Introduction
Breast cancer (BC) is one of the most common
malignancies in women worldwide (1)
and accounts for 25% (1.7 million) of all cancer cases and 15%
(521,900) of all cancer-related mortalities among females, based on
data through 2012 (2). The percentage
of cancer-related mortalities among females in less developed
countries is even higher, for example, female breast cancer
mortality in Belize was 14.0%, while 29.7% in the Cayman Islands in
2013 (3). Despite recent advancements
in BC diagnoses and treatments, prognosis outcomes remain
unsatisfactory due to the highly complex and heterogeneous nature
of this disease (2). Thus,
identification of novel therapeutic targets is required to develop
additional effective treatment strategies.
Scinderin (SCIN), which is also referred to as
adseverin, belongs to the gelsolin super family of actin binding
proteins (4,5). SCIN was originally identified in
chromaffin cells of the adrenal medulla (6), but is typically expressed in a variety
of tissues. For instance, high expression of SCIN was observed in
the kidneys and intestines (7), but
was expressed at lower levels in other tissue types (6,8). Earlier
studies indicated that SCIN serves an important role in the
regulation of various cellular processes, including proliferation,
migration and differentiation. In human prostate cancer and lung
carcinoma cells, SCIN suppression inhibited cell proliferation
(9,10). Conversely, in megakaryoblastic
leukemia, SCIN overexpression inhibited cell proliferation and
tumorigenesis via activating the ras-related C3 botulinum toxin
substrate/p21-activated kinase/mammalian mitogen-activated protein
kinase kinase, mitogen-activated protein kinase kinase 4/c-jun
n-terminal kinase/c-jun, and rapidly accelerated fibrosarcoma
kinase/mitogen-activated protein kinase kinase/extracellular
signal-regulated kinase signaling pathways (11). In addition, manipulation of SCIN in
gastric cancer cells has been linked to increased cell invasion and
metastasis in vivo (12), and
has also been revealed to be involved in the differentiation of
dental pulp cells (13). However, the
role of SCIN in BC is largely unknown.
Thus, in the present study, the biological role of
SCIN in BC was investigated by analyzing its expression in BC
tissue samples, as well as in control mammary fibroadenoma or
fibroadenomatoid hyperplasia tissue samples. In addition, SCIN
expression was ablated in MDA-MB-231 and T-47D BC cells using
lentivirus (LV)-mediated short hairpin (sh)RNA technology to
understand the functional consequences of knocking down SCIN
expression. The results of the present study indicate that SCIN
expression serves an important role in inhibiting proliferation and
promoting apoptosis in BC cells.
Materials and methods
Selection of tumor specimens
This study was approved by the Ethics Committee of
the Second People's Hospital of Shenzhen (Shenzhen, Guangdong,
China), and written consent was obtained from all study
participants. A total of 46 paraffin-embedded BC specimens, with a
median age of 47.54 years (range, 28–64 years) and 21
paraffin-embedded control specimens, with a median age of 29.95
years (range, 17–45 years) collected from patients with mammary
fibroadenoma or fibroadenomatoid hyperplasia were obtained from the
Center for Breast Disease Diagnosis and Treatment at the Second
People's Hospital of Shenzhen between January 2017 and June 2017.
All patients were women. Among the 46 cases, 36 were invasive
ductal carcinoma (IDC), 6 were ductal carcinoma in situ
(DCIS) and 4 were mucoid carcinoma. All diagnoses and
classifications were made according to the World Health
Organization criteria (14). These
resected specimens were 2–3 mm thick, then fixed in 10% neutral
buffered formalin (pH 7.4) at room temperature for 12 h, embedded
in paraffin, and cut into 4-µm sections.
Cell culture
Human BC MDA-MB-231, T-47D and MCF-7 cell lines were
obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). These cells were cultured in Dulbecco's modified
Eagle medium (DMEM; Corning Incorporated, Corning, NY, USA)
supplemented with 10% fetal bovine serum at 37°C in a humidified
atmosphere with 5% CO2.
Immunohistochemistry (IHC)
Selected paraffin-embedded specimens were cut into
4-µm sections and stained using the Dako REAL Envision Detection
system (Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) per
the manufacturer's protocols. 4-µm sections were dewaxed by xylene
and rehydrated in a graded ethanol series (100% ethanol for 5 min,
95% alcohol for 5 min, 90% alcohol for 3 min, 80% alcohol for 3
min, 70% alcohol for 3 min at room temperature), following washing
3 times with Phosphate Buffer Solution (PBS) for 10 min at room
temperature. Sections were boiled in 0.01 mol/l citrate buffer (pH
6.0, MVS-0066, Maixin Biotechnology Development Co., Ltd, Fuzhou,
China) at 100°C for 4 min, then cooled at room temperature for 1 h
for antigen retrieval. The endogenous peroxidase activity was
blocked with 3% H2O2 for 10 min at room
temperature, followed by washing 3 times with PBS for 10 min at
room temperature. Next, slides were immersed in 5% goat serum
(YJ0130, Yanjing Biological Co., Shanghai, China) for 10 min at
room temperature to block nonspecific binding. Following removal of
goat serum, the slides were incubated with primary rabbit anti-SCIN
monoclonal antibody (1:800 dilution; cat. no. ab199723, Abcam,
Cambridge, MA, USA) and mouse anti-Ki67 monoclonal antibody (1:100
dilution; cat. no. MAB-0129, MXB Bio-company, Fuzhou, China) at 4°C
overnight. The next day, the slides were re-heated at room
temperature for 1 h. Then washed 3 times with PBS for 10 min in
room temperature, the sections were then incubated with
poly-horseradish peroxidase (HRP) anti-rabbit/mouse IgG (cat. no
PV-9000, Beijing ZSGB-Bio Company, Beijing, China) for 30 min at
room temperature. DAB staining was performed and later
semi-quantitative evaluation of the immunohistochemical signal was
performed by two pathologists working independently in a blinded
fashion with an Olympus BX-51 microscope (Olympus Corporation,
Tokyo, Japan) at ×200 magnification (15). The staining intensity of SCIN
expression was scored as follows: 0, Negative (no cytoplasmic SCIN
expression); 1, weak (1–25% staining); 2, moderate (26–50%
staining); 3, strong (51–75% staining); and 4, high (76–100%)
staining. The staining scores were analyzed using the X-tile
software program (v3.6.1; Yale University School of Medicine, New
Haven, CT, USA), and a score of 2 was designated as the cutoff
value (16). Thus, samples having
total scores of ≥2 were classified as having high SCIN expression,
while scores of <2 represented no or low SCIN expression.
Samples that exhibited <14% Ki-67 (proliferation marker)
expression were grouped as Ki-67 negative (17).
SCIN expression in breast cancer cells
from GEO
The information about SCIN expression in BC cells
was extracted from GEO (https://www.ncbi.nlm.nih.gov/geo/). The term ‘breast
cancer cells’ was entered and searched in the GEO datasets, and
eligible and high quality profiling expression data was identified,
‘SCIN’ gene was then searched in the GDS4296 datasets and for the
expression of SCIN in BC cells.
Generation of LV expressing SCIN,
shRNA and target cell transfection
Several shRNAs were designed and tested to target
the human SCIN gene in preliminary studies (NM_033128). The shRNA
sequence (5′-CGAGATGAGCTGACAACAT-3′) that optimally ablated SCIN
expression was selected for the present study. A random shRNA
sequence (5′-TTCTCCGAACGTGTCACGT-3′) was used as a control. These
shRNAs were subsequently ligated into the GV115 lentiviral vector
with enhanced green fluorescent protein (GFP) cDNA (GeneChem). To
generate LV, MDA-MB-231 and T-47D cells were transfected with
GV115-SCIN or control shRNA using X-tremeGENE HP DNA Transfection
Reagent (cat no. 6366546001, Roche Diagnostics, Basel,
Switzerland), along with two helper plasmids, pHelper 1.0 and
pHelper 2.0 (GeneChem), per the manufacturer's protocols. At 2 days
post-transfection, the supernatant containing packaged LV was
collected and filtered through a 0.45-µm filter. To infect
MDA-MB-231 and T-47D cells, LV particles, at a multiplicity of
transfection of 20, were added to the plated cells in 6-well plates
and then cultured at 37°C in an incubator. A transfection
efficiency of ~80% was observed via GFP expression using
fluorescence microscopy following 3 days of LV transfection.
Subsequent experimentations were performed 72 h following
transfection.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from BC MDA-MB-231, T-47D
and MCF-7 cell lines respectively using TRIzol reagent (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The cDNA was
retro-transcribed from isolated RNA using the M-MLV kit (cat no.
M1705, Promega Corporation, Madison, WI, USA). The RT-qPCR reaction
conditions were at 95°C for 15 sec, at 95°C for 5 sec, then at 60°C
for 30 sec, and the amplification was for 45 cycles using the
Real-Time PCR Detection system (cat no. MX3000p; Agilent
Technologies, Inc.) with SYBR Premix Ex Taq (cat no. DRR041B,
Takara Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's protocols. The primer sequences for SCIN gene
amplification were as follows: Forward, AGGAAGGTCTGAACTAAT and
reverse, CTGTTACTTATGTCTGCTAT. The primer sequences for GAPDH
amplification were as follows: Forward, TGACTTCAACAGCGACACCCA and
reverse, CACCCTGTTGCTGTAGCCAAA. The relative mRNA expression levels
were determined by the cycle threshold (Ct) normalized to GAPDH
expression, using the 2−ΔΔCq formula (18).
Western blot analysis
The knockdown efficiency of SCIN shRNA in BC
cells transfected with GV115-SCIN shRNA or control shRNA LV was
determined. At 2 days post-LV transfection, the cells were
collected and lysed in radioimmunoprecipitation lysis buffer
(BioTeke Corporation, Beijing, China). Protein concentration was
measured using the BCA Protein assay kit (Beyotime Institute of
Biotechnology, Haimen, China), and 20 µg protein lysates in each
pane were separated by 10% SDS-PAGE, followed by transfer to
polyvinylidene difluoride (PVDF) membranes (EMD Millipore,
Billerica, MA, USA). Subsequently, the PVDF membranes were blocked
in 5% skimmed milk diluted with TBST (cat no. BD232100, Solarbio
Co, Beijing, China) for 1 h at room temperature. The membranes were
then incubated with primary rabbit anti-SCIN monoclonal antibody
(mAb) (80 kDa, 1:200; cat no. HPA020518, Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and GAPDH mouse mAb (37 kDa, 1:1,000; cat no.
SC-32233, Santa Cruz Biotechnology, Dallas TX) at 4°C overnight.
Thereafter, the membranes were incubated with secondary goat
anti-rabbit IgG (1:2,000; cat. no. sc-2004, Santa Cruz
Biotechnology, Inc.) and goat anti-mouse IgG (1:2,000; cat. no.
sc-2005, Santa Cruz Biotechnology, Inc.) antibodies for 1.5 h at
room temperature, respectively. The protein bands were visualized
using the Pierce™ ECL Western Blotting Substrate kit (Thermo Fisher
Scientific, Inc.).
Cell Celigo analysis
LV infected MDA-MB-231 and T-47D cells were seeded
into 96-well plates at a concentration of 2,000 cells/well in
incubator with 4°C and 5% CO2 for 3 days. The cell
nuclei were stained with Hoechst (2.6 µg/ml; Invitrogen; Thermo
Fisher Scientific, Inc.) for 5 min at room temperature to quantify
absolute cell number; green fluorescence-tagged shRNA was measured
by a fluorescence microscope at x100 magnification to quantify
cellular siRNA uptake. All analyses were performed using a Celigo
cytometer (Nexcelom Bioscience, Lawrence, MA, USA) over a period of
5 days. Gross quantitative analysis for each fluorescence channel
was performed, including total counts of gated events.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
BC cells were seeded into a 96-well plate (2,000
cells/well) 3 days after LV transfection. The MTT assay was
performed per the manufacturer's protocols (cat no. JT343;
Gen-view, Beijing, China). Briefly, at the indicated time points
(1, 2, 3, 4 and 5 days), the MTT solution (5 mg/ml) was added to
the wells and the cells were further incubated at 37°C for 4 h. The
formazan dye was dissolved with 100 µl dimethyl sulfoxide at
37°C for 2–5 min. The optical density of the dye was measured using
a microplate reader at 490 nm absorbance and 570 nm as the
wavelength reference.
Caspase-3/7 activity assay
Caspase-3/7 activity was determined using the
Caspase-Glo 3 and 7 kit (cat no. G8091, Promega Corporation),
according to the manufacturer's protocols, following plating
of transfected cells in 96-well plates (in triplicate) for 72
h.
Cell apoptosis
Cell apoptosis was analyzed using the
allophycocyanin (APC)/annexin V kit (cat no. 88-8007, eBioscience;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. After 5 days of transfection, the cells were stained for
10–15 min in 100 µl cell suspension buffer, containing 5 µl annexin
V-APC stain, at room temperature in the dark. Cell apoptosis was
analyzed by flow cytometry (EMD Millipore).
Statistical analysis
All statistical analyses were performed using SPSS
20.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference. Association
between SCIN expression and clinicopathological features of
patients with BC, as well as RT-qPCR experiments, were evaluated
using the χ2 test. All other data sets were evaluated
using Student's t-test and expressed as the mean ± standard error
of the mean.
Results
SCIN expression is increased in breast
cancer tissues
The IHC results revealed that SCIN protein
expression was elevated in BC tissue samples compared with that in
control mammary fibroadenoma or fibroadenomatoid hyperplasia tissue
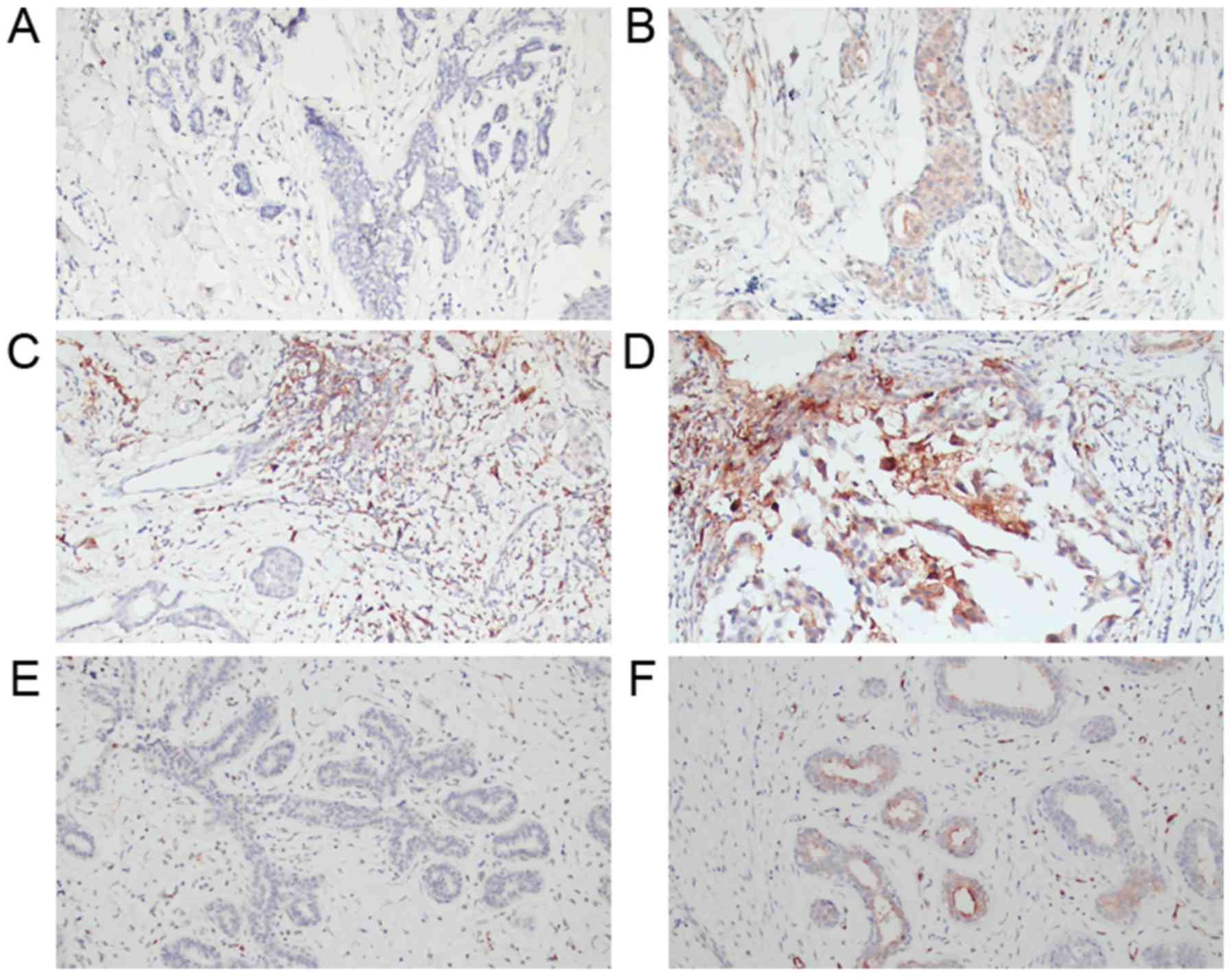
samples (Fig. 1). SCIN expression in
the cytoplasm of BC ductal epithelial hyperplasia cells was
primarily detected with varying intensity (Fig. 1A-D). However, very weak or no SCIN
expression was observed in mammary fibroadenoma or fibroadenomatoid
hyperplasia tissue samples (Fig. 1E and
F). Quantification of SCIN expression in the BC tissue samples
demonstrated that 60.9% of the BC tissue samples (28/46) revealed
high SCIN expression compared with 28.6% of the mammary
fibroadenoma or fibroadenomatoid hyperplasia tissue samples (6/21)
(P=0.014) (Table I; Fig. 1).
 | Table I.Comparison of SCIN expression in
breast fibroadenoma and breast cancer tissues. |
Table I.
Comparison of SCIN expression in
breast fibroadenoma and breast cancer tissues.
|
|
| SCIN expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Type of tissue | n | None/low, n (%) | High, n (%) | χ2 | P-value |
|---|
| Fibroadenoma of the
breast | 21 | 15 (71.4) | 6 (28.6) | 6.017 | 0.014 |
| Breast cancer | 46 | 18 (39.1) | 28 (60.9) |
|
|
Further analysis of the association between SCIN
expression and clinicopathological features demonstrated no
significant differences between high and low expression of SCIN.
The proportion of high SCIN-expressing Ki-67-positive BC tissue
samples (78.6%, 22/28) was higher compared with the Ki-67-negative
BC tissue samples (21.4%, 6/28); however, this difference was not
statistically significant (Table
II). This observation may indicate that SCIN expression in BC
tissue is associated with Ki-67 expression, suggesting that changes
in SCIN expression could affect BC cell proliferation.
 | Table II.Association between SCIN expression
and clinicopathological features in patients with breast
cancer. |
Table II.
Association between SCIN expression
and clinicopathological features in patients with breast
cancer.
|
|
| SCIN
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Pathological
variables | n | None/low, n
(%) | High, n (%) | χ2 | P-value |
|---|
| Pathological
type |
|
|
| 0.634 | 0.728 |
|
IDC | 36 | 13 (36.1) | 23 (63.9) |
|
|
|
DCIS | 6 | 3 (50.0) | 3 (50.0) |
|
|
| Mucoid
carcinoma | 4 | 2 (50.0) | 2 (50.0) |
|
|
| ER |
|
|
| 0.417 | 0.518 |
|
Negative | 18 | 6 (33.3) | 12 (66.7) |
|
|
|
Positive | 28 | 12 (42.9) | 16 (57.1) |
|
|
| PR |
|
|
| 0.225 | 0.635 |
|
Negative | 25 | 9 (36.0) | 16 (64.0) |
|
|
|
Positive | 21 | 9 (42.9) | 12 (57.1) |
|
|
| HER2 |
|
|
| 0.004 | 0.949 |
|
Negative | 36 | 14 (38.9) | 22 (61.1) |
|
|
|
Positive | 10 | 4 (40.0) | 6 (60.0) |
|
|
| Ki-67 |
|
|
| 0.004 | 0.949 |
|
Negative | 10 | 4 (40.0) | 6 (60.0) |
|
|
|
Positive | 36 | 14 (38.9) | 22 (61.1) |
|
|
| Stage |
|
|
| 0.48 | 0.488 |
|
≤II | 38 | 14 (36.8) | 24 (63.2) |
|
|
|
≥III | 8 | 4 (50.0) | 4 (50.0) |
|
|
| Molecular
characteristics |
|
|
| 4.036 | 0.258 |
| Luminal
A | 7 | 5 (71.4) | 2 (28.6) |
|
|
| Luminal
B | 21 | 7 (33.3) | 14 (66.7) |
|
|
| HER2
overexpression | 10 | 4 (40.0) | 6 (60.0) |
|
|
|
Triple-negative | 8 | 2 (25.0) | 6 (75.0) |
|
|
SCIN is expressed in breast cancer
cells
To investigate the functional contribution of SCIN
in BC, the Gene Expression Omnibus database was initially utilized
(GDS4296, Affymetrix Human Genome U133 Plus 2.0 Array; Affmetrix;
Thermo Fisher Scientific, Inc. http://www.ncbi.nlm.nih.gov/geo/tools/profileGraph.cgi?ID=GDS4296%3A222272_x_at&sortby=tissue)
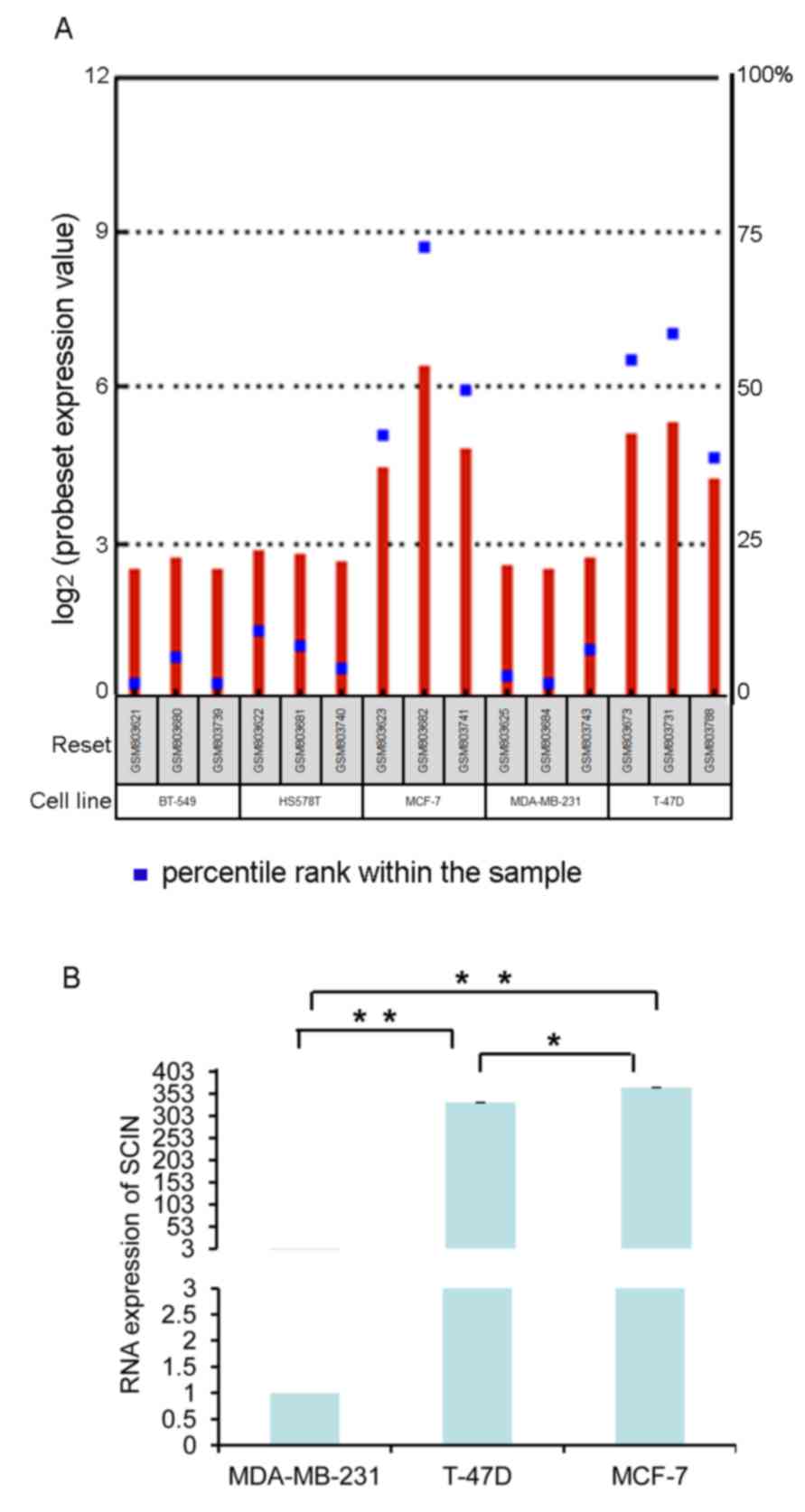
to determine SCIN levels in BC samples (19). Using this database, SCIN was revealed
to be expressed in multiple BC cell lines, including BT-549,
HS578T, MCF-7, MDA-MB-231 and T-47D. As presented in Fig. 2A, SCIN expression was higher in MCF-7
and T-47D cells. SCIN expression in BC cells was further validated
using RT-qPCR (Fig. 2B). The
MDA-MB-231 cell line is a highly invasive and metastatic, while
T-47D and MCF-7 cell lines exhibit comparatively mild invasive and
metastatic capabilities. Therefore, MDA-MB-231 and T-47D cells were
selected to examine the effect of SCIN on different BC cells.
Suppressed SCIN expression inhibits
breast cancer cell proliferation
In order to investigate the molecular function of
SCIN in BC cells, SCIN expression was ablated using LV-mediated
knockdown. Following 3 days of LV transfection, infection
efficiency and good cell morphology were observed by fluorescent
microscope with ×200 magnification (Fig.
3A and B). Then RT-qPCR analysis revealed that SCIN mRNA
expression was significantly lower in MDA-MB-231 and T-47D cells in
the shSCIN group compared with that in the shCtrl group (Fig. 3C and D). Similarly, a reduction in
SCIN protein expression was confirmed in BC cells that were
transfected with shSCIN (Fig. 3E and
F).
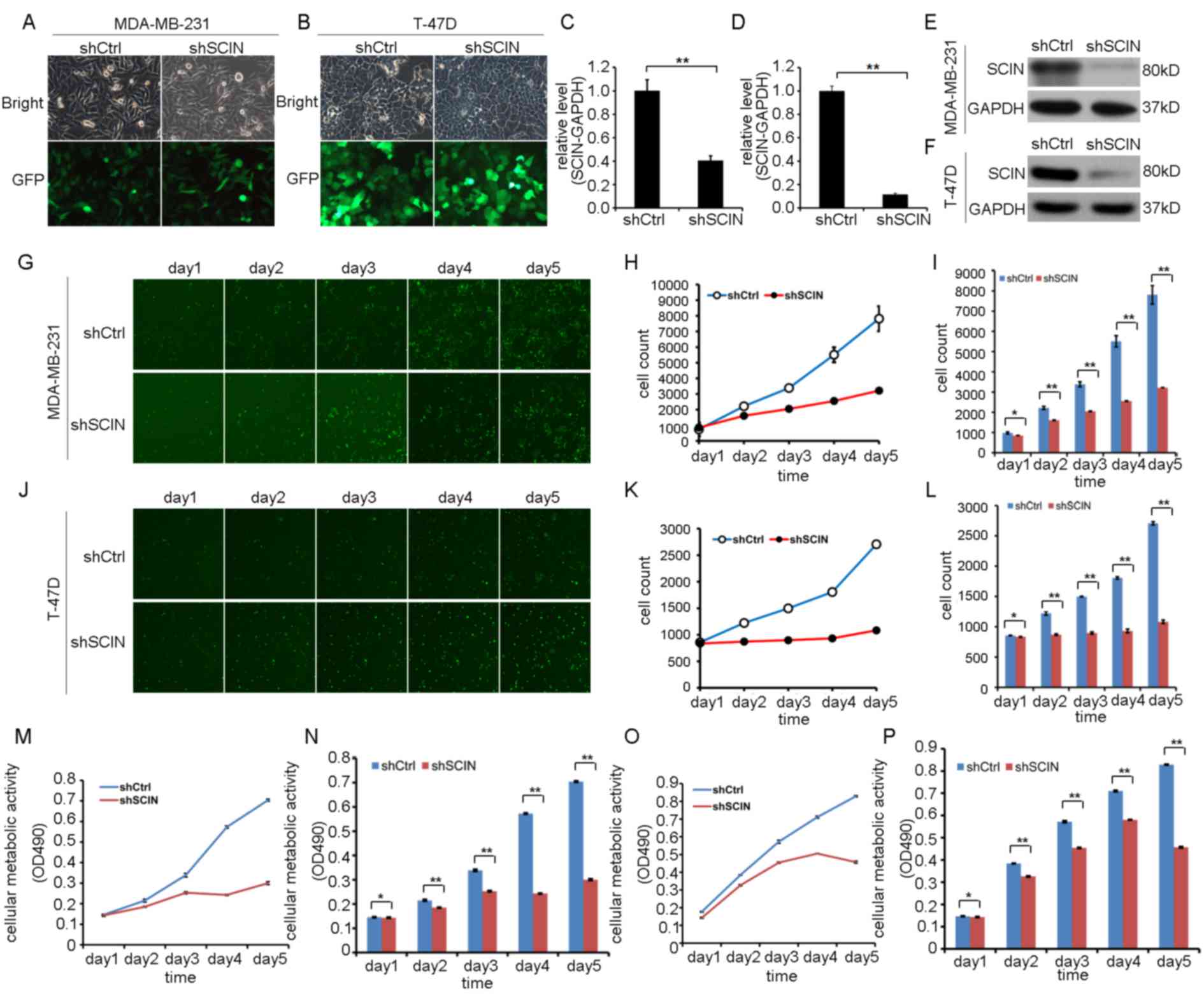 | Figure 3.Knockdown of SCIN expression by
lentivirus-mediated shRNA and its effect on cell proliferation.
Representative images of SCIN-knockdown in (A) MDA-MB-231 and (B)
T-47D cells, assessed by GFP expression using fluorescence
microscopy (×200 magnification). SCIN RNA expression following
knockdown in (C) MDA-MB-231 and (D) T-47D cells, assessed by
reverse transcription-quantitative polymerase chain reaction
analysis. GAPDH expression was used as an internal control. Data
are presented as mean values ± standard error of the mean of three
independent experiments. **P<0.01 compared with the shCtrl
group. Western blot analysis of SCIN-knockdown in (E) MDA-MB-231
and (F) T-47D cells. Representative blots of three separate
experiments. (G) Representative images of Celigo assay (×100
magnification), (H) cumulative data of Celigo assay, and (I) bar
graphs indicate the mean cell count values in MDA-MB-231 cells
transfected with shCtrl or shSCIN were illustrated. (J)
Representative images of Celigo assay (×100 magnification), (K)
cumulative data of Celigo assay, and (L) bar graphs indicate the
mean cell count values in T-47D cells transfected with shCtrl or
shSCIN were presented. Cellular metabolic activity, assessed with
MTT assay, of (M) MDA-MB-231 and (O) T-47D cells. Bar graphs
indicate the mean cellular metabolic activity values in the
MDA-MB-231 (N) and T-47D cells (P). All data represented are mean
values ± standard error of the mean from four independent
experiments. Student's t-test was used for statistical analysis.
**P<0.01. *P>0.05 vs. control, SCIN, scinderin; BC, breast
cancer; shCtrl, short hairpin control; GFP, green fluorescent
protein; OD, optical density. |
Next, proliferation was assessed in MDA-MB-231 and
T-47D cells using the in vitro Celigo assay 3 days after
shSCIN or shCtrl transfection. A marked reduction in proliferation
of MDA-MB-231 and T-47D cells was identified (Fig. 3G-L). A separate experiment using the
MTT assay confirmed that cellular metabolic activity was decreased
in the MDA-MB-231 when silencing SCIN expression (Fig. 3M and N). The lower cellular metabolic
activity was also examined in the T-47D cells with knockdown of
SCIN expression (Fig. 3O and P).
Thus, these results indicate that SCIN-knockdown inhibits BC cell
proliferation and cellular metabolic activity.
SCIN-knockdown induces cell
apoptosis
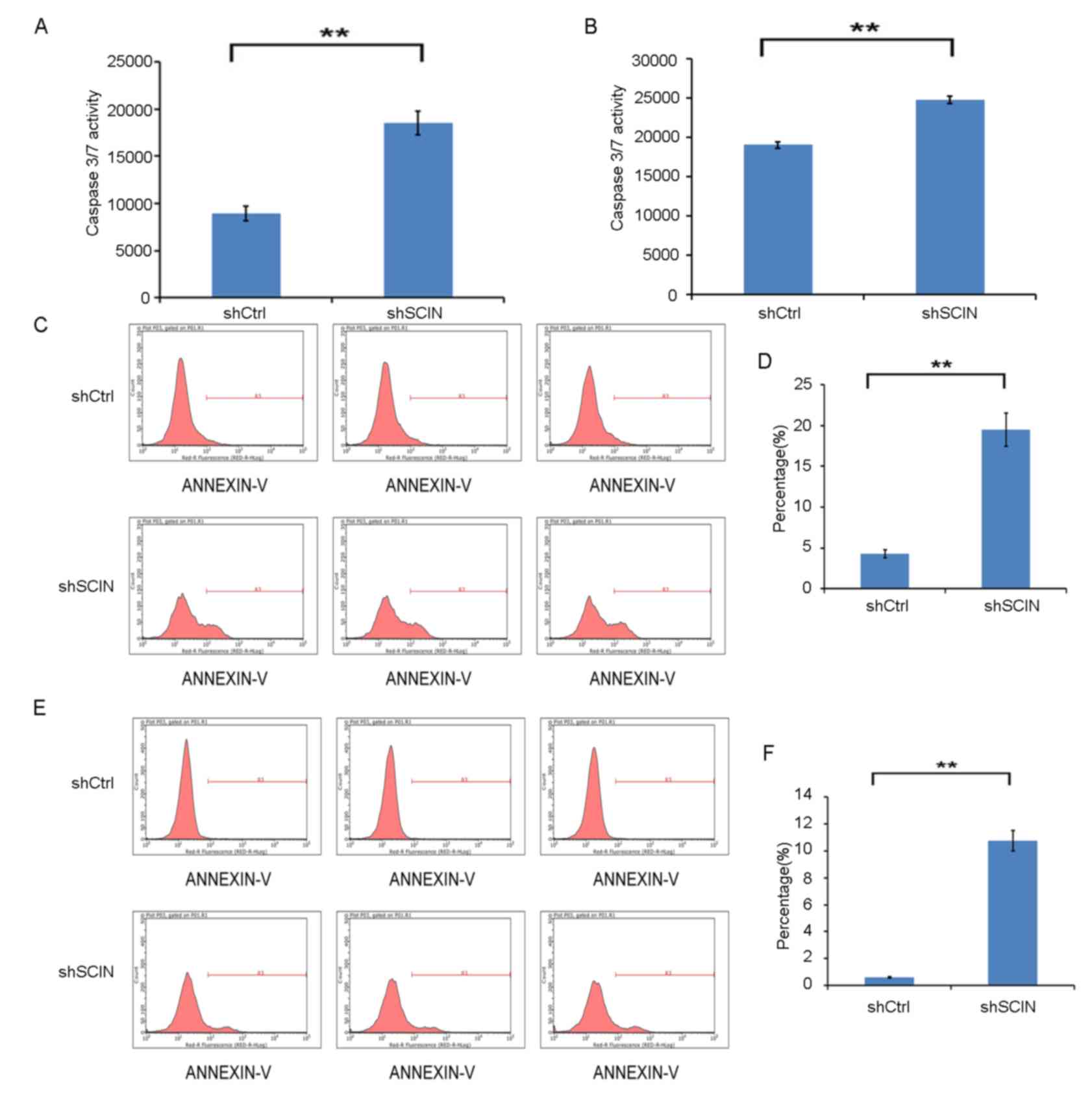
To further investigate the mechanism by which
SCIN-knockdown reduces cell proliferation, Caspase-3/7 activity in
MDA-MB-231 and T-47D cells was analyzed following 72 h of LV
transfection of shSCIN and shCtrl. SCIN-knockdown significantly
enhanced Caspase-3/7 activity in the two cell types (P<0.01;
Fig. 4A and B). Flow cytometric
analysis of cell apoptosis was also performed. As presented in
Fig. 4C-F, apoptosis was
significantly increased in the MDA-MB-231 and T-47D cells in the
shSCIN group (P<0.01) following 5 days of LV transfection.
Discussion
The majority of cases of BC-associated mortality
occur due to metastasis, for which cell proliferation is the
biggest contributor. Cell proliferation is directly and indirectly
associated with BC prognosis (20).
Multiple studies evaluating the role of numerous proliferation
genes have added to the current understanding of BC metastasis
(21–23), but the functional contribution of SCIN
expression in BC proliferation has been relatively under-reported.
Thus, in the present study, the role of SCIN in BC proliferation
and apoptosis was investigated. The results demonstrated that SCIN
expression is increased in BC cells at the protein (IHC) and mRNA
(RT-qPCR) levels. Furthermore, knockdown of SCIN expression in
MDA-MB-231 and T-47D cells, with LV-mediated gene silencing
technology, suggested that SCIN expression regulates cell
proliferation and apoptosis. Inhibition of proliferation and
induction of apoptosis were observed in MDA-MB-231 and T-47D cells
following SCIN-knockdown.
Uncontrolled cell proliferation is a central
hallmark of all types of cancer. Since the actin cytoskeleton
serves a prominent role in cancer cell cycle regulation (24), a number of cytoskeleton-associated
proteins have been studied in reference to BC (25–27). SCIN
contains six gelsolin-like domains, three actin-binding sites and
two calcium-binding sites (6,28). Previous studies have linked abnormal
SCIN expression to various carcinomas, as well as to cell
proliferation. In the present study, SCIN expression was revealed
to be associated with Ki-67-positive BC cells in tissue samples.
Although not significant, this trend does indicate that there may
be an association between SCIN and Ki-67 expression in BC tissues.
Since Ki-67 is a cellular marker of proliferation, the results of
the current study indicate that SCIN may also be involved in the
proliferation of BC cells. Consistent with this conclusion,
SCIN-knockdown was revealed to inhibit breast carcinoma cell
proliferation in vitro in the present study. This
observation was in accordance with studies by Liu et al
(10) and Wang et al (9), who revealed that SCIN overexpression
enhanced human lung and prostate carcinoma cell proliferation.
Therefore, as SCIN governs filamentous actin (F-actin) cytoskeleton
remodeling, it is possible that SCIN's effects on cell
proliferation may be mediated through F-actin. This hypothesis
requires further analysis, for example, observing whether the
F-actin cytoskeleton is changed when SCIN expression is silenced in
BC cells. Further, animal experiments should be performed to
validate the proliferative ability of SCIN.
Apoptosis can significantly contribute to normal
cell physiology. Apoptosis maintains absolute cell numbers, affects
wound healing and regulates remodeling (29). Abnormalities in apoptosis lead to
numerous diseases, including cancer, in which the normal mechanisms
of cell cycle regulation become dysfunctional (30). Inducing apoptosis during
carcinogenesis can contribute to the treatment and prevention of
cancer development and progression (31,32). In
the current study, SCIN-knockdown induced cell apoptosis. Therefore
SCIN may serve an important role in the development and progression
of BC.
In summary, the results of the current study
demonstrated that shRNA-mediated SCIN-knockdown inhibited breast
cancer cell proliferation and induced apoptosis. This sheds new
light on the novel role of SCIN in BC development and
progression.
Acknowledgements
Not applicable
Funding
This study was funded by the following grants: The
Science, Technology and Innovation Committee of Shenzhen
Municipality (grant no. JCYJ20160425103015129) and the Science,
Technology and Innovation Committee of Shenzhen Municipality (grant
no. JSGG20160226202029158).
Availability of data and materials
All data used during the current study are available
from the corresponding author on reasonable request.
Authors' contributions
WJ designed the study and wrote the manuscript. XZ,
JW and YL performed the experiments. CH analyzed data. XW and RL
also contributed to study design, data analysis and modified the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The Second People's Hospital of Shenzhen, and written consent was
obtained from all study participants.
Patient consent for publication
Patients provided written consent for the
publication of data and any associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Harbeck N and Gnant M: Breast cancer.
Lancet. 389:1134–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Razzaghi H, Quesnel-Crooks S, Sherman R,
Joseph R, Kohler B, Andall-Brereton G, Ivey MA, Edwards BK, Mery L,
Gawryszewski V and Saraiya M: Leading causes of cancer
mortality-Caribbean Region, 2003–2013. Morb Mortal Wkly Rep.
65:1395–1400. 2016. View Article : Google Scholar
|
|
4
|
Lejen T, Pene TD, Rosé SD and Trifaró JM:
The role of different Scinderin domains in the control of F-actin
cytoskeleton during exocytosis. Ann N Y Acad Sci. 971:248–250.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen XM, Guo JM, Chen P, Mao LG, Feng WY,
Le DH and Li KQ: Suppression of scinderin modulates
epithelialmesenchymal transition markers in highly metastatic
gastric cancer cell line SGC7901. Mol Med Rep. 10:2327–2333. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Del Castillo Rodriguez A, Lemaire S,
Tchakarov L, Jeyapragasan M, Doucet JP, Vitale ML and Trifaró JM:
Chromaffin cell scinderin, a novel calcium-dependent actin
filament-severing protein. EMBO J. 9:43–52. 1990.PubMed/NCBI
|
|
7
|
Lueck A, Brown D and Kwiatkowski DJ: The
actin-binding proteins adseverin and gelsolin are both highly
expressed but differentially localized in kidney and intestine. J
Cell Sci. 111:3633–3643. 1998.PubMed/NCBI
|
|
8
|
Tchakarov L, Vitale ML, Jeyapragasan M,
Del Castillo Rodriguez A and Trifaró JM: Expression of scinderin,
an actin filament-severing protein, in different tissues. FEBS
Lett. 268:209–212. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang D, Sun SQ, Yu YH, Wu WZ, Yang SL and
Tan JM: Suppression of SCIN inhibits human prostate cancer cell
proliferation and induces G0/G1 phase arrest. Int J Oncol.
44:161–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu H, Shi D, Liu T, Yu Z and Zhou C:
Lentivirus-mediated silencing of SCIN inhibits proliferation of
human lung carcinoma cells. Gene. 554:32–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zunino R, Li Q, Rosé SD, Romero-Benítez
MM, Lejen T, Brandan NC and Trifaró JM: Expression of scinderin in
megakaryoblastic leukemia cells induces differentiation,
maturation, and apoptosis with release of plateletlike particles
and inhibits proliferation and tumorigenesis. Blood. 98:2210–2219.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu JJ, Liu JY, Chen J, Wu YX, Yan P, Ji
CD, Wang YX, Xiang DF, Zhang X, Zhang P, et al: Scinderin promotes
the invasion and metastasis of gastric cancer cells and predicts
the outcome of patients. Cancer Lett. 376:110–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coratti A, Fernandes E, Lombardi A, Di
Marino M, Annecchiarico M, Felicioni L and Giulianotti PC:
Robot-assisted surgery for gastric carcinoma: Five years follow-up
and beyond: A single western center experience and long-term
oncological outcomes. Eur J Surg Oncol. 41:1106–1113. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin H, Huang JF, Qiu JR, Zhang HL, Tang
XJ, Li H, Wang CJ, Wang ZC, Feng ZQ and Zhu J: Significantly
upregulated TACSTD2 and Cyclin D1 correlate with poor prognosis of
invasive ductal breast cancer. Exp Mol Pathol. 94:73–78. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi H, Chen S, Jin H, Xu C, Dong G, Zhao
Q, Wang W, Zhang H, Lin W, Zhang J, et al: Downregulation of MSP58
inhibits growth of human colorectal cancer cells via regulation of
the cyclin D1-cyclin-dependent kinase 4-p21 pathway. Cancer Sci.
100:1585–1590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheang MC, Chia SK, Voduc D, Gao D, Leung
S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67
index, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta (CT)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shankavaram UT, Reinhold WC, Nishizuka S,
Major S, Morita D, Chary KK, Reimers MA, Scherf U, Kahn A, Dolginow
D, et al: Transcript and protein expression profiles of the NCI-60
cancer cell panel: An integromic microarray study. Mol Cancer Ther.
6:820–832. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Diest PJ, van der Wall E and Baak JP:
Prognostic value of proliferation in invasive breast cancer: A
review. J Clin Pathol. 57:675–681. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim K, Son MY, Jung CR, Kim DS and Cho HS:
EHMT2 is a metastasis regulator in breast cancer. Biochem Biophys
Res Commun. 496:758–762. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song L, Liu D, Zhao Y, He J, Kang H, Dai
Z, Wang X, Zhang S, Zan Y and Xue X: Sinomenine reduces growth and
metastasis of breast cancer cells and improves the survival of
tumor-bearing mice through suppressing the SHh pathway. Biomed
Pharmacother. 98:687–693. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao Y, Li F, Zhou H, Yang Y, Wu R, Chen Y,
Li W, Li Y, Xu X, Ke C and Pei Z: Down-regulation of MRPS23
inhibits rat breast cancer proliferation and metastasis.
Oncotarget. 8:71772–71781. 2017.PubMed/NCBI
|
|
24
|
Hall A: The cytoskeleton and cancer.
Cancer Metastasis Rev. 28:5–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kas SM, de Ruiter JR, Schipper K,
Annunziato S, Schut E, Klarenbeek S, Drenth AP, van der Burg E,
Klijn C, Ten Hoeve JJ, et al: Insertional mutagenesis identifies
drivers of a novel oncogenic pathway in invasive lobular breast
carcinoma. Nat Genet. 49:1219–1230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mercado-Matos J, Clark JL, Piper AJ,
Janusis J and Shaw LM: Differential involvement of the microtubule
cytoskeleton in insulin receptor substrate 1 (IRS-1) and IRS-2
signaling to AKT determines the response to microtubule disruption
in breast carcinoma cells. J Biol Chem. 292:7806–7816. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar N, Hati S, Munshi P, Sen S, Sehrawat
S and Singh S: A novel spiroindoline targets cell cycle and
migration via modulation of microtubule cytoskeleton. Mol Cell
Biochem. 429:11–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chumnarnsilpa S, Lee WL, Nag S, Kannan B,
Larsson M, Burtnick LD and Robinson RC: The crystal structure of
the C-terminus of adseverin reveals the actin-binding interface.
Proc Natl Acad Sci USA. 106:13719–13724. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
King KL and Cidlowski JA: Cell cycle
regulation and apoptosis. Annu Rev Physiol. 60:601–617. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jordan VC: The new biology of
estrogen-induced apoptosis applied to treat and prevent breast
cancer. Endocr Relat Cancer. 22:R1–R31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Carew JS, Espitia CM, Zhao W, Kelly KR,
Coffey M, Freeman JW and Nawrocki ST: Reolysin is a novel
reovirus-based agent that induces endoplasmic reticular
stress-mediated apoptosis in pancreatic cancer. Cell Death Dis.
4:e7282013. View Article : Google Scholar : PubMed/NCBI
|