kaplan-Meier curves and analyzed using log-rank test. One-way
factorial ANOVA followed by Fisher's least significant difference
(PLSD) test was used to compare means among multiple groups.
Non-parametric Mann-Whitney U tests and Student's t-tests were used
to compare means between two groups. Spearman's rank correlation
coefficient tests were used to identify significant correlations
between ZEB1 and ZEB2. IBM SPSS statistics version 22 software (IBM
Japan, Tokyo, Japan) was used for all statistical analyses.
Results
Anti-tumor effects of ZEB siRNAs in
mouse glioma
We reported previously that ZEB1 and ZEB2 are highly
expressed and function redundantly in breast cancer cells (6). To investigate the roles of these factors
in glioma, we simultaneously knocked down both ZEB1 and ZEB2 in
mouse glioma GL261 cells. For this purpose, the cells were
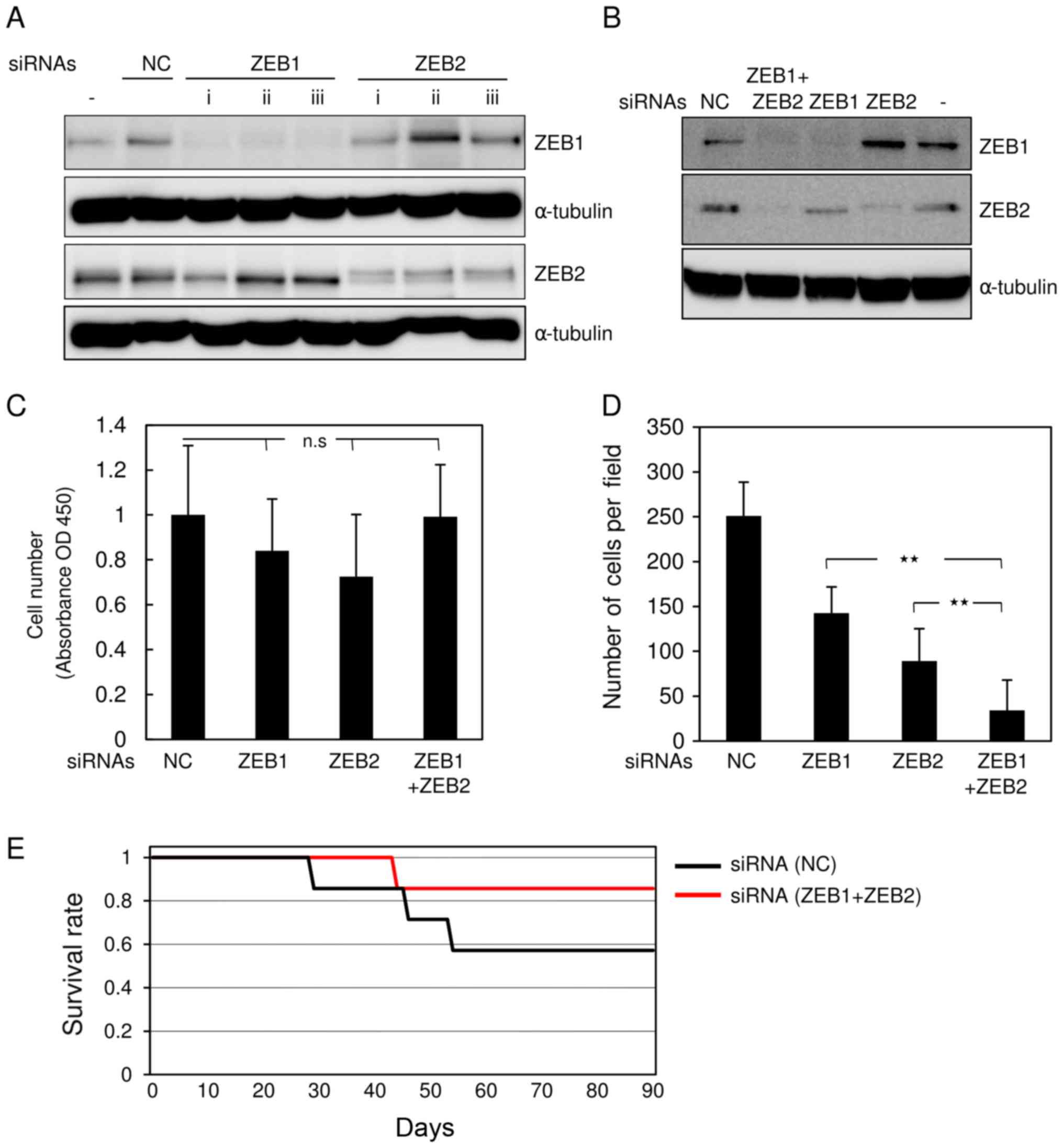
transfected with siRNAs against mouse ZEB1 and ZEB2. All siRNAs
effectively silenced the corresponding genes when transfected alone
(Fig. 1A). Combined transfection with
both siRNAs clearly knocked down the target genes, in comparison
with control siRNA (Fig. 1B). GL261
cells transfected with both siRNAs or either alone proliferated
almost as rapidly as cells transfected with control siRNA (Fig. 1C). Transfection with either ZEB1 or
ZEB2 siRNA alone moderately inhibited invasive properties in
comparison with control siRNA (Fig.
1D). Of the two, ZEB2 siRNA was slightly more effective than
ZEB1 siRNA (Fig. 1D). However,
transfection with both siRNAs had the most potent effects.
Consistent with our previous reports (6), we chose double knockdown using both
siRNAs, rather than either alone, to further evaluate the roles of
ZEBs in glioma cells. Allograft implantation revealed that mice
inoculated with double-knockdown GL261 cells lived comparably
slightly longer than those transfected with control GL261 cells,
but the difference was not significant (Fig. 1E, and data not shown). These findings
suggest that silencing of both ZEB1 and ZEB2 is more effective at
reducing aggressive phenotypes in mouse glioma cells than silencing
of either protein alone.
Anti-tumor effects of ZEBs siRNAs in
human glioma cells
The δEF1 family proteins ZEB1 and ZEB2 are highly
expressed in the basal-like subtype of breast cancer cells
(6,11). Recently, we found that ZEB1 is much
more highly expressed in glioma KG-1-C and U251 cells than in the
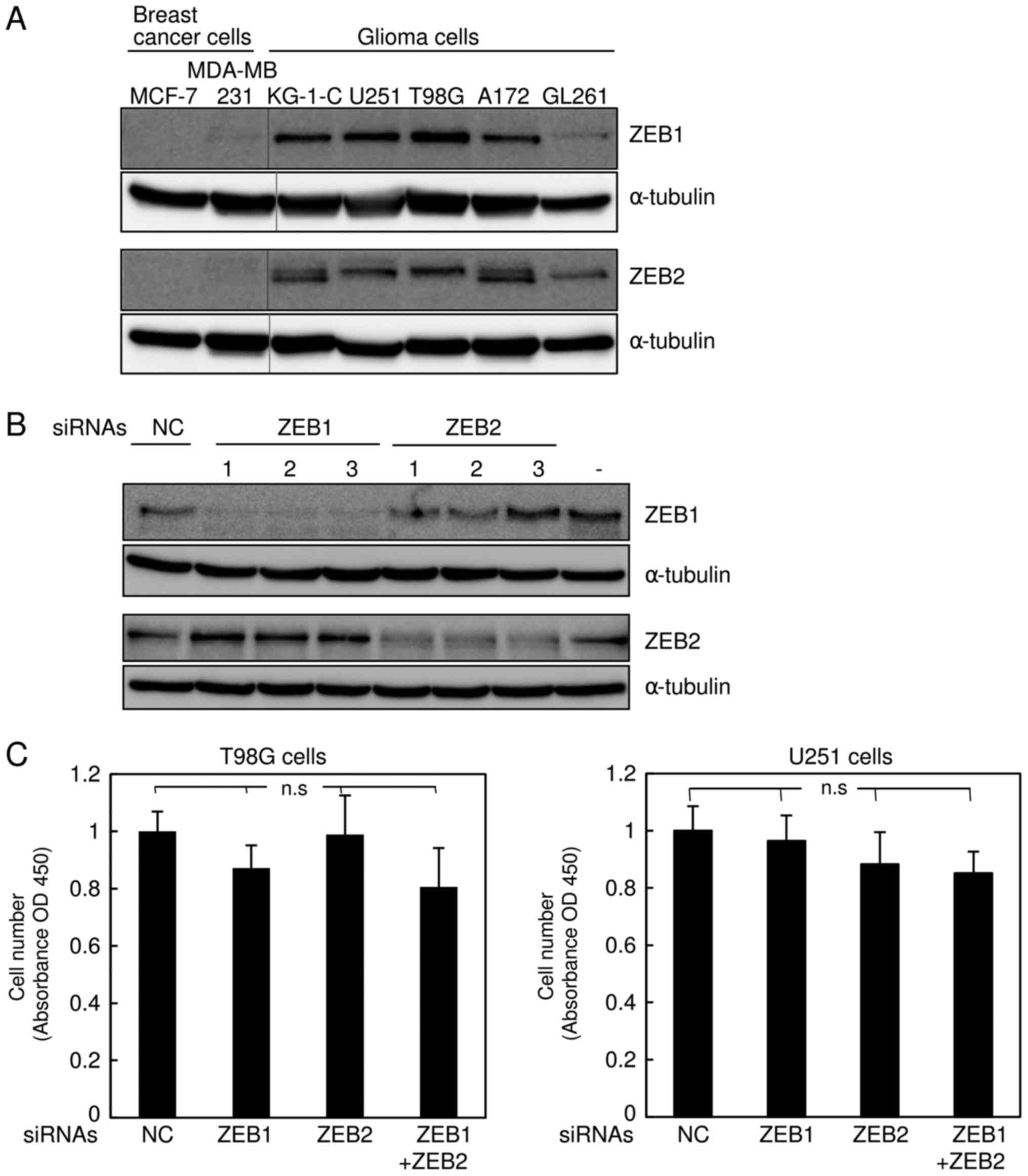
basal-like breast cancer cell line MDA-MB-231 (7). Hence, we performed immunoblot analyses
to examine the protein levels of ZEB2, as well as ZEB1, in several
types of human glioma cells (KG-1-C, U251, T98 G, and A172) and
mouse glioma cells (GL261), and compared them with two subtypes of
human breast cancer cells. Consistent with our previous findings
(6,12), ZEB1 and ZEB2 were negligibly expressed
in the luminal breast cancer cell line MCF7, but detectable in
MDA-MB-231 cells (Fig. 2A).
Importantly, expression of ZEB2, as well as ZEB1, was much higher
in all human glioma cells than in MDA-MB-231 cells (Fig. 2A). The level of ZEB1 in mouse GL261
cells was apparently similar to that in MDA-MB-231 cells, possibly
due to the lower affinity of the ZEB1 antibody to mouse antigen,
whereas ZEB2 was expressed at levels similar to those in other
human glioma cells. Next, we assessed the efficacy of the siRNAs
targeting human ZEB1 and ZEB2 in human glioma T98G and U251 cells
(Fig. 2B and data not shown), and
confirmed that these siRNAs specifically silenced their target
genes. Similar to the findings shown in Fig. 1C, these siRNAs did not affect the
proliferation rate of T98G or U251 cells (Fig. 2C). Cells simultaneously transfected
with both ZEB1 and ZEB2 siRNAs exhibited considerable reduction of
invasive properties in comparison with those transfected with
control or either siRNA alone (Fig.
2D). The anti-tumor effects of both siRNAs in T98G and U251
cells were confirmed by colony formation assay in soft agar
(Fig. 2E). As with the findings shown
in Fig. 1, these observations suggest
that silencing of both ZEBs, which is more potent than that of
either alone, dramatically inhibits invasiveness of human glioma
cells without affecting cell proliferation.
Expression of ZEB1 and ZEB2 in
specimens from glioma patients
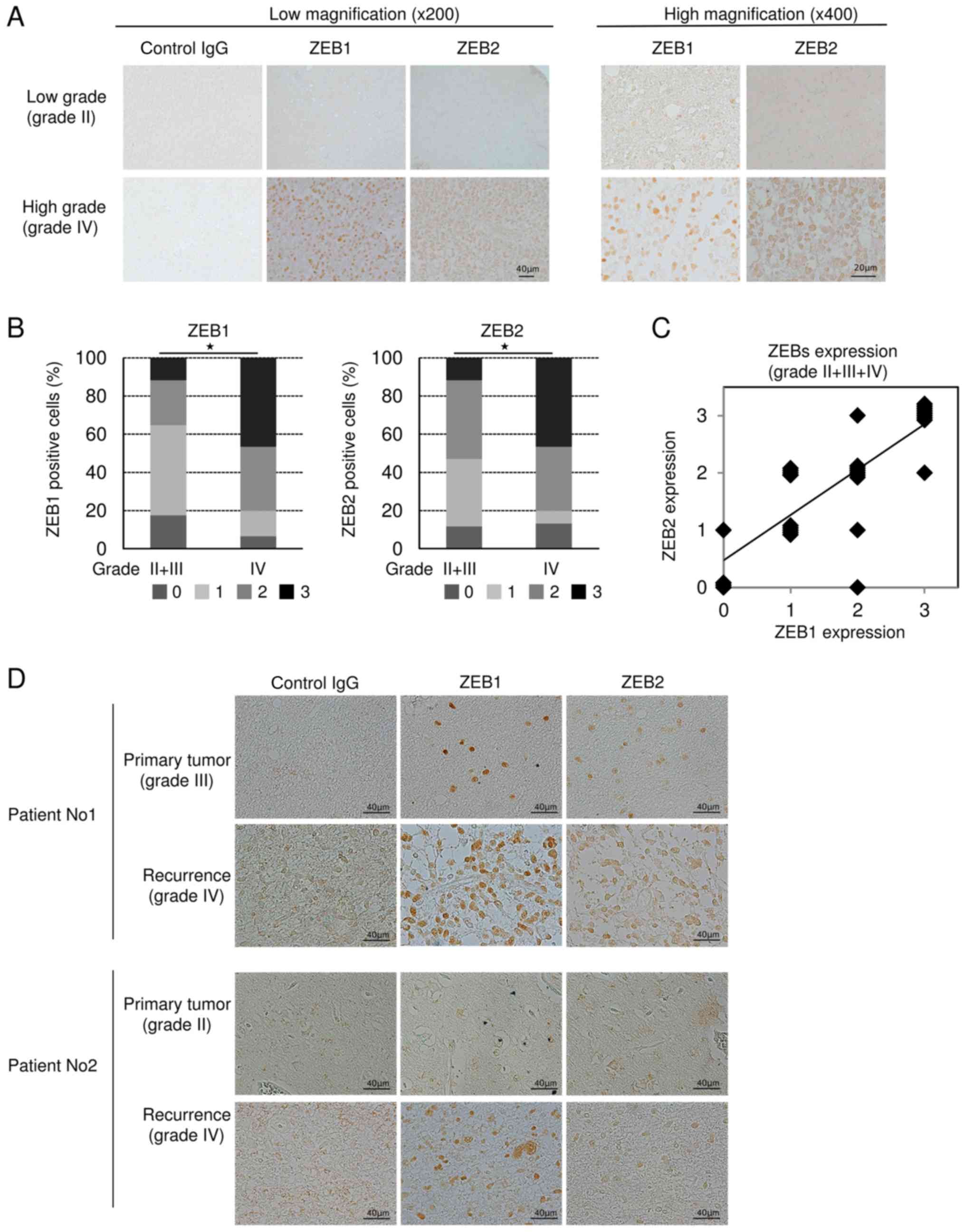
To determine whether expression of ZEBs is
correlated with pathological grade or aggressiveness of glioma
patients, we prepared 32 specimens from 25 patients who had been
treated at our university hospital. In grade II glioma, ZEB1 and
ZEB2 were marginally or negligibly expressed, but were clearly
detectable in grade IV. ZEB1 and ZEB2 were localized in the nucleus
and sometimes in the cytoplasm (Fig.
3A). Statistical analyses revealed that ZEB-positive cells were
significantly more abundant in grade IV glioma than in grade II+III
(Fig. 3B). Similarly, ZEB1-positive
cells were significantly more abundant in grade IV glioma than in
grade II, whereas ZEB2 was more highly, but not significantly,
expressed in grade IV than in grade II (data not shown). However,
the difference in ZEB expression between grades III and IV could
not be statistically analyzed due to the small number of grade III
specimens (n=4). A positive correlation between the levels of ZEB1
and ZEB2 was detected in grades II, III, and IV (Fig. 3C), suggesting that expression of not
only ZEB1 but also ZEB2 is positively correlated with glioma grade.
Importantly, some of our subjects had experienced recurrent glioma
after primary surgery. One of these patients had been diagnosed
initially as grade III glioma, and the other as grade II; both
patients were secondarily diagnosed as grade IV glioblastoma
multiforme at recurrence. IHC analyses using anti-ZEB1 and
anti-ZEB2 antibodies revealed that, in both of these patients, the
number of ZEB-positive cells was dramatically higher in the
specimens following recurrence (Fig.
3D), suggesting that expression of ZEBs is positively
correlated with aggressiveness of human glioma.
Discussion
Malignant glioma exhibits aggressiveness with high
infiltration properties, causing clinical therapeutic difficulties
(1). Pathological grade and invasive
properties are well correlated, and higher pathological grade
corresponds to poorer prognosis. Consequently, chemotherapy in
combination with radiation therapy is a standard postoperative
treatment. In vitro and in vivo experiments have
shown that tumor cells located around the tumor nest or at the
invasion front are resistant to chemo- and radiotherapy and
initiate invasion into adjacent tissue after radiation therapy
(3). These cellular heterogeneities
appear to result from a small proportion of tumor cells undergoing
the EMT, or from cancer stem cells. Although the EMT in glioma has
not been thoroughly assessed, it has been widely studied in other
kinds of tumor cells, including breast, lung, and pancreatic
cancer. Cells undergoing the EMT are extremely resistant to
anti-tumor drugs in breast and pancreatic cancers (13,14).
EMT-like phenotypes in breast cancer cells are largely dependent on
expression of ZEB1 and ZEB2 (11).
However, the molecular mechanisms by which ZEBs are induced only in
cancer cells with high aggressiveness, such as the basal-like
subtype of breast cancer cells, remain unclear. Recently, we found
that activation of MEK-ERK1/2 pathway induces expression of ZEBs,
and that the MEK1/2 inhibitor U0126 decreases ZEB expression in
breast cancer cells (7,11). Although ZEBs were more strongly
expressed in glioma cells than in the basal-like subtype of breast
cancer cells (Fig. 2A), the levels of
phospho-ERK1/2 did not dramatically differ between these two cell
types (7), suggesting that activation
of the ERK1/2 pathway alone is not sufficient to induce ZEBs. Thus,
expression of ZEBs may be regulated by an unknown pathway or
pathways that collaborate with the ERK1/2 pathway and is more
highly activated in glioma cells.
TGF-β dramatically induces ZEBs in many cell types,
and thus acts as a key cytokine in the EMT (5). In glioma cells, pathological grade is
positively correlated with the amounts of secreted TGF-β and
mesenchymal marker proteins (6). When
TGF-β signals in glioma cells are ameliorated by chemical
inhibitors, tumor growth and stemness properties are dramatically
suppressed (15). Consistent with
previous results (15), transient
treatment with siRNA also reduced tumor growth in vivo
(Fig. 1). Moreover,
whole-transcriptome sequencing analyses have revealed that the
frequency of mutations in IDH1 and TP53 (which
encodes p53) is lower in primary glioblastomas, but higher in
recurrent tumors (i.e., grade IV glioblastoma multiforme) (16). In esophageal cancer, mutant p53
contributes to the enrichment of cancer cells undergoing the EMT
via upregulation of ZEB1 (17). In
addition, epidermal growth factor receptor (EGFR) is frequently
amplified and mutated in glioblastomas. Following treatment with an
EGFR-blocking monoclonal antibody, patients with recurrent
glioblastoma have a significantly superior progression-free
survival and better overall survival. Taken together, these
observations indicate that the MEK-ERK1/2 pathway activated by
receptor tyrosine kinases, in cooperation with p53 and/or TGF-β,
determines the aggressiveness of glioma cells by regulating
expression of ZEBs.
The positive correlation between ZEB1 expression and
invasiveness of glioblastoma was reported previously (18). In this study, we found that both ZEB1
and ZEB2 are highly expressed in high-grade and recurrent glioma,
and that their levels are positively correlated (Fig. 3). Thus far, however, we have not
detected any positive correlation between expression of ZEBs and
overall survival. Therefore, we must further investigate this
correlation using more patients with detailed clinical information.
In addition, further analyses, especially of ZEB2, will require
high-quality antibodies with adequate sensitivities for IHC; the
antibodies used in this study were obtained commercially and may
have been of insufficient quality for IHC analysis.
In summary, we showed that ZEB1 and ZEB2 are more
highly expressed in grade IV glioma than in grades II and III.
ZEB-targeted diagnosis and therapy for high grade gliomas would
require development of high-quality antibodies that simultaneously
recognize both ZEB1 and ZEB2, as well as anti-tumor drugs that
simultaneously target both proteins. Our findings show that ZEBs
are promising diagnostic and therapeutic targets for glioma and/or
glioblastoma multiforme.
Acknowledgements
The authors would like to thank Dr T. Nakazawa
(Department of Pathology, University of Yamanashi, Yamanashi,
Japan) for the immunohistochemical analyses and valuable
discussions.
Funding
This work was supported by JSPS KAKENHI (grant nos.
JP15H05018 and 26462178).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KS, TK, and KE performed experiments and analyzed
data. KM, and HK drafted manuscript. TK, and MS conceived and
designed the project, and drafted manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and national research committees and with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards. The study was approved by the Ethics Committee of the
University of Yamanashi and written informed consent was obtained
from all participants. All applicable international, national, and
institutional guidelines for the care and use of animals were
followed. All procedures performed involving animals were in
accordance with the ethical standards of the institution or
practice at which the studies were conducted.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zaravinos A: The regulatory role of
MicroRNAs in EMT and cancer. J Oncol. 2015:8658162015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saitoh M: Epithelial-mesenchymal
transition is regulated at post-transcriptional levels by
transforming growth factor-β signaling during tumor progression.
Cancer Sci. 106:481–488. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Horiguchi K, Sakamoto K, Koinuma D, Semba
K, Inoue A, Inoue S, Fujii H, Yamaguchi A, Miyazawa K, Miyazono K
and Saitoh M: TGF-β drives epithelial-mesenchymal transition
through δEF1-mediated downregulation of ESRP. Oncogene.
31:3190–3201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shirakihara T, Kawasaki T, Fukagawa A,
Semba K, Sakai R, Miyazono K, Miyazawa K and Saitoh M:
Identification of integrin α3 as a molecular marker of cells
undergoing epithelial-mesenchymal transition and of cancer cells
with aggressive phenotypes. Cancer Sci. 104:1189–1197. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawataki T, Yamane T, Naganuma H,
Rousselle P, Andurén I, Tryggvason K and Patarroyo M: Laminin
isoforms and their integrin receptors in glioma cell migration and
invasiveness: Evidence for a role of alpha5-laminin(s) and
alpha3beta1 integrin. Exp Cell Res. 313:3819–3831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shirakihara T, Horiguchi T, Miyazawa M,
Ehata S, Shibata T, Morita I, Miyazono K and Saitoh M: TGF-β
regulates isoform switching of FGF receptors and
epithelial-mesenchymal transition. EMBO J. 30:783–795. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brehar FM, Ciurea AV, Chivu M, Zarnescu O,
Radulescu R and Dragu D: The development of xenograft glioblastoma
implants in nude mice brain. J Med Life. 1:275–286. 2008.PubMed/NCBI
|
|
11
|
Sinh ND, Endo K, Miyazawa K and Saitoh M:
Ets1 and ESE1 reciprocally regulate expression of ZEB1/ZEB2,
dependent on ERK1/2 activity, in breast cancer cells. Cancer Sci.
108:952–960. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fukagawa A, Ishii H, Miyazawa K and Saitoh
M: δEF1 associates with DNMT1 and maintains DNA methylation of the
E-cadherin promoter in breast cancer cells. Cancer Med. 4:125–135.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelial-to-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fischer KR, Durrans A, Lee S, Sheng J, Li
F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al:
Epithelial-to-mesenchymal transition is not required for lung
metastasis but contributes to chemoresistance. Nature. 527:472–476.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ikushima H, Todo T, Ino Y, Takahashi M,
Miyazawa K and Miyazono K: Autocrine TGF-beta signaling maintains
tumorigenicity of glioma-initiating cells through Sry-related
HMG-box factors. Cell Stem Cell. 5:504–514. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li R, Li H, Yan W, Yang P, Bao Z, Zhang C,
Jiang T and You Y: Genetic and clinical characteristics of primary
and secondary glioblastoma is associated with differential
molecular subtype distribution. Oncotarget. 6:7318–7324.
2015.PubMed/NCBI
|
|
17
|
Ohashi S, Natsuizaka M, Wong GS,
Michaylira CZ, Grugan KD, Stairs DB, Kalabis J, Vega ME, Kalman RA,
Nakagawa M, et al: Epidermal growth factor receptor and mutant p53
expand an esophageal cellular subpopulation capable of
epithelial-to-mesenchymal transition through ZEB transcription
factors. Cancer Res. 70:4174–4184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siebzehnrubl FA, Silver DJ, Tugertimur B,
Deleyrolle LP, Siebzehnrubl D, Sarkisian MR, Devers KG, Yachnis AT,
Kupper MD, Neal D, et al: The ZEB1 pathway links glioblastoma
initiation, invasion and chemoresistance. EMBO Mol Med.
5:1196–1212. 2013. View Article : Google Scholar : PubMed/NCBI
|