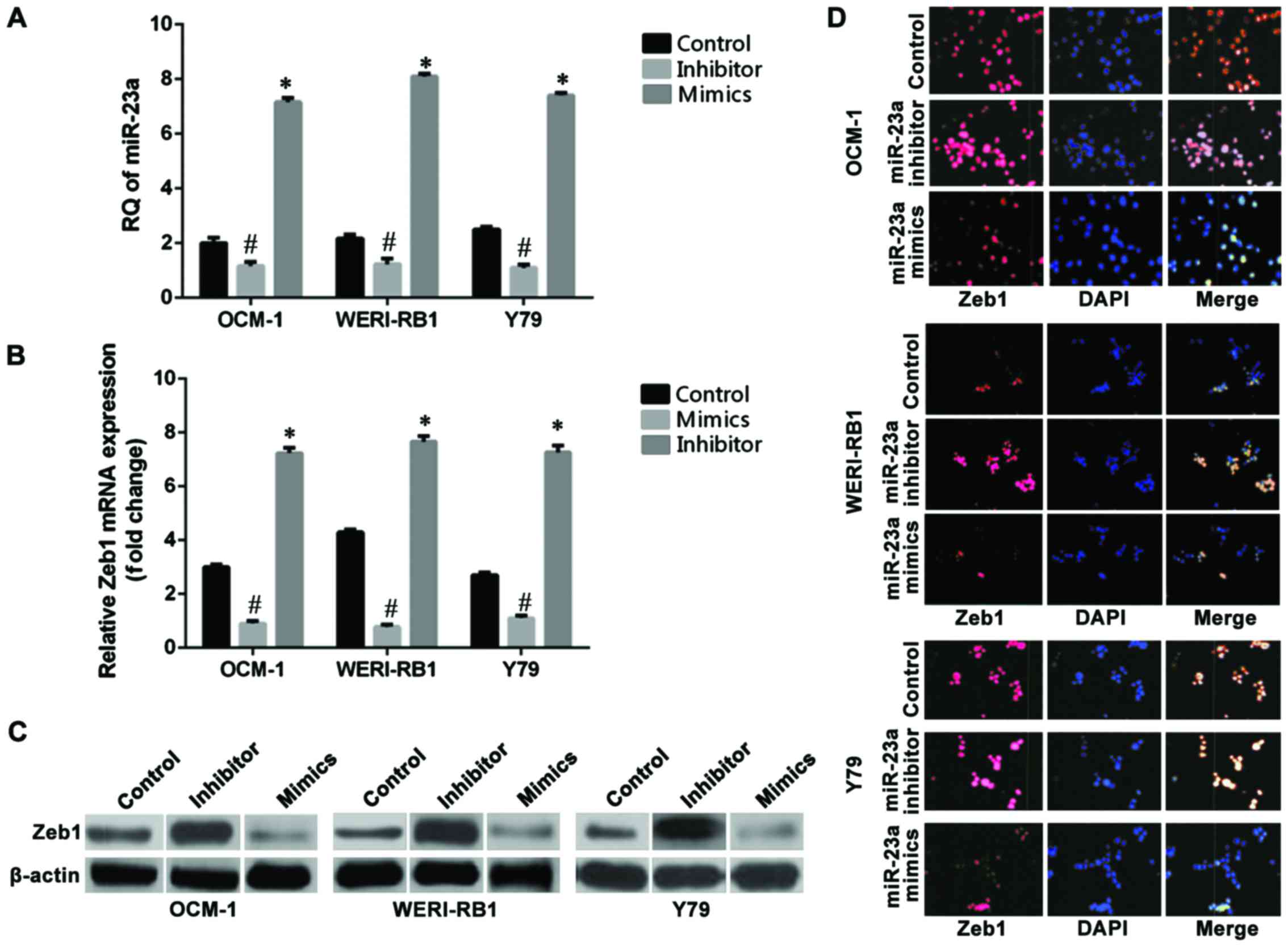
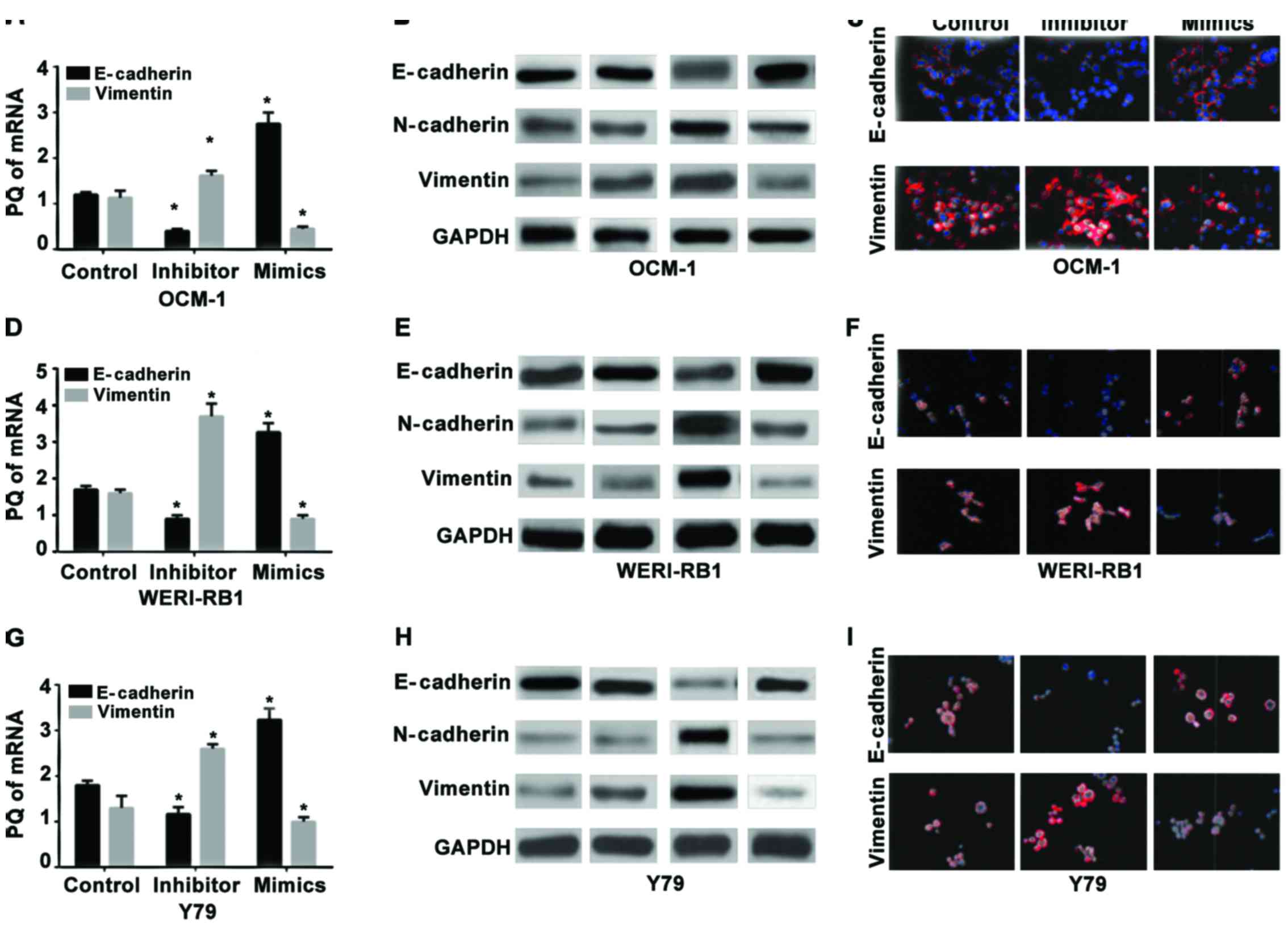
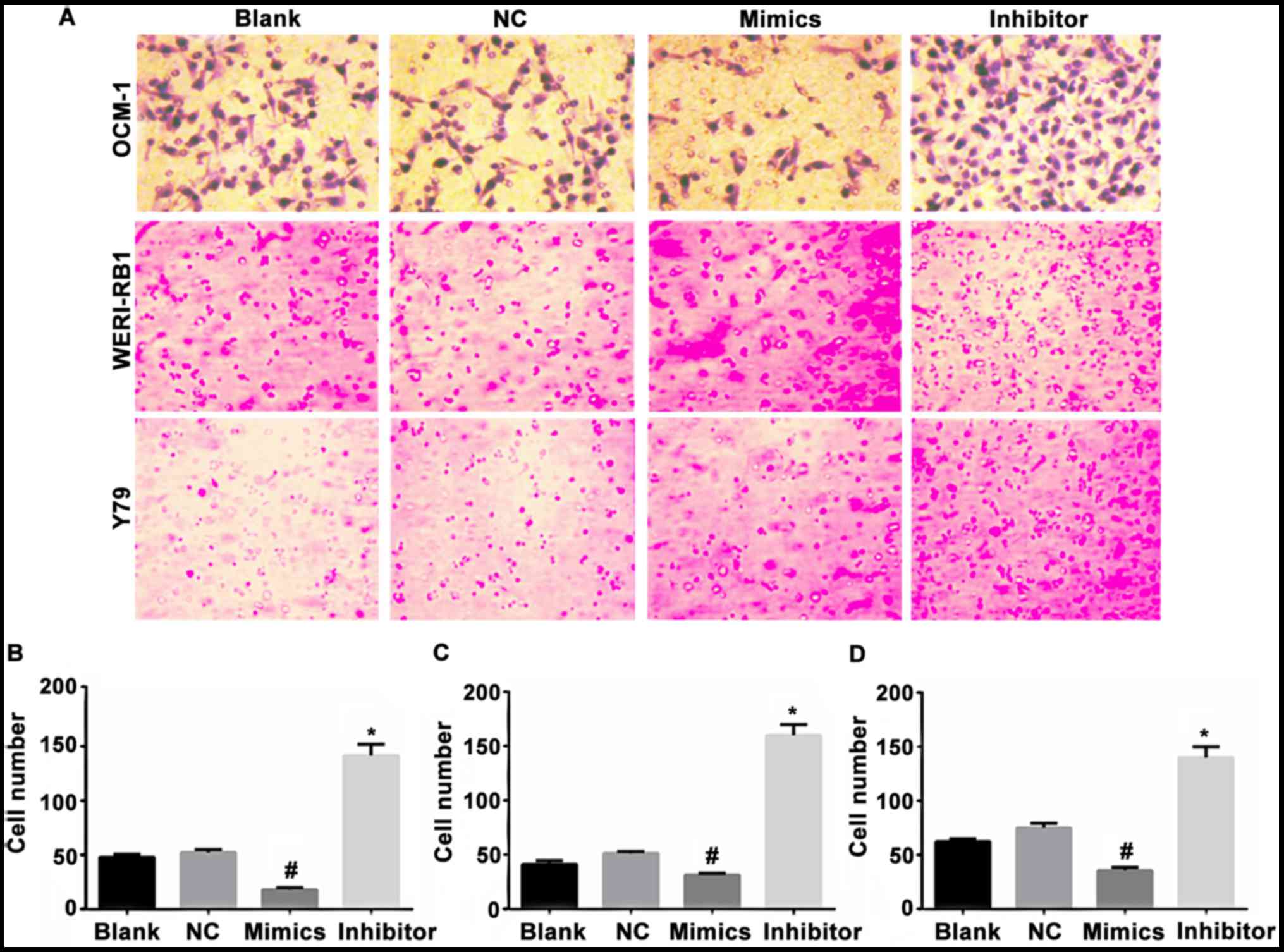
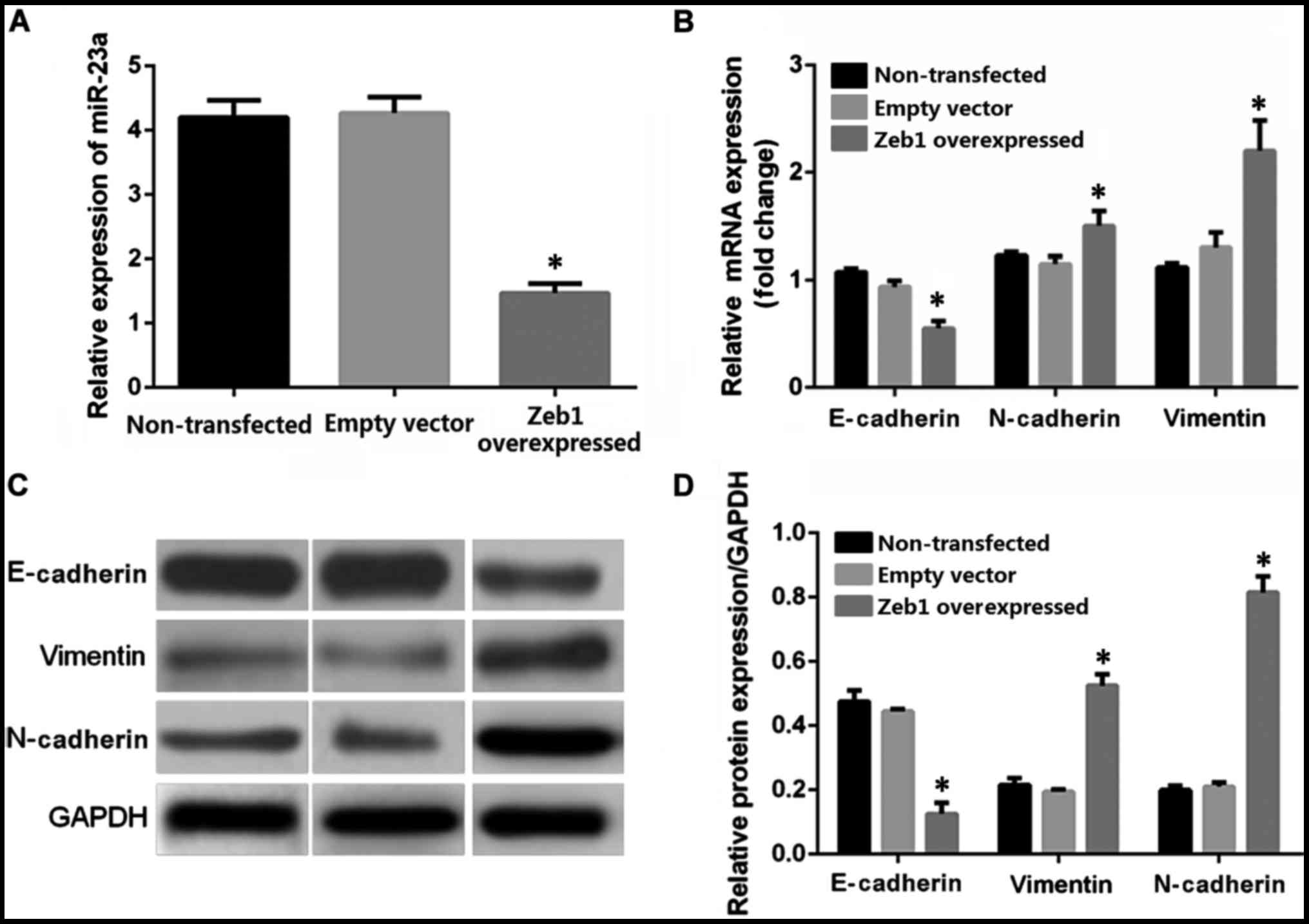
|
1
|
Cho MH, Park JH, Choi HJ, Park MK, Won HY,
Park YJ, Lee CH, Oh SH, Song YS, Kim HS, et al: DOT1L cooperates
with the c-Myc-p300 complex to epigenetically derepress CDH1
transcription factors in breast cancer progression. Nat Commun.
6:78212015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ichikawa K, Kubota Y, Nakamura T, Weng JS,
Tomida T, Saito H and Takekawa M: MCRIP1, an ERK substrate,
mediates ERK-induced gene silencing during epithelial-mesenchymal
transition by regulating the co-repressor CtBP. Mol Cell. 58:35–46.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L, Yang H, Abel EV, Ney GM, Palmbos
PL, Bednar F, Zhang Y, Leflein J, Waghray M, Owens S, et al: ATDC
induces an invasive switch in KRAS-induced pancreatic
tumorigenesis. Genes Dev. 29:171–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen L, Gibbons DL, Goswami S, Cortez MA,
Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, et al: Metastasis
is regulated via microRNA-200/ZEB1 axis control of tumour cell
PD-L1 expression and intratumoral immunosuppression. Nat Commun.
5:52412014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Bu F, Royer C, Serres S, Larkin
JR, Soto MS, Sibson NR, Salter V, Fritzsche F, Turnquist C, et al:
ASPP2 controls epithelial plasticity and inhibits metastasis
through β-catenin-dependent regulation of ZEB1. Nat Cell Biol.
16:1092–1104. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang P, Wei Y, Wang L, Debeb BG, Yuan Y,
Zhang J, Yuan J, Wang M, Chen D, Sun Y, et al: ATM-mediated
stabilization of ZEB1 promotes DNA damage response and
radioresistance through CHK1. Nat Cell Biol. 16:864–875. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chao CH, Chang CC, Wu MJ, Ko HW, Wang D,
Hung MC, Yang JY and Chang CJ: MicroRNA-205 signaling regulates
mammary stem cell fate and tumorigenesis. J Clin Invest.
124:3093–3106. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park KS, Raffeld M, Moon YW, Xi L, Bianco
C, Pham T, Lee LC, Mitsudomi T, Yatabe Y, Okamoto I, et al: CRIPTO1
expression in EGFR-mutant NSCLC elicits intrinsic EGFR-inhibitor
resistance. J Clin Invest. 124:3003–3015. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lehmann W, Mossmann D, Kleemann J, Mock K,
Meisinger C, Brummer T, Herr R, Brabletz S, Stemmler MP and
Brabletz T: ZEB1 turns into a transcriptional activator by
interacting with YAP1 in aggressive cancer types. Nat Commun.
7:104982016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelial-to-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Ahn YH, Chen Y, Tan X, Guo L,
Gibbons DL, Ungewiss C, Peng DH, Liu X, Lin SH, et al: ZEB1
sensitizes lung adenocarcinoma to metastasis suppression by PI3K
antagonism. J Clin Invest. 124:2696–2708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tian K, Di R and Wang L: MicroRNA-23a
enhances migration and invasion through PTEN in osteosarcoma.
Cancer Gene Ther. 22:351–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wen YC, Lee WJ, Tan P, Yang SF, Hsiao M,
Lee LM and Chien MH: By inhibiting snail signaling and miR-23a-3p,
osthole suppresses the EMT-mediated metastatic ability in prostate
cancer. Oncotarget. 6:21120–21136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang J, Zhao H, Cai H and Wu H: Diagnostic
and prognostic potentials of microRNA-27a in osteosarcoma. Biomed
Pharmacother. 71:222–226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang XW, Liu N, Chen S, Wang Y, Zhang ZX,
Sun YY, Qiu GB and Fu WN: High microRNA-23a expression in laryngeal
squamous cell carcinoma is associated with poor patient prognosis.
Diagn Pathol. 10:222015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma G, Dai W, Sang A, Yang X and Gao C:
Upregulation of microRNA-23a/b promotes tumor progression and
confers poor prognosis in patients with gastric cancer. Int J Clin
Exp Pathol. 7:8833–8840. 2014.PubMed/NCBI
|
|
18
|
He Y, Meng C, Shao Z, Wang H and Yang S:
MiR-23a functions as a tumor suppressor in osteosarcoma. Cell
Physiol Biochem. 34:1485–1496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang L, Yang X, Ji W, Deng J, Qiu F, Yang
R, Fang W, Zhang L, Huang D, Xie C, et al: Effects of a functional
variant c.353T>C in snai1 on risk of two contextual diseases.
Chronic obstructive pulmonary disease and lung cancer. Am J Respir
Crit Care Med. 189:139–148. 2014.PubMed/NCBI
|
|
20
|
Li C, Ma H, Wang Y, Cao Z, Graves-Deal R,
Powell AE, Starchenko A, Ayers GD, Washington MK, Kamath V, et al:
Excess PLAC8 promotes an unconventional ERK2-dependent EMT in colon
cancer. J Clin Invest. 124:2172–2187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Caramel J, Papadogeorgakis E, Hill L,
Browne GJ, Richard G, Wierinckx A, Saldanha G, Osborne J,
Hutchinson P, Tse G, et al: A switch in the expression of embryonic
EMT-inducers drives the development of malignant melanoma. Cancer
Cell. 24:466–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hur K, Toiyama Y, Takahashi M, Balaguer F,
Nagasaka T, Koike J, Hemmi H, Koi M, Boland CR and Goel A:
MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT)
in human colorectal cancer metastasis. Gut. 62:1315–1326. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng L, Yang T, Kuang Y, Kong B, Yu S,
Shu H, Zhou H and Gu J: MicroRNA-23a promotes neuroblastoma cell
metastasis by targeting CDH1. Oncol Lett. 7:839–845. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Celià-Terrassa T, Meca-Cortés O, Mateo F,
de Paz Martínez A, Rubio N, Arnal-Estapé A, Ell BJ, Bermudo R, Díaz
A, Guerra-Rebollo M, et al: Epithelial-mesenchymal transition can
suppress major attributes of human epithelial tumor-initiating
cells. J Clin Invest. 122:1849–1868. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mizuguchi Y, Isse K, Specht S, Lunz JG
III, Corbitt N, Takizawa T and Demetris AJ: Small proline rich
protein 2a in benign and malignant liver disease. Hepatology.
59:1130–1143. 2014. View Article : Google Scholar : PubMed/NCBI
|