Introduction
Colorectal cancer (CRC) currently ranks third among
the leading causes of cancer-related mortality worldwide, with
>1.2 million new cases and >600,000 deaths annually (1). Due to the increasing trend in the number
of cases and mortality rate, research has been focused on
investigating potentially effective therapeutic compounds that may
control the malignant progression of CRC. Due to the attempts of
scientists and clinicians to discover therapeutic compounds that
effectively treat CRC while reducing treatment-related toxicity,
there has been a resurgence of interest in investigating the
antitumor activity of medicinal herbs and dietary compounds
(2,3).
Genipin, a medicinal herb compound derived from the
gardenia fruit, has been used as a traditional Chinese medicine
over several decades, and recent reports have demonstrated that
genipin possesses medicinal properties, including anti-inflammatory
(4), anti-angiogenic (5) and anti-thrombotic properties (6). Genipin has also exhibited significant
anititumor activity against several types of cancer, such as
gastric cancer (7), hepatocellular
carcinoma (8), breast cancer
(9) and cervical cancer (10). However, the effect of genipin on colon
cancer has yet to be investigated.
The aim of the present study was to determine the
therapeutic effects of genipin on colon cancer cells and elucidate
the molecular mechanisms underlying the medicinal properties of
genipin in colon cancer, in order to provide the basis for future
research on genipin as a pharmaceutical compound in colon cancer
treatment.
Materials and methods
Genipin, cell lines and nude mice
Genipin (98%) was purchased from Xi'an Kailai
Biology Company (Xi'an, China). HCT116 and SW480 cells were
purchased from the American Type Culture Collection (Manassas, VA,
USA). HCT116 cells were routinely cultured in McCoy's 5A medium
supplemented with 10% fetal calf serum (both Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and SW480 cells were grown in
Dulbecco modified Eagle's medium (DMEM) supplemented with 10% fetal
bovine serum (FBS) and 5% 0.1 mM penicillin-streptomycin, and all
cells were incubated at 37°C in 5% CO2. Male Balb/c nude
mice (180–220 g, 5–6 weeks old) were provided by the Third Military
Medical University (Chongqing, China) and maintained with standard
food and water at 27±2°C. The animal experiments conducted in this
study were approved by the Laboratory Animal Welfare and Ethics
Committee of the Third Military Medical University.
Cell viability assay
HCT116 or SW480 cells were seeded into 96-well
plates at a density of 7,000 cells per well and incubated with
genipin (, 150, 300, 450 or 600 µM) for 12, 24 and 36 h. After
treatment, the culture medium was replaced with 10 µl CCK-8
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) and 90 µl
medium per well and incubated in the dark for 2 h at 37°C. Finally,
the optical density (OD) for each well was measured at 450 nm with
a microplate reader.
Cell morphology observation and DAPI
staining
HCT116 cells (~5×105) were seeded into
each well of a 6-well plate and, following treatment with genipin
for 24 h, the cells were fixed with 4% (v/v) for 10 min and
observed under an inverted light microscope. The cells were then
stained with DAPI (Solarbio, Beijing, China) for 20 min, and images
were captured with a fluorescence microscope. Image-ProPlus 6.0
software was used to calculate the mean fluorescence intensity of
the DAPI-stained cells.
Cell cycle distribution and apoptosis
analysis
For cell cycle analysis, genipin-treated HCT116
cells were fixed with pre-cooled 70% ethanol overnight. Following
incubation with RNaseA (0.25 mg/ml) for 30 min at 37°C, the cells
were incubated with 20 µg/ml propidium iodide (PI; Solarbio) in the
dark for 30 min. Finally, ~10,000 cells per sample were analyzed by
flow cytometry. FlowJo 7.6 software (FlowJo LLC, Ashland, OR, USA)
was used to evaluate the cells at the G1, S and G2 phase. For
apoptotic analysis, double staining with an Annexin V
(FITC-conjugated) and PI (Solarbio) was used to analyze early- and
late-stage apoptosis. HCT116 cells were washed twice with cold
phosphate-buffered saline and suspended in binding buffer with
106 cells/ml. The cells were then stained with Annexin V
(FITC-conjugated, 10 µl/ml) and PI (10 µl/ml) for 25 min in the
dark prior to being analyzed by flow cytometry.
Determination of mitochondrial
membrane potential (MMP) and reactive oxygen species (ROS)
To determine the MMP, genipin-treated HCT116 cells
were stained with 10 µM Rhodamine 123 (Solarbio) for 30 min and
evaluated by fluorescence microscopy. To determine ROS generation,
genipin-treated HCT116 cells were stained with 20 µM DCFH-DA
(Solarbio) for 30 min and evaluated by flow cytometry.
Western blot analysis
Cells or tissue were lysed in RIPA lysis buffer
(Sigma-Aldrich; Merck-KGaA, USA) on ice for 30 min and subsequently
centrifuged to obtain the lysate supernatant. Protein lysates
(30–50 µg total protein), were run on an SDS-PAGE gel and electro
transferred onto PVDF membranes (Thermo Fisher Scientific, Inc.).
Appropriate primary antibodies were added to blocking buffer and
incubated with the membrane overnight at 4°C. After washing, the
membranes were incubated with the corresponding secondary
antibodies (ZSGB-BIO) for 2 h. Finally, the bands were visualized
by the Odyssey Infrared Imaging System (Li-Cor Biosciences,
Lincoln, NE, USA). The antibodies utilized included anti-Bax
(dilution, 1:800; ab53154), anti-cleaved caspase-3 (dilution,
1:500; ab2302), anti-p53 (dilution, 1:800; ab131442), anti-B-cell
lymphoma (Bcl)-2 (dilution, 1:500; ab182858; all Abcam, Cambridge,
UK) and anti-β-actin (dilution, 1:500; sc-130065; Santa Cruz
Biotechnology Inc., Dallas, TX, USA), Goat anti-Rabbit IgG (H+L)
Highly Cross-Adsorbed secondary antibodies, Alexa Fluor Plus 800
(dilution, 1:100,000; A32735), Donkey anti-Mouse IgG (H+L)
Secondary antibodies, WesternDot 800 (dilution, 1:5,000; W10823,
Invitrogen; Thermo Fisher Scientific, Inc.).
Animal experiments
Male Balb/c nude micewere subcutaneously injected
with 5×107 HCT116 or SW480 cells. After ~3 weeks, when
the subcutaneous tumors had grown to 2 mm3, the
xenografted mice were randomly divided into four groups (n=5 per
group). In 4 of the groups, the mice were intragastrically
administered genipin (dissolved by saline, 20, 40, or 80 mg/kg per
day) for 4 weeks, while the control group mice were gavaged with
saline. The diameter of the tumor was measured weekly by a vernier
caliper to calculate the tumor volume (V=L × S × S/2 where V, tumor
volume; L, long diameter; and S, short diameter). For TUNEL
staining, the primary tumors were removed from the nude mice and
subsequently snap-frozen in liquid nitrogen. The frozen sections
(7-mm) were then treated with 4% paraformaldehyde for 30 min and
incubated with 0.3% H2O2 for 20 min. The
sections were then permeabilized with 0.1% Triton X-100 for 2 min
and dyed with TUNEL reaction mixture (5 µl TdT+45 µl
fluorescein-dUTP) for 60 min. Images were immediately captured
using fluorescence microscopy.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS Inc., Chicago, IL, USA). The data are expressed as
the mean ± standard deviation. Comparison of >2 groups was
performed using one-way analysis of variance with Holm-Sidak's post
hoc multiple comparison test, and comparison between two groups was
performed with two-tailed Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Genipin disrupts cell viability and
leads to morphological changes in HCT116 cells
Genipin has shown the ability to disrupt growth in
several cancer cell lines, including human gastric cancer AGS cells
(7), human hepatocellular carcinoma
Hep3B cells (8) and human breast
cancer MDA-MB-231 cells (9). However,
to the best of our knowledge, genipin has not yet been investigated
in colon cancer cells.
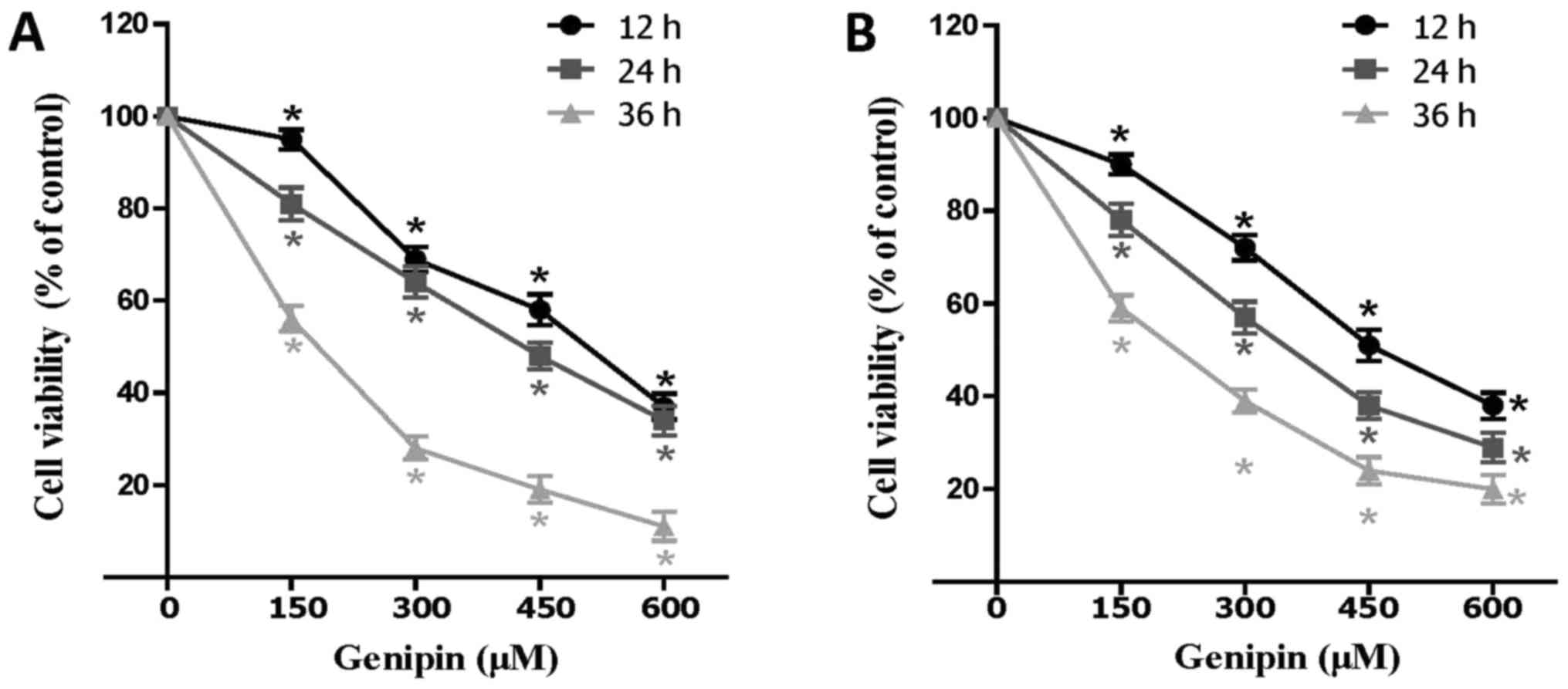
To evaluate the effect of genipin on colon cancer
cells, HCT116 and SW480 cells were treated with different
concentrations of genipin and cell viability was determined. The
results demonstrated that genipin was able to disrupt the viability
in both HCT116 and SW480 cells in a dose- and time-dependent manner
(Fig. 1A and B). Furthermore, the
IC50 values were calculated, with an IC50
value of 463.4 µM at 12 h, 377.6 µM at 24 h, and 168.4 µM at 36 h.
Based on IC50 calculations, further experiments were
performed at concentrations of 200, 400 and 600 µM genipin for 24
h.
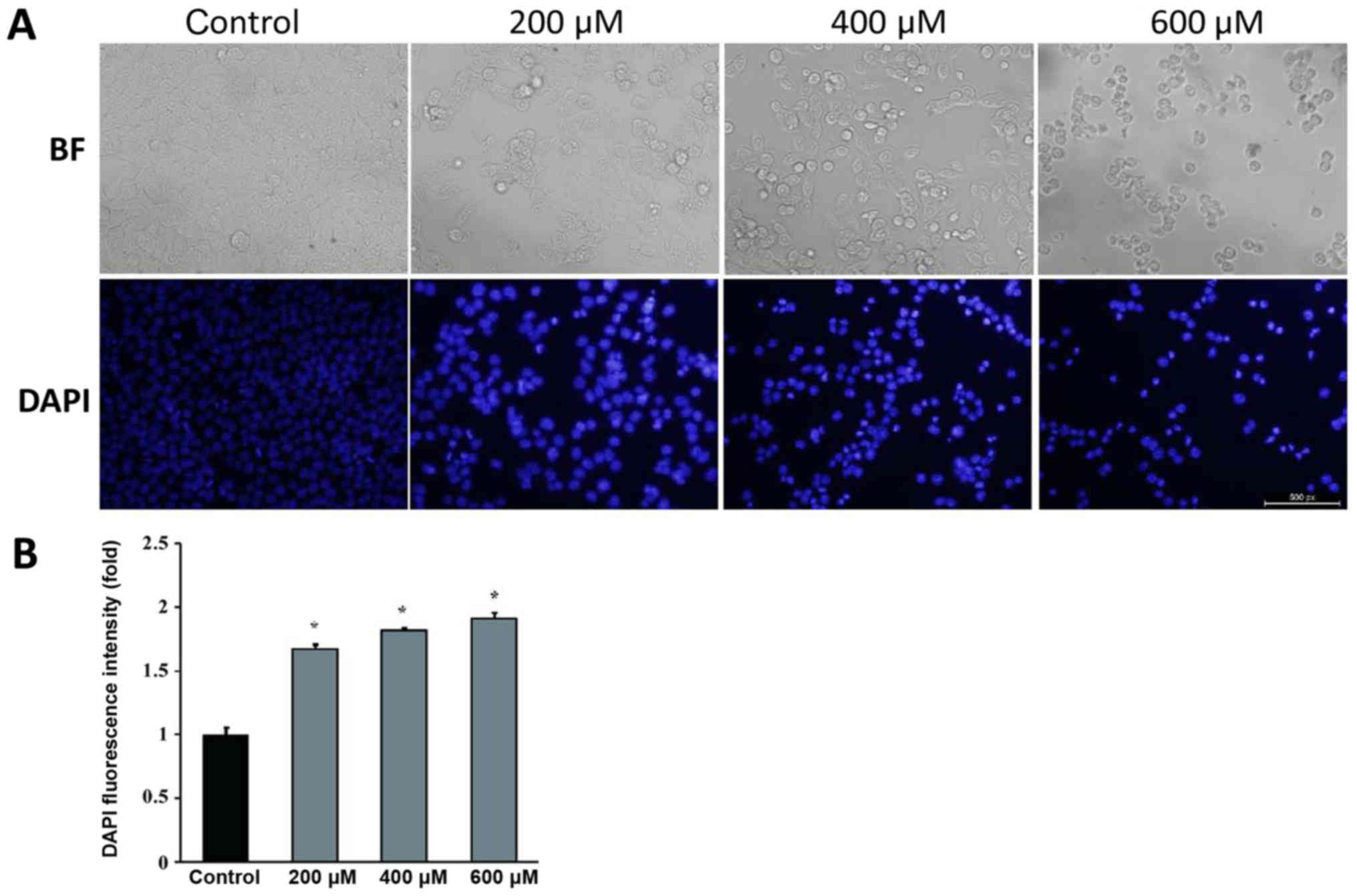
In order to investigate the effect of genipin on the
morphological characteristics of colon cancer cells, DAPI staining
was used in HCT116 cells treated with genipin, and it revealed that
a higher percentage of genipin-treated HCT116 cells exhibited the
typical morphological changes of apoptosis, such as cell atrophy,
membrane bubbling, DNA fragmentation and nucleus shrinkage under
the microscope (Fig. 2A). The
fluorescence intensity of DAPI increased with the increase in the
dose of genipin (Fig. 2A and B),
indicating a higher percentage of apoptotic cells. These results
suggested that genipin restrained the growth of HCT116 cells and
increased the cell death rate in a dose-dependent manner.
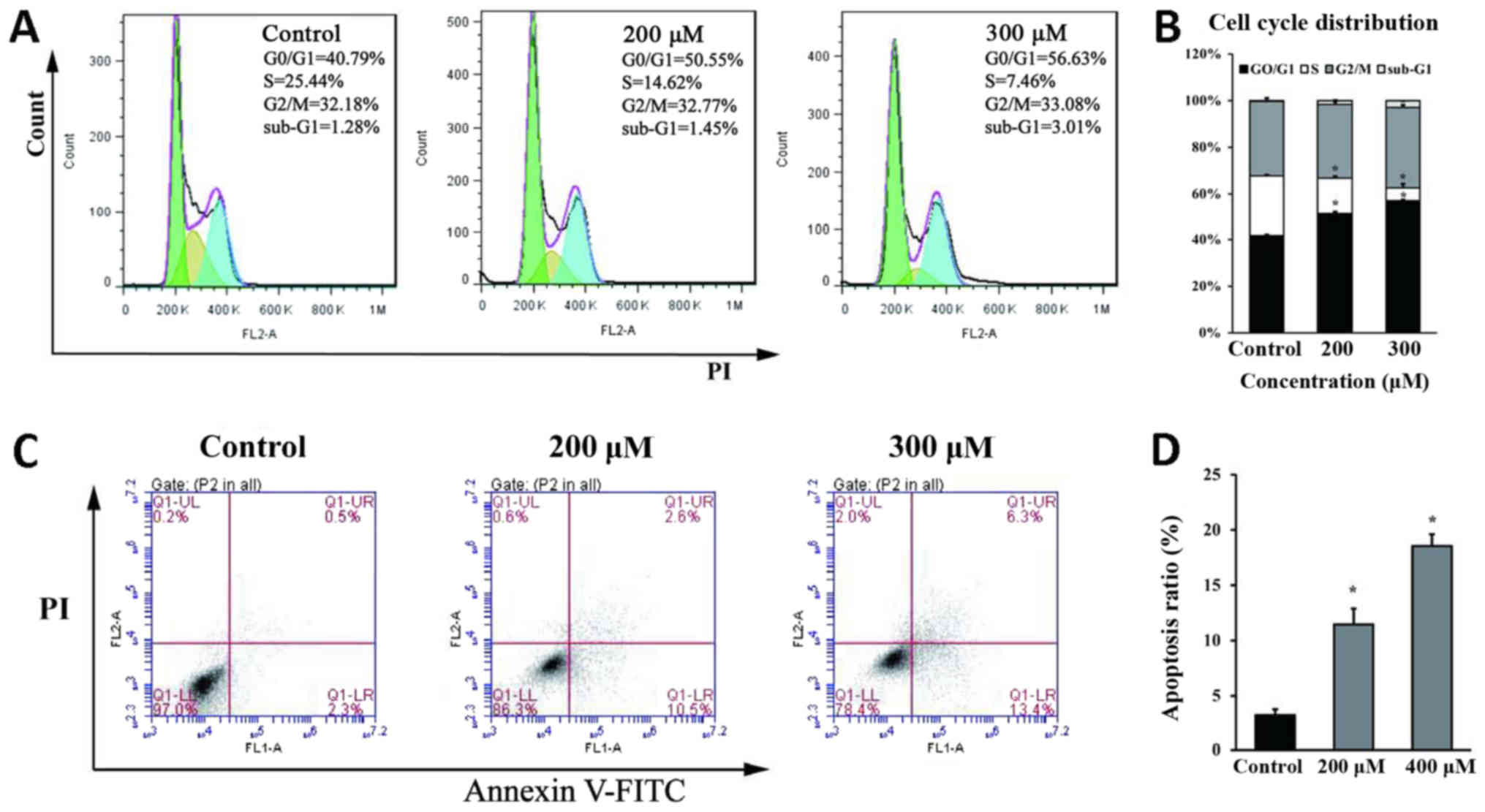
Genipin induces G0/G1 phase arrest and
apoptosis in HCT116 cells
To further elucidate the mechanisms of
genipin-induced disruption of cell growth, cell cycle and apoptosis
analysis were performed in HCT116 cells. Cell cycle analysis
revealed that the percentage of cells in the G0/G1 phase increased
from 40.79% (control) to 50.55% (200 µM) and 56.63% (300 µM),
whereas the percentage of cells in the S phase decreased with the
corresponding treatments (Fig. 3A and
B). The disruption in the cell cycle appeared to correspond to
a higher percentage of apoptotic cells among genipin-treated cells
(13.1% for 200 µM and 19.7% for 300 µM) compared with the control
group (2.8%) (Fig. 3C and D). These
results were consistent with previous reports demonstrating that
genipin significantly disrupted cell cycle progression in rat C6
glioma cells (11) and human leukemia
K562 cells (12), and promoted
apoptosis in rat C6 glioma cells (11).
Genipin reduces MMP and increases ROS
generation in HCT116 cells
ROS generation plays a crucial role in mediating
apoptosis induction. The increase in ROS may lead to loss of MMP
and increase oxidative cell damage, ultimately contributing to
apoptosis (13). Based on this
understanding, MMP and ROS changes in HCT116 cells were evaluated
following genipin treatment, and a dose-dependent decrease in MMP
was observed with genipin treatment (Fig.
4A and B). Intracellular ROS production was also measured and,
as illustrated in Fig. 4C and D,
genipin treatment significantly increased the intracellular ROS
level in HCT116 cells. Collectively, these results indicate that
ROS generation and mitochondrial dysfunction are involved in
genipin-induced apoptosis in HCT116 cells. These results are
consistent with recent reports demonstrating that genipin
significantly interferes with the function of uncoupling protein 2,
ultimately disrupting the protein gradient across the inner
mitochondrial membrane and increasing ROS production (14). In addition, the ROS-antagonizing agent
N-acetyl-L-cysteine (NAC) blunted the disruptive effect of genipin
on the viability in HCT116 cells (Fig.
4E), indicating that genipin-induced disruption of cell
viability may be dependent on ROS generation.
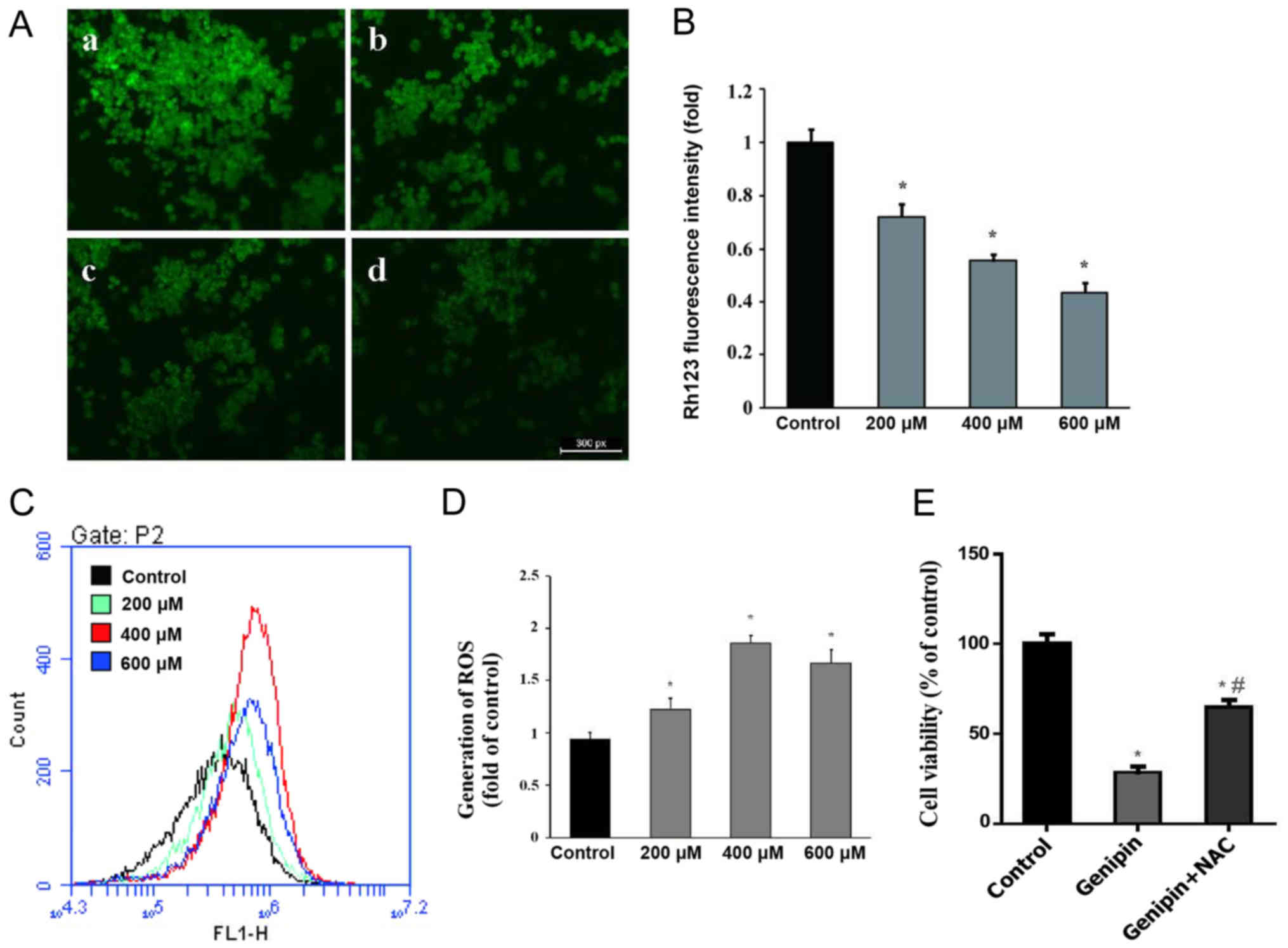 | Figure 4.Effect of genipin on MMP and ROS. (A)
MMP was detected by Rhodamine 123 staining after cells were treated
with (a) 0.1% DMSO, (b) 200 µM, (c) 400 µM and (d) 600 µM genipin.
Scale bar, 25 µm; magnification, ×200. (B) Mean fluorescence
intensity was calculated by Image-ProPlus 6.0 software; n=3. (C)
ROS production was detected by flow cytometric analysis after
DCFH-DA staining. (D) The generation of ROS is shown in a
histogram; n=3. (E) Effect of NAC on the inhibitory effect of
genipin on the viability of HCT116 cells, n=5. Data are expressed
as the mean ± standard deviation. *P<0.05 vs. control.
#P<0.05 vs. genipin. MMP, mitochondrial membrane
potential; ROS, reactive oxygen species; DMSO, dimethyl sulfoxide;
DCFH-DA, dichloro-dihydro-fluorescein diacetate; NAC,
N-acetyl-L-cysteine. |
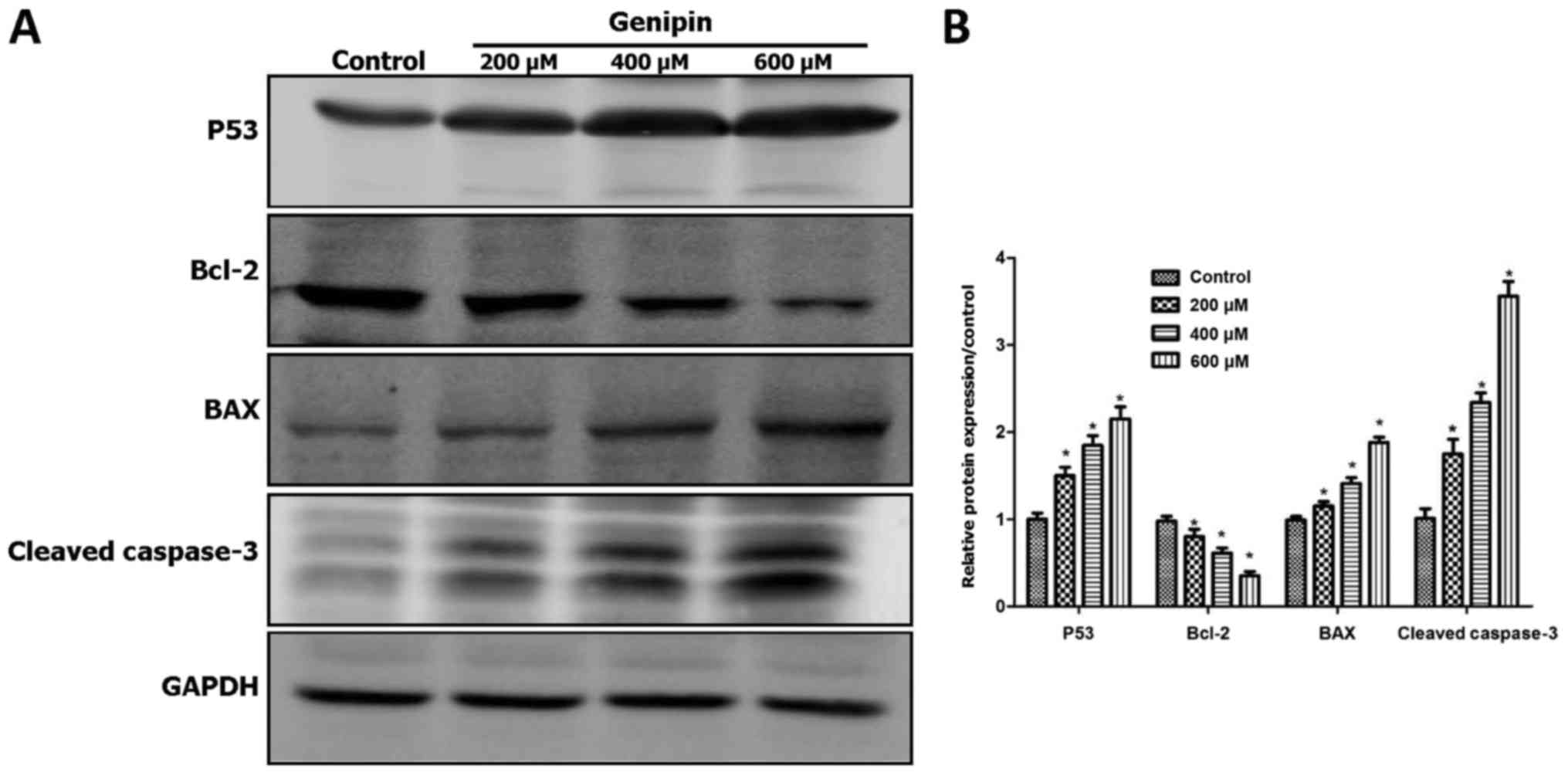
Genipin regulates the expression of
apoptosis-associated proteins in HCT116 cells
The balance between the anti-apoptotic gene Bcl-2
and the pro-apoptotic gene Bax plays a key role in cellular
homeostasis, and abnormal Bax/Bcl-2 ratio may cause apoptosis by
altering the MMP (15). Caspase-3 is
regulated by multiple genes associated with apoptosis and is
considered as the most important terminal cutting enzyme in the
apoptotic process; an increase in cleaved caspase-3 levels plays a
crucial role in apoptosis initiation (16). In addition, Bcl-2 family proteins and
caspase-3 are key regulatory factors in the mitochondrial-mediated
apoptotic induction.
To elucidate whether the mitochondrial apoptotic
pathway is involved in genipin-induced apoptosis in HCT116 cells,
the protein expression of Bcl-2, Bax and cleaved caspase-3 were
evaluated by western blotting (Fig.
5). There was a significant upregulation in the protein
expression of pro-apoptotic Bax and cleaved caspase-3 following
genipin treatment, while the expression of anti-apoptotic Bcl-2 was
noticeably decreased (Fig. 5A and B).
Thus, genipin may promote cell death through Bax-dependent
activation of the mitochondrial pathway in HCT116 cells.
The tumor suppressor p53 regulates cell cycle
arrest, apoptosis and the DNA repair process. In the present study,
genipin treatment significantly increased the expression of the p53
protein (Fig. 5A and B), suggesting
that p53 may play an important role in genipin-induced apoptosis.
Upregulation of p53 may also explain the cell cycle arrest induced
by genipin, due to the canonical ability of p53to activate p21
expression and cause G1 arrest (17).
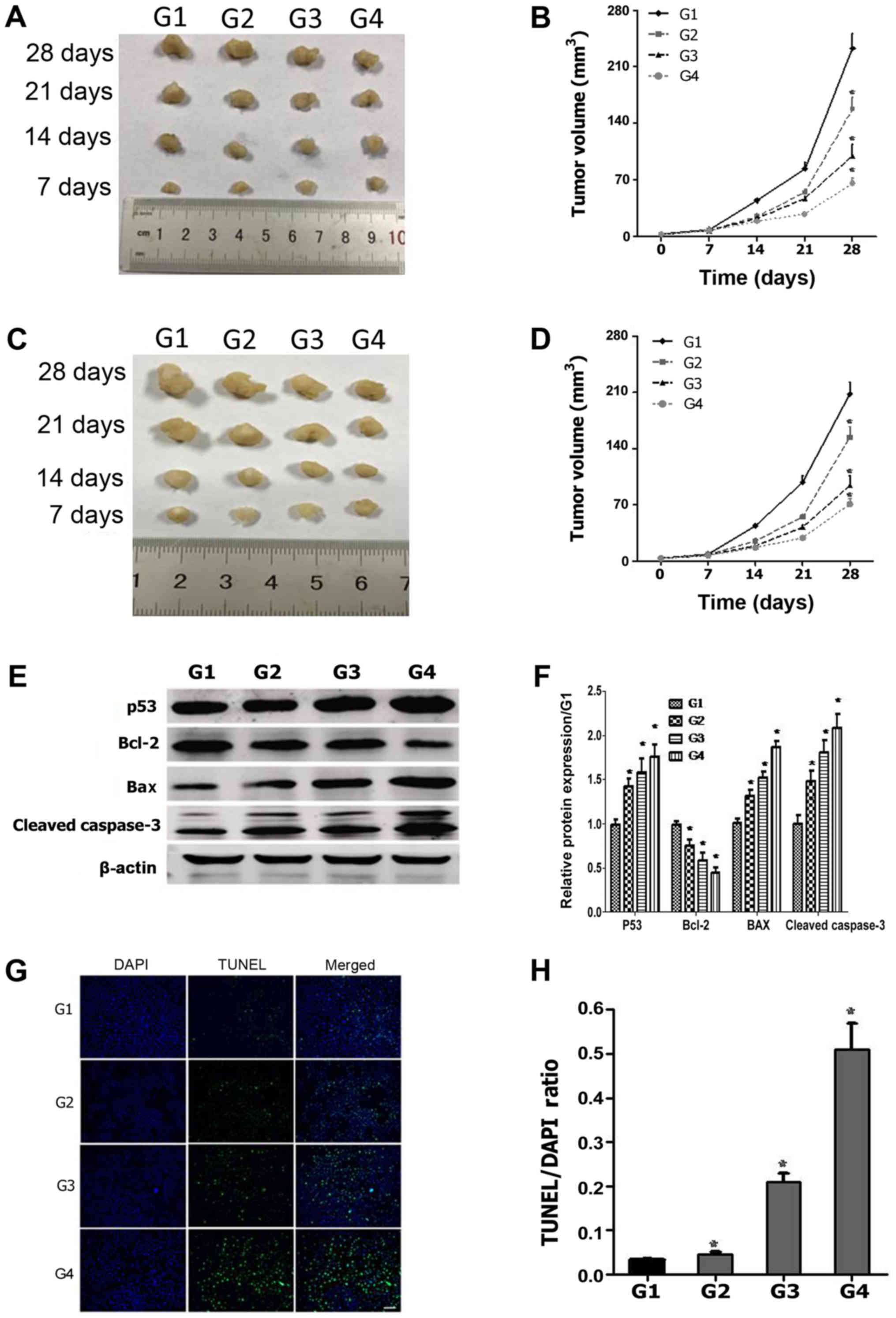
Genipin causes significant tumor
growth inhibition and apoptosis in nude mice
To verify the effect of genipin on HCT116 cells
in vivo, nude mice were subcutaneously xenografted with
HCT116 and SW480 cells, and were then treated with 20, 40 and 80
mg/kg of genipin. Based on tumor volume measurements, genipin
exerted an inhibitory effect on HCT116 tumor growth as indicated by
a decrease in tumor volume by 33% (20 mg/kg), 57% (40 mg/kg) and
73% (80 mg/kg) compared with the vehicle control group (Fig. 6A and B). Furthermore, a similar
inhibitory effect of genipin on tumor growth was observed when nude
mice were subcutaneously xenografted with SW480 cells (Fig. 6C and D). Western blotting analysis
revealed that genipin increased the expression of p53, Bax and
cleaved caspase-3, and reduced the expression of Bcl-2 (Fig. 6E and F), consistently with the results
of in vitro experiments (Fig.
5). TUNEL staining of tumor sections demonstrated that
apoptosis gradually increased with the increase in genipin
concentration (Fig. 6G and H). Taken
together, these results suggest that genipin exerted a significant
antitumor effect on colon tumor xenografts in nude mice, and this
antitumor effect maybe associated with p53 and Bax-mediated
activation of the mitochondrial apoptosis pathway.
Discussion
Genipin arrests the growth of several types of
cancer cells, such as AGS human gastric cancer cells (7), Hep3B human hepatocellular carcinoma
cells (8) and MDA-MB-231 human breast
cancer cells (9). However, the effect
of genipin on colon cancer cells remains unclear. In the present
study, genipin exerted a dose-dependent antitumor effect on HCT116
and SW480 cells in vitro and in vivo, which may be
attributed to genipin-induced G0/G1 cell cycle arrest and
apoptosis. It has been reported that genipin not only significantly
increased the G0/G1 ratio of rat C6 glioma cells (11), but also markedly inhibited G2/M phase
transition in human leukemia K562 cells (12). In this experiment, genipin arrested
cell cycle progression in the G0/G1 phase in HCT116 cells, which
may be associated with activation of p53.
The tumor suppressor p53 performs important
functions in regulating cell growth and cell death: It participates
in the cell cycle checkpoint pathway, which allows cells to repair
both endogenous and exogenous DNA damage; it also regulates
multiple genes, including p21, which is associated with G1 phase
arrest (17). Similarly, the results
of the present study revealed that genipin treatment significantly
increased the expression of the p53 protein in vivo and
in vitro.
ROS lead to MMP loss and oxidative cell damage,
eventually contributing to apoptosis (13). This study suggested that genipin
promoted cell death via the generation of ROS and the reduction of
MMP. Recent research demonstrated that genipin significantly
interferes with the function of uncoupling protein 2, which
dissipates the proton gradient across the inner membrane of the
mitochondria and decreases ROS production (14). Furthermore, ROS-antagonizing agents,
such as NAC, blunted the disruptive effect of genipin on the
viability of HCT116 cells, indicating that genipin-induced
disruption in cell viability may be dependent on ROS
generation.
The balance between the anti-apoptotic gene Bcl-2
and the pro-apoptotic gene Bax plays a key role in cell
development. Abnormal expression of Bax and Bcl-2 triggers
apoptosis via the mitochondrial pathway (15). Caspase-3 is regulated by multiple
genes associated with apoptosis and is considered as the most
important terminal cutting enzyme in the apoptotic process
(16). Additionally, Bcl-2 family
proteins and caspase-3 are key regulatory factors of the
mitochondrial-mediated apoptosis pathway. In the present study, the
levels of Bax and cleaved caspase-3 were markedly upregulated,
while the expression of Bcl-2 decreased significantly following
treatment with genipin, demonstrating that genipin promoted
apoptosis via the Bax-initiated mitochondrial-mediated pathway.
In conclusion, genipin exerted a dose-dependent
inhibitory effect on the growth of HCT116 and SW480 cells. The
inhibitory mechanism was associated with cell cycle arrest at the
G0/G1 phase by induction of the expression of p53. Genipin also
induced ROS generation and MMP decrease, and finally triggered
apoptosis by upregulating the expression of Bcl-2 family proteins
and activating caspase-3. Taken together, these findings
demonstrated that genipin suppressed the proliferation and enhanced
the apoptosis of colon cancer cells; thus, it may prove useful as a
novel drug for the prevention and treatment of colon cancer.
However, the detailed molecular mechanism remains unknown and
further investigation is required to elucidate it.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Science and Technology program of Chongqing (grant no.
cstc2013yykfB10006) and the ‘111 Project’ for Biomechanics and
Tissue Repair Engineering, China (grant no. 32450183).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
XW and LL conceived the project and designed the
experiments. JY and JL conducted the experiments. JY wrote the
manuscript. XW and LL revised the manuscript. All authors have
reviewed and approved the final version of this manuscript.
Ethics approval and consent to
participate
The present study was approved by the Third Military
Medical University Animal Use and Care Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jeyamohan S, Moorthy RK, Kannan MK and
Arockiam AJ: Parthenolide induces apoptosis and autophagy through
the suppression of PI3k/AKT signaling pathway in cervical cancer.
Biotechnol Lett. 38:1251–1260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramezanpour M, Da SK and Sanderson BJ:
Venom present in sea anemone (heteractis magnifica) induces
apoptosis in non-small-cell lung cancer A549 cells through
activation of mitochondria-mediated pathway. Biotechnol Lett.
36:489–495. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koo HJ, Lim KH, Jung HJ and Park EH:
Anti-inflammatory evaluation of gardenia extract, geniposide and
genipin. J Ethnopharmacol. 103:496–500. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park EH, Joo MH, Kim SH and Lim CJ:
Antiangiogenic activity of gardenia jasminoides fruit. Phytother
Res. 17:961–962. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suzuki Y, Kondo K, Ikeda Y and Umemura K:
Antithrombotic effect of geniposide and genipin in the mouse
thrombosis model. Planta Medica. 67:807–810. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim JM, Ko H, Kim SJ, Shim SH, Ha CH and
Chang HI: Chemopreventive properties of genipin on ags cell line
via induction of JNK/Nrf2/are signaling pathway. J Biochem Mol
Toxicol. 30:45–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim BC, Kim HG, Lee SA, Lim S, Park EH,
Kim SJ and Lim CJ: Genipin-induced apoptosis in hepatoma cells is
mediated by reactive oxygen species/c-JUN NH2-terminal
kinase-dependent activation of mitochondrial pathway. Biochem
Pharmacol. 70:1398–1407. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim ES, Jeong CS and Moon A: Genipin, a
constituent of gardenia jasminoides ellis, induces apoptosis and
inhibits invasion in MDA-MB-231 breast cancer cells. Oncol Rep.
27:567–572. 2012.PubMed/NCBI
|
|
10
|
Cao HL, Feng QA, Xu W, Li X, Kang Z, Ren Y
and Du L: Genipin induced apoptosis associated with activation of
the c-JUN NH2-terminal kinase and p53 protein in hela cells. Biol
Pharm Bull. 33:1343–1348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang YC, Chou FP, Huang HP, Hsu JD and
Wang CJ: Inhibition of cell cycle progression by penta-acetyl
geniposide in rat c6 glioma cells. Toxicol Appl Pharmacol.
198:11–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng Q, Cao, Hl, Xu W, Li XR, Ren YQ and
Du LF: Apoptosis induced by genipin in human leukemia k562 cells:
Involvement of c-JUN N-terminal kinase in G(2)/M arrest. Acta
Pharmacol Sin. 32:519–527. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cho YS, Lee JH, Jung KH, Park JW, Moon SH,
Choe YS and Lee KH: Molecular mechanism of (18) F-FDG uptake
reduction induced by genipin in T47D cancer cell and role of
uncoupling protein-2 in cancer cell glucose metabolism. Nucl Med
Biol. 43:587–592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuwana T, Mackey MR, Perkins G, Ellisman
MH, Latterich M, Schneiter R, Green DR and Newmeyer DD: Bid, bax
and lipids cooperate to form supramolecular openings in the outer
mitochondrial membrane. Cell. 111:331–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yun N, Kim C, Cha H, Park WJ, Shibayama H,
Park IS and Oh YJ: Caspase-3-mediated cleavage of picot in
apoptosis. Biochem Biophys Res Commun. 432:533–538. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jayaraman L and Prives C: Covalent and
noncovalent modifiers of the p53 protein. Cell Mol Life Sci.
55:76–87. 1999. View Article : Google Scholar : PubMed/NCBI
|