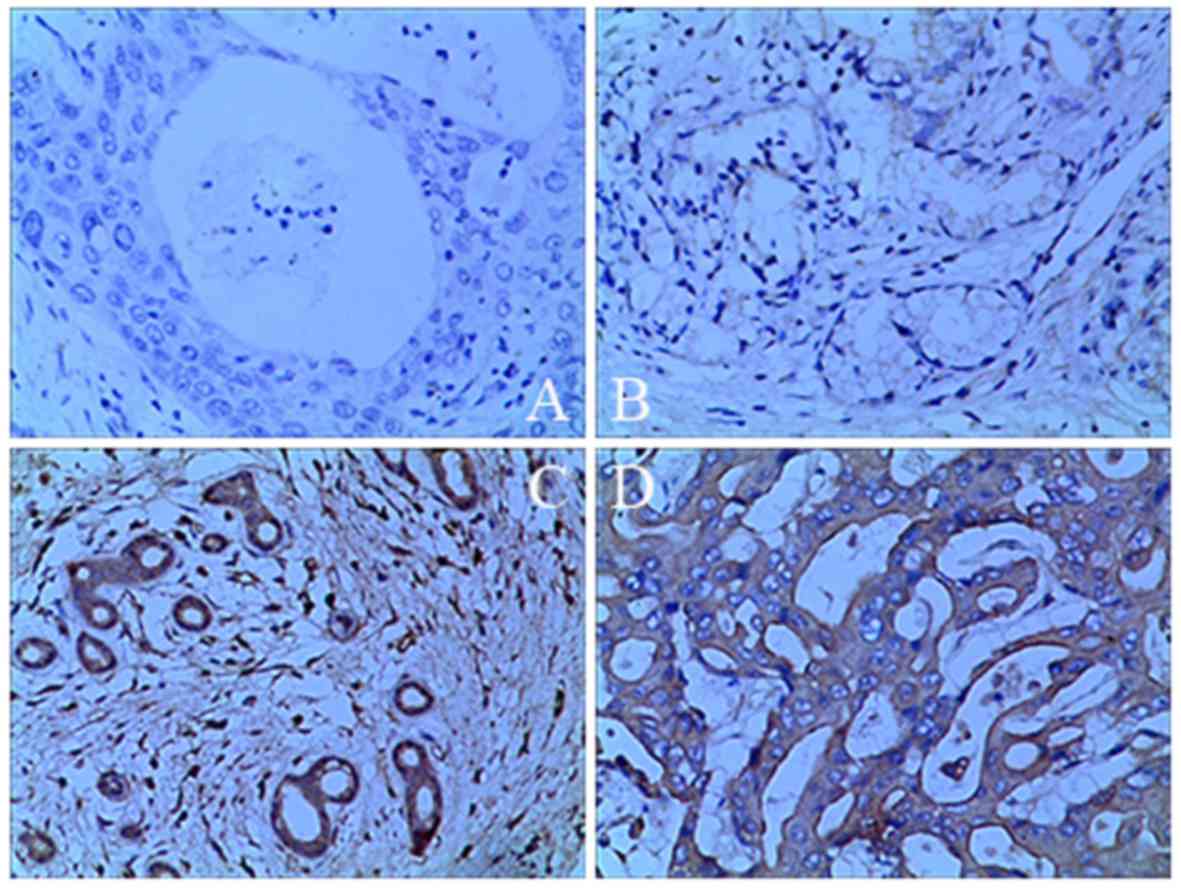
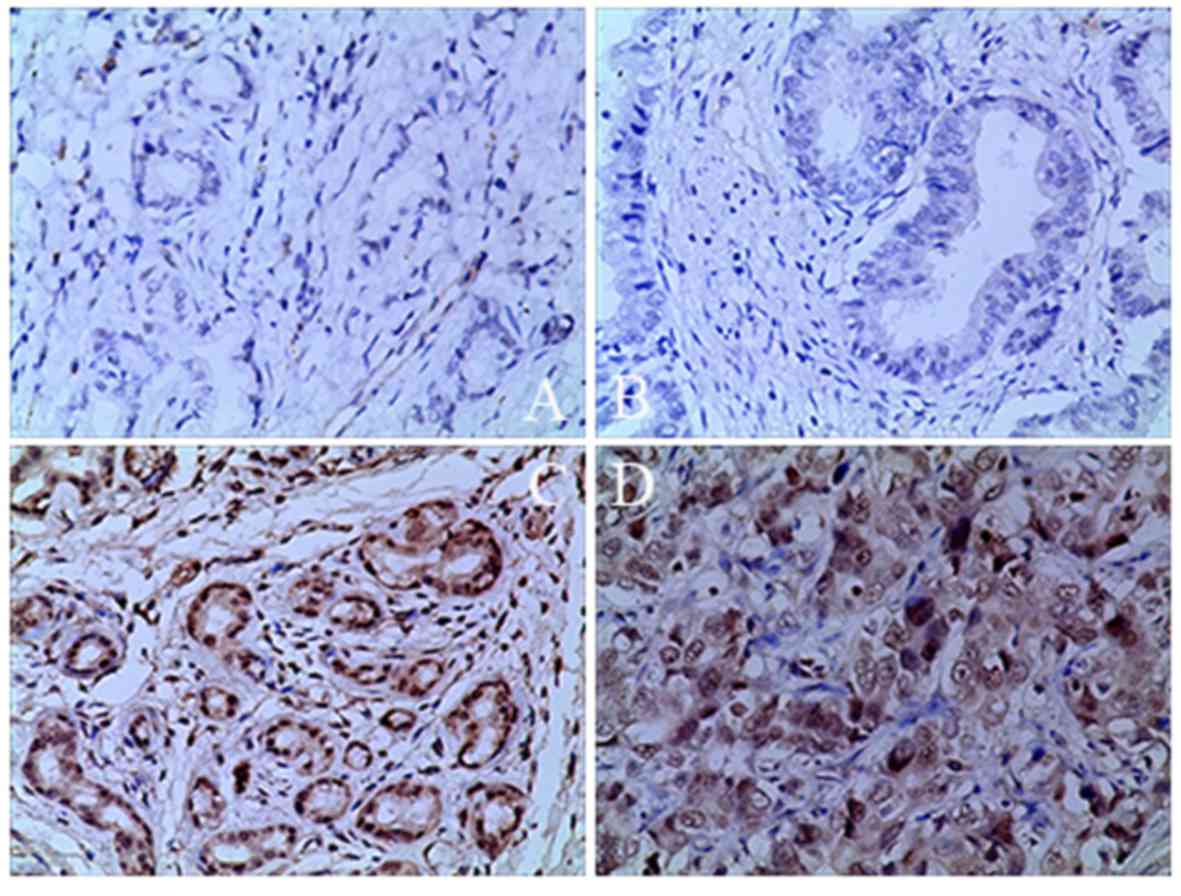
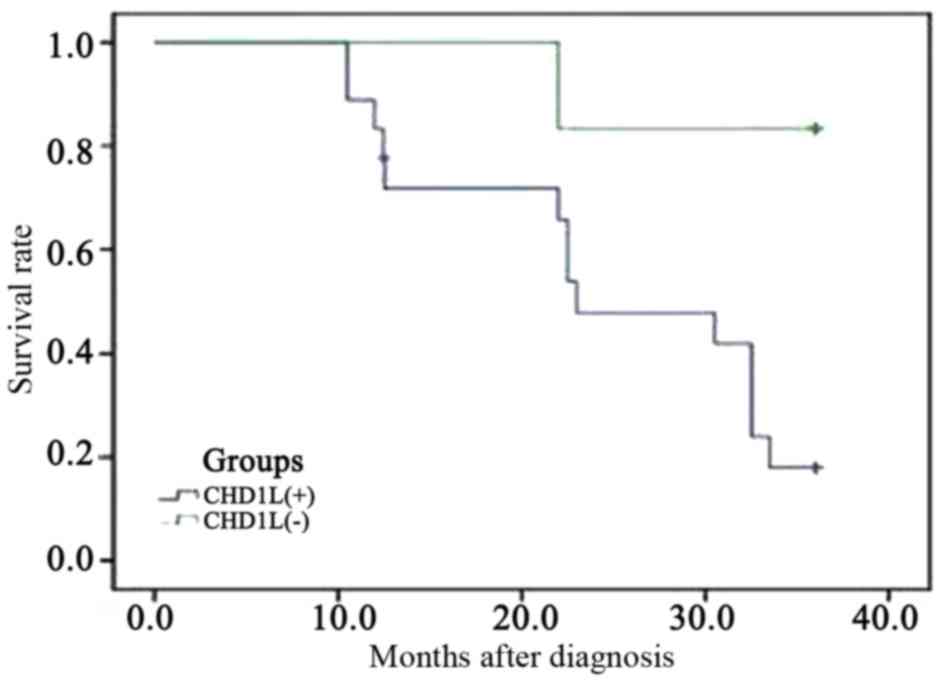
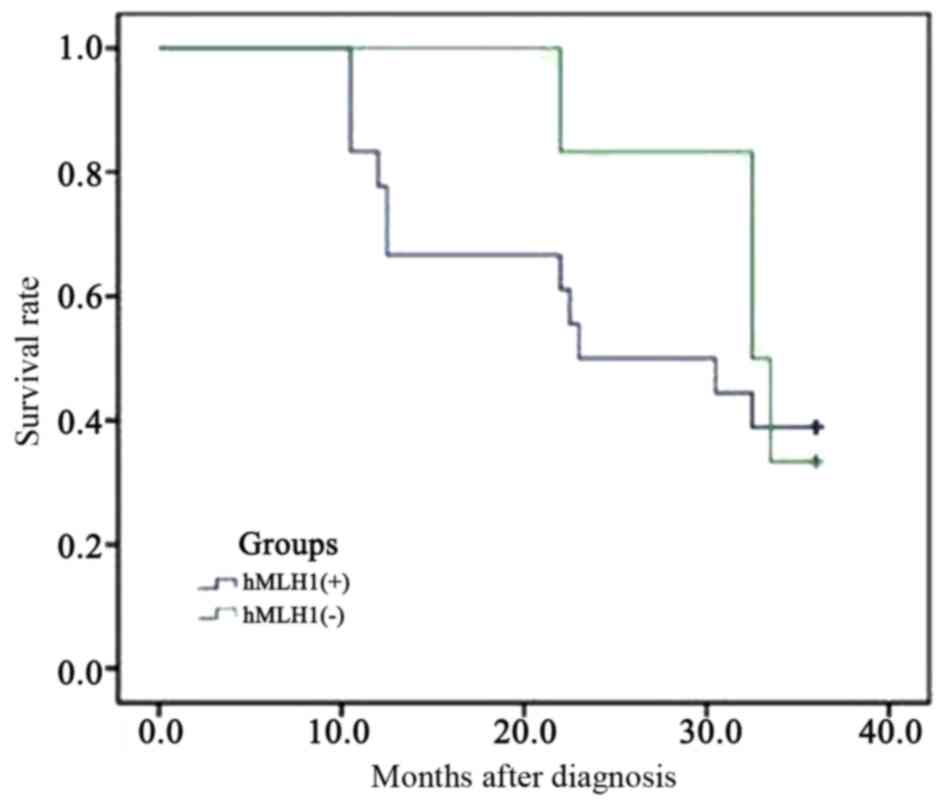
|
1
|
Ghouri YA, Mian I and Blechacz B: Cancer
review: Cholangiocarcinoma. J Carcinog. 14:12015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wadsworth CA, Lim A, Taylor-Robinson SD
and Khan SA: The risk factors and diagnosis of cholangiocarcinoma.
Hepatol Int. 7:377–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eckel F, Brunner T and Jelic S: ESMO
Guidelines Working Group: Biliary cancer: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 22
Suppl 6:vi40–vi44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tyson GL and El-Serag HB: Risk factors for
cholangiocarcinoma. Hepatology. 54:173–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schweitzer N and Vogel A: Systemic therapy
of cholangiocarcinoma: From chemotherapy to targeted therapies.
Best Pract Res Clin Gastroenterol. 29:345–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen L, Chan TH, Yuan YF, Hu L, Huang J,
Ma S, Wang J, Dong SS, Tang KH, Xie D, et al: CHD1L promotes
hepatocellular carcinoma progression and metastasis in mice and is
associated with these processes in human patients. J Clin Invest.
120:1178–1191. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ahel D, Horejsí Z, Wiechens N, Polo SE,
Garcia-Wilson E, Ahel I, Flynn H, Skehel M, West SC, Jackson SP, et
al: Poly(ADP-ribose)-dependent regulation of DNA repair by the
chromatin remodeling enzyme ALC1. Science. 325:1240–1243. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He WP, Zhou J, Cai MY, Xiao XS, Liao YJ,
Kung HF, Guan XY, Xie D and Yang GF: CHD1L protein is overexpressed
in human ovarian carcinomas and is a novel predictive biomarker for
patients survival. BMC Cancer. 12:4372012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tian F, Xu F, Zhang ZY, Ge JP, Wei ZF, Xu
XF and Cheng W: Expression of CHD1L in bladder cancer and its
influence on prognosis and survival. Tumour Biol. 34:3687–3690.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ji X, Li J, Zhu L, Cai J, Zhang J, Qu Y,
Zhang H, Liu B, Zhao R and Zhu Z: CHD1L promotes tumor progression
and predicts survival in colorectal carcinoma. J Surg Res.
185:84–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su Z, Zhao J, Xian G, Geng W, Rong Z, Wu Y
and Qin C: CHD1L is a novel independent prognostic factor for
gastric cancer. Clin Transl Oncol. 16:702–707. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan S, Sorrell M and Berman Z: Functional
interplay between ATM/ATR-mediated DNA damage response and DNA
repair pathways in oxidative stress. Cell Mol Life Sci.
71:3951–3967. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bronner CE, Baker SM, Morrison PT, Warren
G, Smith LG, Lescoe MK, Kane M, Earabino C, Lipford J, Lindblom A,
et al: Mutation in the DNA mismatch repair gene homologue hMLH1 is
associated with hereditary non-polyposis colon cancer. Nature.
368:258–261. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang R, Qin W, Xu GL, Zeng FF and Li CX:
A meta-analysis of the prevalence of somatic mutations in the hMLH1
and hMSH2 genes in colorectal cancer. Colorectal Dis. 14:e80–e89.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gu MJ, Bae YK, Kim A, Hong SM, Yu E, Kim
J, Jang KT, Chang HK, Jung ES, Bae HI, et al: Expression of hMLH1,
hMSH2 and hMSH6 in small intestinal carcinomas.
Hepatogastroenterology. 59:2228–2232. 2012.PubMed/NCBI
|
|
16
|
Kuan JC, Wu CC, Sun CA, Chu CM, Lin FG,
Hsu CH, Kan PC, Lin SC, Yang T and Chou YC: DNA methylation
combinations in adjacent normal colon tissue predict cancer
recurrence: Evidence from a clinical cohort study. PLoS One.
10:e01233962015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamarajah SK, Burns WR, Frankel TL, Cho CS
and Nathan H: Validation of the American Joint Commission on Cancer
(AJCC) 8th Edition Staging System for Patients with Pancreatic
Adenocarcinoma: A Surveillance, Epidemiology and End Results (SEER)
Analysis. Ann Surg Oncol. 24:2023–2030. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Plevová P, Krepelová A, Papezová M,
Sedláková E, Curík R, Foretová L, Navrátilová M, Novotný J,
Zapletalová J, Palas J, et al: Immunohistochemical detection of the
hMLH1 and hMSH2 proteins in hereditary non-polyposis colon cancer
and sporadic colon cancer. Neoplasma. 51:275–284. 2004.PubMed/NCBI
|
|
19
|
Gil-García B and Baladrón V: The complex
role of NOTCH receptors and their ligands in the development of
hepatoblastoma, cholangiocarcinoma and hepatocellular carcinoma.
Biol Cell. 108:29–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun J, Zhang L, Zhao H, Qiu X, Chen W,
Wang D, Ban N, Fan S, Shen C, Xia X, et al: CHD1L regulates cell
cycle, apoptosis, and migration in glioma. Cell Mol Neurobiol.
36:565–576. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He LR, Ma NF, Chen JW, Li BK, Guan XY, Liu
MZ and Xie D: Overexpression of CHD1L is positively associated with
metastasis of lung adenocarcinoma and predicts patients poor
survival. Oncotarget. 6:31181–31190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu J, Zong Y, Fei X, Chen X, Huang O, He
J, Chen W, Li Y, Shen K and Zhu L: Presence of CHD1L
over-expression is associated with aggressive tumor biology and is
a novel prognostic biomarker for patient survival in human breast
cancer. PLoS One. 9:e986732014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Giedl J, Schneckenpointner R, Filbeck T,
Ruemmele P, Hofstaedter F, Burger M, Hartmann A and Stoehr R: Low
frequency of HNPCC-associated microsatellite instability and
aberrant MMR protein expression in early-onset bladder cancer. Am J
Clin Pathol. 142:634–639. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park JW, Chang HJ, Park S, Kim BC, Kim DY,
Baek JY, Kim SY, Oh JH, Choi HS, Park SC and Jeong SY: Absence of
hMLH1 or hMSH2 expression as a stage-dependent prognostic factor in
sporadic colorectal cancers. Ann Surg Oncol. 17:2839–2846. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vageli D, Daniil Z, Dahabreh J, Karagianni
E, Vamvakopoulou DN, Ioannou MG, Scarpinato K, Vamvakopoulos NC,
Gourgoulianis KI and Koukoulis GK: Phenotypic mismatch repair hMSH2
and hMLH1 gene expression profiles in primary non-small cell lung
carcinomas. Lung Cancer. 64:282–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nam TK, Lee JH, Cho SH, Chung IJ, Ahn SJ,
Song JY, Yoon MS, Chung WK and Nah BS: Low hMLH1 expression prior
to definitive chemoradiotherapy predicts poor prognosis in
esophageal squamous cell carcinoma. Cancer Lett. 260:109–117. 2008.
View Article : Google Scholar : PubMed/NCBI
|