Introduction
Central neurocytoma (CN) is a rare but usually
benign neuronal tumor composed of uniform round cells with neuronal
differentiation and typically located in the supratentorial
ventricles in young adults (1,2). Surgery
and radiotherapy are widely considered as the most important
therapeutic approaches for CN. Patients with CN commonly exhibit a
good prognosis, but in certain cases the clinical course is more
aggressive or is followed by recurrence (3). Chemotherapy may be useful for recurrent
CN that cannot be resected and has been radiated. Therefore, it is
necessary to develop effective antitumor drugs for adjuvant therapy
of CN. In particular, the combination of antitumor drugs with
molecular target therapy may greatly improve the prognosis of
CN.
Oridonin, a diterpenoid isolated from the Chinese
medicinal herb Rabdosia rubescens, exhibits potential
anti-inflammatory, anti-tumor, pro-apoptotic and neurological
effects (4,5). Oridonin has been demonstrated to inhibit
proliferation and induce apoptosis in a variety of human cancer
cells, including colon, pancreatic, breast, lung and liver cancer
(6–10). However, the underlying mechanisms
remain poorly understood. Previously, oridonin has been
demonstrated to exert antitumor activities through several
signaling pathways that are associated with cell proliferation and
apoptosis, including c-Jun N-terminal kinase (JNK), p38
mitogen-activated protein kinase (MAPK), extracellular
signal-regulated kinase (ERK) and protein kinase B (Akt) signaling
pathways (11,12). Although oridonin exhibits a
significant antitumor function in multiple types of cancer, its
exact effect on CN and the underlying mechanism remain unclear.
The Wnt/β-catenin signaling pathway is a canonical
Wnt pathway, which serves a critical role in regulating cell
growth, apoptosis, motility, polarity and differentiation (13). Dysregulation of the pathway is
identified in several types of cancer, including liver, colon and
several types of brain tumors (14).
The underlying molecular mechanisms of CN have been largely
examined, but it has been suggested that the receptors and
effectors of the Wnt pathway are differentially overexpressed in CN
cells (15). Additionally, it has
also been demonstrated that oridonin inhibits the proliferation of
human osteosarcoma cells by suppressing Wnt signaling (16). Therefore, the present study
hypothesized whether oridonin may inhibit the growth of CN cells by
affecting Wnt/β-catenin signaling transduction.
In the present study, the roles of oridonin in the
proliferation and apoptosis of CN cells as well as the potential
molecular mechanisms were investigated. It was indicated that
oridonin was able to inhibit proliferation and induce apoptosis,
which might be mediated by repressing the Wnt/β-catenin signaling
pathway in CN cells.
Materials and methods
Cell culture and treatment
Central neurocytoma tissue was obtained from 1
patient diagnosed with CN for cell culture following resection at
the First Affiliated Hospital of Jiamusi University (Jiamusi,
China). Resected tissues were stored in
Mg2+/Ca2+-free Hank's Balanced Salt Solution
(HBSS), and then cut into small pieces in
piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) solution (20 mM
PIPES, 25 mM glucose, 5 mM KCl, 120 mM NaCl) and treated as
previously described (17–19). The tissue samples were resuspended in
Dulbecco's modified Eagle's medium/F-12/N2 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 5%
fetal bovine serum (FBS; Lonza Group, Ltd., Basel, Switzerland),
100 U/ml penicillin, 100 µg/ml streptomycin, 10 ng/ml epidermal
growth factor (EGF) and 20 ng/ml basic fibroblast growth factor
(bFGF). A total of 1×106 dissociated cells/well were
plated into collagen IV (BD Biosciences, Franklin Lakes, NJ, USA)
or fibronectin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
precoated 6-well culture plate. The cells were cultured in a 37°C
incubator with 5% CO2. The present study design was
approved by the Ethics Committee of First Affiliated Hospital of
Jiamusi University, and all patients provided written informed
consent.
RNA interference and overexpression of
β-catenin
Small interfering RNA (siRNA) targeting β-catenin
(siβ-catenin) and recombinant adenoviruses expressing β-catenin
(Ad-β-ctn) were obtained as previously described using the AdEasy
technology (20–22). The sequence of β-catenin siRNA was
5′-CAGGGGGUUGUGGUUAAGCUCUU-3′. A scramble siRNA sequence
(5′-TTCTCCGAACGTGTCACGT-3′) was used as a control (Gima Biol
Engineering Inc., Shanghai, China). A total of 2×104
cultured central neurocytoma cells were seeded into each well of a
12-well plate and were cultured to 80% confluence. Cell
transfections were performed using 100 nmol siRNA and 5 µl
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. Cells
were further cultured for 48 h following transfection, and cells
were subsequently lysed and analyzed for the protein expression of
β-catenin by western blotting. In addition, cells were treated with
oridonin, and then cell proliferation was detected.
Proliferation assay
Cell proliferation was assessed by MTT assay.
Briefly, following transfection, 104 cells were seeded
in a 96-well flat bottom plate. The cells were cultured in a 5%
CO2 incubator at 37°C. Once cell confluence reached 80%,
the supernatant was replaced with fresh medium and the cells were
treated with 0, 5, 10, 15, 20 or 25 µM oridonin (Xi'an Hao-Xuan
Bio-tech Co., Ltd., Xi'an, China) dissolved in dimethyl sulfoxide
(DMSO) for 24, 48 or 72 h followed by an additional 4 h subsequent
to the addition of 20 µl MTT (5 mg/ml) into each well. A total of
200 µl DMSO was added to the wells for cell lysis. Absorbance was
detected using an ELISA spectrophotometer at 490 nm.
Apoptosis assay
A total of 1×106 central neurocytoma
cells were seeded on 60 mm dishes and cultured in Dulbecco's
modified Eagle's/Nutrient Mixture F12 (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 5% fetal bovine serum (Lonza
Group, Ltd., Basel, Switzerland), 100 U/ml penicillin and 100 µg/ml
streptomycin. When cells reached 80% confluence, they were treated
with 0, 10, 15 or 20 µM oridonin for the indicated time. Apoptosis
was quantified by Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) assay (BD Biosciences, Franklin Lakes,
NJ, USA) following the manufacturer's protocol. Briefly, cells were
collected by trypsinization and pooled with cell supernatants from
corresponding culture dishes; 500 µl of this cell suspension was
incubated with 2 µl of Annexin-V-FITC stock solution for 15 min in
the dark. After a short centrifugation (300 × g, 5 min), cells were
washed in PBS before being resuspended in 0.5 ml of 1× binding
buffer supplemented with 1 µl PI (1 µg/ml final concentration). The
Annexin V-FITC/PI assay detects the amount of phosphatidylserine on
the outer surface of the plasma membrane (a biochemical alteration
unique to membranes of apoptotic cells) and the amount of PI, a dye
that easily enters dead cells or cells in the late stages of
apoptosis and binds DNA but does not bind with the plasma membrane
of viable cells. Fluorescence was detected using a FACSCalibur flow
cytometer by fluorescence activated cell sorter (FACS) analysis,
and data were analyzed using CellQuestPro version 5.2 software (BD
Biosciences, San Jose, CA, USA). The cells with phosphatidylserine
on their surface were considered to be apoptotic.
Western blot analysis
The cells were washed twice with PBS and were lysed
with lysis buffer (50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 1% NP40, 150
mM NaCl, 10 mM NaF and 1 mM Na3VO4)
containing a protease inhibitor cocktail (Roche Molecular
Diagnostics, Branchburg, NJ, USA). Following centrifugation at
12,000 × g for 10 min at 4°C, the supernatant was collected and
quantified using a bicinchoninic acid quantification kit (Beyotime
Institute of Biotechnology, Haimen, China). 50 µg proteins were
loaded per lane onto 10% SDS-PAGE gels (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and transferred to Immobilon-P membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked with 5%
skimmed milk in Tris-buffered saline containing 0.05% Tween 20 for
1 h at room temperature, and incubated with the following specific
primary antibodies overnight at 4°C. The antibodies against B-cell
lymphoma-2 (Bcl-2; 1:500; catalogue no. sc7382; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), Bcl-2-like protein 4 (Bax;
1:500; cat no. sc493; Santa Cruz Biotechnology, Inc.), cleaved
caspase-3 (1:1,000; cat no. 9661S; Cell Signaling Technology, Inc.,
Danvers, MA, USA) and cleaved poly (ADP-ribose) polymerase 1
(cleaved PARP; 1:1,000; catalogue no. 9541; Cell Signaling
Technology, Inc.), β-catenin (1:500; catalogue no. sc1496; Santa
Cruz Biotechnology, Inc.), cyclin D1 (1:1,000; catalogue no.
sc20044; Santa Cruz Biotechnology, Inc.), v-myc avian
myelocytomatosis viral oncogene homolog (c-Myc; 1:1,000; catalogue
no. sc788; Santa Cruz Biotechnology, Inc.) and β-actin (1:1,000;
catalogue no. sc47778; Santa Cruz Biotechnology, Inc.) were used
for detection. This was followed by incubation with horseradish
peroxidase-conjugated goat anti-mouse secondary antibody (1:2,000;
catalogue no. sc-2005; Santa Cruz Biotechnology, Inc.) and
anti-rabbit immunoglobulin G antibody (1:2,000; catalogue no.
sc-2004; Santa Cruz Biotechnology, Inc.) for 2 h at room
temperature. The blots were visualized using enhanced
chemiluminescence detection reagent (GE Healthcare Life Sciences,
Little Chalfont, UK). The gray value of the targeted bands was
quantified with QuantityOne software version 4.6.2 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) following incubation, with
β-actin used as the internal reference.
Quantitative reverse-transcriptase
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using TRIzol
reagent according to the manufacturer's protocol (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). cDNA was
generated with reverse transcription using the
RevertAid™ First Strand cDNA synthesis kit (Fermentas,
Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA) and was
amplified using a TaqMan® Gene Expression Assay (Applied
biosystems; Thermo Fisher Scientific, Inc.) with fluorogenic
carboxyfluorescein-labeled probes using specific primers for target
proteins. The specific primers for PCR were forward,
5′-ACCAGTGGATTCTGTGTTGTT-3′ and reverse,
5′-ATTTGAAGGCAGTCTGTCGTA-3′ for β-catenin and forward,
5′-GATCCCTCCAAAATCAAGTG-3′ and reverse, 5′-GAGTCCTTCCACGATACCAA-3′
for GAPDH. Real-time fluorescence detection was performed with the
ABI PRISM 7700 Sequence Detector (Applied Biosystems; Thermo Fisher
Scientific, Inc.). PCR involved 40 amplification cycles of 94°C for
10 sec, 53°C for 30 sec and 72°C for 40 sec, followed by final
extension at 72°C for 10 min. β-catenin expression was normalized
to GAPDH expression and calculated using the 2−ΔΔCt
formula (23). The relative level of
β-catenin mRNA was presented as a percentage of the control.
Statistical analysis
All of the experiments were repeated at least 3
times. SPSS (version 16.0; SPSS, Inc., Chicago, IL, USA) was used
to analyze the experimental data. One-way analysis of variance was
used to assess the differences between the groups. Duncan's
multiple range test was employed for pairwise comparison and
followed by Bonferroni correction. The data are presented as the
mean ± standard error of the mean. P<0.05 (two-tailed) was
considered statistically significant difference.
Results
Oridonin suppresses the proliferation
of CN cells
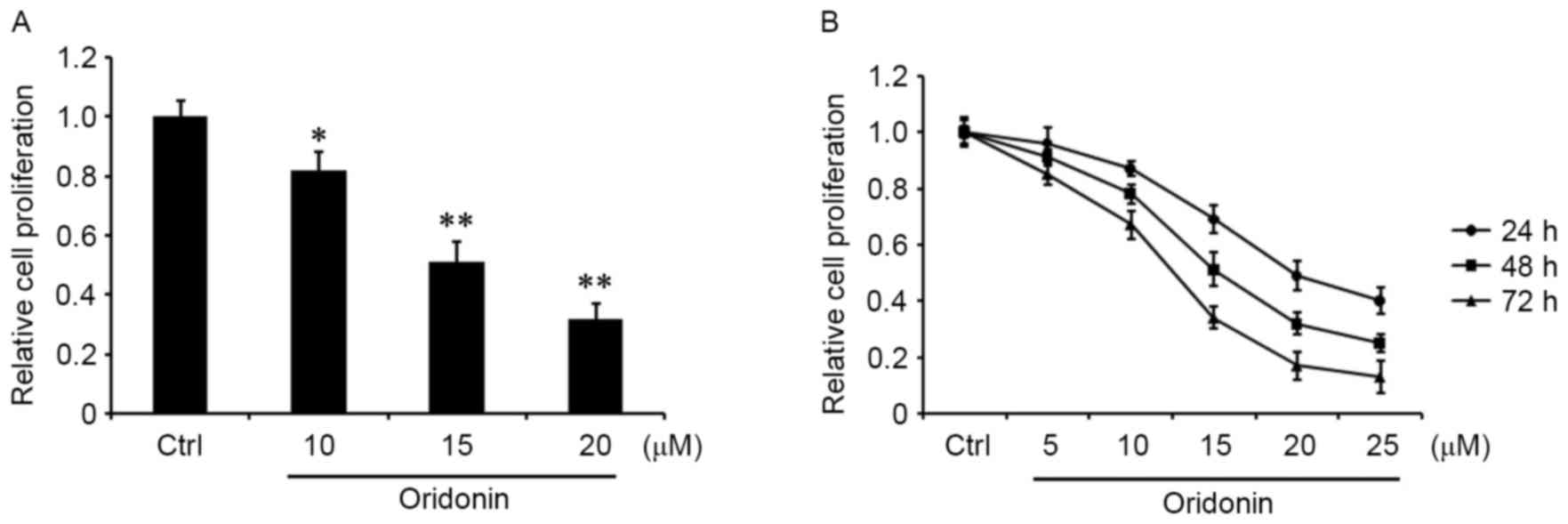
To determine the role of oridonin in CN cells, CN
tissue was obtained from one patient diagnosed with CN following
resection, and a CN cell line was generated by primary culture
(17–19). The cells were grown in the medium for
24 h, and then treated with 10, 15 and 20 µM oridonin for 48 h or
were treated with 5, 10, 15, 20 or 25 µM oridonin for 24, 48 or 72
h. An MTT assay was performed to evaluate cell proliferation. The
results demonstrated that oridonin was able to markedly decrease
the proliferation of CN cells in a concentration- and
time-dependent manner relative to the DMSO-treated control
(Fig. 1A and B).
Oridonin induces the apoptosis of CN
cells
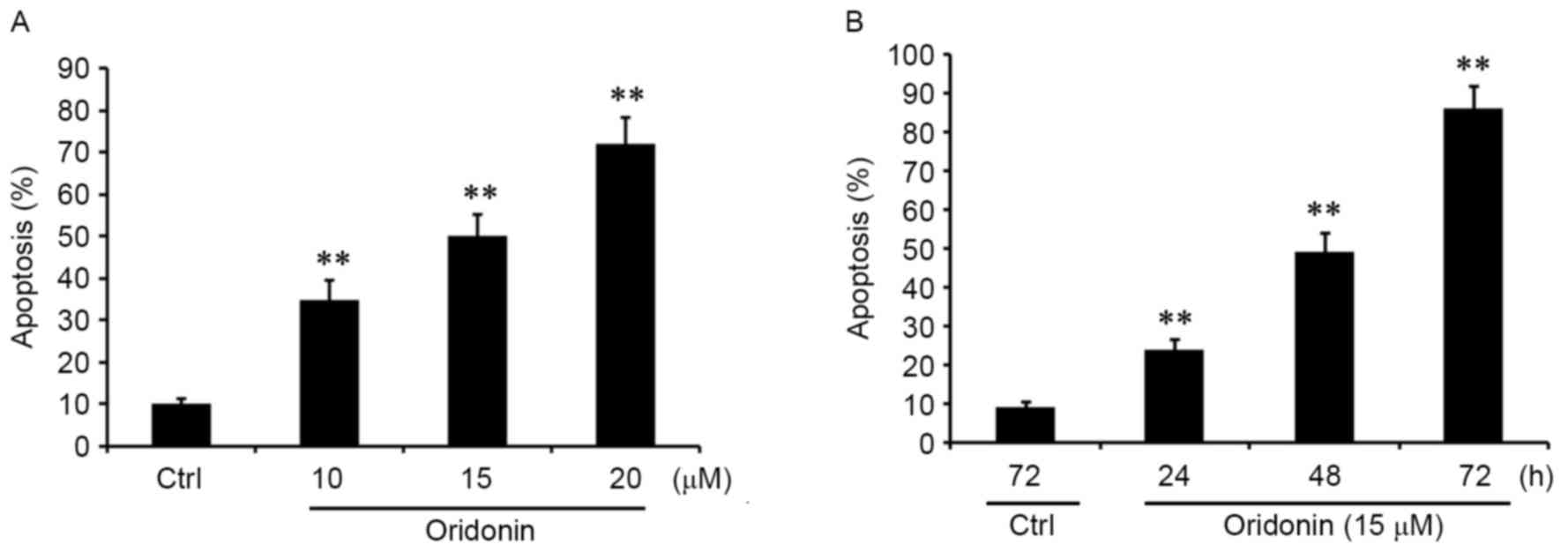
To confirm whether the inhibition of proliferation
that is mediated by oridonin is associated with cell apoptosis, the
cells were treated with 10, 15 and 20 µM oridonin for 48 h.
Apoptosis was quantified by FACS analyses. The results demonstrated
that oridonin treatment was able to significantly promote the
apoptosis of CN cells in a concentration-dependent manner (Fig. 2A). Additionally, CN cells were treated
with 15 µM oridonin for 24, 48 and 72 h. It was identified that
apoptosis was induced by oridonin in a time-dependent manner
(Fig. 2B). These results were
consistent with the pattern observed with the inhibition of
proliferation, suggesting that oridonin acted as a potent apoptotic
inducer in CN cells.
Oridonin affects the expression of
apoptosis-associated proteins in CN cells
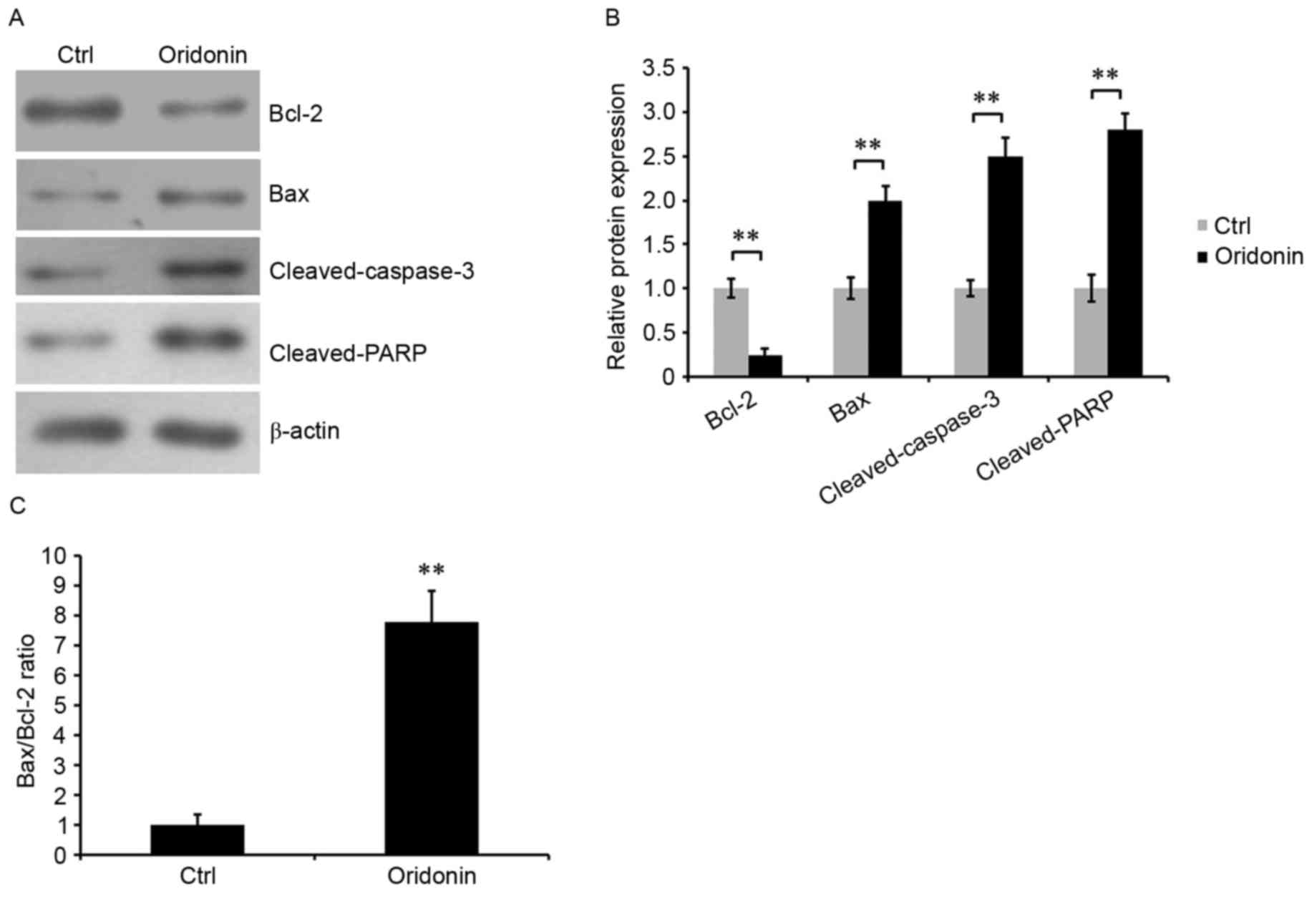
To additionally determine the role of oridonin in
the apoptosis of CN cells, western blotting was performed to assess
the expression levels of apoptosis-associated proteins: Bcl-2, Bax,
cleaved caspase-3 and cleaved PARP. The results demonstrated that
the expression of Bcl-2, an anti-apoptotic protein, was
significantly reduced in oridonin-treated CN cells compared with
control cells. By contrast, the expression levels of pro-apoptosis
protein Bax, cleaved caspase-3 and cleaved PARP were increased
(Fig. 3A and B), and the Bax/Bcl-2
ratio was increased (Fig. 3C). The
Bax/Bcl-2 ratio is a key factor in determining the occurrence and
level of apoptosis (24). These
results indicated that treatment with oridonin was able to alter
cell apoptosis, potentially by modulating Bcl-2, Bax, and cleaved
caspase-3 and cleaved PARP.
Oridonin downregulates the
Wnt/β-catenin signaling pathway
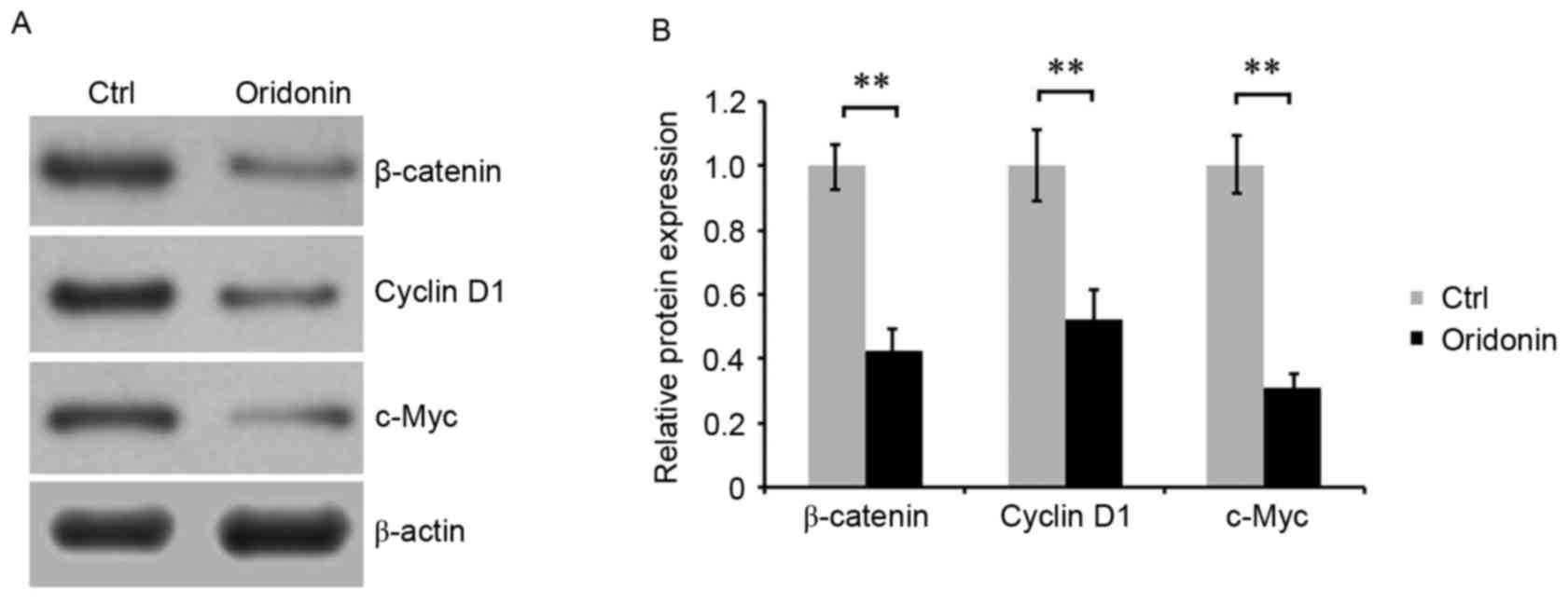
To investigate the molecular mechanism underlying
the effect of oridonin on the growth and apoptosis of CN cells, the
Wnt/β-catenin signaling pathway was evaluated by detecting the
expression of key proteins of this pathway. CN cells were treated
with 15 µM oridonin for 48 h. Western blot analysis was performed,
and the results indicated that treatment with oridonin was able to
downregulate the accumulation of β-catenin, cyclin D1 and c-Myc in
CN cells. Densitometric analysis of the western blot bands
confirmed these results (Fig. 4A and
B), suggesting that oridonin regulated the growth and apoptosis
of CN cells, which is likely to be mediated by Wnt signaling.
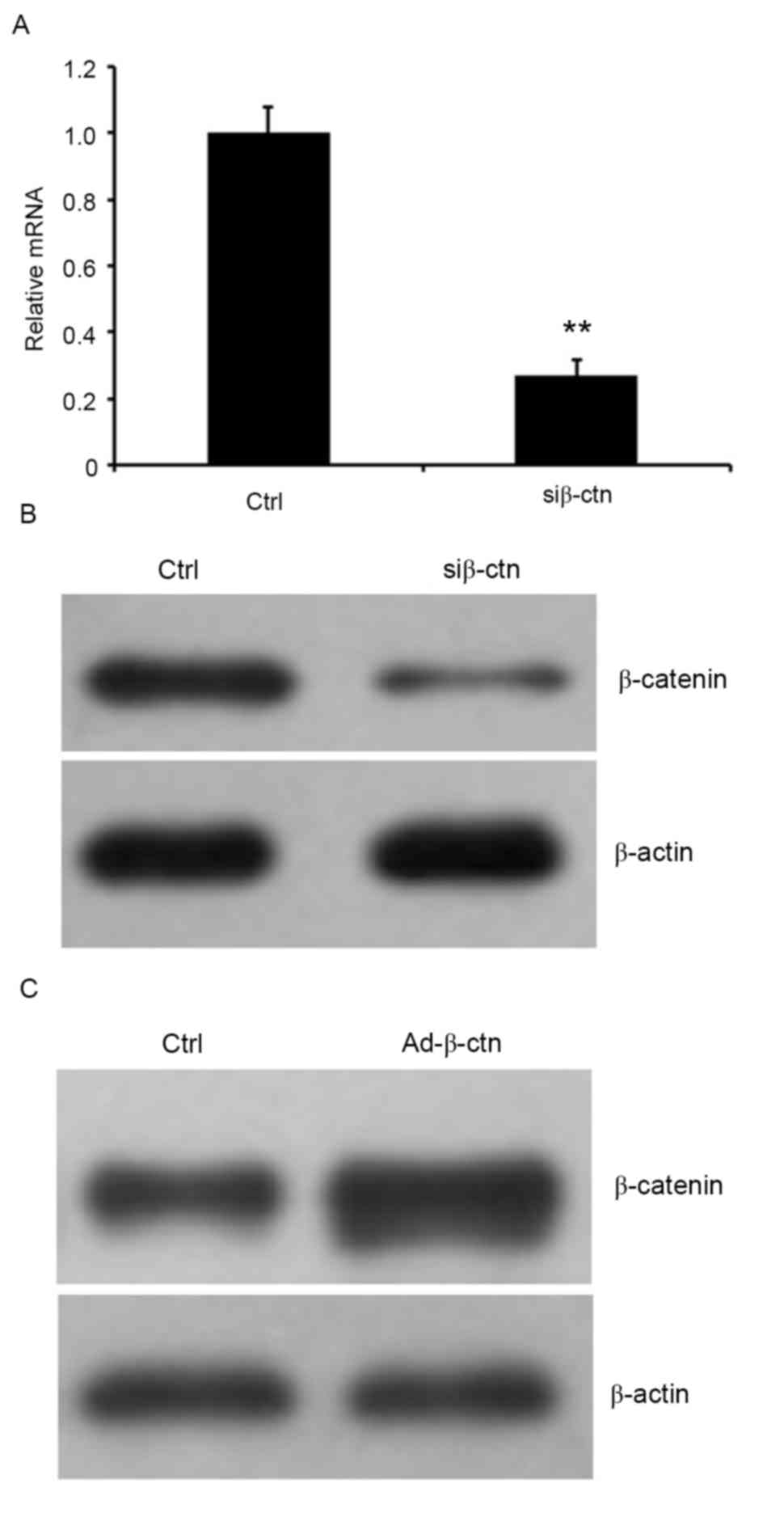
Silencing and overexpressing β-catenin
in CN cells
To verify the involvement of Wnt/β-catenin signaling
pathway in oridonin-mediated inhibition on proliferation of CN
cells, β-catenin was knocked down using siRNA or was overexpressed
by infecting cells with recombinant adenoviruses that expressed
β-catenin in CN cells. The RNA expression level of β-catenin was
examined by RT-qPCR, and protein expression was detected by western
blot analysis. The results indicated that the relative β-catenin
mRNA expression was markedly decreased in cells that were treated
with β-catenin siRNA and the residual protein expression of
β-catenin in the cells was markedly reduced compared with the
control siRNA-treated cells (Fig. 5A and
B). Additionally, western blot analysis indicated that
β-catenin was significantly elevated in cells that overexpressed
β-catenin (Fig. 5C).
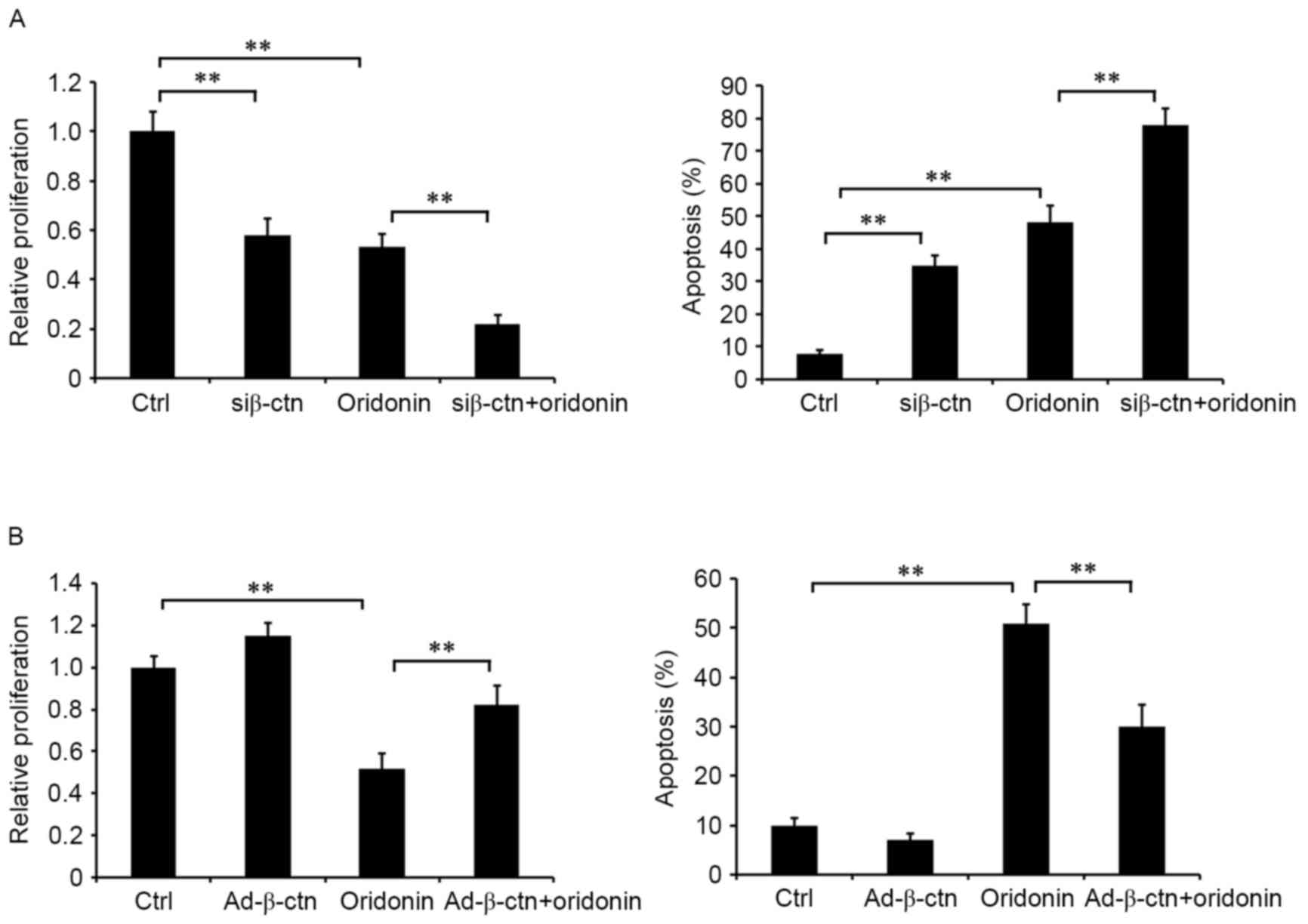
Role of β-catenin in oridonin-mediated
inhibition of cell proliferation
The effect of β-catenin, a key member of the Wnt
signaling pathway, on oridonin-mediated inhibition of cell
proliferation in CN cells was examined in the aforementioned cell
lines. β-catenin-silenced and non-silenced CN cells were treated
with or without 15 µM oridonin for 48 h. Detection of cell
proliferation indicated that treatment with oridonin and silencing
of β-catenin inhibited cell proliferation and induced apoptosis
(Fig. 6A). The combination of
oridonin treatment and silencing of β-catenin markedly augmented
the effects on cell proliferation and apoptosis compared with
oridonin single treatment (Fig. 6A).
Additionally, the overexpression of β-catenin attenuated the
effects of oridonin on proliferation and apoptosis (Fig. 6B). These data suggested that the
Wnt/β-catenin signaling pathway served an important role in the
antitumor activities of oridonin in CN cells, and that oridonin
regulated the growth of CN cells, which might be via the
Wnt/β-catenin signaling pathway.
Discussion
Oridonin is a diterpenoid compound isolated from the
Chinese traditional medicine herb Rabdosia rubescens
(25), which has attracted attraction
due to its antitumor activities (26). Oridonin has been demonstrated to exert
antitumor effects by inhibiting cell growth, proliferation and
inducing apoptosis in multiple types of human cancer (27). However, the role of oridonin in the
regulation of biological function of CN cells and the underlying
molecular mechanisms remain unclear. The molecular regulatory
mechanisms in CN cells are predominantly unexplored. In the present
study, the data suggested that oridonin may suppress cell
proliferation and induce apoptosis in CN cells, and that the
function may be mediated by altering the Wnt/β-catenin signaling
pathway. To investigate the molecular mechanisms underlying the
inhibition of cell proliferation and induction of apoptosis in CN
cells, the expression levels of apoptosis-associated proteins,
Bcl-2, Bax, cleaved caspase-3 and cleaved PARP, were detected by
western blotting in the present study.
Anti-apoptotic Bcl-2 and pro-apoptotic Bax as well
as Bcl-2 family proteins regulate mitochondrial permeability to
alter apoptosis via an intrinsic pathway (28). In the present study, treatment with
oridonin was able to decrease Bcl-2 expression and increase Bax
expression in CN cells. Furthermore, the Bax/Bcl-2 ratio (an
important parameter to measure the occurrence and levels of
apoptosis) was significantly elevated.
Cleaved caspase-3, also known as mature or activated
caspase-3, is a critical mediator of cell apoptosis (29). Pro-caspases require cleavage after
aspartic acid residues, which result in one large and one small
subunit. These subunits associate into an a2b2 tetramer to form the
active enzyme (30,31). Caspase-3 is able to cleave PARP to a
specific 85-kDa form, which is observed during apoptosis (30,31). In
the present study, treatment with oridonin was able to increase the
levels of cleaved caspase-3 and cleaved PARP, which was consistent
with the promotion of apoptosis. These data indicated that
oridonin-induced apoptosis in CN cells is associated with a
decrease in Bcl-2 expression and an increase in Bax expression and
activation of caspase-3.
The canonical Wnt signaling pathway serves an
important role in the regulation of cell proliferation and
apoptosis. It has been indicated that downstream target genes of
the Wnt/β-catenin pathway, including c-Myc and cyclin
D1, are associated with apoptosis (32,33). The
Wnt signaling pathway is activated by interactions between the Wnt
ligand and the Frizzled family receptor and the low-density
lipoprotein receptor-related protein 5/6. The interaction leads to
an accumulation of β-catenin in the cytoplasm and translocation
into the nucleus due to the inactivation of the β-catenin
destruction complex, Axin/adenomatous polyposis coli/glycogen
synthase kinase-3β (34–37). Subsequently, β-catenin interacts with
TCF4/LEF to activate the transcription of the target genes
Bcl-2, cyclin D1 and c-Myc, which control the
transition from G1 to S, resulting in abnormal cellular
proliferation and apoptosis (38–40). The
deregulation of Wnt signaling has been identified to be involved in
tumorigenesis and the development of various types of cancer
(41). It has been demonstrated that
the Wnt pathway receptor, Frizzled-1, and the effector, T cell
transcription factor 4 (TCF4), are highly expressed in CN cells and
involved in the origin and expansion of neurocytoma from native
subependymal progenitor cells (15),
suggesting that Wnt/β-catenin signaling is activated in CN cells.
Combined with the reported finding that oridonin inhibits Wnt
signaling in osteosarcoma cells (16), it is possible that oridonin may affect
Wnt signaling in CN cells.
The stability of β-catenin is commonly used to
evaluate the activity of the Wnt/β-catenin signaling pathway. In
the present study, it was revealed that oridonin was able to
downregulate the level of β-catenin protein in CN cells in a
concentration- and time-dependent manner. It has been previously
reported that β-catenin is able to bind TCFs to stimulate cellular
growth and proliferation in tumorigenesis by triggering the cell
cycle regulator cyclin D1 (42).
c-Myc, as the target of β-catenin protein, also serves a critical
role in tumor prognosis (43). In the
present study, it was demonstrated that cyclin D1 and c-Myc, the
downstream targets of β-catenin, were reduced in CN cells,
indicating that the Wnt/β-catenin pathway was inhibited.
Additionally, silencing β-catenin was able to augment
oridonin-mediated inhibition of proliferation, whereas the
overexpression of β-catenin was able to attenuate these effects in
CN cells. These findings indicated that the antitumor activity of
oridonin is mediated via the Wnt/β-catenin signaling pathway in CN
cells.
The potential molecular mechanism underlying the
antitumor activities of oridonin has been investigated. Several
studies indicated that certain signaling pathways and key genes
associated with cell apoptosis and cells cycle were regulated by
oridonin (44). For instance,
oridonin is able to induce autophagy and apoptosis by upregulating
p21 in prostate cancer cells (43) or
can downregulate the phosphoinositide 3-kinase/Akt signaling
pathway to suppress proliferation and induce caspase-dependent
apoptosis in cervical cancer HeLa cells (45). In addition, oridonin may downregulate
the activities of ERK and Akt and stimulate c-Jun and MAPK pathways
to suppress proliferation and induce apoptosis in osteosarcoma
cells (46). Oridonin has also been
suggested to suppress the protein tyrosine kinase-Ras-Raf-JNK
survival pathway and activate the ERK-p53 apoptotic pathway,
resulting in cell cycle arrest and apoptosis in murine fibrosarcoma
cells (47). Therefore, whether other
signaling pathways or proteins take part in the regulation of
antitumor activities of oridonin in CN cells remains to be
elucidated.
In conclusion, the results of the present study
indicated that oridonin exerts its antitumor function in CN cells
by downregulating the activity of the Wnt/β-catenin signaling
pathway, suggesting that oridonin and other compounds from Chinese
herbal medicines targeting the Wnt/β-catenin signaling may be
alternative drugs for CN therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key
Research and Development Program of China (grant no.
2016YFE0126000), China National Natural Science Foundation (grant
no.81570392), China Postdoctoral Science Foundation funded project
(grant no.2016M591937), Natural Science Fund for Colleges and
Universities in Jiangsu Province (grant no.16KJB320017), and High
Level Talent Support Program of Yangzhou University (grant no.
137080077).
Availability of data and materials
The data sets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
JL and YW were major contributors in the conception
and design of the research and revision of the manuscript.
Acquisition of data was performed by WW, SG and LW were the major
contributors in the analysis and interpretation of data and
statistical analysis. Drafting the manuscript was performed by
JL.
Ethics approval and consent to
participate
Ethics Committee of Jiamusi University approved this
study.
Patient consent for publication
Written informed consent was obtained from patient
for publication of this research article and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim DG, Paek SH, Kim IH, Chi JG, Jung HW,
Han DH, Choi KS and Cho BK: Central neurocytoma: The role of
radiation therapy and long term outcome. Cancer. 79:1995–2002.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schild SE, Scheithauer BW, Haddock MG,
Schiff D, Burger PC, Wong WW and Lyons MK: Central neurocytomas.
Cancer. 79:790–795. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schmidt MH, Gottfried ON, von Koch CS,
Chang SM and McDermott MW: Central neurocytoma: A review. J
Neurooncol. 66:377–384. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuo LM, Kuo CY, Lin CY, Hung MF, Shen JJ
and Hwang TL: Intracellular glutathione depletion by oridonin leads
to apoptosis in hepatic stellate cells. Molecules. 19:3327–3344.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kadota S, Basnet P, Ishii E, Tamura T and
Namba T: Antibacterial activity of trichorabdal A from Rabdosia
trichocarpa against Helicobacter pylori. Zentralblatt fur
Bakteriologie: Int J Med Microbiol. 286:63–67. 1997. View Article : Google Scholar
|
|
6
|
Yang J, Jiang H, Wang C, Yang B, Zhao L,
Hu D, Qiu G, Dong X and Xiao B: Oridonin triggers apoptosis in
colorectal carcinoma cells and suppression of microRNA-32
expression augments oridonin-mediated apoptotic effects. Biomed
Pharmacother. 72:125–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H, Wang YF, Liu TG, Xiang XL and
Huang SL: Experimental study on anti-pancreatic cancer effect of
oridonin. Zhong Yao Cai. 37:1230–1233. 2014.(In Chinese).
PubMed/NCBI
|
|
8
|
Li Y, Wang Y, Wang S, Gao Y, Zhang X and
Lu C: Oridonin phosphate-induced autophagy effectively enhances
cell apoptosis of human breast cancer cells. Med Oncol. 32:3652015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang YY, Lv YF, Lu L and Cai L: Oridonin
inhibits mTOR signaling and the growth of lung cancer tumors.
Anticancer Drugs. 25:1192–1200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bohanon FJ, Wang X, Ding C, Ding Y,
Radhakrishnan GL, Rastellini C, Zhou J and Radhakrishnan RS:
Oridonin inhibits hepatic stellate cell proliferation and
fibrogenesis. J Surg Res. 190:55–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bu HQ, Liu DL, Wei WT, Chen L, Huang H, Li
Y and Cui JH: Oridonin induces apoptosis in SW1990 pancreatic
cancer cells via p53- and caspase-dependent induction of p38 MAPK.
Oncol Rep. 31:975–982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang CL, Wu LJ, Tashiro S, Onodera S and
Ikejima T: Oridonin induces a caspase-independent but mitochondria-
and MAPKdependent cell death in the murine fibrosarcoma cell line
L929. Biol Pharm Bull. 27:1527–1531. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Behrens J and Lustig B: The Wnt connection
to tumorigenesis. Int J Dev Biol. 48:477–487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Knoepfler PS, Cheng PF and Eisenman RN:
N-myc is essential during neurogenesis for the rapid expansion of
progenitor cell populations and the inhibition of neuronal
differentiation. Genes Dev. 16:2699–2712. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sim FJ, Keyoung HM, Goldman JE, Kim DK,
Jung HW, Roy NS and Goldman SA: Neurocytoma is a tumor of adult
neuronal progenitor cells. J Neurosci. 26:12544–12555. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Liu YZ, Zhang RX, Wang X, Meng ZJ,
Huang J, Wu K, Luo JY, Zuo GW, Chen L, et al: Oridonin inhibits the
proliferation of human osteosarcoma cells by suppressing
Wnt/β-catenin signaling. Int J Oncol. 45:795–803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roy NS, Benraiss A, Wang S, Fraser RA,
Goodman R, Couldwell WT, Nedergaard M, Kawaguchi A, Okano H and
Goldman SA: Promoter-targeted selection and isolation of neural
progenitor cells from the adult human ventricular zone. J Neurosci
Res. 59:321–331. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roy NS, Wang S, Jiang L, Kang J, Benraiss
A, Harrison-Restelli C, Fraser RA, Couldwell WT, Kawaguchi A, Okano
H, et al: In vitro neurogenesis by progenitor cells isolated from
the adult human hippocampus. Nat Med. 6:271–277. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee SJ, Kim JE, Paek SH, Keyoung HM, Kim
DG and Jung HW: Primary cell culture of central neurocytomas. J
Korean Neurosurg Soc. 34:238–244. 2003.
|
|
20
|
Wu K, Yang Q, Mu Y, Zhou L, Liu Y, Zhou Q
and He B: Berberine inhibits the proliferation of colon cancer
cells by inactivating Wnt/beta-catenin signaling. Int J Oncol.
41:292–298. 2012.PubMed/NCBI
|
|
21
|
He TC, Zhou S, da Costa LT, Yu J, Kinzler
KW and Vogelstein B: A simplified system for generating recombinant
adenoviruses. Proc Natl Acad Sci USA. 95:2509–2514. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang N, Song WX, Luo J, Luo X, Chen J,
Sharff KA, Bi Y, He BC, Huang JY, Zhu GH, et al: BMP-9-induced
osteogenic differentiation of mesenchymal progenitors requires
functional canonical Wnt/beta-catenin signalling. J Cell Mol Med.
13:2448–2464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(t)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng M, Li J, Wang J, Ma C, Jiao Y, Wang
Y, Zhang J, Sun Q, Ju Y, Gao L1 and Zhao Y: High glucose increases
LPS-induced DC apoptosis through modulation of ERK1/2, AKT and
Bax/Bcl-2. BMC Gastroenterol. 14:982014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abelson PH: Medicine from plants. Science.
247:5131990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu H, Qian C and Shen Z: Anti-tumor
activity of oridonin on SNU-5 subcutaneous xenograft model via
regulation of c-Met pathway. Tumour Biol. 35:9139–9146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu YQ, Mu ZQ, You S, Tashiro S, Onodera S
and Ikejima T: Fas/FasL signaling allows extracelluar-signal
regulated kinase to regulate cytochrome c release in
oridonin-induced apoptotic U937 cells. Biol Pharm Bull.
29:1873–1879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin S, Shen JN, Wang J, Huang G and Zhou
JG: Oridonin induced apoptosis through Akt and MAPKs signaling
pathways in human osteosarcoma cells. Cancer Biol Ther. 6:261–268.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng Y, Qiu F, Ye YC, Tashiro S, Onodera
S and Ikejima T: Oridonin induces G2/M arrest and apoptosis via
activating ERK-p53 apoptotic pathway and inhibiting PTK-Ras-Raf-JNK
survival pathway in murine fibrosarcoma L929 cells. Arch Biochem
Biophys. 490:70–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stennicke HR and Salvesen GS:
Caspases-controlling intracellular signals by protease zymogen
activation. Biochim Biophys Acta. 1477:299–306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gray DC, Mahrus S and Wells JA: Activation
of specific apoptotic caspases with an engineered small
molecule-activated protease. Cell. 142:637–646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wharton KA Jr: Runnin' with the Dvl:
Proteins that associate with Dsh/Dvl and their significance to Wnt
signal transduction. Dev Biol. 253:1–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rao TP and Kühl M: An updated overview on
Wnt signaling pathways: A prelude for more. Circ Res.
106:1798–1806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Minde DP, Anvarian Z, Rüdiger SG and
Maurice MM: Messing up disorder: How do missense mutations in the
tumor suppressor protein APC lead to cancer? Mol Cancer.
10:1012011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Minde DP, Radli M, Forneris F, Maurice MM
and Stefan Rüdiger GD: large extent of disorder in adenomatous
polyposis coli offers a strategy to guard Wnt signalling against
point mutations. PLoS One. 8:e772572013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lopez J and Tait SW: Mitochondrial
apoptosis: Killing cancer using the enemy within. Br J Cancer.
112:957–962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cohen GM: Caspases: The executioners of
apoptosis. Biochem J. 326:1–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tetsu O and McCormick F: Beta-catenin
regulates expression of cyclin D1 in colon carcinoma cells. Nature.
398:422–426. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Damalas A, Ben-Ze'ev A, Simcha I, Shtutman
M, Leal JF, Zhurinsky J, Geiger B and Oren M: Excess beta-catenin
promotes accumulation of transcriptionally active p53. EMBO J.
18:3054–3063. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He TC, Sparks AB, Rago C, Hermeking H,
Zawel L, da Costa LT, Morin PJ, Vogelstein B and Kinzler KW:
Identification of c-MYC as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li CY, Wang EQ, Cheng Y and Bao JK:
Oridonin: An active diterpenoid targeting cell cycle arrest,
apoptotic and autophagic pathways for cancer therapeutics. Int J
Biochem Cell Biol. 43:701–704. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li X, Li X, Wang J, Ye Z and Li JC:
Oridonin up-regulates expression of P21 and induces autophagy and
apoptosis in human prostate cancer cells. Int J Biol Sci.
8:901–912. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hu HZ, Yang YB and Xu XD: Oridonin induces
apoptosis via PI3K/Akt pathway in cervical carcinoma HeLa cell
line. Acta Pharmacol Sin. 28:1819–1826. 2007. View Article : Google Scholar : PubMed/NCBI
|