Introduction
In many countries, screening programs based on
periodic mammography are provided to women aged 45–50 to 70–75
years, to diagnose breast cancer in an early stage (1). Breast glandular doses are low and
typically 3–5 mGy for a two-view mammography (2–4). However,
the use of ionizing radiation always implies a risk for
radiation-induced breast cancer.
Mammography radiation consists of low-energy X-rays
with a typical peak and mean photon energy of 28–30 kV and 15–20
keV respectively. 30 kV X-rays have a more-dense ionization pattern
resulting in a higher linear energy transfer (LET) of 4.34 keV/µm
compared to high energy photons such as e.g., 60Co
γ-rays (LET 0.3 keV/µm, energy 1.25 MeV). The biological impact of
the higher LET of low-energy mammography X-rays compared to high
energy photons is still a matter of debate. The International
Commission on Radiological Protection (ICRP) acknowledges that,
based on in vitro experiments on cells, there seems to be
significant differences in relative biological effectiveness (RBE)
of different low LET radiation qualities, but still recommends the
use of an RBE of 1 for 30 kV X-rays (5).
In the high dose range, starting from 100 mGy, there
is a linear relationship with the dose received and the long term
effects of radiation such as induction of cancer (6). The effects of low (<100 mGy) and very
low doses (<10 mGy), typically applied in medical diagnostics,
are debated, as the published data are more dispersed; on the
contrary, for doses higher than 100 mGy more consistent data are
available (7–9). Furthermore, epidemiological data on the
low dose range do not have sufficient statistical power to assess
the long term radiation risks of the low and very low exposure
levels (3). However, the inability to
quantify these risks does not imply that the risk to the population
is negligible. A very small risk, if applied to a large number of
healthy individuals, can result in a significant public health
problem (3).
In vitro studies investigating cellular and
genetic effects can highlight the consequences of low and very low
doses of ionizing radiation. After an exposure to just a few mGy
damage to the DNA, such as DNA double strand breaks (DSB) evidenced
by γH2AX foci, can be demonstrated (10–12) even
in primary breast epithelial cells (13), and changes in transcription level of
genes can also be detected (14–16). Doses
as low as a few mGy have an impact on the cell physiology and gene
expression analysis demonstrated that different gene profiles are
activated after low and high doses of X-rays in whole blood
(17). However, how these effects
translate into low dose risks, and whether they have detrimental or
beneficial effects, is still a matter of debate. In radiation
protection practice a linear no threshold (LNT) extrapolation is
used to calculate the risks at low doses using data from higher
doses. However, this is just a working hypothesis which might
underestimate or overestimate the effects of low dose radiation
(3).
Most data on the low dose effects of mammography
X-rays are derived from blood lymphocytes, primary fibroblasts or
cell lines (18–20). However, the breast is a unique tissue
in terms of sensitivity to radiation, due to the presence of
reproductive hormones like estrogen. Estrogens can act as complete
carcinogens as they stimulate both estrogen receptor-mediated cell
proliferation and induce DNA damage through the formation of
genotoxic metabolites such as reactive oxygen species (ROS)
(21–24).
In this pilot study we investigated the effect of
mammography X-rays in the dose range of 0–500 mGy, with special
emphasis on the very low doses (0–20 mGy), in mammary epithelial
cells present in freshly resected healthy breast tissue. By using
the γH2AX-foci assay we quantified the number of DNA DSB induced by
radiation in the glandular epithelial tissue (25). To study the RBE of mammography X-rays,
60Co γ-rays were used as reference radiation
quality.
Materials and methods
Subjects, preparation of the breast
tissue and irradiations
Non-cancerous, freshly resected breast tissue was
collected during surgery in the department of plastic surgery and
breast clinic of the Ghent University Hospital (Ghent, Belgium).
Ethical clearance was received from the commission for medical
ethics from the Ghent University hospital. Signed informed consent,
allowing the analysis of the effects of irradiation on breast
tissue, was obtained from each donor.
From a total of 18 specimens, 15 breast
tissue-samples were of good quality (see further): 11 out of 15
were from women without an increased risk for breast cancer
(mammectomy for esthetical reasons), whereas 4 were from women with
a high risk profile for breast cancer of which 3 had a confirmed
BRCA mutation (we do not have the information if it was a BRCA1 or
2 mutation). Mean age of the women was 41 years. None of the women
had breast cancer.
Immediately after resection, the tissue samples were
processed for irradiation by first removing the fat tissue. The
remaining connective tissue containing the mammary epithelial cells
was cut into slices with a thickness between 1.5 and 2 mm, while
being kept in Ringer's solution (9 g/l NaCl (Sigma-Aldrich, Bornem,
Belgium), 0.42 g/l CaCl2 and 0.24 g/l KCl (VWR, Leuven,
Belgium), pH 7.2). The slices of breast tissue were kept until
irradiation in a 5% CO2-incubator. Once the slices of breast tissue
were ready for irradiation, they were kept in a 5%
CO2-incubator at 37°C in DMEM/F12-ham medium
(Invitrogen, Merelbeke, Belgium) supplemented with 5% fetal calf
serum (Invitrogen), antibiotics and growth factors [10 µg/ml
insulin (Sigma, Belgium); 0.5 µg/ml hydrocortisone (Sigma); 20
ng/ml epidermal growth factor (Tebu-bio, Boechout, Belgium); 50
U/ml penicillin and 50 µg/ml streptomycin (Invitrogen)].
Samples from each donor were irradiated with
mammography X-rays, with doses ranging from 2 to 500 mGy (doses: 2,
4, 10, 20, 40, 100 and 500 mGy). Samples from the same donors were
also irradiated with identical doses of 60Co γ-rays,
used as reference radiation. Mammography-irradiations were
performed with a Siemens Mammomat3 producing a 30 kV Mo/Mo X-ray
spectrum at a dose rate of 0.125 Gy/min. As this mammography X-ray
device is designed to deliver very low doses, the number of doses
in the higher dose range (20–500 mGy) had to be kept to a minimum.
A layer of exactly 2 mm medium, containing the slices of breast
tissue, was irradiated at 37°C. The irradiations with
60Co γ-rays were performed in a water bath at 37°C at a
dose rate of 5 mGy/min for doses up to 40 mGy and at a dose rate of
0.6 Gy/min for higher doses. Within each experiment sham-irradiated
controls were included.
Fixation, embedding and
haematoxylin-eosin staining
Immediately after irradiation, the tissue samples
were incubated at 37°C for 30 min, the time for maximal γH2AX
foci-formation (26). Thereafter,
samples were transferred to cold ringer's solution and kept on
ice-water to inhibit repair processes. Subsequently they were
transferred to paraformaldehyde (PFA; 4%; VWR) for fixation.
After 24 h in PFA the tissue-samples were dehydrated
and embedded in paraffin. Tissue sections of 5 µm were cut with a
microtome. Two tissue sections for each specimen were placed on the
same slide and multiple slides were made.
The quality of the tissue and the presence of
mammary glands was inspected by performing a haematoxylin-eosin
staining using a multipurpose slide staining device (Robot stainer:
Microm HMS 740; Microm, Walldorf, Germany).
γH2AX immunostaining
Prior to the γH2AX immunostaining, tissue sections
were deparaffinised and rehydrated using the Robot stainer (Microm
HMS 740; Microm).
Antigen retrieval was performed in sodium citrate
buffer (0.2 g/l citric acid, pH 6; Merck KGaA, Darmstadt, Germany).
The tissue sections were brought in the buffer and boiled twice
during 5 min using a microwave. After washing of the sections in
PBS, they were pretreated in 3% H2O2 (VWR) to
inactivate the endogenous peroxidases. Sections were further
incubated in blocking serum (BS) (PBS 5 ml, BSA 50 mg (bovine serum
albumin; Roche Diagnostics, Vilvoorde, Belgium); NRS 0.250 ml
[Normal rabbit serum; Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA); Tween-20 (1 ml 10% Tween-20 (VWR)] for 30 min to
avoid nonspecific binding and to permeabilize the membranes.
Immunostaining was done using a tri-step reaction
using consecutively a mouse-anti-γH2AX primary antibody [Biolegend,
Trembodegem, Belgium; 2 h, 1/1,000 in PBS with 10% BS at room
temperature (RT)], a biotinylated rabbit-anti-mouse secondary
antibody (Dako; Agilent Technologies, Inc.; 30 min, 1/200 in PBS
with 10% BS at RT) and streptavidine-horse radish peroxidase (Dako;
Agilent Technologies, Inc.; 30 min, 1/200 in PBS at RT). Between
the steps the tissue-sections were washed in PBS (2×5 min). Next,
the sections were incubated with DAB-NiCl2 (stock
solution (ss) DAB: 25 mg/1 ml AD; ss NiCl2: 4 g/50 ml
AD; work solution: 200 µl DAB ss; 50 µl NiCl2 ss; 10 ml
PBS; 5 µl H2O2) during 10 min in the dark to
visualize the γH2AX foci. Slides were washed during 10 min in
running tap water before dehydrating and mounting the sections with
mounting medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Slides were sealed with a cover slip.
Foci scoring
γH2AX-foci scoring was done manually, using a light
microscope (Leica LEITZ-DMRB; Leica, Diegem, Belgium) at 63×
magnification (Fluotar 63×/1.25 Oil; Leica). Per breast tissue
sample and per radiation condition, at least two tissue-sections
were scored by two experienced researchers. In each section the
number of γH2AX foci in 100 nuclei was scored, if possible in at
least 4 different glandular groups. Only the epithelial cells from
the inner luminar layer of the glands were taken into account.
Analysis
For the analysis of the γH2AX foci data, two linear
fittings (Y=c+αD) were performed for both radiation qualities (30
kV X-rays and γ-rays): one in the very low dose range of 0–20 mGy
and one in the higher range of 20–500 mGy.
To compare the effects of the different radiation
qualities, the RBE of 30 kV X-rays compared to γ-rays was
calculated using the following formula:
‘RBE=αx/αγ’. The RBE is defined as a ratio,
between two absorbed doses delivered with two radiation qualities,
one of which is a ‘reference radiation’, that result in the same
effect in a given biological system, under identical
conditions.
Statistical analysis was done using the program
SigmaPlot (SigmaPlot for Windows Version 13.0; Systat. Software,
Inc., San Jose, CA, USA). To compare two groups of data, a Student
t-test or a rank sum test was used, depending on the normal
distribution of the data.
Results
Hematoxylin and eosin (H&E)
staining
Inspection of the tissue samples by H&E staining
revealed that for some donors, the samples contained cells with an
abnormal morphology. In those cases, all tissue samples of that
donor were excluded from the study.
Furthermore, in a number of tissue samples no glands
were present. Especially samples taken from elderly women tended to
contain a very limited amount of glandular epithelial tissue.
Tissue samples containing less than 100 epithelial cells were
discarded. As a result, per donor not all dose points and
sham-irradiated controls could be analyzed for foci formation.
γH2AX foci assay
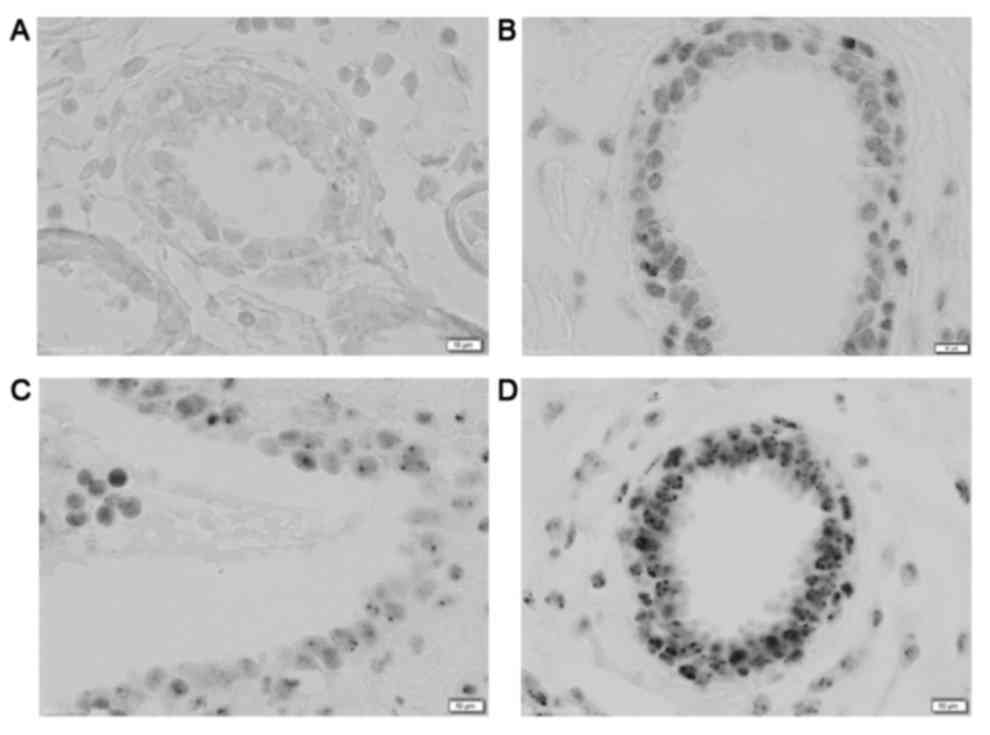
Examples of the foci staining are given in Fig. 1. The use of DAB-NiCl2
resulted in very distinct black foci and a light background
staining of the tissue. No counterstain was used, since the
immunostaining already resulted in sufficient contrast to the
tissue and counterstaining tended to darken the nuclei, reducing
the ability to distinguish the foci.
The number of background foci could be measured in
all 4 high-risk women and in 5 women with a normal risk profile. No
difference in the mean number of background foci could be detected
between women at high risk for breast cancer (0.44 foci/cell ±
0.15) and women without an elevated risk (0.36 foci/cell ± 0.10)
(P=0.68). Also for all irradiated samples (2–500 mGy), no
significant differences between women with and without elevated
risk could be observed.
As no differences were observed between women with
and without elevated genetic breast cancer risk, all the data were
pooled (Table I). The mean number of
background foci in the pooled samples was 0.38 foci/cell ± 0.08.
The threshold detection dose, leading to a significant increase in
number of foci per cell, was 10 mGy after irradiation with 30 kV
X-rays (P=0.0487) while this was 20 mGy after irradiation with
60Co γ-rays (P=0.0358).
 | Table I.Yield of γH2AX foci in cells exposed
to increasing doses of 30 kV X-rays and γ-rays. |
Table I.
Yield of γH2AX foci in cells exposed
to increasing doses of 30 kV X-rays and γ-rays.
|
| 30 kV X-rays | γ-rays |
|---|
|
|
|
|
|---|
| Dose (mGy) | Number of scored
samples | Mean number of foci
per cell | SEM | Number of scored
samples | Mean number of foci
per cell | SEM |
|---|
| 0 | 9 | 0.38 | 0.08 | 9 | 0.38 | 0.08 |
| 2 | 4 | 0.55 | 0.14 | 6 | 0.33 | 0.10 |
| 4 | 6 | 0.50 | 0.11 | 10 | 0.45 | 0.09 |
| 10 | 4 | 0.60 | 0.08 | 5 | 0.41 | 0.07 |
| 20 | 2 | 0.94 | 0.34 | 4 | 0.73 | 0.07 |
| 40 | 8 | 0.65 | 0.07 | 8 | 0.59 | 0.06 |
| 100 | 5 | 1.06 | 0.19 | 5 | 1.06 | 0.11 |
| 500 | 4 | 2.29 | 0.82 | 4 | 1.96 | 0.62 |
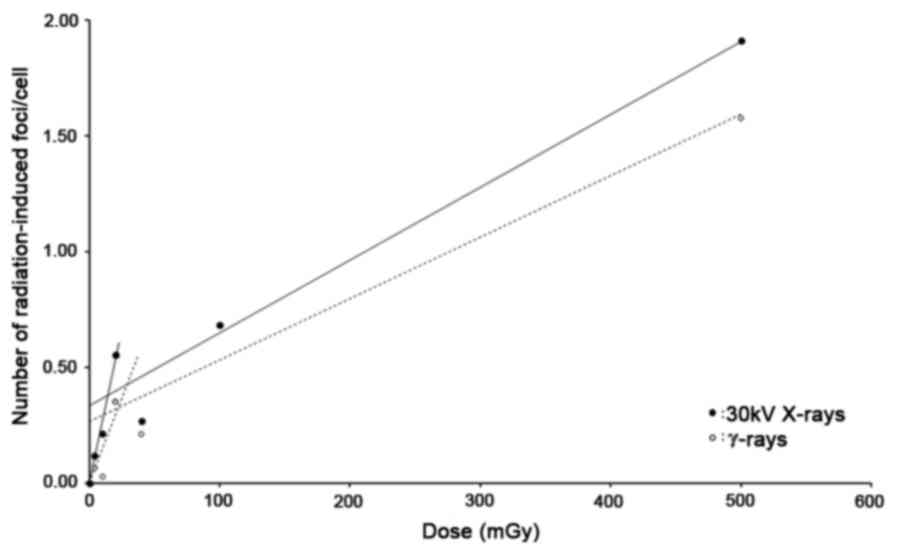
The dose response relationship over the whole dose
range shows a biphasic linear behavior for both 30 kV X-rays and
γ-rays, with the very low dose range (0–20 mGy) being characterized
by a steeper slope than the higher dose range (20–500 mGy)
(Fig. 2). An overview of the α-values
or slopes of the linear fittings performed in both dose ranges are
given in Table II. It can be
observed that the slope in the very low dose range is 8.71, resp.
5.77 times steeper than the slope in the higher dose range for 30
kV X-rays, resp. γ-rays. Table I also
lists the RBE (=α30kV/αγ) values. In the dose range 0–20
mGy, 30 kV X-rays are more effective in inducing DNA DSB than
γ-rays (RBE=1.82). In the higher dose range an RBE close to 1 was
obtained.
 | Table II.Overview of the slopes or α-values of
the linear dose response fittings (Y=c+αD) performed in the 0–20
and 20–500 mGy dose-range. |
Table II.
Overview of the slopes or α-values of
the linear dose response fittings (Y=c+αD) performed in the 0–20
and 20–500 mGy dose-range.
|
| 0–20 mGy | 95% CI | 20–500 mGy | 95% CI |
αL/αH | 95% CI |
|---|
| α30kV X-rays | 0.027 | 0.023–0.031 | 0.0031 | 0.0012–0.0050 | 8.71 | 4.64–25.20 |
| αγ-rays | 0.015 | 0.004–0.020 | 0.0026 | 0.0009–0.0044 | 5.77 | 0.94–22.14 |
| RBE | 1.82 | 1.17–7.56 | 1.19 | 0.28–5.58 |
|
|
Discussion
A mammography examination consists of 2 views of
approximately 2 mGy each resulting in a glandular dose of 3–5 mGy.
In most screening programs 10 examinations are performed during a
screening period of about 20 years. Although the dose per
examination is very small, the risk for radiation-induced breast
cancer cannot be neglected in view of the large population size and
the repetitive character involved in this type of asymptomatic
screening. At low doses, phenomena such as hypersensitivity,
bystander effect, adaptive response, threshold hypothesis and
hormetic response can play a role (3,6,27) and extrapolation of radiation-effects
from the high-dose range to the low-dose range by the LNT model can
lead to both an underestimation and overestimation of the low-dose
effects of radiation.
In this study we wanted to investigate the
biological efficacy of mammography X-rays for DNA DSB induction in
glandular epithelial cells present in resected breast tissue of
healthy women and ex vivo irradiated with very low doses.
For this, our study is unique as the set-up corresponds as close as
possible to the exact physiological conditions of mammography
screening.
To focus on a high number of data points in the high
dose range was beyond the scope of this study, as we and others
have previously published such data (19,28–30).
Nevertheless, the lack of details between 100 and 500 mGy may be a
possible limitation of our study.
As our population included 4 women with an increased
genetic risk for breast cancer, we first investigated if a
significant difference could be observed between those women and
women without a high risk for breast cancer in the number of
background γH2AX foci, and in the number of radiation-induced foci
at any dose. No significant differences were detected. These
results are in contrast with the results of Colin et al who
found an increased background number of γH2AX and an increased
radiation-induced number of foci (2, 4 mGy and fractionated 2+2
mGy) in cell cultures of primary mammary epithelial cells of
high-risk patients compared to low-risk patients (13). Since γH2AX functions upstream of BRCA1
and BRCA2, it is not surprising that no differences are found in
γH2AX induction between donors with and without a BRCA mutation 30
min after irradiation (31). Analysis
of residual DSB by scoring γH2AX foci at later time points after
irradiation (e.g., 24 h) is better suited to detect deficiencies in
DSB-repair, that may be related to cancer induction, but this was
not the aim of our study. In the paper of van Oorschot et al
(32), radiation-induced foci were
analyzed in repair deficient cell lines immediately and 24 h after
irradiation. Differences in foci number were only observed when the
cells were allowed to repair the DNA DSB for 24 h after
irradiation. At 30 min post irradiation no differences were
observed, in agreement with our findings.
As no differences were observed the data of all
donors were pooled for further analysis. The threshold detection
dose obtained with the γH2AX foci-assay after irradiation with 30
kV X-rays was 10 mGy. In a previous study using lymphocytes, we
also found a significant increase in γH2AX foci after 10 mGy of 30
kV X-rays (19). Other research
groups showed a statistical significant increase of DNA DSB in
MCF-10A cells after 9 mGy of 30 kV X-rays (20) and in cultured primary mammary
epithelial cells a threshold detection dose of 4 mGy and of a
fractionated 2+2 mGy was reported (13). These results suggest that the
threshold detection dose of mammography X-rays for DSB induction is
around or below 10 mGy.
Although some literature data report a linear dose
response for γH2AX, pointing to a proportionally identical
biological response to low and high radiation doses, other studies
demonstrated that for very low doses, foci induction is much higher
than at higher doses pointing to the phenomenon of low dose
hypersensitivity (for a review see Beels et al (33,34)).
Beels et al (33) found a
hypersensitive response in whole blood in pediatric patients
exposed in vivo to low doses of X-rays for cardiac
catheterization (mean dose 6 mSv). They further confirmed these
results with a study on γH2AX-induction in whole blood and isolated
T-lymphocytes irradiated in vitro with 100 kVp X-rays and
γ-rays (34). When whole blood was
irradiated with X-rays, a biphasic dose response was observed,
characterized by a very steep response in the 0–10 mGy dose range,
which became less steep if doses higher than 20 mGy were used.
After irradiation with γ-rays, the biphasic effect was still there,
but much less pronounced. The effects were also less pronounced
when isolated lymphocytes instead of whole blood was used. They
concluded that both cellular environment and radiation quality play
a role in this hypersensitive response. A strong correlation
between cell culture conditions and a low dose hypersensitive
response was also found by Groesser et al (35).
The results obtained in this study are comparable
with those of Beels et al (33,34). A
clear biphasic dose response was noted, with a hypersensitive
component for both 30 kV X-rays and γ-rays in the very low dose
range of 0–20 mGy, indicating that the LNT-model results in an
underestimation of the induced DNA damage. In the very low dose
range, 30 kV X-rays were also more effective in inducing DNA DSB
than γ-rays resulting in an RBE of 1.82. In the higher dose range
of 20–500 mGy an RBE close to 1 was obtained.
The low dose hypersensitivity observed in our study
is probably caused by the bystander effect. The bystander effect is
largely propagated by damaged cells which release signaling
molecules to neighboring cells via gap-junction mediated
intercellular communication and via the release of diffusible
factors such as ROS into the extra-cellular environment (36–40). In
our study, 2 mm thick slices of breast tissue, including epithelial
glandular structures, were irradiated and as such, gap-junction
mediated communication between cells in their natural
micro-environment is still intact. Also, estrogens, which are
highly available in breast tissue, are an important source of
ROS.
Mills et al on the other hand, did not find
an increased RBE in the low dose range (0–30 mGy) for mammography
X-rays, on the contrary, they found an RBE value of only 1.1
(20). The difference between our
study - performed on freshly resected breast tissue containing
mammary glands - and the study of Mills et al could be due
to the fact that Mills et al worked with an MCF10A cell line
and not with breast tissue sections. In their study, MCF-10A cells
were grown in monolayers, which could influence gap-junction
mediated intercellular communication. Furthermore, the cells were
kept at 0°C some time before, during and after the irradiation.
From literature it is known that hypothermia is a known
radioprotectans and has a protective role against the damaging
effects of ROS (41,42). Moreover, hypothermia could influence
cell processes such as cellular communication.
Overall, it seems that the bystander effect might
induce a hypersensitive response in breast tissue and that this
response is also dependent on the energy deposition which differs
between 30 kV X-rays and γ-rays, tissue culture conditions and
temperature.
In conclusion, our results indicate the existence of
a low dose (0–20 mGy) hypersensitive response in glandular
epithelial cells of breast tissue, with an RBE=1.82 for mammography
X-rays. The impact of these findings on the radiation risk
calculations for women participating in mammography screenings is
unclear, since several parameters will affect the repair of these
radiation-induced DSB. However, in our previous study, we have
shown that DSB induced by mammography X-rays are more difficult to
repair than those induced by γ-rays, and that the same number of
mammography induced DSB resulted in a higher number of chromosomal
aberrations which are a hallmark for cancer (19).
These results are based on a limited
patient-population and a future larger study, including ROS
measurements, will be needed to confirm the obtained results.
Acknowledgements
The authors would like to thank Mrs. Leen Pieters
from the Department of Basic Medical Sciences, University of Ghent
(Ghent, Belgium) for her technical assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JD performed the experimental research and
contributed to the analysis and interpretation of the data and
manuscript preparation; TV performed experimental research; PB, NR
and RVDB were responsible for the clinical part and provided
patient material; HT was responsible for the conception and design
of the radiation procedures and contributed to the interpretation
of the radiation experiments; AV was responsible for the conception
and design of the study, the interpretation of the data, the
critical revision of the manuscript draft and the final approval of
the submitted version.
Ethics approval and consent to
participate
All samples were taken from patients that signed an
informed consent stating that their material could be used for
anonymous analysis and publication of data. The handling of patient
material and data was approved and performed according to the
guidelines of the Ghent University Hospital's Ethical Committee and
the Helsinki Declaration on medical ethics and later
amendments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Altobelli E and Lattanzi A: Breast cancer
in European Union: An update of screening programmes as of March
2014 (review). Int J Oncol. 45:1785–1792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klein R, Aichinger H, Dierker J, Jansen
JT, Joite-Barfuss S, Säbel M, Schulz-Wendtland R and Zoetelief J:
Determination of average glandular dose with modern mammography
units for two large groups of patients. Phys Med Biol. 42:651–671.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brenner DJ, Doll R, Goodhead DT, Hall EJ,
Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Puskin JS, et
al: Cancer risks attributable to low doses of ionizing radiation:
Assessing what we really know. Proc Natl Acad Sci USA.
100:13761–13766. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hendrick RE, Pisano ED, Averbukh A, Moran
C, Berns EA, Yaffe MJ, Herman B, Acharyya S and Gatsonis C:
Comparison of acquisition parameters and breast dose in digital
mammography and screen-film mammography in the American College of
Radiology Imaging Network digital mammographic imaging screening
trial. AJR Am J Roentgenol. 194:362–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
The 2007 recommendations of the
International Commission on Radiological Protection: ICRP
publication 103. Ann ICRP. 37:1–332. 2007.
|
|
6
|
Averbeck D: Does scientific evidence
support a change from the LNT model for low-dose radiation risk
extrapolation? Health Phys. 97:493–504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heyes GJ, Mill AJ and Charles MW:
Mammography-oncogenecity at low doses. J Radiol Prot. 29:A123–A132.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hunter N and Muirhead CR: Review of
relative biological effectiveness dependence on linear energy
transfer for low-LET radiations. J Radiol Prot. 29:5–21. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leenhouts HP and Chadwick KH: Dose-effect
relationships, epidemiological analysis and the derivation of low
dose risk. J Radiol Prot. 31:95–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rothkamm K and Löbrich M: Evidence for a
lack of DNA double-strand break repair in human cells exposed to
very low x-ray doses. Proc Natl Acad Sci USA. 100:5057–5062. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bakkenist CJ and Kastan MB: Initiating
cellular stress responses. Cell. 118:9–17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grudzenski S, Raths A, Conrad S, Rübe CE
and Löbrich M: Inducible response required for repair of low-dose
radiation damage in human fibroblasts. Proc Natl Acad Sci USA.
107:14205–14210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Colin C, Devic C, Noël A, Rabilloud M,
Zabot MT, Pinet-Isaac S, Giraud S, Riche B, Valette PJ,
Rodriguez-Lafrasse C and Foray N: DNA double-strand breaks induced
by mammographic screening procedures in human mammary epithelial
cells. Int J Radiat Biol. 87:1103–1112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amundson SA, Lee RA, Koch-Paiz CA, Bittner
ML, Meltzer P, Trent JM and Fornace AJ Jr: Differential responses
of stress genes to low dose-rate gamma irradiation. Mol Cancer Res.
1:445–452. 2003.PubMed/NCBI
|
|
15
|
Franco N, Lamartine J, Frouin V, Le Minter
P, Petat C, Leplat JJ, Libert F, Gidrol X and Martin MT: Low-dose
exposure to gamma rays induces specific gene regulations in normal
human keratinocytes. Radiat Res. 163:623–635. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang F, Stenoien DL, Strittmatter EF, Wang
J, Ding L, Lipton MS, Monroe ME, Nicora CD, Gristenko MA, Tang K,
et al: Phosphoproteome profiling of human skin fibroblast cells in
response to low- and high-dose irradiation. J Proteome Res.
5:1252–1260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
El-Saghire H, Thierens H, Monsieurs P,
Michaux A, Vandevoorde C and Baatout S: Gene set enrichment
analysis highlights different gene expression profiles in whole
blood samples X-irradiated with low and high doses. Int J Radiat
Biol. 89:628–638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kühne M, Urban G, Frankenberg D and
Löbrich M: DNA double-strand break misrejoining after exposure of
primary human fibroblasts to CK characteristic X rays, 29 kVp X
rays and 60Co gamma rays. Radiat Res. 164:669–676. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Depuydt J, Baert A, Vandersickel V,
Thierens H and Vral A: Relative biological effectiveness of
mammography X-rays at the level of DNA and chromosomes in
lymphocytes. Int J Radiat Biol. 89:532–538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mills CE, Thome C, Koff D, Andrews DW and
Boreham DR: The relative biological effectiveness of low-dose
mammography quality X rays in the human breast MCF-10A cell line.
Radiat Res. 183:42–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fucic A and Gamulin M: Interaction between
ionizing radiation and estrogen: What we are missing? Med
Hypotheses. 77:966–969. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shao C, Folkard M, Held KD and Prise KM:
Estrogen enhanced cell-cell signalling in breast cancer cells
exposed to targeted irradiation. BMC Cancer. 8:1842008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liehr JG: Genotoxicity of the steroidal
oestrogens oestrone and oestradiol: Possible mechanism of uterine
and mammary cancer development. Hum Reprod Update. 7:273–281. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cavalieri E, Chakravarti D, Guttenplan J,
Hart E, Ingle J, Jankowiak R, Muti P, Rogan E, Russo J, Santen R
and Sutter T: Catechol estrogen quinones as initiators of breast
and other human cancers: Implications for biomarkers of
susceptibility and cancer prevention. Biochim Biophys Acta.
1766:63–78. 2006.PubMed/NCBI
|
|
25
|
Rogakou EP, Pilch DR, Orr AH, Ivanova VS
and Bonner WM: DNA double-stranded breaks induce histone H2AX
phosphorylation on serine 139. J Biol Chem. 273:5858–5868. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rothkamm K and Horn S: gamma-H2AX as
protein biomarker for radiation exposure. Ann Ist Super Sanita.
45:265–271. 2009.PubMed/NCBI
|
|
27
|
Averbeck D: Non-targeted effects as a
paradigm breaking evidence. Mutat Res. 687:7–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beyreuther E, Dörr W, Lehnert A, Lessmann
E and Pawelke J: Relative biological effectiveness of 25 and 10 kV
X-rays for the induction of chromosomal aberrations in two human
mammary epithelial cell lines. Radiat Environ Biophys. 48:333–340.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Beyreuther E, Lessmann E, Pawelke J and
Pieck S: DNA double-strand break signalling: X-ray energy
dependence of residual co-localised foci of gamma-H2AX and 53BP1.
Int J Radiat Biol. 85:1042–1050. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lehnert A, Lessmann E, Pawelke J and Dörr
W: RBE of 25 kV X-rays for the survival and induction of
micronuclei in the human mammary epithelial cell line MCF-12A.
Radiat Environ Biophys. 45:253–260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roy R, Chun J and Powell SN: BRCA1 and
BRCA2: Different roles in a common pathway of genome protection.
Nat Rev Cancer. 12:68–78. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
van Oorschot B, Hovingh S, Dekker A,
Stalpers LJ and Franken NA: Predicting radiosensitivity with
gamma-H2AX foci assay after single high-dose-rate and pulsed
dose-rate ionizing irradiation. Radiat Res. 185:190–198. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Beels L, Bacher K, De Wolf D, Werbrouck J
and Thierens H: gamma-H2AX foci as a biomarker for patient X-ray
exposure in pediatric cardiac catheterization: Are we
underestimating radiation risks? Circulation. 120:1903–1909. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Beels L, Werbrouck J and Thierens H: Dose
response and repair kinetics of gamma-H2AX foci induced by in vitro
irradiation of whole blood and T-lymphocytes with X- and
gamma-radiation. Int J Radiat Biol. 86:760–768. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Groesser T, Cooper B and Rydberg B: Lack
of bystander effects from high-LET radiation for early cytogenetic
end points. Radiat Res. 170:794–802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Azzam EI, de Toledo SM and Little JB:
Direct evidence for the participation of gap junction-mediated
intercellular communication in the transmission of damage signals
from alpha-particle irradiated to nonirradiated cells. Proc Natl
Acad Sci USA. 98:473–478. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Azzam EI, de Toledo SM and Little JB:
Stress signaling from irradiated to non-irradiated cells. Curr
Cancer Drug Targets. 4:53–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hanot M, Hoarau J, Carrière M, Angulo JF
and Khodja H: Membrane-dependent bystander effect contributes to
amplification of the response to alpha-particle irradiation in
targeted and nontargeted cells. Int J Radiat Oncol Biol Phys.
75:1247–1253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mothersill C and Seymour C:
Radiation-induced bystander effects: Past history and future
directions. Radiat Res. 155:759–767. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou H, Suzuki M, Geard CR and Hei TK:
Effects of irradiated medium with or without cells on bystander
cell responses. Mutat Res. 499:135–141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lisowska H, Wegierek-Ciuk A, Banasik-Nowak
A, Braziewicz J, Wojewodzka M, Wojcik A and Lankoff A: The
dose-response relationship for dicentric chromosomes and γ-H2AX
foci in human peripheral blood lymphocytes: Influence of
temperature during exposure and intra- and inter-individual
variability of donors. Int J Radiat Biol. 89:191–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dang L, Lisowska H, Manesh SS, Sollazzo A,
Deperas-Kaminska M, Staaf E, Haghdoost S, Brehwens K and Wojcik A:
Radioprotective effect of hypothermia on cells-a multiparametric
approach to delineate the mechanisms. Int J Radiat Biol.
88:507–514. 2012. View Article : Google Scholar : PubMed/NCBI
|