Introduction
Breast cancer is the most common type of malignant
cancer among women worldwide, and the second leading cause of
cancer-associated mortality among women in Asia in 2012 (1). In 2012, figures published by the
International Agency for Research on Cancer (IARC), the specialized
cancer agency of the World Health Organization, indicate ~1.7
million women globally are diagnosed with breast cancer annually,
which attributes for an estimated 520,000 mortalities each year
(accounting for 15% of all cancer associated-loss of life)
(2). Metastasis, together with
recurrence and resistance to chemotherapy, are the leading causes
of breast cancer mortality (3);
however, the underlying mechanism of breast cancer metastasis is
still poorly understood and warrants extensive study.
MicroRNAs (miRNAs), single-stranded noncoding RNAs,
play pivotal roles in numerous biological processes, ranging from
development, organogenesis, proliferation, apoptosis, and migration
to invasion (4). In general, miRNAs
attenuate or repress the expression of target genes at the
post-transcriptional level through sequence-specific binding to the
3′untranslated region (3′UTR) of target genes (5). Aberrant miRNA expression is associated
with diverse pathophysiological diseases, including cancer.
Accumulating evidence demonstrates that miRNAs are implicated in
tumor metastasis and control central metastasis regulators
(6). miR-10b, one of the metastasis
miRs, is upregulated in breast cancer tissues and cells (7,8). It
positively regulates invasion and metastasis by inhibition of
HOXD10 and resulting in increased expression of ras homolog family
member C (RHOC) (8), negatively
regulates phosphatase and tensin homolog (PTEN) and ultimately
results in protein kinase B (AKT) activation in breast cancer stem
cells (9). Furthermore, miR-10b plays
a critical role in transforming growth factor (TGF)-β1-induced
epithelial-mesenchymal transition (EMT) in breast cancer (7).
Abundant core fucosylation was reported in numerous
types of cancer, including breast cancer (10), hepatocellular carcinoma (11), non-small cell lung cancer (12) and colon carcinoma (13). Fucosyltranferase 8 (FUT8) specifically
catalyzes the transfer of a fucose residue to the innermost GlcNAc
residue of N-linked type complex glycopeptide via the α1,6-linkage.
Upregulated levels of FUT8 were revealed in the EMT process
(12) and in activation of the
β-catenin pathway, the PI3K/AKT signaling pathway and hypoxia
condition (14). miR-26a, miR-34a and
miR-146a repress FUT8 expression by binding to the 3′UTR of
FUT8 in hepatocellular carcinoma (11). However, thus far, the regulation of
FUT8 in breast cancer was not previously documented.
In the present study, it was confirmed that miR-10b
promoted the motility and proliferation of breast cancer cells, by
enhancing FUT8 expression, resulting in the activation of AKT
signaling.
Materials and methods
Cells and cell culture
The immortalized human mammary epithelial cell line
MCF10A and human breast cancer cell line MDA-MB-231 were obtained
from the American Type Culture Collection (Manassas, VA, USA).
MCF10A cells were cultured in Dulbecco's modified Eagle's medium
(DMEM)/F12 complete medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), containing epidermal growth factor (20 ng/ml),
hydrocortisone (0.5 mg/ml), cholera toxin (100 ng/ml) purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany), insulin (10
µg/ml), penicillin (100 units/ml) and streptomycin (100 µg/ml;
Gibco), at 37°C in 5% CO2. MDA-MB-231 cells were
cultured in DMEM (Hyclone; GE Healthcare, Chicago, IL, USA) at 37°C
in 5% CO2. All cell cultures were supplemented with 10%
fetal bovine serum (FBS; Hyclone; GE Healthcare), 100 IU/ml
penicillin and 100 µg/ml streptomycin.
Antibodies and reagents
Primary antibodies used were as follows: Mouse
anti-epithelial (E)-cadherin immunoglobulin (Ig)G2a mAb (1:50,000;
cat. no. 610181) (BD Biosciences; San Jose, CA, USA), mouse
anti-Fut8 IgG1 mAb (1:1,000; cat. no. sc-271244), mouse anti-Twist
(Twist2C1a; 1:1000; cat. no. sc-81417), mouse anti-N-cadherin IgG1
mAb (1:1,000; cat. no. sc-8424) (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), rabbit anti-GAPDH (1:100,000; cat. no. G9545) and
anti-Fibronectin polyclonal antibodies (FN; 1:10,000; cat. no.
F3648) (Sigma-Aldrich; Merck KGaA), rabbit anti-AKT (1:1,000; cat.
no. 9272) and anti-phosphorylated (p)-AKT (Ser473; D9E)
XP® mAb (1:1,000; cat. no. 4060) (Cell Signaling
Technology, Inc., Danvers, MA, USA). The secondary antibodies were
horseradish peroxides (HRP)-labeled goat anti-mouse IgG (1:5,000;
cat. no. A0216) and goat anti-rabbit IgG (1:5,000; cat. no. A0208)
(Beyotime Institute of Biotechnology, Haimen, China).
The reagents used in the present study were as
follows: MK2206 (Sigma-Aldrich; Merck KGaA) and TGF-β1 (BD
Biosciences). MK2206, a PI3K/AKT signaling inhibitor, can
persistently reduce the expression of p-AKT. MK2206 (working
concentration 10 nM) was added to miR-10b-overexpressing MCF10A
cells, using DMSO as a negative control, to confirm whether miR-10b
promotes cell motility and proliferation via activating AKT. For
induction of EMT, MCF10A cells (~30% confluence) were incubated
with 5 ng/ml TGF-β1 at 37°C for 48 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA, including miRNA, was extracted using
TRIzol reagent (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols. The concentration was determined using a
NanoDrop ND-1000 (Thermo Fisher Scientific, Inc.) and the RNA
sample (A260/A280>1.8) was reversed transcribed using ReverTra
Ace-α-® kit (Toyobo; Shanghai, China), according to the
manufacturer's protocols. Specific primers used for multiple genes
were as follows: FUT8 forward, 5′-TCCATGACCCTAATGGTCTTTT-3′; and
reverse, 5′-TGTCCTGTACTTCATGCGCT-3′; β-actin forward,
5′-GCACAGAGCCTCGCCTT-3′; and reverse, 5′-GTTGTCGACGACGAGCG-3′;
RNU6B forward, 5′-CTCGCTTCGGCAGCACA-3′; and reverse,
5′-AACGCTTCACGAATTTGCGT-3′. The specific hairpin-itTM miRNA primers
for miR-10b were designed and synthesized by GenePharma (Suzhou,
China; cat. no. F02001). RT-qPCR was performed using UltraSYBR
Mixture (Beijing CoWin Biotech Co., Ltd., Beijing) and run on the
CFX96 RT-PCR detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The relative expression levels of the target
genes were quantified using 2−ΔΔCq method from
triplicate experiments (15).
Plasmid construction
The plasmid pBABE-Puro-Twist was purchased from
Addgene, Inc. (Cambridge, MA, USA) and for the plasmid
pLVX-AcGFP1-FUT8, human gene FUT8 was amplified with the
following primer: Sense, 5′-CCGCTCGAGCGGGCCACCATGCGGCCATGGACTG, and
antisense, 5′-CGCGGATCCGCGGATCAGAGCCCTCTTCATCTACAG, using the same
PCR conditions as aforementioned. The PCR product was digested with
restriction enzyme XhoI and BamHI, and ligated into
the vector pLVX-AcGFP1-N1 (Addgene Inc.) at the corresponding
sites, according to the manufacturer's instructions. All the
plasmids were confirmed by DNA sequencing.
Transfection and RNA interference
MCF10A cells were stably transfected with plasmids
pBABE-Puro-Twist and the negative control vector, pBABE-puro, using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), selected with puro (0.5 µg/ml) and designated as
MCF10A/Twist and MCF10A/Mock. Lentiviral vector pLVX-AcGFP1-FUT8 or
pLVX-AcGFP1-N1 (negative control) and packaging plasmids were
co-transfected into 293T cells using Lipofectamine®
2000. After 48 h post-transfection, supernatants containing
lentiviruses were harvested and purified with 0.22 µM filters
(Merck KGaA). Then, the lentiviruses titer were determined and
infected into MCF10A cells. The stable clones were selected with
puro and designated as MCF10A/FUT8 and MCF10A/Mock2 (negative
control). All the stably transfected cells were confirmed by
western blot analysis. For RNA interference assay, MDA-MB-231 cells
were transfected with small interfering (si)RNAs for FUT8 and
negative control (NC), and expression of FUT8 was detected by
RT-qPCR and western blot. The sequences of validated siRNA for
FUT8 were: Forward, 5′-GUGUCUCAGUUUGUCAAAUTT-3′; and
reverse, 5′-AUUUGACAAACUGAGACACTT-3′; for si-1; forward,
5′-GGUGUGUAAUAUCAACAAATT-3′; and reverse,
5′-UUUGUUGAUAUUACACACCTT-3′ for si-2.
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
buffer on ice for 30 min, supplemented with protease inhibitor and
phosphorylase inhibitor (Sigma-Aldrich; Merck KGaA), and
centrifuged at 14,000 × g at 4°C for 15 min. Total protein samples
(30 µg/lane) were separated on a 10% gel using SDS-PAGE and
transferred onto polyvinylidene fluoride membranes by
electrophoresis. After 2 h of blocking with 5% bovine serum albumin
(BSA; cat. no. B2064; Sigma-Aldrich) at 37°C, membranes were
incubated with E-cadherin, N-cadherin, Vimentin, FUT8, AKT, p-AKT,
Fibronectin, Twist and GADPH primary antibodies overnight at 4°C,
and blotted with appropriate HRP-conjugated secondary antibody at
room temperature for 30 min. Protein bands were analyzed by
ChemiDoc XRS+ system (Bio-Rad).
Wound healing
For the wound healing assay, cells were seeded into
6-well plates at a density of 5×105 cells/well for 24 h.
Linear wounds were scratched on the 100% confluent monolayer using
a pipette tip. Cells were cultured in medium without FBS; images
were captured under an inverted microscope (×40, magnification) at
0 and 24 h and analyzed using the Scion image software (version
4.0.3.2; Scion Corporation, Frederick, MD, USA).
Transwell assays
Transwell assays were performed in Transwell inserts
with an 8.0 µm-pore polycarbonate membrane (Costar; Corning
Incorporated, Corning, NY, USA). Briefly, pretreated cells were
suspended in serum-free media and seeded into the upper chamber
(1.5×105 cells/chamber). The complete growth medium
supplemented with 10% FBS was deposited in the lower chamber. After
incubation for 16 h, cells on the inside of the membrane were
removed with a cotton swab and the migrated cells on the outside of
the membrane were fixed and stained with 0.1% crystal violet in
methanol for 15 min, and imaged under a light microscope. The
stained cells were lysed with 2% SDS in PBS and subjected to
spectrophotometric analysis at 570 nm.
MTT assay
Cells at a density of 2×103 cells were
plated in 96-well plates and at various hours of culturing (0, 24,
48, 72 and 92 h), 10 µl MTT solution (Cers, Yantai, China) was
added to form formazan. The reaction was stopped by the addition of
100 µl DMSO. The absorbance was measured at 490 nm and assessed as
previously described (16).
Statistical analysis
Data were presented as the mean ± standard deviation
from 3 repeated experiments using GraphPad Prism 5 software
(GraphPad Software, Inc., La Jolla, CA, USA). Data between groups
were analyzed by paired or unpaired Student's t-test and multiple
testing was performed using one-way ANOVA and a t-test with
Bonferroni's correction as a post-hoc test. Comparisons between
means with P<0.05 were considered to indicate a statistically
significant difference.
Results
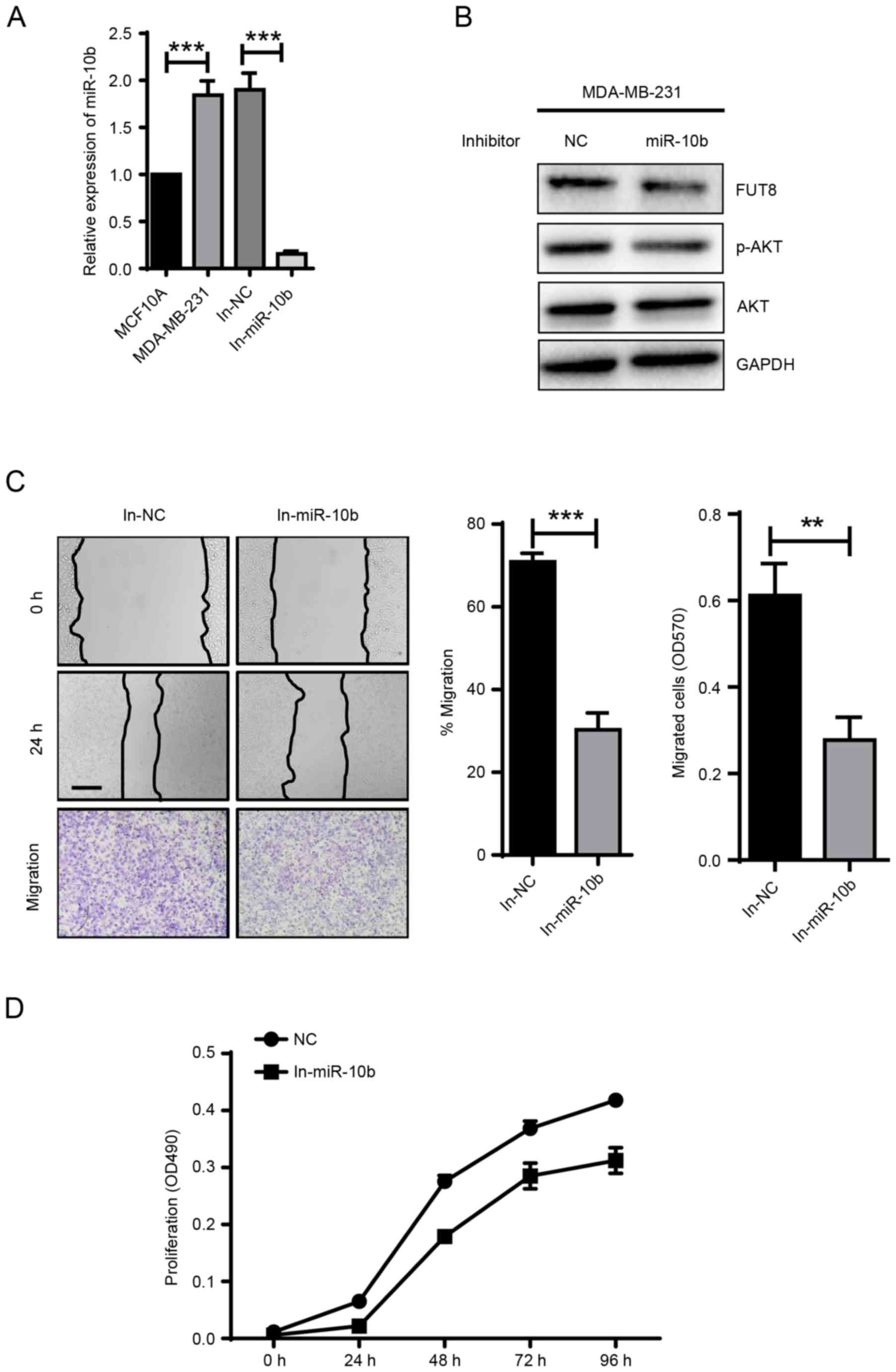
miR-10b promotes cell motility and
proliferation via activating AKT
It has been revealed that miR-10b exerts positive
effect on cell metastasis and invasion, in vitro and in
vivo (8). Critical role of
miR-10b is also demonstrated in TGF-β1-induced EMT process in
breast cancer (7). In the present
study, miR-10b was transfected into MCF10A cells, which
demonstrated a 5.6×104-fold increase, compared with NC
(Fig. 1A), and the impact of
overexpressed miR-10b on cell motility and proliferation were
examined. Compared with NC-mimic, cell motility evaluated by wound
healing and transwell assays, and cell proliferation evaluated by
MTT assay were significantly promoted for ~1.7-fold (P<0.05) and
~2-fold (P<0.01) with miR-10b overexpression, respectively,
(Fig. 1B and C). The expression of
the epithelial marker E-cadherin, mesenchymal markers N-cadherin
and vimentin were detected to confirm the effects of miR-10b on
EMT. miR-10b induced increased vimentin expression, however, no
effects on E-cadherin and N-cadherin expression were observed
(Fig. 1D). In addition, an increase
in p-AKT expression was observed in miR-10b-overexpressed MCF10A
cells (Fig. 1D). Conversely, the
motility and proliferation of MCF10A cells were significantly
reduced when p-AKT was inhibited (Fig. 1B
and C) with an AKT antagonist MK2206, compared with
miR-10b-transfected cells (Fig. 1E).
Following the addition of the MK2206 inhibitor, the data indicated
that miR-10b may promote the cell motility and proliferation via
activating AKT.
 | Figure 1.miR-10b promotes the motility and
proliferation of BC cells via activation of AKT. (A) Fold-changes
of miR-10b were assessed in miR-10b transiently overexpressed
MCF10A cells. (B) MCF10A cells transiently transfected with miR-10b
and treated with or without MK2206 were subjected to wound healing
assay (Scale bar, 200 µm) and transwell assay (×100 magnification).
(C) Analysis of proliferation of miR-10b transiently overexpressed
MCF10A cells cultured ± MK2206 inhibitor. (D) Western blot analysis
of E-cadherin, N-cadherin, vimentin, p-AKT and AKT in miR-10b
transiently overexpressed MCF10A cells. (E) Western blot analysis
of p-AKT and AKT in miR-10b transiently overexpressed MCF10A cells
± MK2206 inhibitor. **P<0.01, ***P<0.001 vs. NC. BC, breast
cancer; AKT, protein kinase B; p-AKT, phosphorylated protein kinase
B; miR, microRNA; NC, negative control; E, epithelial; N,
neural. |
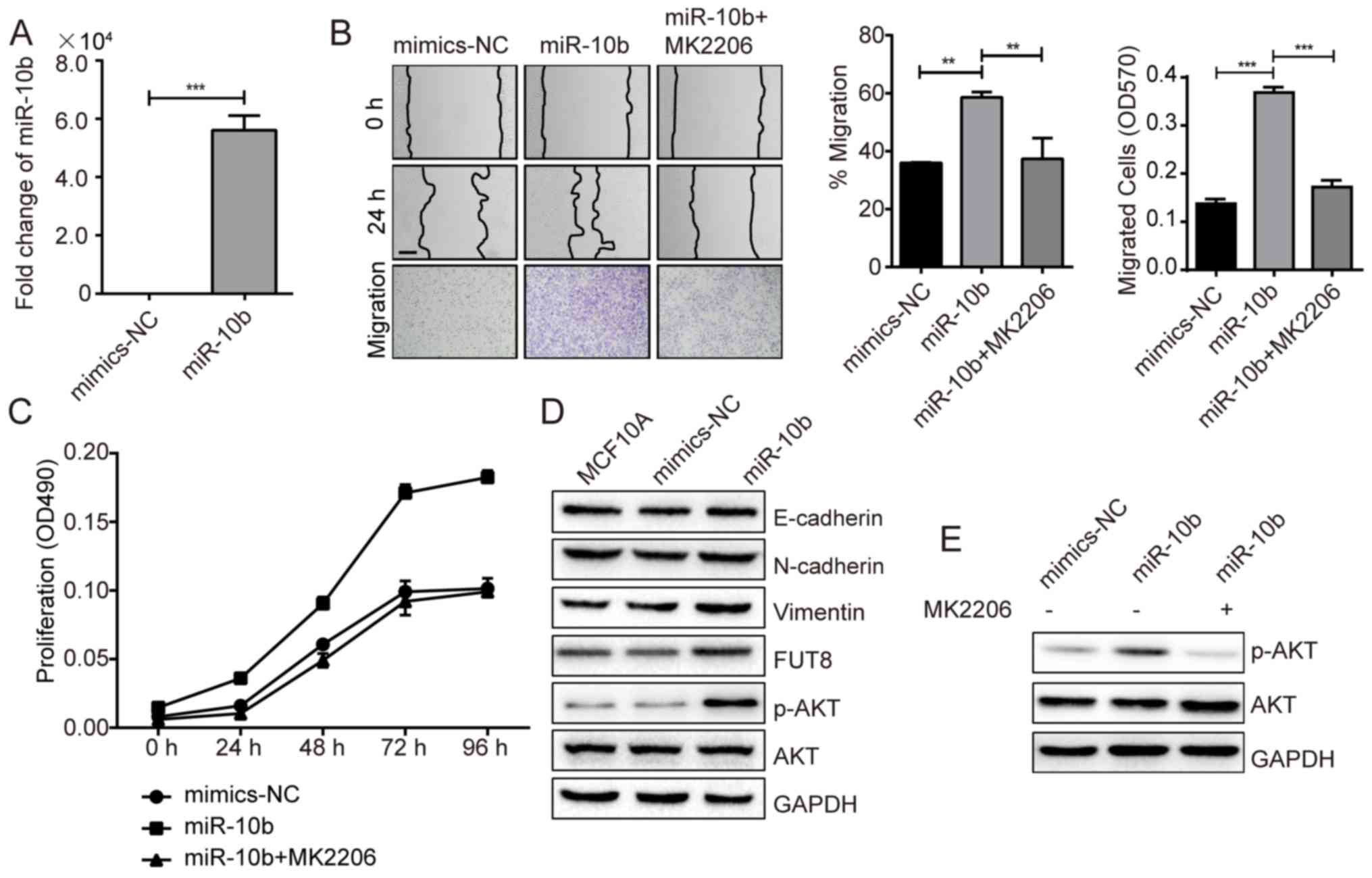
Roles of FUT8 on cell motility and
proliferation
Accumulated evidence has highlighted the important
role of glycosylation in cancer progression (17). In a previous study, we performed a
gene array to analyze the changes of glycogenes in
miR-10b-overexpressed MCF10A cells. Specifically, it was notable
that FUT8 was significantly upregulated by miR-10b (Fig. 1D). It was thus proposed that miR-10b
may function through the regulation of the target gene, FUT8.
To validate our hypothesis, we established stable
FUT8-overexpressed cells MCF10A/FUT8 (Fig. 2A). There was a 2.5-fold increase
(P<0.05) of p-AKT expression in MCF10A/FUT8 cells, compared with
Mock2 cells. In addition, overexpressed FUT8 resulted in decreased
E-cadherin and enhanced Fibronectin (FN) and N-cadherin expression
(Fig. 2A), though no obvious change
on cell morphology was observed (data not shown). The effects of
FUT8 overexpression significantly promoted the motility and
proliferation of MCF10A cells at 24 h, compared with
Mock2-transfected cells (Fig. 2B and
C). Metastatic MDA-MB-231 cells, in comparison with MCF10A
cells, displayed increased p-AKT and FUT8 expression (Fig. 2D). Transient silencing of FUT8 in
MDA-MB-231 cells attenuated FUT8 and p-AKT expression (Fig. 2E), and decreased cell motility and
proliferation, compared with NC cells (Fig. 2F and G).
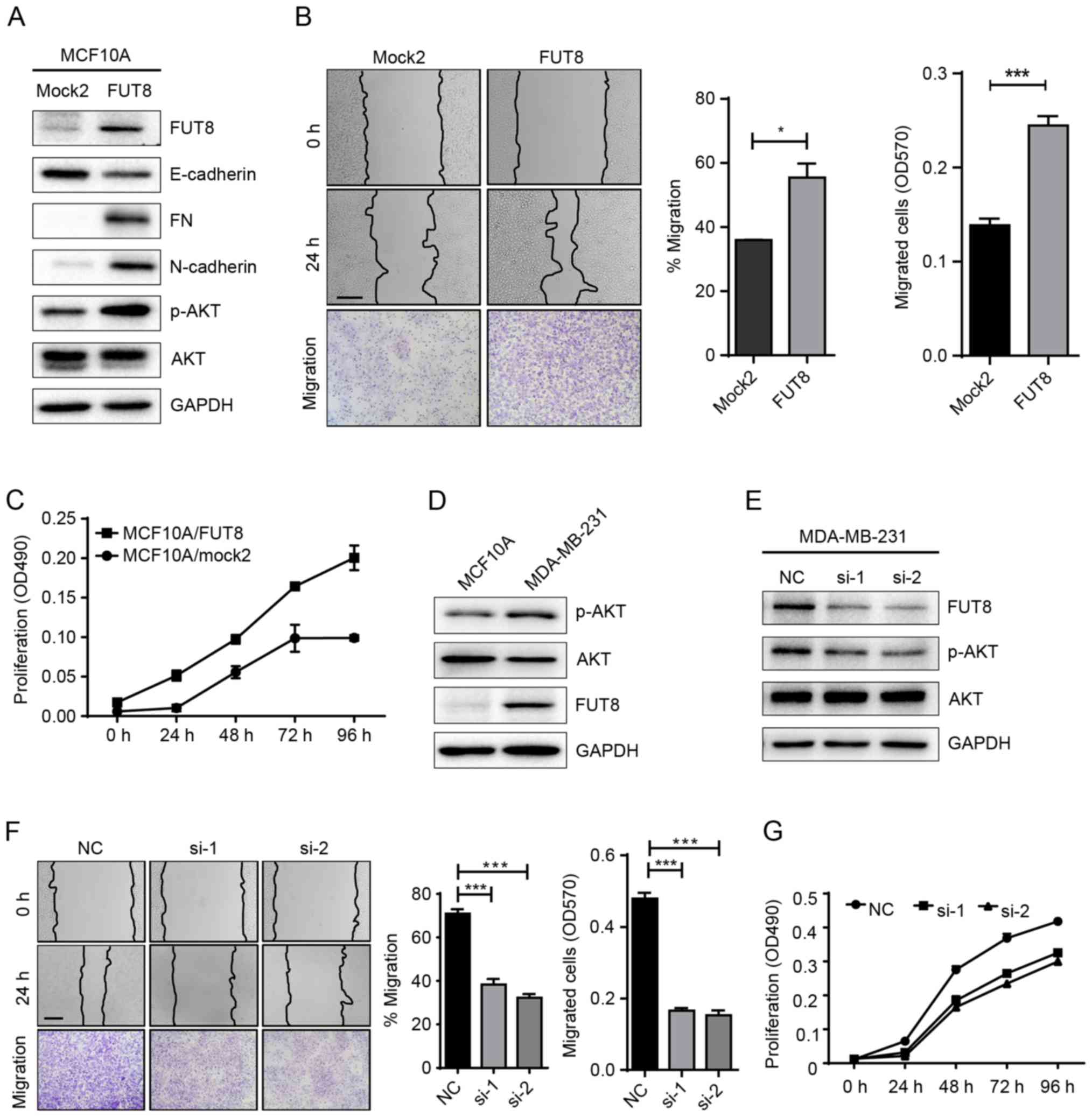 | Figure 2.Effects of FUT8 on cell motility and
proliferation. (A) Western blot analysis of EMT markers, p-AKT and
AKT, detected in FUT8-overexpressed MCF10A cells. (B)
FUT8-overexpressed MCF10A cells were subjected to wound healing and
Transwell assay. *P<0.05, ***P<0.001 vs. Mock. (C)
Proliferation analysis of FUT8-overexpressed MCF10A cells. (D)
Western blot analysis of p-AKT, AKT and FUT8 expression in MCF10A
and MDA-MB-231 cells. (E) Western blot analysis of p-AKT, AKT and
FUT8 in FUT8-knockdown MDA-MB-231 cells. (F) Wound healing and
Transwell assay (Scale bar, 200 µm; ×100 magnification) in
FUT8-knockdown MDA-MB-231 cells. (G) MTT was performed in
FUT8-knockdown MDA-MB-231 cells. ***P<0.001 vs. NC. FUT8,
fucosyltranferase 8; p-AKT, phosphorylated protein kinase B; AKT,
protein kinase B; NC, negative control. |
The effects of miR-10b on p-AKT
expression in TGF-β-treated and Twist-overexpressed MCF10A
cells
Transcriptional factor Twist has been revealed to
induce the EMT process in multiple cell models (18). Twist directly binds to an E-box
proximal to the putative promoter of miR-10b and regulates its
transcription (8). Herein, a stable
Twist-overexpressed cell MCF10A/Twist was established, and the
expression of miR-10b, FUT8 and p-AKT were detected. It was
demonstrated that overexpressed Twist significantly upregulated
miR-10b and FUT8 and activated the phosphorylation of AKT (Fig. 3A and B). As expected, increased cell
motility and proliferation were also observed, compared with MCF10A
cells (Fig. 3C and D). Conversely,
repression of miR-10b by its inhibitor, resulted in significantly
decreased FUT8 and p-AKT expression in Twist-overexpressed MCF10A
cells (Fig. 3E). Consistently, cell
motility and proliferation were decreased (Fig. 3C and D).
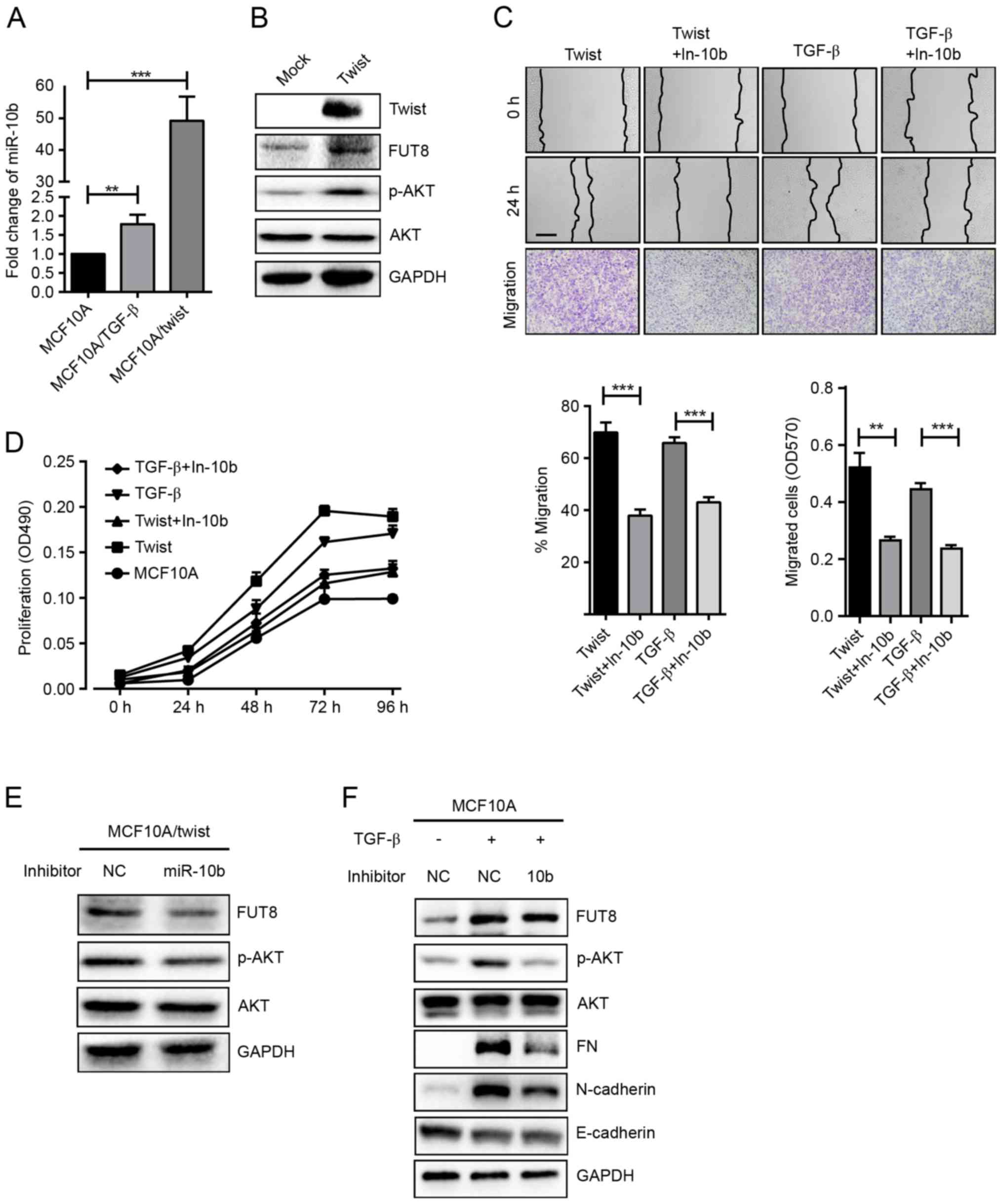 | Figure 3.Effects of miR-10b on p-AKT expression
in TGF-β-treated and Twist-overexpressed MCF10A cells. (A) miR-10b
expression was detected in TGF-β-treated and Twist-overexpressed
MCF10A cells and presented as fold-changes. **P<0.01,
***P<0.001 vs. MCF10A. (B) Western blot analysis of FUT8, p-AKT
and AKT, detected in Twist-overexpressed MCF10A cells. (C)
TGF-β-treated and Twist-overexpressed MCF10A cells were subjected
to wound healing and transwell assay ± inhibitor of miR-10b.
**P<0.01; ***P<0.001 vs. TGF-β-treated or Twist-overexpressed
MCF10A cells. (D) Proliferation analysis in TGF-β-treated and
Twist-overexpressed MCF10A cells ± inhibitor of miR-10b. (E)
Western blot analysis of FUT8, p-AKT and AKT protein in
Twist-overexpressed MCF10A cells, ± inhibitor of miR-10b. (F)
Western blot analysis of EMT markers, FUT8, p-AKT and AKT, detected
in TGF-β-treated MCF10A cells, ± inhibitor of miR-10b. (Scale bar,
200 µm; ×100 magnification). p-AKT, phospho- protein kinase B;
TGF-β, transforming growth factor-β; FUT8, fucosyltranferase;
p-AKT, phosphorylated protein kinase B; AKT, protein kinase B; EMT,
epithelial-mesenchymal transition; miR, microRNA. |
The same phenomenon was observed in TGF-β-induced
cells. TGF-β repressed the expression of E-cadherin, enhanced the
expression of Fibronectin, N-cadherin and FUT8, and activated the
AKT pathway (Fig. 3A). When
TGF-β-induced MCF10A cells were treated with miR-10b inhibitor,
cell motility and proliferation were decreased (Fig. 3C and D). In addition, expression of
Fibronectin, N-cadherin and p-AKT were decreased, while the
expression of FUT8 was slightly attenuated (Fig. 3F).
Inhibitor of miR-10b attenuated p-AKT
expression and reduced the migration and proliferation capacity in
MDA-MB-231 cells
miR-10b is reported to be abundant in breast cancer
cells. Notably, higher miR-10b expression was observed in
triple-negative breast cancer cell lines compared with 6 human
breast cancer-derived cell lines (19). Consistent with the previous report,
miR-10b expression in MDA-MB-231 cells was significantly higher
than in MCF10A cells (Fig. 4A). In
MDA-MB-231 cells treated with miR-10b inhibitor for 48 h, miR-10b
expression was significantly repressed (Fig. 4A). In response to the downregulation
of miR-10b, FUT8 and p-AKT expression were also decreased (Fig. 4B). These results were consistent with
the observation in both Twist-overexpressed and TGF-β-induced
MCF10A cells. Furthermore, downregulated cell motility and
proliferation were detected with the addition of miR-10b inhibitor
in MDA-MB-231 cells, compared with NC cells (Fig. 4C and D).
Discussion
Altered glycosylation is frequently associated with
tumor development and progression and the therapeutics and
diagnostics based on glycans have been investigated (20–22). In
addition, miRNAs are potential therapeutic targets for cancer due
to the key regulatory roles, identified as oncogenes and tumor
suppressors in previous years (23).
miRNA dysregulation has been identified as a frequent phenomenon in
breast cancer and certain dysregulated miRNAs have been validated
(24,25). However, the roles of miRNAs in
glycosylation during tumor progression were rarely reported.
miR-10b, which is upregulated in breast cancer
tissues and breast cancer cells, may contribute to lymph node
metastases in breast cancer (26). In
the present study, a glycan-related gene array was performed to
analyze the variation of glycosylation in miR-10b-overexpressed
MCF10A cells. It was observed that FUT8 expression was
significantly increased at the mRNA and protein levels, and the AKT
pathway was significantly activated. However, there were no
significant changes in E-cadherin and N-cadherin detected, although
a previous report demonstrated that miR-10b exerted a critical
function in TGF-β-induced EMT process in breast cancer (7).
Upregulated core fucosylation has been reported in
multiple types of cancer, including, prostate (27), non-small cell lung (28) and breast cancer (10). Increased core fucosylation was also
detected in EMT (12), consistent
with the result in the present study in TGF-β-induced MCF10A cells.
In the present study, a decline in the expression of epithelial
molecule E-cadherin and an increase in that of mesenchymal markers
(Fibronectin and N-cadherin) were observed in FUT8-overexpressed
MCF10A cells. Furthermore, upregulated p-AKT expression, increased
cell migration and proliferation were also demonstrated, consistent
with the role of FUT8 in regulating the activity of the PI3K/AKT
signaling pathway (29). Similar
results were discovered when miR-10b or FUT8 were knocked-down in
MDA-MB-231 cells, revealing a potential association between
miR-10b, FUT8 and the AKT pathway. In addition, the analysis of
inhibited miR-10b in TGF-β1-induced and Twist-overexpressed MCF10A
cells, led to the following observations: (i) EMT process was
partially reversed and the level of p-AKT was significantly
attenuated, although FUT8 was slightly altered in TGF-β1-induced
MCF10A cells. (ii) FUT8 and p-AKT were significantly attenuated
with inhibition of miR-10b in Twist-overexpressed MCF10A cells.
(iii) Cell migration and proliferation were decreased in response
to the inhibition of miR-10b.
miRNAs regulate the expression of target genes at
the post-transcriptional level through sequence-specific binding to
the 3′UTR of target genes (5).
miR-26a, miR-34a and miR-146a repress FUT8 expression by binding to
the 3′UTR of FUT8 in hepatocellular carcinoma (11). However, the exact mechanism by which
miR-10b manipulates FUT8 expression remains unclear. A potential
miR-10b/TFAP2C/STAT3 pathway is proposed by querying the Pathway
Commons (http://www.pathwaycommons.org).
Taken together, the results of the present study
demonstrated that miR-10b upregulates FUT8 expression, which
further activates the AKT pathway, enhancing the migration and
proliferation of breast cancer cells. Further follow-up studies
will be focused on the mechanism of miR-10b on the regulation of
FUT8 in breast cancer cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81672537), the
Natural Science Foundation of Jiangsu Province, China (grant nos.
BK20160173 and BK20161132) and the Fundamental Research Funds for
the Central Universities (grant nos. JUSRP51619B and
JUSRP116032).
Availability of data and materials
All data generated or analyzed during current study
are available from the corresponding author on reasonable
request.
Authors' contributions
FG and DG designed the experiments. DG, JG and XL
performed the experiments and analyzed the data. DG and FG wrote
the paper.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fan L, Goss PE and Strasser-Weippl K:
Current status and future projections of breast cancer in asia.
Breast Care (Basel). 10:372–378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peart O: Metastatic breast cancer. Radiol
Technol. 88:519M–539M. 2017.PubMed/NCBI
|
|
4
|
Bushati N and Cohen SM: microRNA
functions. Ann Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar
|
|
5
|
Samantarrai D, Dash S, Chhetri B and
Mallick B: Genomic and epigenomic cross-talks in the regulatory
landscape of miRNAs in breast cancer. Mol Cancer Res. 11:315–328.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang Q, Wang Y, Hao Y, Juan L, Teng M,
Zhang X, Li M, Wang G and Liu Y: miR2Disease: A manually curated
database for microRNA deregulation in human disease. Nucleic Acids
Res. 37:D98–D104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han X, Yan S, Weijie Z, Feng W, Liuxing W,
Mengquan L and Qingxia F: Critical role of miR-10b in transforming
growth factor-β1-induced epithelial-mesenchymal transition in
breast cancer. Cancer Gene Ther. 21:60–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bahena-Ocampo I, Espinosa M,
Ceballos-Cancino G, Lizarraga F, Campos-Arroyo D, Schwarz A,
Maldonado V, Melendez-Zajgla J and Garcia-Lopez P: miR-10b
expression in breast cancer stem cells supports self-renewal
through negative PTEN regulation and sustained AKT activation. EMBO
Rep. 17:648–658. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yue L, Han C, Li Z, Li X, Liu D, Liu S and
Yu H: Fucosyltransferase 8 expression in breast cancer patients: A
high throughput tissue microarray analysis. Histol Histopathol.
31:547–555. 2016.PubMed/NCBI
|
|
11
|
Cheng L, Gao S, Song X, Dong W, Zhou H,
Zhao L and Jia L: Comprehensive N-glycan profiles of hepatocellular
carcinoma reveal association of fucosylation with tumor progression
and regulation of FUT8 by microRNAs. Oncotarget. 7:61199–61214.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen CY, Jan YH, Juan YH, Yang CJ, Huang
MS, Yu CJ, Yang PC, Hsiao M, Hsu TL and Wong CH: Fucosyltransferase
8 as a functional regulator of nonsmall cell lung cancer. Proc Natl
Acad Sci USA. 110:630–635. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Osumi D, Takahashi M, Miyoshi E, Yokoe S,
Lee SH, Noda K, Nakamori S, Gu J, Ikeda Y, Kuroki Y, et al: Core
fucosylation of E-cadherin enhances cell-cell adhesion in human
colon carcinoma WiDr cells. Cancer Sci. 100:888–895. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mehta A, Comunale MA, Rawat S, Casciano
JC, Lamontagne J, Herrera H, Ramanathan A, Betesh L, Wang M, Norton
P, et al: Intrinsic hepatocyte dedifferentiation is accompanied by
upregulation of mesenchymal markers, protein sialylation and core
alpha 1,6 linked fucosylation. Sci Rep. 6:279652016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo D, Guo J, Li X and Guan F:
Differential effects of Pax3 on expression of
polysialyltransferases STX and PST in TGF-β-treated normal murine
mammary gland cells. Exp Biol Med (Maywood). 242:177–183. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Munkley J and Elliott DJ: Hallmarks of
glycosylation in cancer. Oncotarget. 7:35478–35489. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teng Y and Li X: The roles of HLH
transcription factors in epithelial mesenchymal transition and
multiple molecular mechanisms. Clin Exp Metastasis. 31:367–377.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fkih M'hamed I, Privat M, Ponelle F,
Penault-Llorca F, Kenani A and Bignon YJ: Identification of
miR-10b, miR-26a, miR-146a and miR-153 as potential triple-negative
breast cancer biomarkers. Cell Oncol (Dordr). 38:433–442. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stuchlova Horynova M, Raska M, Clausen H
and Novak J: Aberrant O-glycosylation and anti-glycan antibodies in
an autoimmune disease IgA nephropathy and breast adenocarcinoma.
Cell Mol Life Sci. 70:829–839. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ho WL, Hsu WM, Huang MC, Kadomatsu K and
Nakagawara A: Protein glycosylation in cancers and its potential
therapeutic applications in neuroblastoma. J Hematol Oncol.
9:1002016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Leoz ML, Young LJ, An HJ, Kronewitter
SR, Kim J, Miyamoto S, Borowsky AD, Chew HK and Lebrilla CB:
High-mannose glycans are elevated during breast cancer progression.
Mol Cell Proteomics. 10:M110.0027172011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kwan JY, Psarianos P, Bruce JP, Yip KW and
Liu FF: The complexity of microRNAs in human cancer. J Radiat Res.
57(Suppl 1): i106–i111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piva R, Spandidos DA and Gambari R: From
microRNA functions to microRNA therapeutics: Novel targets and
novel drugs in breast cancer research and treatment (Review). Int J
Oncol. 43:985–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Christodoulatos GS and Dalamaga M:
Micro-RNAs as clinical biomarkers and therapeutic targets in breast
cancer: Quo vadis? World J Clin Oncol. 5:71–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Min W, Wang B, Li J, Han J, Zhao Y, Su W,
Dai Z, Wang X and Ma Q: The expression and significance of five
types of miRNAs in breast cancer. Med Sci Monit Basic Res.
20:97–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Chen J, Li QK Peskoe SB, Zhang B,
Choi C, Platz EA and Zhang H: Overexpression of α (1,6)
fucosyltransferase associated with aggressive prostate cancer.
Glycobiology. 24:935–944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Honma R, Kinoshita I, Miyoshi E, Tomaru U,
Matsuno Y, Shimizu Y, Takeuchi S, Kobayashi Y, Kaga K, Taniguchi N
and Dosaka-Akita H: Expression of fucosyltransferase 8 is
associated with an unfavorable clinical outcome in non-small cell
lung cancers. Oncology. 88:298–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng L, Luo S, Jin C, Ma H, Zhou H and
Jia L: FUT family mediates the multidrug resistance of human
hepatocellular carcinoma via the PI3K/Akt signaling pathway. Cell
Death Dis. 4:e9232013. View Article : Google Scholar : PubMed/NCBI
|