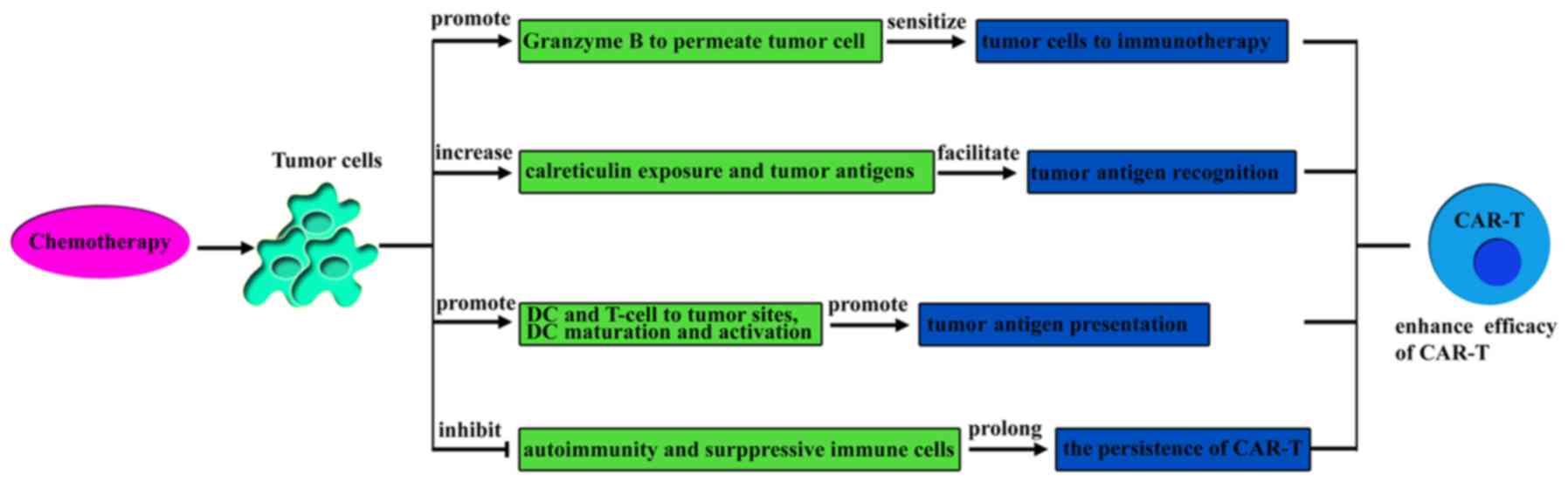
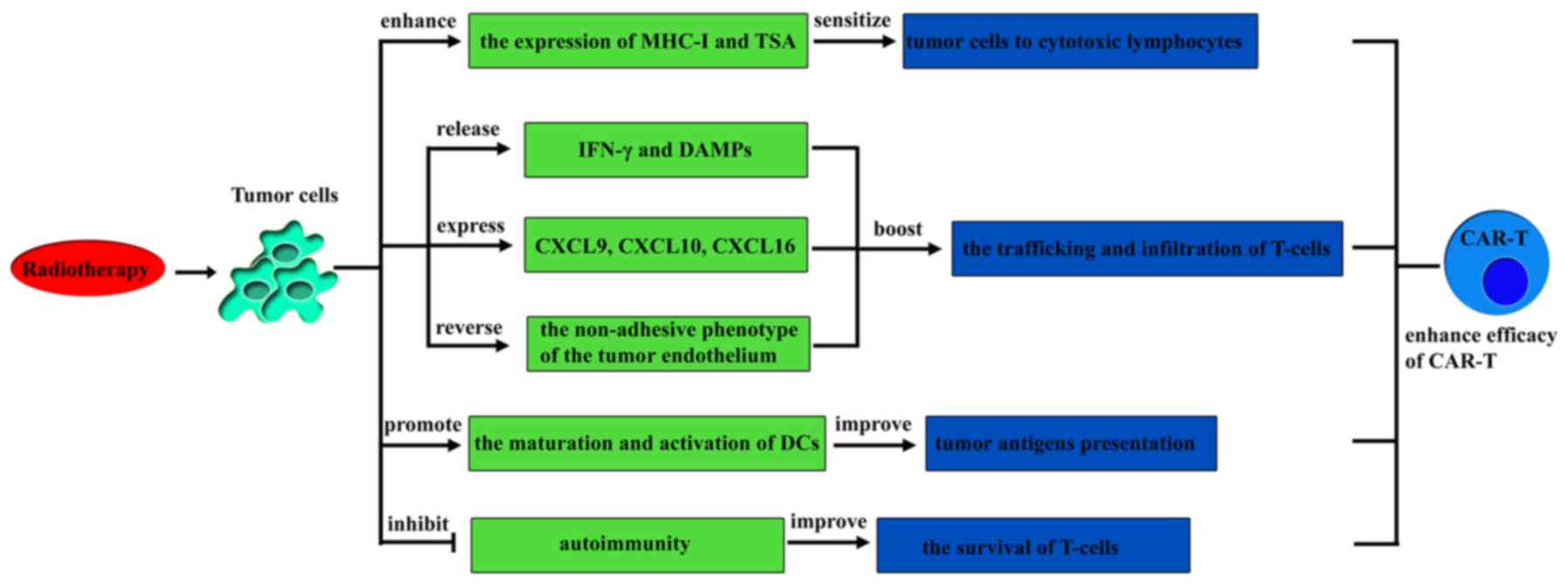
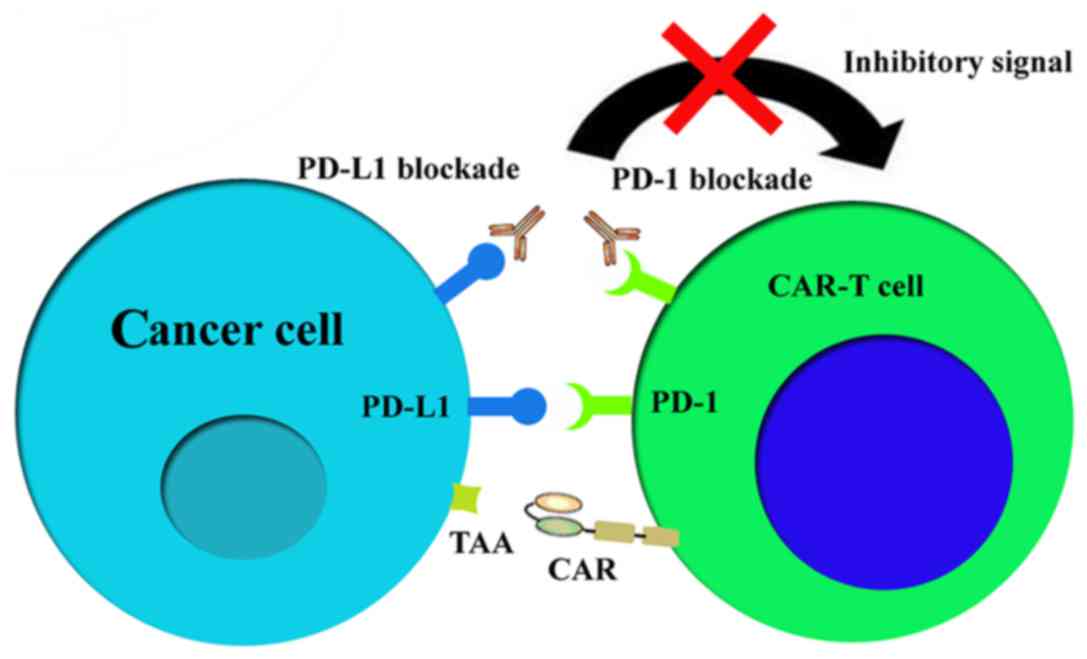
|
1
|
Yang JC and Rosenberg SA: Adoptive T-cell
therapy for cancer. Adv Immunol. 130:279–294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maus MV and June CH: Making better
chimeric antigen receptors for adoptive T-cell therapy. Clin Cancer
Res. 22:1875–1884. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whilding LM and Maher J: CAR T-cell
immunotherapy: The path from the by-road to the freeway? Mol Oncol.
9:1994–2018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haji-Fatahaliha M, Hosseini M, Akbarian A,
Sadreddini S, Jadidi-Niaragh F and Yousefi M: CAR-modified T-cell
therapy for cancer: An updated review. Artif Cells Nanomed
Biotechnol. 44:1339–1349. 2016.PubMed/NCBI
|
|
5
|
Sadelain M, Brentjens R and Rivière I: The
basic principles of chimeric antigen receptor design. Cancer
Discov. 3:388–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seliger B: Different regulation of MHC
class I antigen processing components in human tumors. J
Immunotoxicol. 5:361–367. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan J, Yang JF, Deng BP, Zhao XJ, Zhang X,
Lin YH, Wu YN, Deng ZL, Zhang YL, Liu SH, et al: High efficacy and
safety of low-dose CD19-directed CAR-T cell therapy in 51
refractory or relapsed B acute lymphoblastic leukemia patients.
Leukemia. 31:2587–2593. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tiberghien P, Deconinck E and Adotevi O:
More on anti-CD19 CAR T cells in CNS diffuse large-B-cell lymphoma.
N Engl J Med. 377:2101–2102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lamers CH, Sleijfer S, van Steenbergen S,
van Elzakker P, van Krimpen B, Groot C, Vulto A, den Bakker M,
Oosterwijk E, Debets R and Gratama JW: Treatment of metastatic
renal cell carcinoma with CAIX CAR-engineered T cells: Clinical
evaluation and management of on-target toxicity. Mol Ther.
21:904–912. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thistlethwaite FC, Gilham DE, Guest RD,
Rothwell DG, Pillai M, Burt DJ, Byatte AJ, Kirillova N, Valle JW,
Sharma SK, et al: The clinical efficacy of first-generation
carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited
by poor persistence and transient pre-conditioning-dependent
respiratory toxicity. Cancer Immunol Immunother. 66:1425–1436.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng KC, Guo YL, Liu Y, Dai HR, Wang Y, Lv
HY, Huang JH, Yang QM and Han WD: Cocktail treatment with
EGFR-specific and CD133-specific chimeric antigen receptor-modified
T cells in a patient with advanced cholangiocarcinoma. J Hematol
Oncol. 10:42017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lamers CH, Klaver Y, Gratama JW, Sleijfer
S and Debets R: Treatment of metastatic renal cell carcinoma (mRCC)
with CAIX CAR-engineered T-cells-a completed study overview.
Biochem Soc Trans. 44:951–959. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park JR, Digiusto DL, Slovak M, Wright C,
Naranjo A, Wagner J, Meechoovet HB, Bautista C, Chang WC, Ostberg
JR and Jensen MC: Adoptive transfer of chimeric antigen receptor
re-directed cytolytic T lymphocyte clones in patients with
neuroblastoma. Mol Ther. 15:825–833. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sheng Q and Liu J: The therapeutic
potential of targeting the EGFR family in epithelial ovarian
cancer. Br J Cancer. 104:1241–1245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kershaw MH, Westwood JA, Parker LL, Wang
G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S,
Rogers-Freezer L, et al: A phase I study on adoptive immunotherapy
using gene-modified T cells for ovarian cancer. Clin Cancer Res.
12:6106–6115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morgan RA, Yang JC, Kitano M, Dudley ME,
Laurencot CM and Rosenberg SA: Case report of a serious adverse
event following the administration of T cells transduced with a
chimeric antigen receptor recognizing ERBB2. Mol Ther. 18:843–851.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Louis CU, Savoldo B, Dotti G, Pule M, Yvon
E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, et al: Antitumor
activity and long-term fate of chimeric antigen receptor-positive T
cells in patients with neuroblastoma. Blood. 118:6050–6056. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anampa J, Chen A, Wright J, Patel M,
Pellegrino C, Fehn K, Sparano JA and Andreopoulou E: Phase I trial
of veliparib, a poly ADP ribose polymerase inhibitor, plus
metronomic cyclophosphamide in metastatic HER2-negative breast
cancer. Clin Breast Cancer. 18:e135–e142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bracci L, Schiavoni G, Sistigu A and
Belardelli F: Immune-based mechanisms of cytotoxic chemotherapy:
Implications for the design of novel and rationale-based combined
treatments against cancer. Cell Death Differ. 21:15–25. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vierboom MP, Bos GM, Ooms M, Offringa R
and Melief CJ: Cyclophosphamide enhances anti-tumor effect of
wild-type p53-specific CTL. Int J Cancer. 87:253–260. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Michaud M, Martins I, Sukkurwala AQ,
Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot
G, et al: Autophagy-dependent anticancer immune responses induced
by chemotherapeutic agents in mice. Science. 334:1573–1577. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sistigu A, Yamazaki T, Vacchelli E, Chaba
K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remédios C, et
al: Cancer cell-autonomous contribution of type I interferon
signaling to the efficacy of chemotherapy. Nat Med. 20:1301–1309.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alizadeh D, Trad M, Hanke NT, Larmonier
CB, Janikashvili N, Bonnotte B, Katsanis E and Larmonier N:
Doxorubicin eliminates myeloid-derived suppressor cells and
enhances the efficacy of adoptive T-cell transfer in breast cancer.
Cancer Res. 74:104–118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramakrishnan R, Huang C, Cho HI, Lloyd M,
Johnson J, Ren X, Altiok S, Sullivan D, Weber J, Celis E and
Gabrilovich DI: Autophagy induced by conventional chemotherapy
mediates tumor cell sensitivity to immunotherapy. Cancer Res.
72:5483–5493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Motyka B, Korbutt G, Pinkoski MJ, Heibein
JA, Caputo A, Hobman M, Barry M, Shostak I, Sawchuk T, Holmes CF,
et al: Mannose 6-phosphate/insulin-like growth factor II receptor
is a death receptor for granzyme B during cytotoxic T cell-induced
apoptosis. Cell. 103:491–500. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Trapani JA, Sutton VR, Thia KY, Li YQ,
Froelich CJ, Jans DA, Sandrin MS and Browne KA: A clathrin/dynamin-
and mannose-6-phosphate receptor-independent pathway for granzyme
B-induced cell death. J Cell Biol. 160:223–233. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Parente-Pereira AC, Whilding LM, Brewig N,
van der Stegen SJ, Davies DM, Wilkie S, van Schalkwyk MC,
Ghaem-Maghami S and Maher J: Synergistic chemoimmunotherapy of
epithelial ovarian cancer using ErbB-retargeted T cells combined
with carboplatin. J Immunol. 191:2437–2445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Whilding LM and Maher J: ErbB-targeted CAR
T-cell immunotherapy of cancer. Immunotherapy. 7:229–241. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Proietti E, Moschella F, Capone I and
Belardelli F: Exploitation of the propulsive force of chemotherapy
for improving the response to cancer immunotherapy. Mol Oncol.
6:1–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Senovilla L, Vitale I, Martins I, Tailler
M, Pailleret C, Michaud M, Galluzzi L, Adjemian S, Kepp O,
Niso-Santano M, et al: An immunosurveillance mechanism controls
cancer cell ploidy. Science. 337:1678–1684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma Y, Adjemian S, Mattarollo SR, Yamazaki
T, Aymeric L, Yang H, Catani Portela JP, Hannani D, Duret H, Steegh
K, et al: Anticancer chemotherapy-induced intratumoral recruitment
and differentiation of antigen-presenting cells. Immunity.
38:729–741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Michaud M, Xie X, Bravo-San Pedro JM,
Zitvogel L, White E and Kroemer G: An autophagy-dependent
anticancer immune response determines the efficacy of melanoma
chemotherapy. Oncoimmunology. 3:e9440472014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martins I, Tesniere A, Kepp O, Michaud M,
Schlemmer F, Senovilla L, Séror C, Métivier D, Perfettini JL,
Zitvogel L and Kroemer G: Chemotherapy induces ATP release from
tumor cells. Cell Cycle. 8:3723–3728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Apetoh L, Ghiringhelli F, Tesniere A,
Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E,
Saulnier P, et al: Toll-like receptor 4-dependent contribution of
the immune system to anticancer chemotherapy and radiotherapy. Nat
Med. 13:1050–1059. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Obeid M, Tesniere A, Ghiringhelli F, Fimia
GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T,
Casares N, et al: Calreticulin exposure dictates the immunogenicity
of cancer cell death. Nat Med. 13:54–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Garg AD, Krysko DV, Verfaillie T,
Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu
C, Roebroek AJ, et al: A novel pathway combining calreticulin
exposure and ATP secretion in immunogenic cancer cell death. EMBO
J. 31:1062–1079. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Garg AD, Galluzzi L, Apetoh L, Baert T,
Birge RB, Pedro Bravo-San JM, Breckpot K, Brough D, Chaurio R,
Cirone M, et al: Molecular and translational classifications of
DAMPs in immunogenic cell death. Front Immunol. 6:5882015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Proietti E, Greco G, Garrone B, Baccarini
S, Mauri C, Venditti M, Carlei D and Belardelli F: Importance of
cyclophosphamide-induced bystander effect on T cells for a
successful tumor eradication in response to adoptive immunotherapy
in mice. J Clin Invest. 101:429–441. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lutsiak ME, Semnani RT, De Pascalis R,
Kashmiri SV, Schlom J and Sabzevari H: Inhibition of CD4(+)25+ T
regulatory cell function implicated in enhanced immune response by
low-dose cyclophosphamide. Blood. 105:2862–2868. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heylmann D, Bauer M, Becker H, van Gool S,
Bacher N, Steinbrink K and Kaina B: Human CD4+CD25+ regulatory T
cells are sensitive to low dose cyclophosphamide: Implications for
the immune response. PLoS One. 8:e833842013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Traverso I, Fenoglio D, Negrini S, Parodi
A, Battaglia F, Kalli F, Conteduca G, Tardito S, Traverso P,
Indiveri F and Filaci G: Cyclophosphamide inhibits the generation
and function of CD8(+) regulatory T cells. Hum Immunol. 73:207–213.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ghiringhelli F, Larmonier N, Schmitt E,
Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E,
Bonnotte B and Martin F: CD4+CD25+ regulatory T cells suppress
tumor immunity but are sensitive to cyclophosphamide which allows
immunotherapy of established tumors to be curative. Eur J Immunol.
34:336–344. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kwa M, Li X, Novik Y, Oratz R, Jhaveri K,
Wu J, Gu P, Meyers M, Muggia F, Speyer J, et al: Serial
immunological parameters in a phase II trial of exemestane and
low-dose oral cyclophosphamide in advanced hormone
receptor-positive breast cancer. Breast Cancer Res Treat.
168:57–67. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mumtaz IM, Hoyer BF, Panne D, Moser K,
Winter O, Cheng QY, Yoshida T, Burmester GR, Radbruch A, Manz RA
and Hiepe F: Bone marrow of NZB/W mice is the major site for plasma
cells resistant to dexamethasone and cyclophosphamide: Implications
for the treatment of autoimmunity. J Autoimmun. 39:180–188. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brodsky RA: High-dose cyclophosphamide for
autoimmunity and alloimmunity. Immunol Res. 47:179–184. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lamers CH, Willemsen R, van Elzakker P,
van Steenbergen-Langeveld S, Broertjes M, Oosterwijk-Wakka J,
Oosterwijk E, Sleijfer S, Debets R and Gratama JW: Immune responses
to transgene and retroviral vector in patients treated with ex
vivo-engineered T cells. Blood. 117:72–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Muranski P, Boni A, Wrzesinski C, Citrin
DE, Rosenberg SA, Childs R and Restifo NP: Increased intensity
lymphodepletion and adoptive immunotherapy-how far can we go? Nat
Clin Pract Oncol. 3:668–681. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rosenberg SA and Dudley ME: Adoptive cell
therapy for the treatment of patients with metastatic melanoma.
Curr Opin Immunol. 21:233–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Qiao J, Liu Z and Fu YX: Adapting
conventional cancer treatment for immunotherapy. J Mol Med (Berl).
94:489–495. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang W, Kryczek I, Dostál L, Lin H, Tan L,
Zhao L, Lu F, Wei S, Maj T, Peng D, et al: Effector T cells
abrogate stroma-mediated chemoresistance in ovarian cancer. Cell.
165:1092–1105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Reits EA, Hodge JW, Herberts CA, Groothuis
TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH,
Neijssen J, et al: Radiation modulates the peptide repertoire,
enhances MHC class I expression, and induces successful antitumor
immunotherapy. J Exp Med. 203:1259–1271. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gaipl US, Multhoff G, Scheithauer H,
Lauber K, Hehlgans S, Frey B and Rödel F: Kill and spread the word:
Stimulation of antitumor immune responses in the context of
radiotherapy. Immunotherapy. 6:597–610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Higgins JP, Bernstein MB and Hodge JW:
Enhancing immune responses to tumor-associated antigens. Cancer
Biol Ther. 8:1440–1449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sharma A, Bode B, Wenger RH, Lehmann K,
Sartori AA, Moch H, Knuth A, Boehmer Lv and Broek Mv: γ-Radiation
promotes immunological recognition of cancer cells through
increased expression of cancer-testis antigens in vitro and in
vivo. PLoS One. 6:e282172011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee Y, Auh SL, Wang Y, Burnette B, Wang Y,
Meng Y, Beckett M, Sharma R, Chin R, Tu T, et al: Therapeutic
effects of ablative radiation on local tumor require CD8+ T cells:
Changing strategies for cancer treatment. Blood. 114:589–595. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lugade AA, Sorensen EW, Gerber SA, Moran
JP, Frelinger JG and Lord EM: Radiation-induced IFN-gamma
production within the tumor microenvironment influences antitumor
immunity. J Immunol. 180:3132–3139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ganss R, Ryschich E, Klar E, Arnold B and
Hämmerling GJ: Combination of T-cell therapy and trigger of
inflammation induces remodeling of the vasculature and tumor
eradication. Cancer Res. 62:1462–1470. 2002.PubMed/NCBI
|
|
58
|
Aymeric L, Apetoh L, Ghiringhelli F,
Tesniere A, Martins I, Kroemer G, Smyth MJ and Zitvogel L: Tumor
cell death and ATP release prime dendritic cells and efficient
anticancer immunity. Cancer Res. 70:855–858. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang B, Bowerman NA, Salama JK, Schmidt
H, Spiotto MT, Schietinger A, Yu P, Fu YX, Weichselbaum RR, Rowley
DA, et al: Induced sensitization of tumor stroma leads to
eradication of established cancer by T cells. J Exp Med. 204:49–55.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Matsumura S, Wang B, Kawashima N,
Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti
SC, Dustin ML and Demaria S: Radiation-induced CXCL16 release by
breast cancer cells attracts effector T cells. J Immunol.
181:3099–3107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liao YP, Wang CC, Butterfield LH, Economou
JS, Ribas A, Meng WS, Iwamoto KS and McBride WH: Ionizing radiation
affects human MART-1 melanoma antigen processing and presentation
by dendritic cells. J Immunol. 173:2462–2469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Crouse J, Kalinke U and Oxenius A:
Regulation of antiviral T cell responses by type I interferons. Nat
Rev Immunol. 15:231–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Burnette BC, Liang H, Lee Y, Chlewicki L,
Khodarev NN, Weichselbaum RR, Fu YX and Auh SL: The efficacy of
radiotherapy relies upon induction of type i interferon-dependent
innate and adaptive immunity. Cancer Res. 71:2488–2496. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Deng L, Liang H, Xu M, Yang X, Burnette B,
Arina A, Li XD, Mauceri H, Beckett M, Darga T, et al:
STING-Dependent Cytosolic DNA sensing promotes radiation-induced
type I interferon-dependent antitumor immunity in immunogenic
tumors. Immunity. 41:843–852. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Diamond MS, Kinder M, Matsushita H,
Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM,
Kalinke U, et al: Type I interferon is selectively required by
dendritic cells for immune rejection of tumors. J Exp Med.
208:1989–2003. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fuertes MB, Kacha AK, Kline J, Woo SR,
Kranz DM, Murphy KM and Gajewski TF: Host type I IFN signals are
required for antitumor CD8+ T cell responses through CD8{alpha}+
dendritic cells. J Exp Med. 208:2005–2016. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kang J, Demaria S and Formenti S: Current
clinical trials testing the combination of immunotherapy with
radiotherapy. J Immunother Cancer. 4:512016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rodriguez-Ruiz ME, Rodriguez I, Garasa S,
Barbes B, Solorzano JL, Perez-Gracia JL, Labiano S, Sanmamed MF,
Azpilikueta A, Bolaños E, et al: Abscopal effects of radiotherapy
are enhanced by combined immunostimulatory mAbs and Are dependent
on CD8 T cells and crosspriming. Cancer Res. 76:5994–6005. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Demaria S, Ng B, Devitt ML, Babb JS,
Kawashima N, Liebes L and Formenti SC: Ionizing radiation
inhibition of distant untreated tumors (abscopal effect) is immune
mediated. Int J Radiat Oncol Biol Phys. 58:862–870. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Weiss T, Weller M, Guckenberger M, Sentman
CL and Roth P: NKG2D-based CAR T cells and radiotherapy exert
synergistic efficacy in glioblastoma. Cancer Res. 78:1031–1043.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yovino S and Grossman SA: Severity,
etiology and possible consequences of treatment-related lymphopenia
in patients with newly diagnosed high-grade gliomas. CNS Oncol.
1:149–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Baniyash M: TCR zeta-chain downregulation:
Curtailing an excessive inflammatory immune response. Nat Rev
Immunol. 4:675–687. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Alanio C, Lemaitre F, Law HK, Hasan M and
Albert ML: Enumeration of human antigen-specific naive CD8+ T cells
reveals conserved precursor frequencies. Blood. 115:3718–3725.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yoo EJ, Park JC, Kim EH, Park CH, Shim CN,
Lee HJ, Chung HS, Lee H, Shin SK, Lee SK, et al: Prognostic value
of neutrophil-to-lymphocyte ratio in patients treated with
concurrent chemoradiotherapy for locally advanced oesophageal
cancer. Dig Liver Dis. 46:846–853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gazdic M, Markovic Simovic B, Jovicic N,
Misirkic-Marjanovic M, Djonov V, Jakovljevic V, Arsenijevic N,
Lukic ML and Volarevic V: Mesenchymal stem cells promote metastasis
of lung cancer cells by downregulating systemic antitumor immune
response. Stem Cells Int. 2017:62947172017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Nelson A, Nair S and Nagaraj S: CD4(+) T
cells suppress immune response to cancer: Novel targets for
antitumor efforts. Expert Rev Clin Immunol. 8:401–403. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang Q, Yang XJ, Kundu SD, Pins M,
Javonovic B, Meyer R, Kim SJ, Greenberg NM, Kuzel T, Meagher R, et
al: Blockade of transforming growth factor-{beta} signaling in
tumor-reactive CD8(+) T cells activates the antitumor immune
response cycle. Mol Cancer Ther. 5:1733–1743. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Tang C, Liao Z, Gomez D, Levy L, Zhuang Y,
Gebremichael RA, Hong DS, Komaki R and Welsh JW: Lymphopenia
association with gross tumor volume and lung V5 and its effects on
non-small cell lung cancer patient outcomes. Int J Radiat Oncol
Biol Phys. 89:1084–1091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kitayama J, Yasuda K, Kawai K, Sunami E
and Nagawa H: Circulating lymphocyte number has a positive
association with tumor response in neoadjuvant chemoradiotherapy
for advanced rectal cancer. Radiat Oncol. 5:472010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Schueneman AJ, Sugar EA, Uram J, Bigelow
E, Herman JM, Edil BH, Jaffee EM, Zheng L and Laheru DA: Low total
lymphocyte count is associated with poor survival in patients with
resected pancreatic adenocarcinoma receiving a GM-CSF secreting
pancreatic tumor vaccine. Ann Surg Oncol. 20 Suppl 3:S725–S730.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Buka D, Dvořák J, Sitorová V, Hátlová J,
Richter I and Sirák I: Changes in the CD8+ density of tumor
infiltrating lymphocytes after neoadjuvant radiochemotherapy in
patients with rectal adenocarcinom. Klin Onkol. 29:204–209. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zitvogel L, Kepp O and Kroemer G: Immune
parameters affecting the efficacy of chemotherapeutic regimens. Nat
Rev Clin Oncol. 8:151–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Aranda F, Buqué A, Bloy N, Castoldi F,
Eggermont A, Cremer I, Fridman WH, Fucikova J, Galon J, Spisek R,
et al: Trial watch: Adoptive cell transfer for oncological
indications. Oncoimmunology. 4:e10466732015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Domschke C, Schneeweiss A, Stefanovic S,
Wallwiener M, Heil J, Rom J, Sohn C, Beckhove P and Schuetz F:
Cellular immune responses and immune escape mechanisms in breast
cancer: Determinants of immunotherapy. Breast Care (Basel).
11:102–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ruella M and Kalos M: Adoptive
immunotherapy for cancer. Immunol Rev. 257:14–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Henick BS, Herbst RS and Goldberg SB: The
PD-1 pathway as a therapeutic target to overcome immune escape
mechanisms in cancer. Expert Opin Ther Targets. 18:1407–1420.
2014.PubMed/NCBI
|
|
88
|
Steinert G, Schölch S, Niemietz T, Iwata
N, García SA, Behrens B, Voigt A, Kloor M, Benner A, Bork U, et al:
Immune escape and survival mechanisms in circulating tumor cells of
colorectal cancer. Cancer Res. 74:1694–1704. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Radziewicz H, Ibegbu CC, Fernandez ML,
Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust
D, Wherry EJ, et al: Liver-infiltrating lymphocytes in chronic
human hepatitis C virus infection display an exhausted phenotype
with high levels of PD-1 and low levels of CD127 expression. J
Virol. 81:2545–2553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chauvin JM, Pagliano O, Fourcade J, Sun Z,
Wang H, Sander C, Kirkwood JM, Chen TH, Maurer M, Korman AJ and
Zarour HM: TIGIT and PD-1 impair tumor antigen-specific CD8+ T
cells in melanoma patients. J Clin Invest. 125:2046–2058. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Neagu MR and Reardon DA: An update on the
role of immunotherapy and vaccine strategies for primary brain
tumors. Curr Treat Options Oncol. 16:542015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
John LB, Devaud C, Duong CP, Yong CS,
Beavis PA, Haynes NM, Chow MT, Smyth MJ, Kershaw MH and Darcy PK:
Anti-PD-1 antibody therapy potently enhances the eradication of
established tumors by gene-modified T cells. Clin Cancer Res.
19:5636–5646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liu X, Ranganathan R, Jiang S, Fang C, Sun
J, Kim S, Newick K, Lo A, June CH, Zhao Y and Moon EK: A chimeric
switch-receptor targeting PD1 augments the efficacy of
second-generation CAR T cells in advanced solid tumors. Cancer Res.
76:1578–1590. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Cogdill AP, Andrews MC and Wargo JA:
Hallmarks of response to immune checkpoint blockade. Br J Cancer.
117:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Moon EK, Wang LC, Dolfi DV, Wilson CB,
Ranganathan R, Sun J, Kapoor V, Scholler J, Puré E, Milone MC, et
al: Multifactorial T-cell hypofunction that is reversible can limit
the efficacy of chimeric antigen receptor-transduced human T cells
in solid tumors. Clin Cancer Res. 20:4262–4273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Burga RA, Thorn M, Point GR, Guha P,
Nguyen CT, Licata LA, DeMatteo RP, Ayala A, Espat Joseph N,
Junghans RP and Katz SC: Liver myeloid-derived suppressor cells
expand in response to liver metastases in mice and inhibit the
anti-tumor efficacy of anti-CEA CAR-T. Cancer Immunol Immunother.
64:817–829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Suarez ER, de Chang K, Sun J, Sui J,
Freeman GJ, Signoretti S, Zhu Q and Marasco WA: Chimeric antigen
receptor T cells secreting anti-PD-L1 antibodies more effectively
regress renal cell carcinoma in a humanized mouse model.
Oncotarget. 7:34341–34355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Shaw Rosewell A, Porter CE, Watanabe N,
Tanoue K, Sikora A, Gottschalk S, Brenner MK and Suzuki M:
Adenovirotherapy delivering cytokine and checkpoint inhibitor
augments CAR T cells against metastatic head and neck cancer. Mol
Ther. 25:2440–2451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li S, Siriwon N, Zhang X, Yang S, Jin T,
He F, Kim YJ, Mac J, Lu Z, Wang S, et al: Enhanced cancer
immunotherapy by chimeric antigen receptor-modified T cells
engineered to secrete checkpoint inhibitors. Clin Cancer Res.
23:6982–6992. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Serganova I, Moroz E, Cohen I, Moroz M,
Mane M, Zurita J, Shenker L, Ponomarev V and Blasberg R:
Enhancement of PSMA-directed CAR adoptive immunotherapy by
PD-1/PD-L1 blockade. Mol Ther Oncolytics. 4:41–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Gargett T, Yu W, Dotti G, Yvon ES, Christo
SN, Hayball JD, Lewis ID, Brenner MK and Brown MP: GD2-specific CAR
T cells undergo potent activation and deletion following antigen
encounter but can be protected from activation-induced cell death
by PD-1 blockade. Mol Ther. 24:1135–1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Scarfò I and Maus MV: Current approaches
to increase CAR T cell potency in solid tumors: Targeting the tumor
microenvironment. J Immunother Cancer. 5:282017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Di Stasi A, De Angelis B, Rooney CM, Zhang
L, Mahendravada A, Foster AE, Heslop HE, Brenner MK, Dotti G and
Savoldo B: T lymphocytes coexpressing CCR4 and a chimeric antigen
receptor targeting CD30 have improved homing and antitumor activity
in a Hodgkin tumor model. Blood. 113:6392–6402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kershaw MH, Wang G, Westwood JA, Pachynski
RK, Tiffany HL, Marincola FM, Wang E, Young HA, Murphy PM and Hwu
P: Redirecting migration of T cells to chemokine secreted from
tumors by genetic modification with CXCR2. Hum Gene Ther.
13:1971–1980. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Xia T, Konno H and Barber GN: Recurrent
loss of STING signaling in melanoma correlates with susceptibility
to viral oncolysis. Cancer Res. 76:6747–6759. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ajina A and Maher J: Prospects for
combined use of oncolytic viruses and CAR T-cells. J Immunother
Cancer. 5:902017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kim DS, Dastidar H, Zhang C, Zemp FJ, Lau
K, Ernst M, Rakic A, Sikdar S, Rajwani J, Naumenko V, et al: Smac
mimetics and oncolytic viruses synergize in driving anticancer
T-cell responses through complementary mechanisms. Nat Commun.
8:3442017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Scott EM, Duffy MR, Freedman JD, Fisher KD
and Seymour LW: Solid tumor immunotherapy with T cell engager-armed
oncolytic viruses. Macromol Biosci. 18:Jan;2018.doi:
10.1002/mabi.201700187. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Nishio N and Dotti G: Oncolytic virus
expressing RANTES and IL-15 enhances function of CAR-modified T
cells in solid tumors. Oncoimmunology. 4:e9880982015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Nishio N, Diaconu I, Liu H, Cerullo V,
Caruana I, Hoyos V, Bouchier-Hayes L, Savoldo B and Dotti G: Armed
oncolytic virus enhances immune functions of chimeric antigen
receptor-modified T cells in solid tumors. Cancer Res.
74:5195–5205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Gilham DE, Debets R, Pule M, Hawkins RE
and Abken H: CAR-T cells and solid tumors: Tuning T cells to
challenge an inveterate foe. Trends Mol Med. 18:377–384. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Sathyanarayanan V and Neelapu SS: Cancer
immunotherapy: Strategies for personalization and combinatorial
approaches. Mol Oncol. 9:2043–2053. 2015. View Article : Google Scholar : PubMed/NCBI
|