Introduction
Condyloma acuminata (CA), a common sexually
transmitted disease, is caused by human papillomavirus (HPV)
(1). CA grows rapidly and recurs
easily and it causes physical pain and psychological issues in
patients. HPV is a type of epithelial DNA virus, which has many
subtypes, among which HPV6 and HPV11 cause CA (2,3). CA is
caused by the epithelial-associated proteins found in the genomes
of each subtype (2,3).
HPV promotes latency and consequently does not cause
a disease phenotype following initial infection by an immune
evasion response reaction, featuring binding to epithelial cells
and integrating into the host genome, resulting in the
proliferation of epidermal cells containing the viral genome and
neovascularization (3). However, the
regulatory mechanisms of HPV infection and consequent immune escape
reaction, persistent infection and cell proliferation and the
regulatory mechanisms of vascular proliferation are poorly
understood and require additional investigation (4).
MicroRNA (miRNA) molecules, a series of
single-stranded non-coding RNA chains measuring 20–25 nucleotides
in length, regulate gene expression at the transcriptional level by
complementary pairing with target gene mRNA (5). The expression of genes through miRNA is
regulated by endogenous regulatory pathways, resulting in high
stability and biocompatibility (5).
miRNA provides a unique source of gene therapy as a broad-spectrum
molecule against viruses (5).
Ubiquitin-protein ligase E3A (UBE3A; E6-AP) is an
important member of the ubiquitin proteasome system and a type of
ubiquitin protein ligase (E3 enzyme) (6). UBE3A is associated with cervical cancer
and may combine with the E6 proto-oncogene encoded by HPV16 within
cervical cancer cells to form the E6/E6-AP protein complex through
the ubiquitin proteasome pathway (4).
Previous studies have identified that UBE3A exhibits abnormal
expression in numerous tumor cells, including prostate, cervical
and breast cancer (6,7). In addition, numerous important cellular
proteins, such as B-cell lymphoma-2 homologous antagonist/killer,
Myc proto-oncogene protein, cyclin-dependent kinase inhibitor 1B,
DNA replication licensing factor MCM-7, retinoblastoma 1 and
Annexin A1, are degenerated through the UBE3A-mediated ubiquitin
proteasome pathway (7).
Insulin-like growth factor-1 (IGF-1), synthesized
and secreted by human hepatocytes, is the primary regulator of
insulin and serves an important function in regulating the growth
and development of the body (8). It
affects cell proliferation, differentiation and inhibits apoptosis,
and its role in tumor development has received attention (9). Overexpression of IGF-1 in serum and
tissue alters the growth of normal cells and causes uncontrolled
proliferation, inhibits differentiation and reduces apoptosis,
resulting in the incidence and development of malignant tumors
(10). Therefore, detecting the
expression of IGF-1 assists in determining the biological behavior
of tumors (10). The present study
attempted to identify and characterize the probable anti-CA
mechanism of miRNA-375 on HPV.
Materials and methods
Cell culture
HPV-18-positive (+) HeLa cervical cancer cell were
obtained from the Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China) and cultured with Dulbecco's
modified Eagle's medium (DMEM; Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS; Hyclone; GE Healthcare Life Sciences), 100 U/ml penicillin
and 100 mg/ml streptomycin at 37°C with 5% CO2.
miRNA and transfections
miRNA-375 mimics and the negative control were
obtained from the Union Medical University Center for Basic Medical
Cells (Beijing, China). HeLa cells were transfected with 50–200 ng
miRNA-375 mimics (sense, 5′-UUUGUUCGUUCGGCUCGCGUG-3′ and antisense,
5′-ACGCGAGCCGAACGAACAA-3′) and the negative control (sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGCGAGCCGAACGAACAA-3′) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
37°C. After transfection for 4 h, the old medium was removed,
replaced with DMEM and cultivated at 24, 48 and 72 h for MTT assay,
cultivated at 48 h for lactate dehydrogenase (LDH) release
activity, apoptosis analysis, caspase activity and western blot
analysis.
MTT assay and LDH release
activity
Following transfection at 4 h, HeLa cells
(1×103 cells/well) were plated with DMEM supplemented
with 10% FBS in 96-well plates for 24, 48 and 72 h at 37°C. MTT
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany; 5 mg/ml) was added
to cells for 4 h at 37°C. Subsequently, 150 µl dimethyl sulfoxide
(99.99%) was added to the cells for dissolution at 37°C for 20 min.
The optical density values were measured using a microplate reader
(model no. 680; Bio-Rad Laboratories, Inc., Hercules, CA, USA) at
492 nm. LDH release was measured using an LDH Cytotoxicity Assay
kit (C0016; Beyotime Institute of Biotechnology, Haimen, China) at
600 nm.
Apoptosis analysis and caspase
activity
Following transfection, HeLa cells (1×105
cells/well) were plated at in 6-well plates for 48 h. Cells were
stained with 10 µl Annexin V-fluorescein isothiocyanate Apoptosis
Detection kit and 5 µl propidium iodide (both BD Biosciences;
Franklin Lakes, NJ, USA) in darkness for 15 min at room
temperature. Cell apoptosis was measured by using a flow cytometer
(FACSCalibur; BD Biosciences) and analyzed using FlowJo software
(version 7.6.1; FlowJo LLC, Ashland, OR, USA).
Following transfection, HeLa cells (1×105
cells/well) were plated in 6-well plates for 48 h. Total protein
from cells was extracted using radioimmunoprecipitation (RIPA)
buffer and total protein concentration was measured using a BCA
protein assay (both Beyotime Institute of Biotechnology). A total
of 5 µg total protein from each sample was used to analyze
caspase-3/9 activity using Caspase 3 Activity Assay kit (cat. no.
C1116) and Caspase 9 Activity Assay kit (cat. no. C1158; both
Beyotime Institute of Biotechnology) according to the protocol of
the manufacturer. The optical density values were measured using a
microplate reader (model no. 680; Bio-Rad Laboratories, Inc.) at
405 nm.
Western blot analysis
Following transfection, HeLa cells (1×105
cells/well) were plated in 6-well plates for 48 h. Total protein
from cells was extracted using RIPA buffer at 4°C for 15 min, and
total protein concentration was measured using a BCA protein assay
(both Beyotime Institute of Biotechnology, Haimen, China). A total
of 50–60 µg total protein from each sample was separated on an
8–12% gel using SDS-PAGE and transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). B-cell
lymphoma 2 (Bcl-2; sc-783; 1:1,000), Bcl-2-associated X protein
(Bax; sc-6236; 1:1,000), tumor protein (p)53 (sc-6243; 1:1,000),
cyclin-dependent kinase inhibitor 1 (p21; sc-397; 1:1,000; all
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), UBE3A (ab126765;
1:1,000; Abcam, Cambridge, USA) and Insulin-like growth factor-1
receptor (IGF-1R; sc-7952; 1:1,000) and GAPDH (cat no. sc-25778;
1:5,000; both Santa Cruz Biotechnology) antibodies were incubated
with the membrane overnight at 4°C subsequent to blocking with
5%-skim milk powder TBST for 1 h at 37°C. Horseradish
peroxidase-conjugated anti-rabbit immunoglobulin G was used as a
secondary antibody (cat no. sc-2004; 1:5,000; Santa Cruz
Biotechnology) and incubated for 1 h at 37°C. Protein bands were
visualized using enhanced chemiluminescent Blotting Detection
Reagents (cat no. NCI4106; Pierce; Thermo Fisher Scientific, Inc.),
the ImageQuant™ LAS 4000 mini system (GE Healthcare Life Sciences,
Chalfont, UK) and analyzed using Image Lab 3.0 (Bio-Rad
Laboratories, Inc.).
Statistical analysis
Data are presented as mean ± standard deviation. All
data were analyzed with GraphPad Prism 5 software (Graph Pad, Inc.,
La Jolla, CA, USA) using one-way analysis of variance and a Tukey's
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
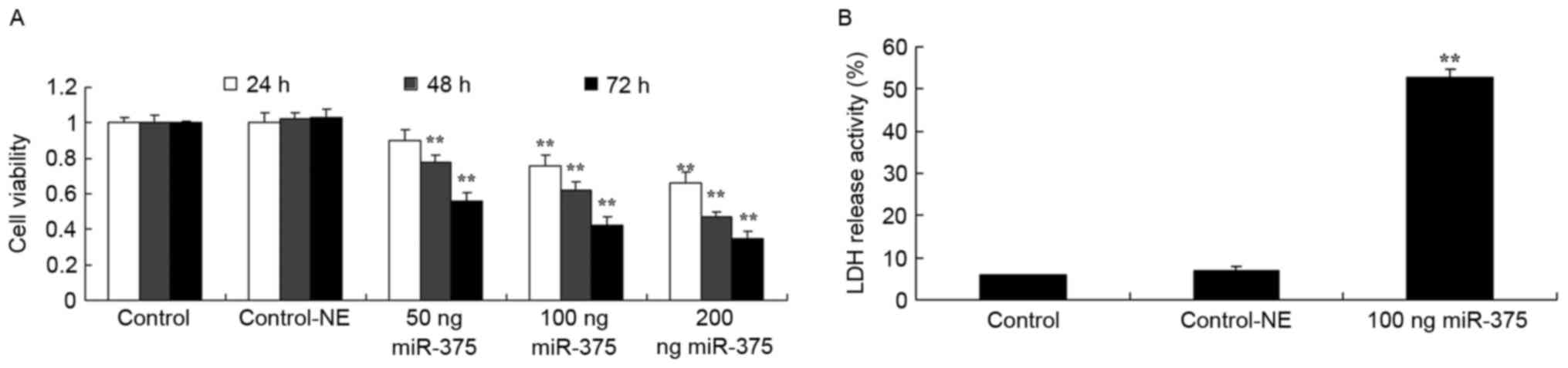
Overexpression of miRNA-375 suppresses cell
viability and LDH activity of HPV-18(+) cervical cancer cells.
Firstly, it was determined if the overexpression of miRNA-375
suppressed cell proliferation and induced apoptosis in HPV-18(+)
cervical cancer cells. As demonstrated in Fig. 1A, 100 (P=0.0098 for 24 h, 0.0085 for
48 h and 0.0056 for 72 h), 200 ng miRNA-375 mimics (P=0.0088 for 24
h, 0.0073 for 48 h and 0.0037 for 72 h) decreased cell viability of
HPV-18(+) cervical cancer cells for 24, 48 and 72 h, and 50 ng
(P=0.0121 for 24 h, 0.0078 for 48 h and 0.0056 for 72 h) miRNA-375
mimics also decreased cell proliferation of HPV-18(+) cervical
cancer cells at 48 and 72 h. Concurrently, 100 ng miRNA-375 mimics
increased LDH activity (P=0.0036) of HPV-18(+) cervical cancer
cells for 48 h, compared with the negative group (Fig. 1B).
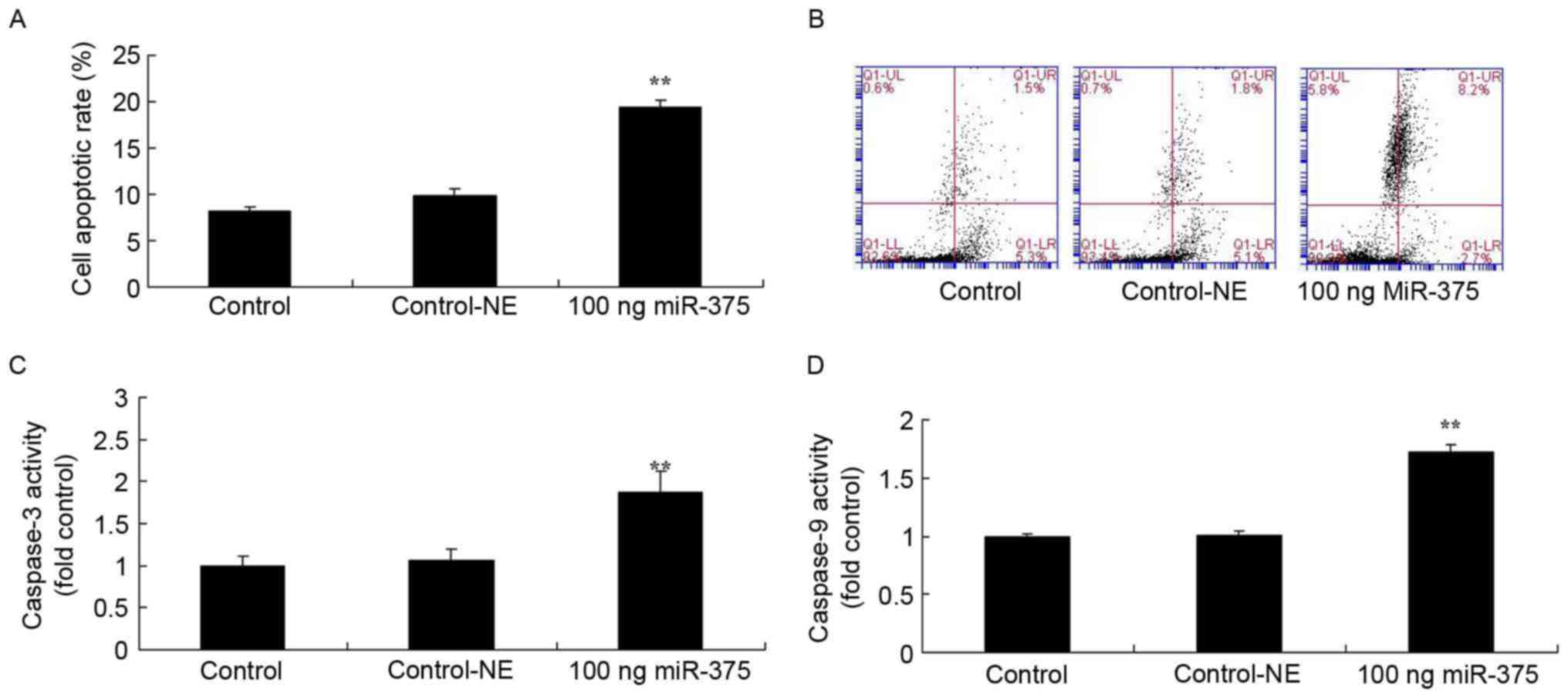
Overexpression of miRNA-375 increases rates of
apoptosis, caspase-3 and caspase-9 activities of HPV-18(+) cervical
cancer cells. To identify the effects of miRNA-375 on cell
apoptosis of HPV-18(+) cervical cancer cells, the rates of
apoptosis, caspase-3 and caspase-9 activities were analyzed. A
total of 100 ng miRNA-375 mimics increased the rate of apoptosis
(P=0.0078) and promoted caspase-3 (P=0.0082) and caspase-9
activities (P=0.0094) of HPV-18(+) cervical cancer cells for 48 h,
compared with the negative group (Fig.
2).
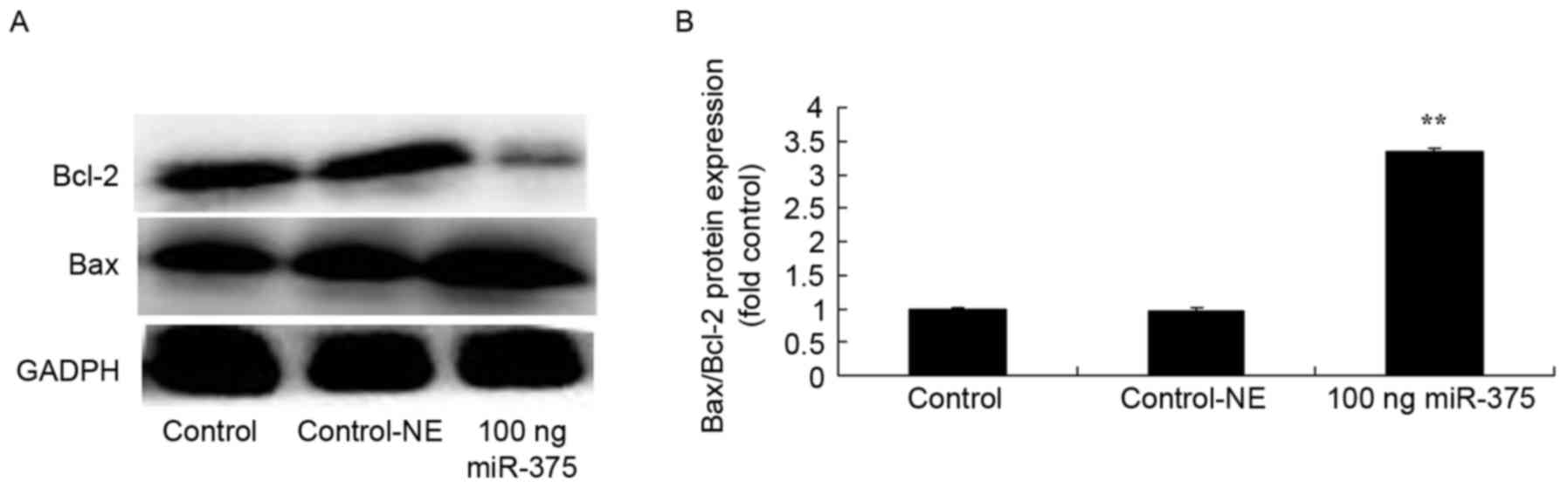
Overexpression of miRNA-375-induced Bax/Bcl-2 of
HPV-18(+) cervical cancer cells. To study the molecular mechanisms
associating miRNA-375 expression with apoptosis in HPV-18(+)
cervical cancer cells, Bax/Bcl-2 protein expression was assessed.
Fig. 3 demonstrates that treatment
with 100 ng miRNA-375 mimics for 48 h increased Bax/Bcl-2 ratio
(P=0.0038), compared with the negative group.
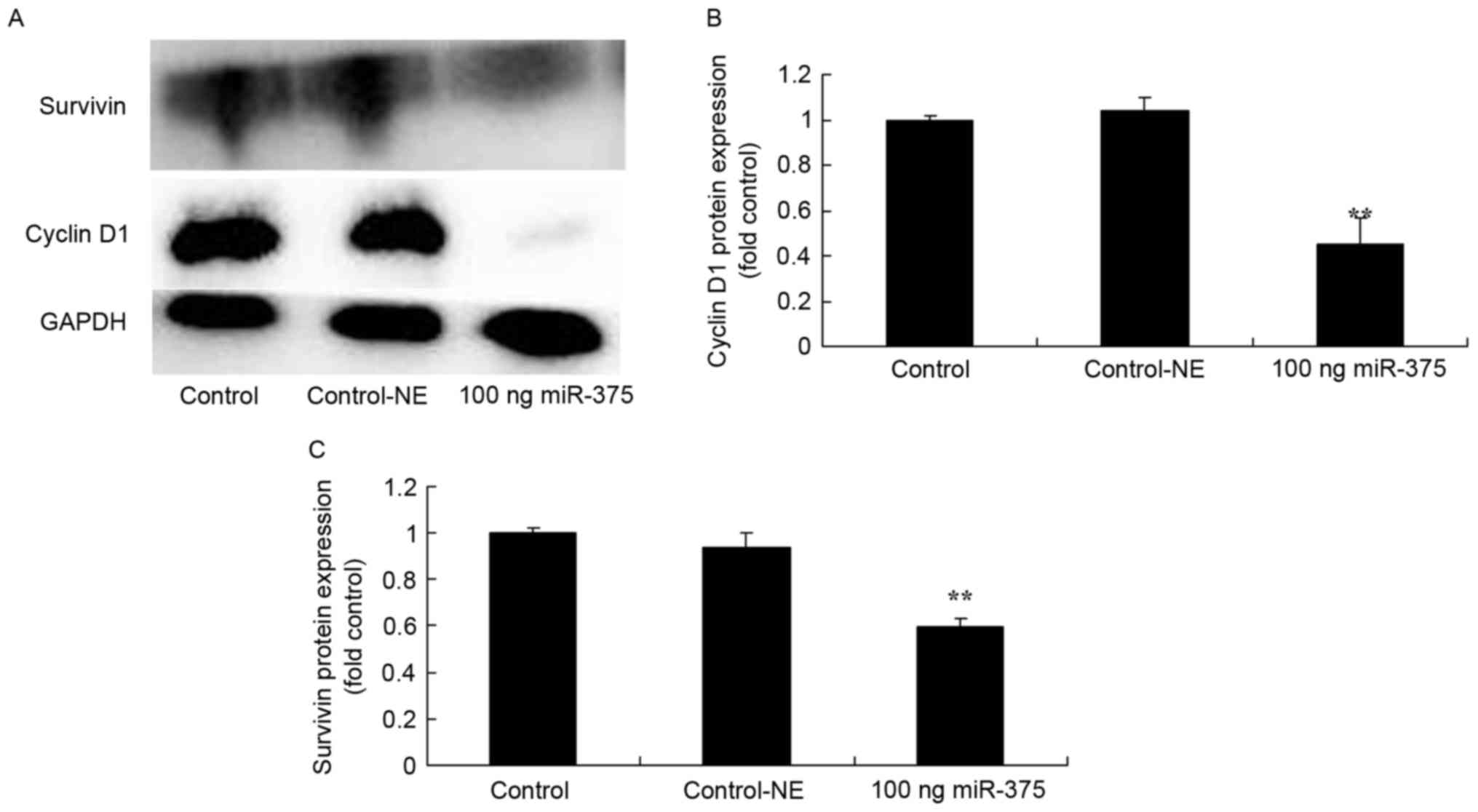
Overexpression of miRNA-375 suppressed cyclin D1 and
survivin protein expression in HPV-18(+) cervical cancer cells. To
assess the effects of miRNA-375 on apoptosis in HPV-18(+) cervical
cancer cells, levels of cyclin D1 and survivin protein expression
in HPV-18(+) cervical cancer cells were measured using western blot
analysis. The overexpression of miRNA-375 suppressed cyclin D1
(P=0.0089) and survivin (P=0.0008) protein expression in the
HPV-18(+) cervical cancer cells for 48 h, compared with the
negative group (Fig. 4).
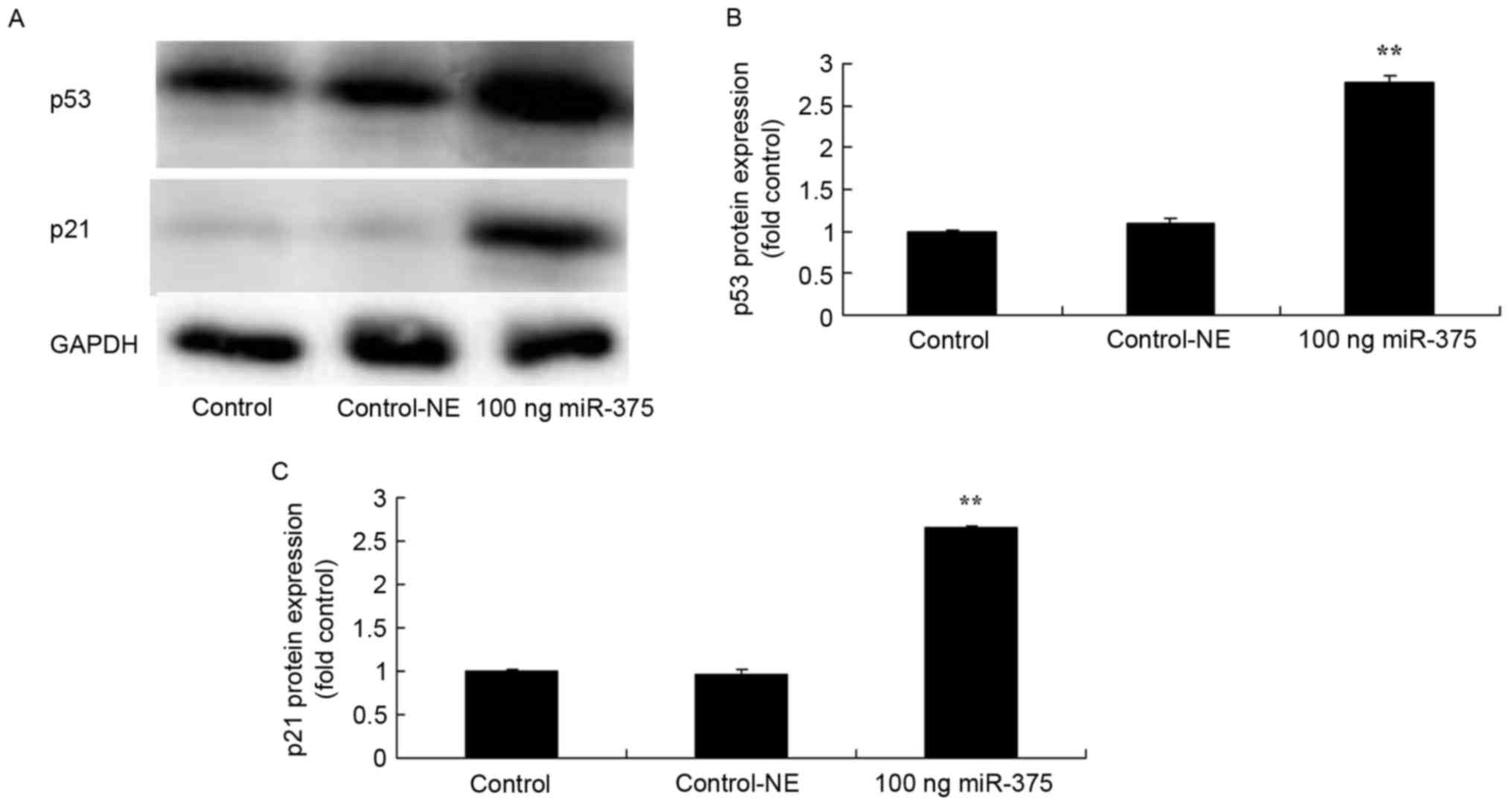
Overexpression of miRNA-375 induced p53 and p21
protein expression in HPV-18(+) cervical cancer cells. To evaluate
the molecular mechanisms associating miRNA-375 expression with
apoptosis in HPV-18(+) cervical cancer cells, p53 and p21 protein
expression of HPV-18(+) cervical cancer cells was analyzed using
western blot analysis. The results indicated that the
overexpression of miRNA-375 induced p53 (P=0.0069) and p21
(P=0.0016) protein expression of HPV-18(+) cervical cancer cells
(Fig. 5).
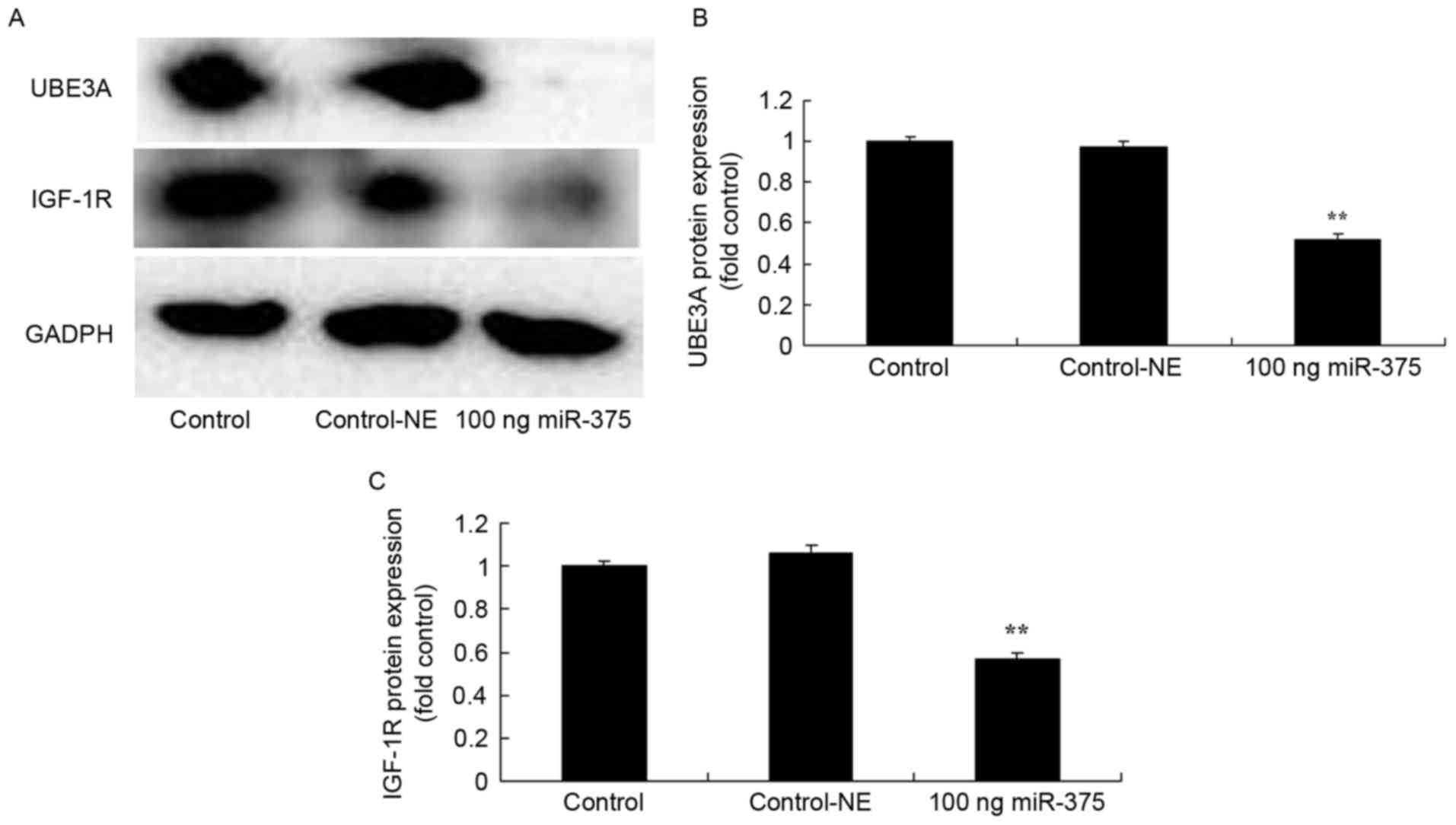
Overexpression of miRNA-375 suppressed UBE3A and
IGF-1R protein expression of HPV-18(+) cervical cancer cells. To
evaluate the effects of miRNA-375 on apoptosis in HPV-18(+)
cervical cancer cells, the levels of UBE3A and IGF-1R protein
expression in HPV-18(+) cervical cancer cells were measured using
western blot analysis. A total of 100 ng miRNA-375 mimics
suppressed UBE3A (P=0.0000) and IGF-1R (P=0.0011) protein
expression in HPV-18(+) cervical cancer cells for 48 h, compared
with the negative group (Fig. 6).
Discussion
CA, also known as Condyloma acuminata and Condyloma
accuminatum, is a proliferative lesion in the skin mucosa of
genital and perianal areas caused by HPV infection (5). HPV is a type of common epithelial DNA
virus 50–55 nm in diameter (11).
Modern molecular biology technology has identified >100 HPV
subtypes, 34 of which are associated with CA; the most common
subtypes include HPV6, HPV11, HPV16 and HPV18 (12). Based on differences in pathogenicity,
HPV subtypes are divided into low-risk and high-risk genotype
groups, with low-risk genotypes (HPV6 and 11) primarily causing
genital warts, including Condyloma, and high-risk genotypes (HPV16
and 18) being associated with genital neoplasms (2). In the present study, miRNA-375
overexpression suppressed cell proliferation and increased LDH
activity in HPV-18(+) cervical cancer cells, compared with the
negative group.
miRNA molecules regulate gene expression at the
transcriptional level through complementary pairing with target
miRNA, consequently resulting in the degradation or inhibition of
the translation of mRNA (5). A
previous study demonstrated that miRNAs serve important functions
in gene regulatory networks, being associated with cell
proliferation and apoptosis, differentiation, development and the
stress response, virus pathogenicity and the incidence of tumors
(13). Therefore, identifying these
miRNA molecules and their target genes is becoming an important
avenue of research and an important topic in the field of gene
regulation (13). In the present
study, it was observed that miRNA-375 overexpression significantly
increased the apoptosis rate and promoted caspase-3 and caspase-9
activities in HPV-18(+) cervical cancer cells.
The UBE3A gene, also known as human papillomavirus
E6-associated protein (E6-AP) gene, is an important component of
the ubiquitin proteasome system (6).
The human UBE3A gene is located on chromosome 15 with a protein
molecular weight of ~100 kDa. Its gene products primarily exist in
the nucleus and cytoplasm, and is expressed in normal tissues of
the prostate, testis, ovary, uterus, breast and brain (14). The UBE3A gene serves an important
function in the incidence and development of multiple diseases; its
abnormal change may result in the incidence and development of
tumors (6). The present study
identified that miRNA-375 significantly suppressed levels of
survivin and UBE3A protein expression in HPV-18(+) cervical cancer
cells. Jung et al (15)
verified that miRNA-375 is a key tumor suppressor of HPV-associated
carcinogenesis, through the suppression of p53, p21 and UBE3A
protein expression.
The ubiquitin proteasome system is associated with
multiple metabolic processes in eukaryotic cells, including
carcinomatous changes, tumor progression, immunological
surveillance escape and tumor drug resistance (16). E3 is an enzyme that specifically
regulates the degradation of its target proteins by recognizing and
binding to specific target protein sequences in the ubiquitin
proteasome system; the E3 enzyme is associated with the selection
and specificity of target proteins (17,18). E6
protein binds to intracellular UBE3A to form complexes that exhibit
ubiquitin protein ligase activity and specifically binds with wild
type p53 and then is degenerated by the ubiquitin-mediated pathway,
consequently blocking p53 to inhibit apoptosis and induce cell
proliferation (16). The present
study also demonstrated that the overexpression of miRNA-375
significantly induced Bax, p53 and p21 protein expression, compared
with the negative group. Liu et al (4) demonstrated that miRNA-375 targeted p53
to regulate the response to ionizing radiation and etoposide
treatment.
As for the mechanism of IGF-1 promoting tumor
invasion and metastasis, there are 3 major hypotheses: i) IGF-1
significantly increases the concentration of vascular endothelial
growth factor in tumor cells and promotes tumor angiogenesis,
therefore facilitating tumor invasion and metastasis; ii) IGF-1
directly activates urokinase plasminogen activator-1 (UPA) and
upregulates its expression, thereby improving the invasiveness of
malignant tumors; iii) IGF-1 promotes the synthesis of cadherin,
laminin and other adhesion molecules in tumor cells to increase the
adhesion of tumor cells to endothelial cells and bone marrow, so as
to promote the metastasis of tumor cells (19,20). In
the present study, it was identified that miRNA-375 overexpression
significantly suppressed IGF-1R protein expression in HPV-18(+)
cervical cancer cells, compared with the negative group. Meng et
al (21) indicated that
miRNA-30a-5p overexpression decreased non-small cell lung cancer
cell growth regulation through the IGF-1R and phosphoinositide
3-kinase/protein kinase B signaling pathway.
To conclude, the present study demonstrated that the
anti-CA mechanism of miRNA-375 may result in the suppression of
cell proliferation and induction of apoptosis, an increase in
caspase-3 and caspase-9 activities, an induction of Bax, p53 and
p21 protein expression and a suppression of survivin protein
expression in HPV-18(+) cervical cancer cells. The present in
vitro study described a suitable model for anti-CA study in
HPV-18(+).
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
HC designed the experiment, SW and HC performed the
experiments, SW and HC analyzed the data and HC wrote the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mukhamedshina YO, Akhmetzyanova ER,
Kostennikov AA, Zakirova EY, Galieva LR, Garanina EE, Rogozin AA,
Kiassov AP and Rizvanov AA: Adipose-derived mesenchymal stem cell
application combined with fibrin matrix promotes structural and
functional recovery following spinal cord injury in rats. Front
Pharmacol. 9:3432018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakazawa M, Matsubara H, Matsushita Y,
Watanabe M, Vo N, Yoshida H, Yamaguchi M and Kataoka T: The human
Bcl-2 family member Bcl-rambo localizes to mitochondria and induces
apoptosis and morphological aberrations in drosophila. PLoS One.
11:e01578232016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhuo SM, Li SC, Lin YQ, Yu HB and Li N:
The effects of anti-Fas ribozyme on T lymphocyte apoptosis in mice
model with chronic obstructive pulmonary disease. Iran J Basic Med
Sci. 20:1102–1108. 2017.PubMed/NCBI
|
|
4
|
Liu Y, Xing R, Zhang X, Dong W, Zhang J,
Yan Z, Li W, Cui J and Lu Y: miR-375 targets the p53 gene to
regulate cellular response to ionizing radiation and etoposide in
gastric cancer cells. DNA Repair (Amst). 12:741–750. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo J, Zhang CD, An JX, Xiao YY, Shao S,
Zhou NM and Dai DQ: Expression of miR-634 in gastric carcinoma and
its effects on proliferation, migration and invasion of gastric
cancer cells. Cancer Med. 7:776–787. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao S, Fang L, Phan LM, Qdaisat A, Yeung
SC and Lee MH: COP9 signalosome subunit 6 (CSN6) regulates
E6AP/UBE3A in cervical cancer. Oncotarget. 6:28026–28041. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu Y, Ye F, Lu W, Hong D, Wan X and Xie X:
HPV16 E6-induced and E6AP-dependent inhibition of the
transcriptional coactivator hADA3 in human cervical carcinoma
cells. Cancer Invest. 27:298–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuramoto H, Hongo A, Liu YX, Ojima Y,
Nakamura K, Seki N, Kodama J and Hiramatsu Y: Immunohistochemical
evaluation of insulin-like growth factor I receptor status in
cervical cancer specimens. Acta Med Okayama. 62:251–259.
2008.PubMed/NCBI
|
|
9
|
Kwasniewski W, Gozdzicka-Jozefiak A,
Kotarska M, Polak G, Barczynski B, Broniarczyk J, Nowak W,
Wolun-Cholewa M, Kwasniewska A and Kotarski J: Analysis of
cytosine-adenine repeats in P1 promoter region of IGF-1 gene in
peripheral blood cells and cervical tissue samples of females with
cervical intraepithelial lesions and squamous cervical cancer. Mol
Med Rep. 11:766–774. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jozefiak A, Pacholska-Bogalska J,
Myga-Nowak M, Kedzia W, Kwasniewska A, Luczak M, Kedzia H and
Gozdzicka-Jozefiak A: Serum and tissue levels of insulin-like
growth factor-I in women with dysplasia and HPV-positive cervical
cancer. Mol Med Rep. 1:231–237. 2008.PubMed/NCBI
|
|
11
|
Zhang MJ, Su H, Yan JY, Li N, Song ZY,
Wang HJ, Huo LG, Wang F, Ji WS, Qu XJ and Qu MH: Chemopreventive
effect of Myricetin, a natural occurring compound, on colonic
chronic inflammation and inflammation-driven tumorigenesis in mice.
Biomed Pharmacother. 97:1131–1137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui X, Wang S, Cai H, Lin Y, Zheng X,
Zhang B and Xia C: Overexpression of microRNA-634 suppresses
survival and matrix synthesis of human osteoarthritis chondrocytes
by targeting PIK3R1. Sci Rep. 6:231172016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujiwara N, Inoue J, Kawano T, Tanimoto K,
Kozaki K and Inazawa J: miR-634 activates the mitochondrial
apoptosis pathway and enhances chemotherapy-induced cytotoxicity.
Cancer Res. 75:3890–3901. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bailus BJ, Pyles B, McAlister MM, O'Geen
H, Lockwood SH, Adams AN, Nguyen JT, Yu A, Berman RF and Segal DJ:
Protein delivery of an artificial transcription factor restores
widespread ube3a expression in an angelman syndrome mouse brain.
Mol Ther. 24:548–555. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung HM, Phillips BL and Chan EK: miR-375
activates p21 and suppresses telomerase activity by coordinately
regulating HPV E6/E7, E6AP, CIP2A and 14-3-3zeta. Mol Cancer.
13:802014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
LaSalle JM, Reiter LT and Chamberlain SJ:
Epigenetic regulation of UBE3A and roles in human
neurodevelopmental disorders. Epigenomics. 7:1213–1228. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ansari T, Brimer N and Vande Pol SB:
Peptide interactions stabilize and restructure human papillomavirus
type 16 E6 to interact with p53. J Virol. 86:11386–11391. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chan AL, Grossman T, Zuckerman V, Di
Giammartino Campigli D, Moshel O, Scheffner M, Monahan B, Pilling
P, Jiang YH, Haupt S, et al: c-Abl phosphorylates E6AP and
regulates its E3 ubiquitin ligase activity. Biochemistry.
52:3119–3129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amichay K, Kidron D, Attias-Geva Z,
Schayek H, Sarfstein R, Fishman A, Werner H and Bruchim I: BRCA1 is
expressed in uterine serous carcinoma (USC) and controls
insulin-like growth factor I receptor (IGF-IR) gene expression in
USC cell lines. Int J Gynecol Cancer. 22:748–754. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee SW, Lee SY, Lee SR, Ju W and Kim SC:
Plasma levels of insulin-like growth factor-1 and insulin-like
growth factor binding protein-3 in women with cervical neoplasia. J
Gynecol Oncol. 21:174–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng F, Wang F, Wang L, Wong SC, Cho WC
and Chan LW: MiR-30a-5p overexpression may overcome EGFR-inhibitor
resistance through regulating PI3K/AKT signaling pathway in
non-small cell lung cancer cell lines. Front Genet. 7:1972016.
View Article : Google Scholar : PubMed/NCBI
|