Introduction
Malignancy is a common and frequently occurring
disease that seriously threatens human health. Malignancy is the
second leading cause of death worldwide, and is the first leading
cause of death in some countries. In the past several decades,
morbidity and mortality rate of lung cancer is the highest among
all types of malignancies (1). The
incidence of lung cancer is expected to be significantly increased
not only in developed countries, such as USA, but also in
developing countries, such as China (2), exerting enormous pressure on the society
(3). According to the lung tumor
histological classification established by WHO in 2015, lung cancer
originates from epithelial and mesenchymal tissues, and epithelial
tissue is the more common origin. Non-small cell lung cancer
(NSCLC) accounts for 85% of lung cancers (4,5). Pulmonary
adenocarcinoma is one of the main types of NSCLC. Among patients
with lung cancer, prognosis for patients with different TNM
clinical stages is significantly different. Early stage (IA-IIB)
NSCLC accounts for only 25–30% of the total number of lung cancers
(6). The 5-year survival rate for
stage I NSCLC is 73%, whereas for stage IV patients is only 13%
(7). Therefore, early diagnosis of
lung adenocarcinoma is particularly important.
At present, low-dose spiral computed tomography
(CT), liquid biopsy and fiberoptic bronchoscopy are mainly used for
early screening of lung cancer. Traditional serum tumor markers in
liquid biopsy have shown certain specificity and sensitivity in the
diagnosis of lung cancer, and the combination of several markers
can significantly improve the accuracy (8,9), such as
CEA, CA125, CA153, and Cyfra21-1, which is more sensitive to the
diagnosis of middle and advanced-stage lung cancer. Circulating
tumor cells (CTCs) refer to the tumor cells in circulating blood
from the primary tumor or metastatic lesions formed by passive
migration, invasion or interference of external factors after
passive shedding (10). CTCs are
present in peripheral blood even in patients with early-stage lung
cancer even before the formation of minimal lesions. However, the
detection of CTCs is not easy. As an observation in patients with
chronic obstructive pulmonary disease (COPD), chest CT and CTCs
detection results of 5 patients were compared every year to see if
there is a shift from COPD to lung cancer. The results showed that
when CTCs were found in the peripheral blood, no small pulmonary
nodules were detected on the chest CT scan; the nodules were
gradually found by chest CT scan in 1–4 years of subsequent
follow-up monitoring, and stage I lung cancer was confirmed by
postoperative pathology (11). CTCs
can also be used as a joint detection method to distinguish between
benign and malignant pulmonary nodules, and the detection rate is
higher than that of traditional tumor markers (12). At the same time, detection of CTCs can
also be used to guide clinical medication and prediction of
prognosis (13,14). Detection of circulating tumor nucleic
acids, including circulating tumor DNA (ctDNA) and circulating
tumor RNA (ctRNA), which were released by tumors into peripheral
blood in the early stages of tumorigenesis, can also be used to
guide the diagnosis. However, different examination methods provide
different results (15).
New bronchoscopic techniques, such as
electromagnetic navigation bronchoscopy (ENB), autofluorescence
bronchoscopy (AFB) and narrow band imaging (NBI) expanded the
diagnostic vision to a certain extent and increased the diagnostic
rate of lung cancer (especially early-stage lung cancer). However,
novel bronchoscopic techniques include invasive procedures and
patients' compliance is poor (16).
With the advantages of convenient operation,
reasonable cost, no obvious trauma and side-effects, low-dose
spiral CT has been widely used in the early diagnosis of lung
cancer (17,18). However, this technique also has some
limitations. For example, the false positive rate is high (19). Therefore, combined diagnosis is always
needed. For now, it has become a hot research field to analyze the
risk factors of stage I lung adenocarcinoma by low-dose
high-resolution spiral CT.
Materials and methods
Subjects
Patients were selected from June 2014 to June 2017
in Guangxing Hospital Affiliated to Zhejiang Chinese Medical
University, the First Affiliated Hospital of Zhejiang University
(Hangzhou, China), and Peking University People's Hospital
(Beijing, China). Patients without complete clinical records and
patients younger than 18 years were excluded. The patients were
further diagnosed in the Department of Thoracic Surgery in three
hospitals, and patients with stage I lung adenocarcinoma were
included to serve as case group. At the same time, benign pulmonary
nodule patients with similar age and sex distributions were also
included to serve as control group.
Diagnostic criteria
Patients in case group (stage I lung adenocarcinoma
patients) were diagnosed according to the diagnostic and treatment
guidelines for primary lung cancer clinics (2011 edition)
established by the Chinese Society of Clinical Oncology (CSCO)
(20). TNM staging was performed
according to the criteria established by the International
Association for the Study of Lung Cancer (IASLC) in 2009: T1a,
greatest diameter of tumor ≤2 cm; T1b, greatest diameter of tumor
>2 and ≤3 cm; N0, no regional lymph node metastasis; M0, no
distant metastasis (21). Patients in
control group were diagnosed as benign pulmonary nodules by
pathological examination and CT imaging (22).
Inclusion and exclusion criteria
Inclusion criteria: Patients in case group were
diagnosed with stage IA and IB lung adenocarcinoma. Patients
without serious cardiovascular, digestive and other diseases,
without liver and kidney dysfunction, age >18 years and <80
years, Karnofsky score >80 points, volunteered to participate in
the study and could cooperate with the researchers. Exclusion
criteria: Patients with a history of malignancy, received lobectomy
or pneumonectomy, severe infections, severe heart diseases,
complicated by liver, kidney and other serious primary diseases,
hematopoietic system, nervous system and mental illness, as well as
pregnant women were excluded from both case and control group.
Patients in control group diagnosed with malignant pulmonary
nodules were also excluded.
Data collection
General demographic information (sex, age,
ethnicity, marital status and education level) and self-reports of
dietary habits, tobacco and alcohol addiction, exercise habits,
past history of related diseases and family disease history were
collected. All patients were subjected to low-dose high-resolution
(512×512 matrix) 128-row 256 iCT imaging.
Ethics approval
This study was approved by the Ethics Committee
(approval no. 2014LL001) of Guangxing Hospital Affiliated to
Zhejiang Chinese Medical University (Ηangzhou, China). Signed
informed consents were obtained from the patients or the
guardians.
Statistical analyses
χ2 test was used to determine the
baseline of the two groups of subjects. Continuous variables were
compared by using ANOVA; Dunnett's method was used when the
variance was homogeneous, and Mann-Whitney U test was used when the
variance was not homogeneous in comparison between two groups.
Multivariate correlation analysis was performed by using logistic
regression analysis. Logistic regression analysis of malignancy
probability, nodule size and percentage of solid nodules in
patients with stage I lung adenocarcinoma were performed by using R
language. ROC curve was drawn and AUC was calculated to evaluate
the diagnostic value of the nodular site (X3), nodule
type (X4), nodule size (X5), spicule sign
(X7), lobulation sign (X8) to lung
adenocarcinoma status. The sensitivity and specificity were
calculated according to the cut-off value.
Results
General information
Case group included 82 males and 112 females, with
an average age of 57.54 years. Control group included 44 males and
46 females, with an average age of 56.28 years. After examination,
no significant differences in sex, age, ethnicity, marital status
and educational level were found between case and control group
(Table I).
 | Table I.Comparison of the basic information
between two groups. |
Table I.
Comparison of the basic information
between two groups.
| Variables | Cases | Case group
(n=194) | Control group
(n=90) | χ2
value | P-value |
|---|
| Sex |
|
Male | 126 | 82 | 44 | 1.092 | 0.296 |
|
Female | 158 | 112 | 46 |
|
|
| Age (years) |
|
<60 | 153 | 101 | 52 | 0.808 | 0.369 |
|
≥60 | 131 | 93 | 38 |
|
|
| Ethnicity |
| Han
nationality | 267 | 183 | 84 | 0.108 | 0.742 |
|
Minority | 17 | 11 | 6 |
|
|
| Marriage
status |
|
Married | 258 | 178 | 80 | 0.606 | 0.436 |
|
Unmarried and divorced | 26 | 16 | 10 |
|
|
| Degree of
education |
| Junior
high school and below | 151 | 104 | 57 | 2.482 | 0.289 |
| High
school, technical secondary |
| school
and college | 74 | 55 | 19 |
|
|
| College
and above | 49 | 35 | 14 |
|
|
| Smoking |
|
Yes | 85 | 56 | 29 | 0.330 | 0.566 |
| No | 199 | 138 | 61 |
|
|
| Family history of
cancer |
|
Yes | 52 | 35 | 17 | 0.030 | 0.864 |
| No | 232 | 159 | 73 |
|
|
Logistic regression analysis
Logistic regression analysis was performed by using
stage I lung adenocarcinoma (1= stage IA and IB lung
adenocarcinoma, 0= benign pulmonary nodules) as dependent variable,
and low-dose high-resolution CT imaging features including nodule
type, nodule size, vacuolar sign, bronchial inflatable sign,
bronchial truncated sign, smooth sign, spicule sign, lobulated sign
and pleural indentation as independent variables. Using stepwise
regression, equation for the final model showed that stage I lung
adenocarcinoma was associated with nodular site (X3,
upper left lobe) [95% CI (1.796, 54.695), p=0.008], nodule type
(X4) (p<0.001), nodule size (X5) [95% CI
(0.614, 0.803), p<0.001], spicule sign (X7) [95% CI
(0.029, 0.580), p=0.008] and lobulation sign (X8) [95%
CI (0.048, 0.673), p=0.011] (Table
II). Stepwise regression equation is: Logistic (p) =−12.009 +
2.294X3 - 0.327X4 - 0.354X5 -
2.042X7 - 1.713X8. Logistic regression
analysis of malignant probability, nodule size, and solid nodule
percentage in patients with stage I lung adenocarcinoma are shown
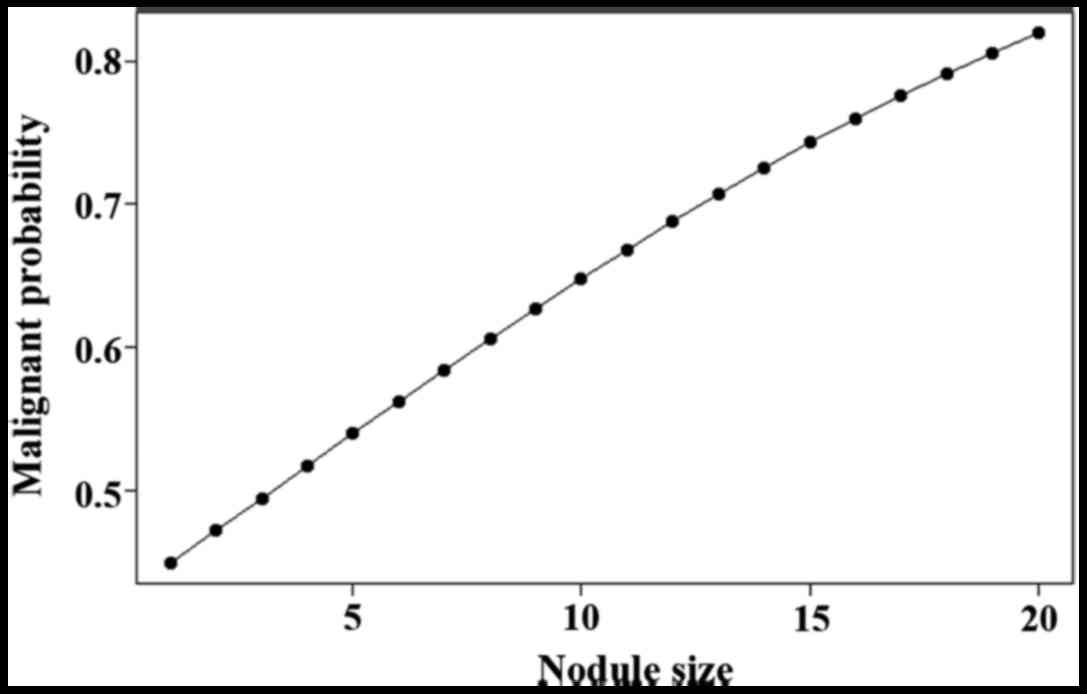
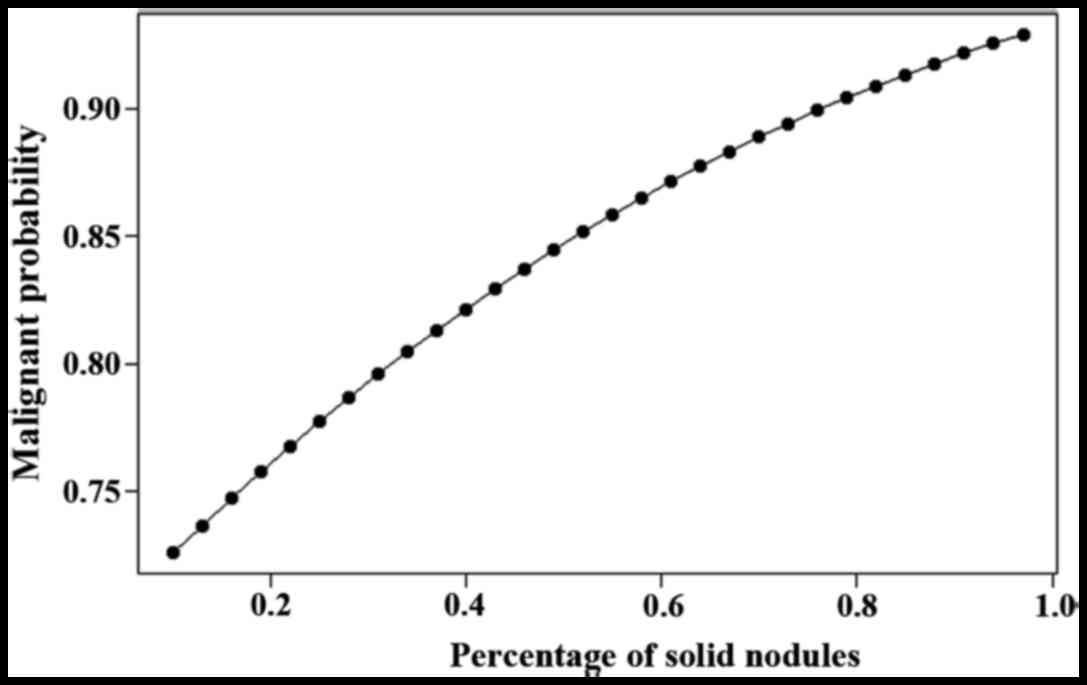
in Figs. 1 and 2.
 | Table II.Logistic regression analysis of
low-dose high-resolution CT imaging features on stage I lung
adenocarcinoma. |
Table II.
Logistic regression analysis of
low-dose high-resolution CT imaging features on stage I lung
adenocarcinoma.
| Variables | Types | B | SE | Wald | Odd ratio | 95% CI | P-value |
|---|
| Smoking
(X1) |
| 0.738 | 0.560 | 1.739 | 2.093 | 0.698–6.271 | 0.187 |
| Family history of
cancer (X2) |
| −1.042 | 0.736 | 2.007 | 0.353 | 0.083–1.491 | 0.157 |
| Nodular site
(X3) | Left upper
lobe | 2.294 | 0.872 | 6.925 | 9.910 | 1.796–54.695 | 0.008 |
|
| Left lower
lobe | 0.049 | 1.166 | 0.002 | 1.050 | 0.107–10.334 | 0.996 |
|
| Right upper
lobe | 1.240 | 0.832 | 2.223 | 3.455 | 0.677–17.633 | 0.136 |
|
| Right middle
lobe | 2.164 | 0.899 | 5.794 | 8.708 | 1.495–50.726 | 0.016 |
| Nodule type
(X4) |
|
|
| 44.188 |
|
| <0.001 |
|
| Partial solid
nodules | 24.995 | 27,265.515 | 0.001 | 0.001 | 0.001 | 0.999 |
|
| Solid nodules | 21.016 | 27,265.515 | 0.001 | 0.001 | 0.001 | 0.999 |
| Nodule size
(X5) |
| 19.901 | 27,265.515 | 0.001 | 0.001 | 0.001 | 0.999 |
|
| Purely ground
glass-like density nodules | −0.354 | 0.069 | 26.443 | 0.702 | 0.614–0.803 | <0.001 |
|
| Bronchial
inflatable sign (X6) | −1.326 | 0.749 | 3.129 | 0.266 | 0.061–1.154 | 0.077 |
|
| Burr sign
(X7) | −2.042 | 0.764 | 7.143 | 0.130 | 0.029–0.580 | 0.008 |
|
| Lobulation sign
(X8) | −1.713 | 0.672 | 6.495 | 0.180 | 0.048–0.673 | 0.011 |
| Constant |
| −20.009 | – | 0.001 | 0.001 |
| 0.999 |
The diagnostic value of CT imaging
risk factors to stage I lung adenocarcinoma in the predictive
model
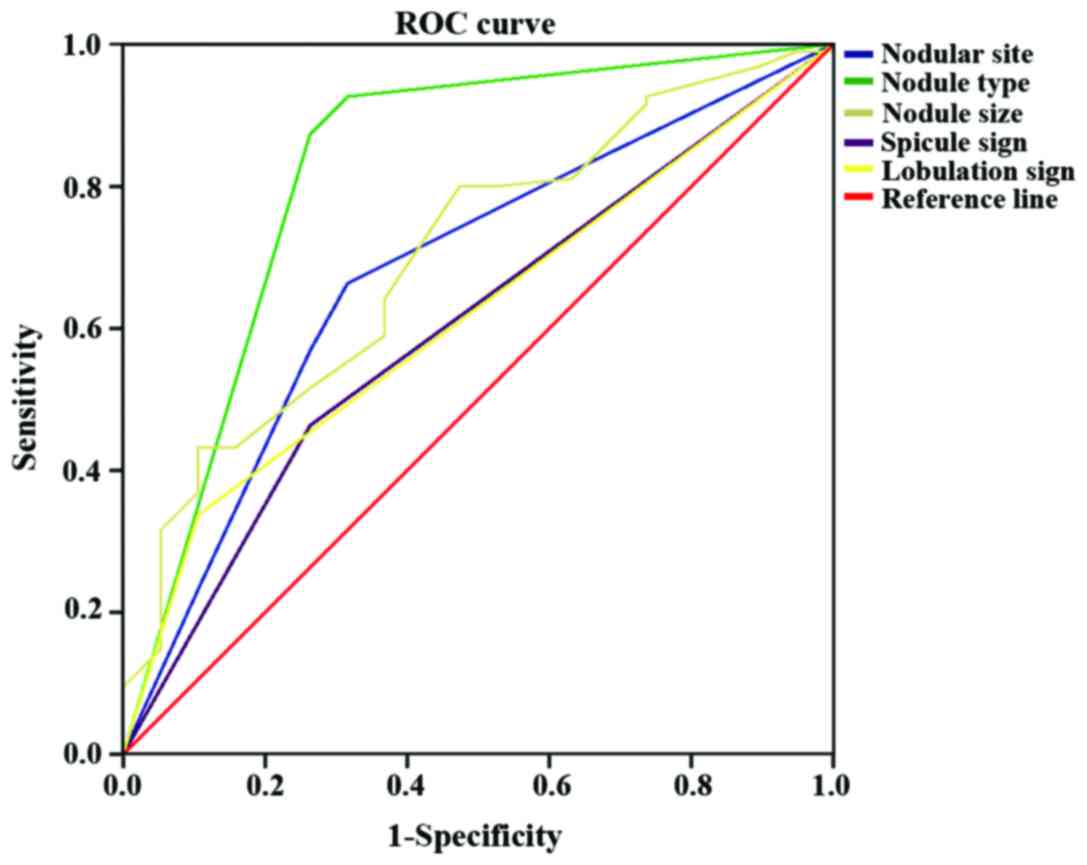
The AUC of nodular site (X3), nodule type
(X4), nodule size (X5), spicule sign
(X7), lobulation sign (X8) were respectively
0.678 (0.545–0.810), 0.821 (0.697–0.945), 0.702 (0.579–0.825),
0.600 (0.465–0.735) and 0.616 (0.490–0.742). The sensitivity and
specificity of nodular site (X3), nodule type
(X4), nodule size (X5), spicule sign
(X7), lobulation sign (X8) in the diagnosis
of stage I lung adenocarcinoma were 66.3 and 31.6%, 92.6 and 31.6%,
80 and 47.4%, 46.3 and 26.3%, 33.7 and 10.5%, respectively
(Table III and Fig. 3).
 | Table III.The sensitivity and specificity of CT
imaging risk factors in the diagnosis of stage I lung
adenocarcinoma in the predictive model. |
Table III.
The sensitivity and specificity of CT
imaging risk factors in the diagnosis of stage I lung
adenocarcinoma in the predictive model.
|
|
|
|
| 95% CI |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Test result
variables | Area under the
curve | SEa | Asymptotic
sigb | Lower bound | Upper bound | Sensitivity | 1-Sensitivity | Youden index |
|---|
| Nodular site
(X3) | 0.678 | 0.067 | 0.015 | 0.545 | 0.810 | 0.663 | 0.316 | 0.347 |
| Nodule type
(X4) | 0.821 | 0.063 | 0.001 | 0.697 | 0.945 | 0.926 | 0.316 | 0.611 |
| Nodule size
(X5) | 0.702 | 0.063 | 0.006 | 0.579 | 0.825 | 0.800 | 0.474 | 0.326 |
| Spicule sign
(X7) | 0.600 | 0.069 | 0.170 | 0.465 | 0.735 | 0.463 | 0.263 | 0.200 |
| Lobulation sign
(X8) | 0.616 | 0.064 | 0.112 | 0.490 | 0.742 | 0.337 | 0.105 | 0.232 |
Discussion
Early lung cancer usually has no typical symptoms,
but it can be detected as pulmonary nodules in imaging. The nodules
in CT imaging or chest X-ray are <30 mm in diameter with round
or irregular shape and show high density shadow, the border is
clear or unclear (23). With the
development of imaging technology, low-dose high-resolution CT
imaging has gradually become the primary means of early screening
of lung cancer, and different screening guidelines, such as
National Comprehensive Cancer Network (NCCN) and the American
College of Chest Physicians (ACCP) (24,25), have
been established by different academic organizations. Based on the
characteristics of Asian people, guidelines for the assessment of
patients with pulmonary nodules were established in February 2016
(26). Subsequently, guidelines for
the classification, diagnosis and treatment of pulmonary nodules
has also been updated (2016 edition) in China (27). Those guidelines all suggest that the
characteristics of lung CT and risk factors of the patients shall
be screened. Age and smoking history were mentioned as a potential
factor for lung cancer, however, the morbidity prediction model and
the follow-up were different in these guidelines.
Studies on the lung cancer prediction model have a
history of >20 years, and the earliest model was the Mayo model
proposed in 1997. The area under the ROC curve (>0.75) in Mayo
model has been verified by multiple external verifications, this
model is currently the most widely used prediction model (28). In recent years, many scholars have put
forward relevant prediction models based on the results of their
own clinical studies, such as the VA model, and the Brock
University model (29,30). Among them, the accuracy of Brock
University model is the highest due to the large data included, and
the area under the ROC curve can reach at least 0.94. For Chinese
population, the relatively mature model is Peking University model
(31). Because the sample size and
risk factors included in each model are different, reported
accuracy is not the same. Therefore, it is important to combine the
epidemiological characteristics of lung cancer in various regions,
establish a corresponding prediction model for the guidance of
local lung cancer treatment.
According to their internal density, pulmonary
nodules are simply divided into solid, partial solid and ground
glass density nodules. Solid nodules are nodules with uniform soft
tissue density, the density is uniform, and the blood vessel and
bronchial images are covered during the period. Partial solid
nodules refer to the nodules that contain both ground glass density
and solid soft tissue density. Ground glass density nodules refer
to vague nodules in the lungs, with nodular density slightly
increased compared to the surrounding lung parenchyma, but the
profile of the internal blood vessels and bronchi are still visible
(32). Ground glass density nodules
are non-specific imaging manifestations that can be caused by a
variety of pathologies including inflammation, and atypical
adenomatous hyperplasia. Highest degree of malignancy was observed
in partial solid nodules, followed by ground glass density nodules
and solid nodules.
Some researchers believe that the diameter of
partial solid nodules is positively correlated with the degree of
malignancy. In the 2013 version of ACCP, the recommended treatment
path for solid nodules is as follows: For partial solid pulmonary
nodules with a diameter ≤8 mm, CT should be routinely performed,
and non-surgical biopsy or surgical treatment should be performed
immediately in the case of enlargement. For partial solid pulmonary
nodules with a diameter >8 mm, CT should be performed at 3
months after the initial examination, and PET scan, non-surgical
biopsy and surgical treatment should be performed actively if
lesion persists. For partial solid pulmonary nodules with a
diameter >15 mm, active treatment should be performed
immediately (33). As a result, the
greater the partial diameter of partial solid nodules is, the
higher the risk of malignancy will be. This study also draws
similar conclusions (Fig. 2).
The size of pulmonary nodules and its pathological
properties are closely correlated with each other. Some studies
even concluded that the size of the diameter is an independent risk
factor for the diagnosis of benign and malignant lesions, while the
diameter of benign nodules is relatively small, and consistent
findings were also found in this study. Occurrence of lung cancer
includes a series of stages of tumor growth including hyperplasia,
atypical hyperplasia, carcinoma in situ and invasive cancer
(34). This study shows that the size
of the nodules is closely related to the degree of malignancy, the
larger the nodules, the higher the degree of malignancy (Fig. 1). This shows that follow-up is
crucial, and some guidelines suggest that follow-up is not
necessary for nodules with a diameter <6 mm, but the results of
this study show that attention should also be paid to some nodules
with small diameters.
Characteristics of the pulmonary nodules are also
important basis for the judgement of the nature of nodules. Due to
differences in the growth rate of various cells in malignant
pulmonary nodules, or bronchial and vascular blockage of the growth
of cancer cells, CT morphological lobulation symptoms arise
(35). Lobulation sign is positively
correlated with the growth rate of cells and degree of malignancy
(36). Spicule sign is caused by the
infiltration of tumor into adjacent bronchus, sheath or local lymph
node. Lobulation sign is also a characteristic of malignant
pulmonary nodules (31). Bronchial
inflatable sign is caused by the growth of tumor cells attached to
the wall, and alveolar bronchia is still preserved in lesions; at
the same time, the tumor cells and peripheral fibrous tissue
proliferate, and the bronchiectasis is formed (37). Bronchial inflatable sign is common in
malignant tumors, but can also be observed in benign tumors
(38). This study also showed that
spicule and lobulation sign were closely correlated with
pathological features.
Incidence of lung cancer is different in different
sites. In terms of lung adenocarcinoma, most cases were found in
upper lung lobes (39). This study
also showed that stage I lung adenocarcinoma mainly affected upper
left lung, followed by upper right lung. However, the mechanism
remains unclear.
In conclusion, in this study, the risk factors of
disease were combined with CT imaging characteristics of pulmonary
nodules to diagnose stage I lung adenocarcinoma. The subjects were
with pulmonary nodules but without obvious symptoms. Therefore, our
study achieved early diagnosis and treatment. Results show that the
size of nodules is closely correlated with the incidence of stage I
lung adenocarcinoma. With the increase of the diameter of pulmonary
nodules, the possibility of malignancy increases. For smaller
nodules, their own risk factors should be combined for follow-up.
The nature inside the nodules is correlated with the degree of
malignancy, and highest malignancy was observed in partial solid
nodules. Lobulation and spicule signs are typical manifestations of
early lung adenocarcinoma, and the probability of malignancy is
relatively high for bronchial inflatable sign. In addition,
incidence of stage I lung adenocarcinoma also showed predilection
in the left upper lung. The sample size in this study is small, and
most patients were from local region. Future studies with larger
sample size are needed to further confirm the conclusion. Our
future study will try to combine liquid biopsy to identify the risk
factors for stage I lung adenocarcinoma, and establish a more
reliable disease prediction model, so as to improve the diagnosis
and treatment of this disease.
Acknowledgements
Not applicable.
Funding
This study was supported by the Science and
Technology Project of Zhejiang Province (Major Science and
Technology Program) (no. 2013C03044-3), and the Agricultural and
Social Development Scientific Research Program of Hangzhou (no.
20180417A03).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RF, YY, HH, XF and LD conceived and designed the
study. BX, WL, CM, FC, JH and JW collected, analyzed and
interpreted the patient data. RF and YY wrote the manuscript. BX,
JH and JW revised the manuscript for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Guangxing Hospital Affiliated to Zhejiang Chinese Medical
University (Hangzhou, China). Patients who participated in this
research had complete clinical data. Signed informed consents were
obtained from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu F, Lin GZ and Zhang JX: An overview of
cancer incidence and trend in China. Chin Cancer. 21:81–85.
2012.
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brawley OW: Avoidable cancer deaths
globally. CA Cancer J Clin. 61:67–68. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang R, Zhang Y, Wen F, Wu K and Zhao S:
Analysis of pathological types and clinical epidemiology of 6,058
patients with lung cancer. Zhongguo Fei Ai Za Zhi. 19:129–135.
2016.(In Chinese). PubMed/NCBI
|
|
6
|
Scott WJ, Howington J, Feigenberg S,
Movsas B and Pisters K: American College of Chest Physicians:
Treatment of non-small cell lung cancer stage I and stage II: ACCP
evidence-based clinical practice guidelines (2nd edition). Chest.
132 3 Suppl:234S–242S. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Woodard GA, Jones KD and Jablons DM: Lung
cancer staging and prognosis. Cancer Treat Res. 170:47–75. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee JH and Chang JH: Diagnostic utility of
serum and pleural fluid carcinoembryonic antigen, neuron-specific
enolase, and cytokeratin 19 fragments in patients with effusions
from primary lung cancer. Chest. 128:2298–2303. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okamura K, Takayama K, Izumi M, Harada T,
Furuyama K and Nakanishi Y: Diagnostic value of CEA and CYFRA 21-1
tumor markers in primary lung cancer. Lung Cancer. 80:45–49. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin M, Chen J-F, Lu Y-T, Zhang Y, Song J,
Hou S, Ke Z and Tseng H-R: Nanostructure embedded microchips for
detection, isolation, and characterization of circulating tumor
cells. Acc Chem Res. 47:2941–2950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ilie M, Hofman V, Long-Mira E, Selva E,
Vignaud JM, Padovani B, Mouroux J, Marquette CH and Hofman P:
‘Sentinel’ circulating tumor cells allow early diagnosis of lung
cancer in patients with chronic obstructive pulmonary disease. PLoS
One. 9:e1115972014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen YY and Xu GB: Erratum to: Effect of
circulating tumor cells combined with negative enrichment and
CD45-FISH identification in diagnosis, therapy monitoring and
prognosis of primary lung cancer. Med Oncol. 31:2402014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gorges TM, Penkalla N, Schalk T, Joosse
SA, Riethdorf S, Tucholski J, Lücke K, Wikman H, Jackson S, Brychta
N, et al: Enumeration and molecular characterization of tumor cells
in lung cancer patients using a novel in vivo device for capturing
circulating tumor cells. Clin Cancer Res. 22:2197–2206. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen X, Wang X, He H, Liu Z, Hu JF and Li
W: Combination of circulating tumor cells with serum
carcinoembryonic antigen enhances clinical prediction of non-small
cell lung cancer. PLoS One. 10:e01262762015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hulbert A, Jusue-Torres I, Stark A, Chen
C, Rodgers K, Lee B, Griffin C, Yang A, Huang P, Wrangle J, et al:
Early detection of lung cancer using DNA promoter hypermethylation
in plasma and sputum. Clin Cancer Res. 23:1998–2005. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iftikhar IH and Musani AI: Narrow-band
imaging bronchoscopy in the detection of premalignant airway
lesions: A meta-analysis of diagnostic test accuracy. Ther Adv
Respir Dis. 9:207–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garg K, Keith RL, Byers T, Kelly K,
Kerzner AL, Lynch DA and Miller YE: Randomized controlled trial
with low-dose spiral CT for lung cancer screening: Feasibility
study and preliminary results. Radiology. 225:506–510. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ettinger DS, Wood DE, Akerley W, Bazhenova
LA, Borghaei H, Camidge DR, Cheney RT, Chirieac LR, D'Amico TA,
Demmy TL, et al: Non-small cell lung cancer, version 1.2015. J Natl
Compr Canc Netw. 12:1738–1761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Croswell JM, Baker SG, Marcus PM, Clapp JD
and Kramer BS: Cumulative incidence of false-positive test results
in lung cancer screening: A randomized trial. Ann Intern Med.
152(505–512): W176–W180. 2010.
|
|
20
|
Zhi X, Wu Y, Ma S, Wang T, Wang C, Wang J,
Shi Y, Lu Y, Liu L, Liu D, et al: Chinese guidelines on the
diagnosis and treatment of primary lung cancer (2011 version).
Zhongguo Fei Ai Za Zhi. 15:677–688. 2012.(In Chinese). PubMed/NCBI
|
|
21
|
Vallières E, Shepherd FA, Crowley J, Van
Houtte P, Postmus PE, Carney D, Chansky K, Shaikh Z and Goldstraw
P: International Association for the Study of Lung Cancer
International Staging Committee and Participating Institutions: The
IASLC Lung Cancer Staging Project: Proposals regarding the
relevance of TNM in the pathologic staging of small cell lung
cancer in the forthcoming (seventh) edition of the TNM
classification for lung cancer. J Thorac Oncol. 4:1049–1059. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsushima Y, Tateishi U, Uno H, Takeuchi M,
Terauchi T, Goya T and Kim EE: Diagnostic performance of PET/CT in
differentiation of malignant and benign non-solid solitary
pulmonary nodules. Ann Nucl Med. 22:571–577. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hansell DM, Bankier AA, MacMahon H, McLoud
TC, Müller NL and Remy J: Fleischner Society: Glossary of terms for
thoracic imaging. Radiology. 246:697–722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wood DE: National Comprehensive Cancer
Network (NCCN) Clinical practice guidelines for lung cancer
screening. Thorac Surg Clin. 25:185–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gould MK, Fletcher J, Iannettoni MD, Lynch
WR, Midthun DE, Naidich DP and Ost DE: American College of Chest
Physicians: Evaluation of patients with pulmonary nodules: When is
it lung cancer?: ACCP evidence-based clinical practice guidelines
(2nd edition). Chest. 132 3 Suppl:108S–130S. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim YK, Lee SH, Seo JH, Kim JH, Kim SD and
Kim GK: A comprehensive model of factors affecting adoption of
clinical practice guidelines in Korea. J Korean Med Sci.
25:1568–1573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Q, Fan Y, Wang Y, Qiao Y, Wang G,
Huang Y, Wang X, Wu N, Zhang G, Zheng X, et al: [China National
Guideline of Classification, Diagnosis and Treatment for Lung
Nodules (2016 version)]. Zhongguo Fei Ai Za Zhi. 19:793–798.
2016.(In Chinese). PubMed/NCBI
|
|
28
|
Swensen SJ, Silverstein MD, Ilstrup DM,
Schleck CD and Edell ES: The probability of malignancy in solitary
pulmonary nodules. Application to small radiologically
indeterminate nodules. Arch Intern Med. 157:849–855. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gould MK, Ananth L and Barnett PG:
Veterans Affairs SNAP Cooperative Study Group: A clinical model to
estimate the pretest probability of lung cancer in patients with
solitary pulmonary nodules. Chest. 131:383–388. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McWilliams A, Tammemagi MC, Mayo JR,
Roberts H, Liu G, Soghrati K, Yasufuku K, Martel S, Laberge F,
Gingras M, et al: Probability of cancer in pulmonary nodules
detected on first screening CT. N Engl J Med. 369:910–919. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Chen KZ and Wang J: Development and
validation of a clinical prediction model to estimate the
probability of malignancy in solitary pulmonary nodules in Chinese
people. Clin Lung Cancer. 12:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wormanns D and Hamer OW: Glossary of terms
for thoracic imaging - German version of the Fleischner Society
Recommendations. ROFO. 187:638–661. 2015.(In German). PubMed/NCBI
|
|
33
|
Ost DE and Gould MK: Decision making in
patients with pulmonary nodules. Am J Respir Crit Care Med.
185:363–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aoki T, Nakata H, Watanabe H, Nakamura K,
Kasai T, Hashimoto H, Yasumoto K and Kido M: Evolution of
peripheral lung adenocarcinomas: CT findings correlated with
histology and tumor doubling time. AJR Am J Roentgenol.
174:763–768. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Patel VK, Naik SK, Naidich DP, Travis WD,
Weingarten JA, Lazzaro R, Gutterman DD, Wentowski C, Grosu HB and
Raoof S: A practical algorithmic approach to the diagnosis and
management of solitary pulmonary nodules: part 2: pretest
probability and algorithm. Chest. 143:840–846. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ost D, Fein AM and Feinsilver SH: Clinical
practice. The solitary pulmonary nodule. N Engl J Med.
348:2535–2542. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Haro A, Yano T, Kohno M, Yoshida T,
Okamoto T and Maehara Y: Ground-glass opacity lesions on computed
tomography during postoperative surveillance for primary non-small
cell lung cancer. Lung Cancer. 76:56–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oda S, Awai K, Murao K, Ozawa A, Yanaga Y,
Kawanaka K and Yamashita Y: Computer-aided volumetry of pulmonary
nodules exhibiting ground-glass opacity at MDCT. AJR Am J
Roentgenol. 194:398–406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Collisson EA, Campbell JD, Brooks AN,
Berger AH, Lee W, Chmielecki J, Beer DG, Cope L, Creighton CJ,
Danilova L, et al: Cancer Genome Atlas Research Network:
Comprehensive molecular profiling of lung adenocarcinoma. Nature.
511:543–550. 2014. View Article : Google Scholar : PubMed/NCBI
|