Introduction
Cervical cancer is one of the most common
malignancies globally and is one of the leading causes of mortality
resulting from gynecologic malignancies (1). In recent years, the incidence of
cervical cancer has decreased. However, the rate of cervical
cancer-associated morbidity in young adult women has increased.
The members of the Piwi protein family are highly
conserved during evolution and serve notable roles in cell
proliferation, gametogenesis, germ cell proliferation and
translational regulation (2–4). Hiwi is a human homologue of the Piwi
family, located on chromosome 12q24.33 and encoding a 98.5 kDa
protein. Hiwi is expressed in CD34+ hematopoietic stem
cells, but not in well-differentiated cell populations (5). Hiwi serves a function in the development
of hematopoietic stem cells (5).
Prior studies have demonstrated that Hiwi is also expressed in
various types of cancer cells and affects the differentiation and
proliferation of tumor cells (2,6–11). Overexpression of Hiwi is reported to
lead to tumors (11).
Hiwi exhibits an increased level of expression in
cervical cancer cells (12,13). However, the function of Hiwi in
cervical cancer cells remains unclear. In the present study, the
effects of siRNA induced-knockdown of Hiwi on the growth and
epithelial-mesenchymal transition of cervical cancer cells were
investigated. The present study reveals that Hiwi may act as an
oncogene in cervical cancer cells and may therefore be a novel
target for the treatment of cervical cancer.
Materials and methods
Cell culture
Human cervical cancer cells, HeLa, were obtained
from the American Type Culture Collection (Manassas, VA, USA). The
cells were grown in Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and cultured in a humidified atmosphere at 37°C
with 5% CO2.
Transfection
Hiwi specific small interfering RNA (siRNA)
(sequence, 5′-GCCGUUCAUACAAGACUAATT-3′ and
5′-UUAGUCUUGUAUGAACGGCTT-3′) and a scrambled negative control
(sequence, 5′-UUCUCCGAACGUGUCACGUTT-3′ and
5′-ACGUGACACGUUCGGAGAATT-3′) were obtained from Biomics
Biotechnologies Co., Ltd. (Nantong, China). The cells were seeded
into 6-well plates (1×105 cells/well). After 24 h, the
cell medium was changed to serum-free medium for an additional 6 h.
Then 100 pmol siRNA or its corresponding negative control was
transfected into the cells using Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. At 6 h post-transfection, the cell medium
was changed to fresh DMEM supplemented with 10% FBS. After
culturing at 37°C for additional 48 h, the cells in each group were
collected for subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells of each group
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The total RNA was reverse
transcribed to cDNA using Moloney murine leukemia virus reverse
transcriptase (Promega Corporation, Madison, WI, USA). Then the
level of Hiwi mRNA was measured using RT-qPCR with the cDNA as the
template and primers as follows: Hiwi forward,
5′-ATGGCCATCTACAAGCAGTC-3′ and reverse, 5′-GACAGTGCTCGCTTAGTGC-3′;
and GAPDH forward, 5′-CACCCACTCCTCCACCTTTG-3′ and reverse,
5′-CCACCACCCTGTTGCTGTAG-3′. The qPCR was performed on a StepOne PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 95°C for 10 min; 40
cycles of 95°C for 10 sec, 60°C for 30 sec, 72°C for 30 sec; and
then kept at 4°C for 10 min. SYBR-Green reagent was obtained from
Biomics Biotechnologies Co., Ltd. The level of Hiwi mRNA was
normalized to GAPDH, and the relative mRNA level of Hiwi was
calculated using the 2−∆∆Cq method (14).
Western blot analysis
The cells transfected with negative control or siRNA
were harvested and proteins in cells were extracted on ice using
radioimmunoprecipitation assay lysis buffer. The concentration of
the proteins was measured using a Enhanced BCA Protein Assay kit
(Beyotime Institute of Biotechnology, Haimen, China). 40 µg
proteins were separated using 12% SDS-PAGE and transferred onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). Following blocking with 5% skimmed milk at 37°C for 1 h, the
membranes were incubated with primary antibodies against Hiwi
(1:1,000; cat. no. sc-22685; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), proliferating cell nuclear antigen (PCNA;
1:2,000; cat. no. sc-25280; Santa Cruz Biotechnology, Inc.), cyclin
A (1:1,000; cat. no. sc-751; Santa Cruz Biotechnology, Inc.),
cyclin D1 (1:1,000; cat. no. sc-70899; Santa Cruz Biotechnology,
Inc.), E-cadherin (1:1,000; cat. no. sc-71009; Santa Cruz
Biotechnology, Inc.), N-cadherin (1:1,000; cat. no. sc-59987; Santa
Cruz Biotechnology, Inc.), vimentin (1:1,000; cat. no. sc-5565;
Santa Cruz Biotechnology, Inc.), snail family transcriptional
repressor 1 (SNAIL; 1:1,000; cat. no. sc-28199; Santa Cruz
Biotechnology, Inc.) and β-actin (1:2,000; cat. no. sc-130065;
Santa Cruz Biotechnology, Inc.) at 4°C overnight. Following washing
with Tris-buffered saline with Tween-20 (TBST), the membranes were
incubated with corresponding horseradish peroxidase (HRP)-labeled
goat anti-rabbit IgG (H+L) (cat. no. A0208), HRP-labeled goat
anti-mouse IgG (H+L) (cat. no. A0216), or HRP-labeled donkey
anti-goat IgG (H+L) (cat. no. A0181) (1:5,000; Beyotime Institute
of Biotechnology) at 37°C for 1 h. Following washing with TBST, the
targeted proteins were visualized using an Chemiluminescent HRP
substrate (ECL) (EMD Millipore). The relative protein level was
analyzed by Quantity One 4.6 (Bio-Rad, Hercules, CA, USA) and
calculated as targeted protein level/reference protein level.
MTT assay
Following transfection, the cells (3×103
cells/well) were seeded into 96-wells plates. The cell viability
was measured using MTT assay. Briefly, MTT at a final concentration
of 0.5 mg/ml was added into the cells in each group at 4, 12, 24,
36 and 48 h and cultured at 37°C for an additional 4 h. The
supernatant was removed, and 200 µl dimethyl sulfoxide
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added into each
well. The absorbance at 490 nm was measured with a microplate
reader.
Cell cycle detection
The cells were transfectged with neative control or
siRNA, and cell cycle of the cells in each group was evaluated
using flow cytometry at 48 h with a Cell Cycle and Apoptosis
Analysis kit (Beyotime Institute of Biotechnology). Briefly, the
cells in each group were collected, washed with ice-cold
phosphate-buffered saline (PBS) and fixed with ice-cold 70% ethanol
at 4°C overnight. Following washing with ice-cold PBS, the cells
were resuspended in 500 µl binding buffer and then 25 µl propidium
iodide and 10 µl RNaseA were added into cells in each group. The
cells were incubated at 37°C for 30 min and then analyzed using a
flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Transwell assay
Following transfection with Hiwi siRNA or negative
control, the cells were collected and suspended (1×105
cells/ml). The Transwell inserts (Corning Life Sciences, Tewksbury,
MA, USA) were inserted into a 24-well plate. Subsequently, 200 µl
cell suspension was added into the upper chambers, and 600 µl DMEM
containing 20% FBS was added into the lower chambers. The cells
were allowed to migrate at 37°C for 24 h. Subsequently, the cells
above the membranes were removed with cotton swabs. The cells below
the membranes were fixed with 4% paraformaldehyde and stained with
0.5% crystal violet at room temperature for 15 min. The cells were
observed via light microscopy using a ×200 magnification. The cells
in each field of view were counted, and the average number of cells
in five randomly selected fields of view was calculated as the
number of migratory cells.
Statistical analysis
The results are presented as the mean ± standard
deviation. Differences between groups were analyzed using Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
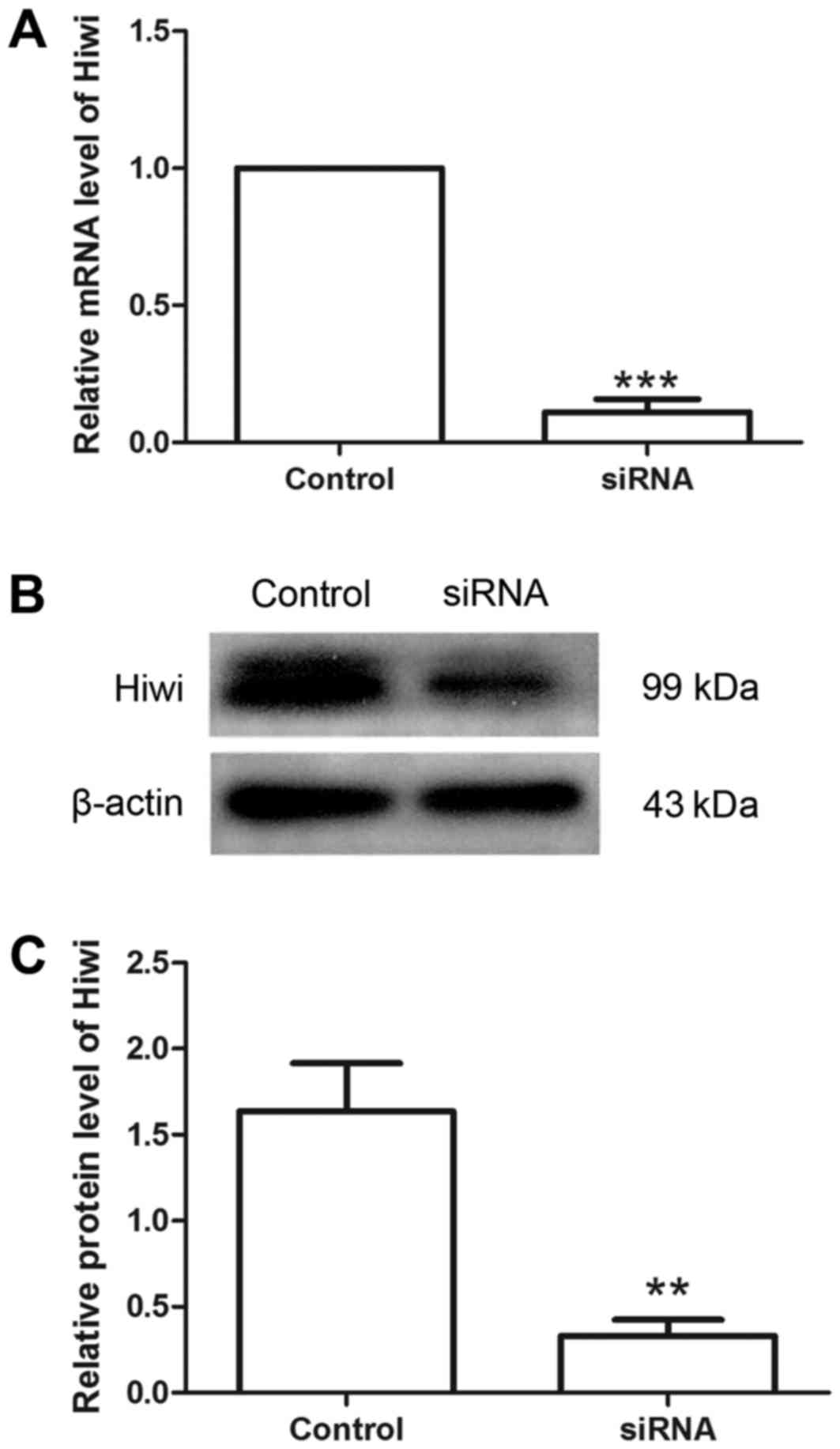
Hiwi siRNA decreases the level of Hiwi
in cervical cancer cells
Hiwi siRNA was used to investigate the effects of
Hiwi on cervical cancer cells. Following transfection with Hiwi
siRNA or the negative control, the relative level of Hiwi mRNA was
detected using RT-qPCR. The results revealed that the relative
level of Hiwi mRNA was decreased to 11.01±4.78% following
transfection with Hiwi siRNA (Fig.
1A). Western blot analysis demonstrated similar results to
RT-qPCR. Compared with the control group, the relative protein
level of Hiwi was decreased from 1.64±0.28 to 0.33±0.09 following
transfection with Hiwi siRNA (Fig. 1B and
C). These results demonstrated that Hiwi siRNA was able to
effectively decrease the levels of mRNA and protein in cervical
cancer cells.
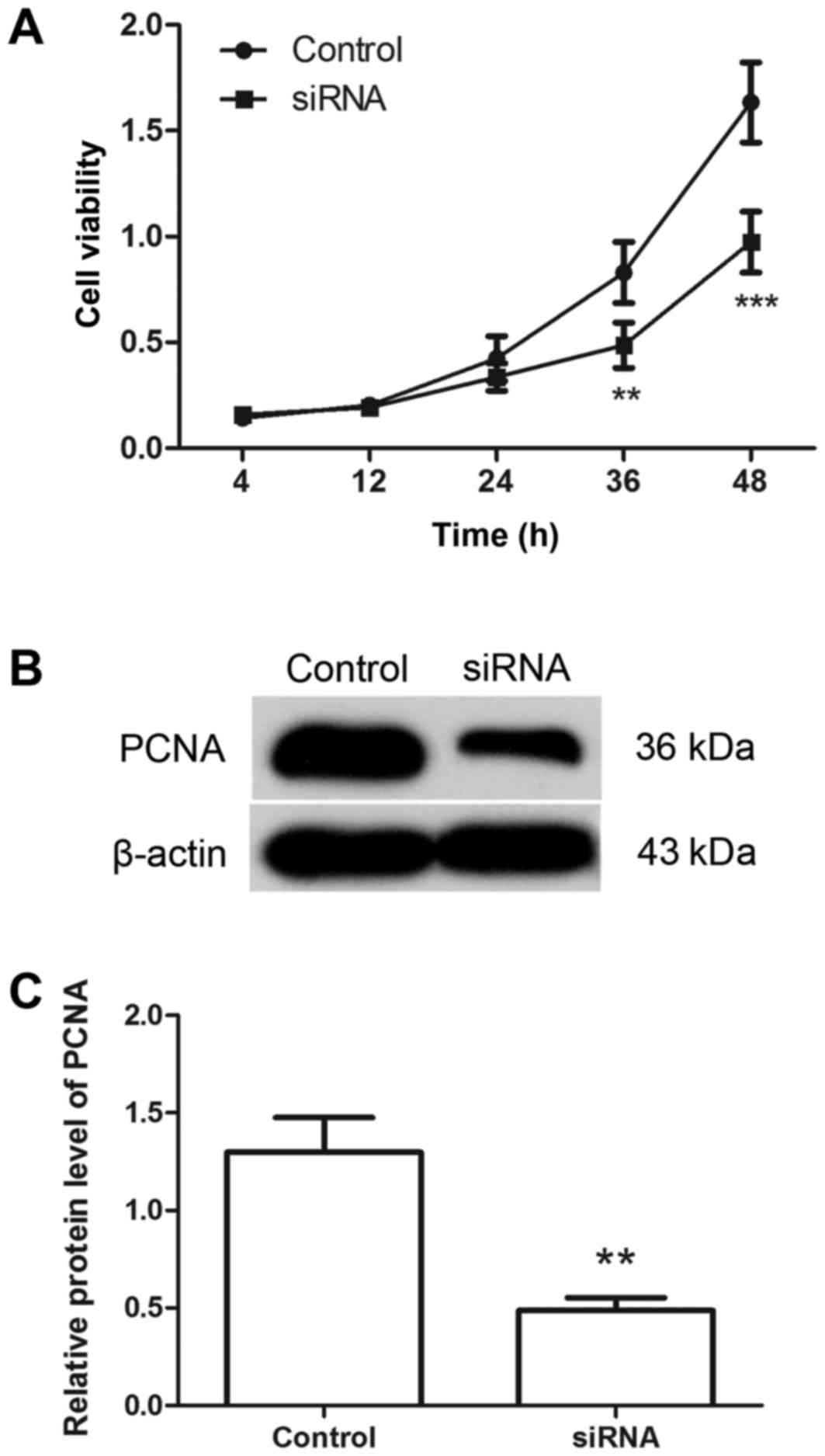
Downregulation of Hiwi inhibits the
proliferation of cervical cancer cells
Following transfection with Hiwi siRNA or the
negative control, the proliferation of cervical cancer cells was
detected using MTT assay. As presented in Fig. 2A, compared with the control group, the
proliferation of the cells was significantly inhibited by
transfection with Hiwi siRNA. The level of PCNA protein was
detected by western blotting. The results of western blot analysis
revealed that, following transfection with Hiwi siRNA, the relative
level of PCNA protein was decreased from 1.30±0.178 to 0.49±0.06
(Fig. 2B and C). These results
support the hypothesis that transfection with Hiwi siRNA inhibits
the proliferation of cervical cancer cells.
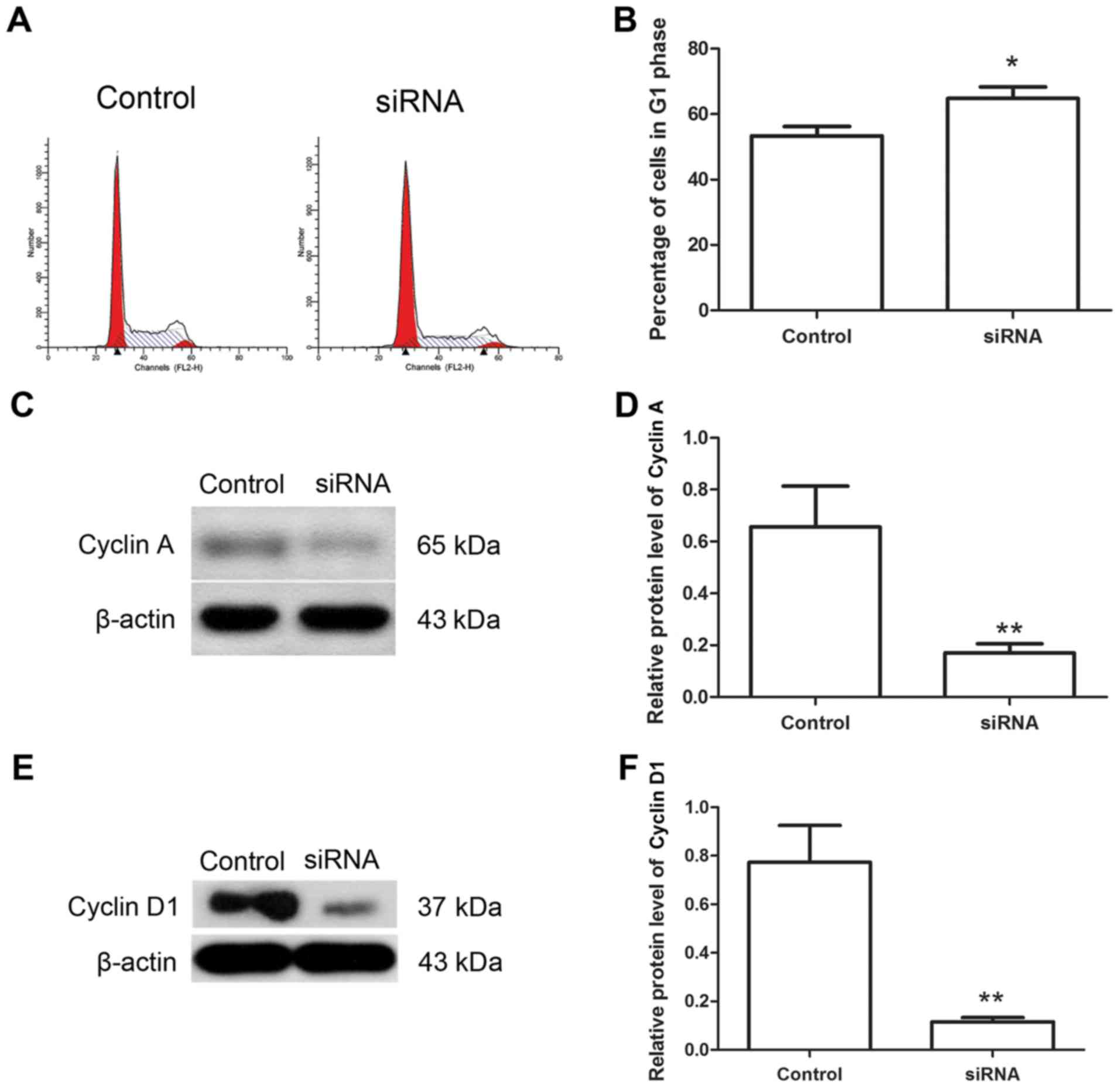
Downregulation of Hiwi inhibits the
cell cycle of cervical cancer cells
The cell cycle has an important function in the
growth of cancer cells, thus the effects of Hiwi siRNA on the cell
cycle of cervical cancer cells were investigated using flow
cytometry. The results of flow cytometry revealed that following
transfection with Hiwi siRNA, the percentage of cells in the G1
phase was increased from 53.37±2.87 to 64.78±3.49% (Fig. 3A and B). The levels of cyclin A and
cyclin D1 proteins, which are important cytokines regulating the
cell cycle progress, were also detected using western blotting.
Western blot analysis revealed that following transfection with
Hiwi siRNA, the relative level of cyclin A protein was decreased
from 0.66±0.16 to 0.17±0.03 (Fig. 3C and
D). The relative level of cyclin D1 protein was also decreased
from 0.77±0.15 to 0.12±0.02 (Fig. 3E and
F). These results demonstrated that Hiwi siRNA may have an
inhibitory effect on the cell cycle of cervical cancer cells.
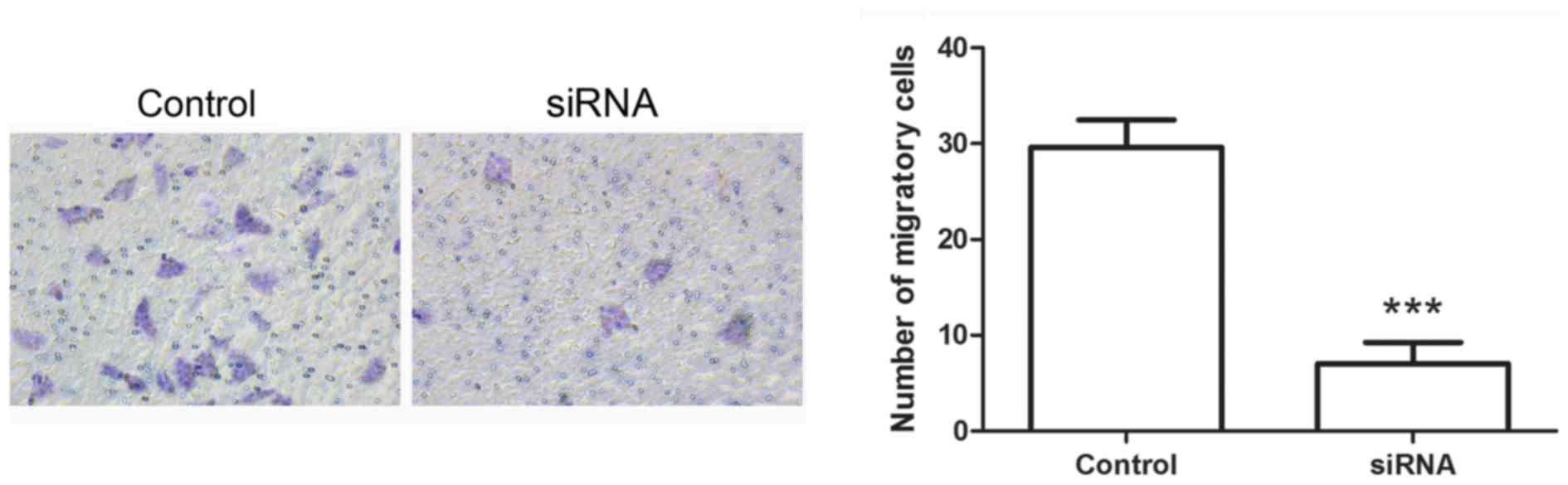
Hiwi siRNA inhibits the
epithelial-mesenchymal transition of cervical cancer cells
Following transfection with Hiwi siRNA, the
migration of cervical cancer cells was evaluated using Transwell
assay. The results of the Transwell assay identified that the
number of migratory cells was decreased significantly in cells
transfected with Hiwi siRNA compared with the control cells
(Fig. 4A and B). The levels of
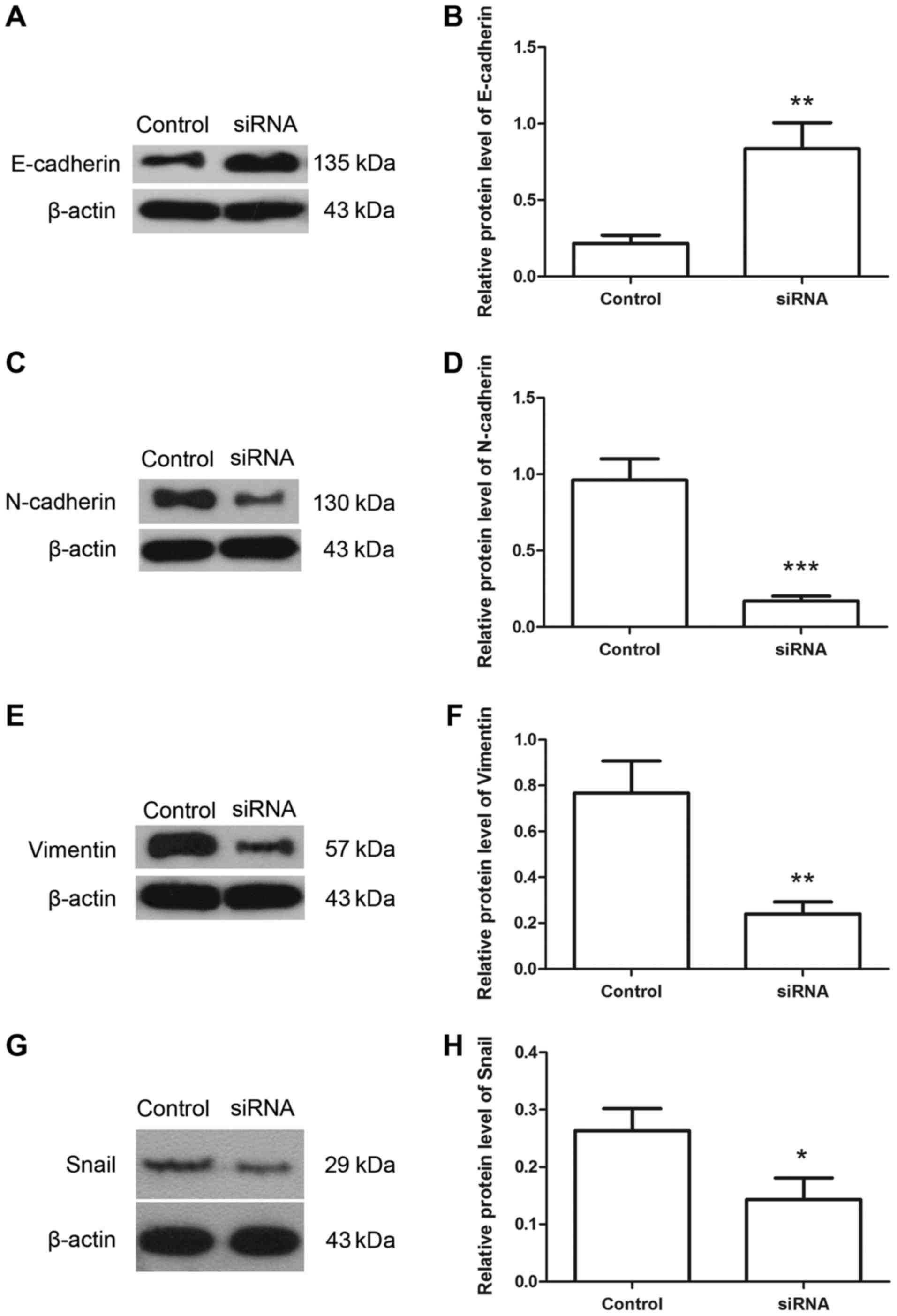
E-cadherin, N-cadherin, vimentin and snail proteins were detected
using western blotting. The results of western blotting revealed
that following transfection with Hiwi siRNA, the relative level of
E-cadherin protein was increased from 0.22±0.05 to 0.84±0.17
(Fig. 5A and B). However the relative
level of N-cadherin protein was decreased from 0.96±0.14 to
0.17±0.03 (Fig. 5C and D). The
relative level of vimentin protein was decreased from 0.77±0.14 to
0.24±0.05 (Fig. 5E and F), and the
relative level of snail protein was decreased from 0.26±0.04 to
0.14±0.04 (Fig. 5G and H). These
results indicate the inhibitory effects of Hiwi siRNA on
epithelial-mesenchymal transition of cervical cancer cells.
Discussion
In the present study, the effects of downregulating
Hiwi in cervical cancer cells were investigated. The results of the
present study demonstrated that suppression of Hiwi was able to
inhibit the proliferation of cervical cancer cells and arrest cell
cycle at the G1 phase. The downregulation of Hiwi was also able to
inhibit the epithelial-mesenchymal transition process. Taken
together, these results suggest that Hiwi may be a potential target
for cervical cancer therapy.
Hiwi is overexpressed in various types of cancer,
including colon (15,16), liver (17), stomach (2) and pancreatic cancer (7), and is associated with the growth,
migration and angiogenesis of cancer cells (11,18). Hiwi
contributes to tumorigenesis, serves as a potential biomarker of
certain types of cancer (17) and is
regarded to be an indicator of poor prognosis (2,6–8,19).
Previous studies have demonstrated that the expression of Hiwi is
associated with the histological grade of cancer, however
associations with age, gender, tumor size or location were not
observed (10,17). Additionally, Hiwi was revealed to be
highly expressed in cervical cancer tissues (12,13), which
indicates that Hiwi may exhibit an association with cervical cancer
progression. The results of the present study demonstrated that the
downregulation of Hiwi was able to inhibit the growth and
epithelial-mesenchymal transition of cervical cancer cells. These
data indicate that Hiwi may serve an oncogenic function in cervical
cancer cells. However, the present study was performed in only one
cervical cancer cell line, which is a limitation of the study.
Additional in vitro and in vivo experiments are
required to further strengthen the findings of the present
study.
Liu et al (12)
demonstrated that Hiwi may be involved in cervical cancer
carcinogenesis and may be a potential therapeutic target for the
treatment of cervical cancer. Liu et al (12) demonstrated that the expression of Hiwi
was associated with the stage of cervical cancer, but exhibited no
association with the other clinical characteristics. Furthermore,
another study (2) also demonstrated
that by blocking endogenous Hiwi expression, it was able to inhibit
the proliferation of gastric cancer cells. Liang et al
(11) demonstrated that the
proliferation of lung cancer cells was inhibited by downregulating
Hiwi.
In the present study, the effects of silencing Hiwi
on the growth of cervical cancer cells were investigated. The
results revealed that the proliferation of cervical cancer cells
was inhibited by transfection with Hiwi siRNA, with decreased
levels of PCNA following transfection. Consistently, Liu et
al (12) demonstrated that Hiwi
promoted tumorigenicity of cervical cancer in vitro and
in vivo. However, when it came to the growth of cervical
cancer cell lines in vitro, Hiwi was revealed to inhibit the
viability of the cervical cancer cell lines, SiHa (12). The results of the present study did
not verify these findings, but the results were consistent with the
results of previous studies (2,11,18). This difference may require further
investigation.
Cell cycle analysis of the cervical cancer cells was
also undertaken in the present study. The results of the present
study demonstrated that cell cycle was arrested upon silencing of
Hiwi. Additionally, the levels of cyclin A and cyclin D1 proteins,
which are regulators of the cell cycle (20), were also decreased following
transfection with Hiwi siRNA. This suggests that the cell cycle of
cervical cancer cells was arrested at the G1 phase by
downregulation of Hiwi. Consistent with the present study, the
results of Wang et al (18)
also identified that the downregulation of Hiwi was able to induce
cell cycle arrest of glioma cells. However, the results of Liu
et al (2) demonstrated that
the cell cycle of gastric cancer cells was arrested by Hiwi
suppression at the G2/M phase. The molecular mechanisms underlying
these differences should be investigated further.
The assays investigating the effects of Hiwi
downregulation on the proliferation and cell cycle demonstrated
that the growth of cervical cancer cells was inhibited by silencing
of Hiwi. In addition to the effects on proliferation and cell
cycle, Hiwi is also able to affect cell apoptosis. The results from
Wang et al (18) demonstrated
that Hiwi suppression induced cell apoptosis in glioma cells. Liang
et al (11) also reported that
cell apoptosis in lung cancer cells was promoted by suppression of
Hiwi.
In the present study, the effects of Hiwi
downregulation on the epithelial-mesenchymal transition process in
cervical cancer cells were also detected. The results of the
present study demonstrated that the downregulation of Hiwi was able
to increase the level of E-cadherin, and decrease the level of
N-cadherin, vimentin and snail, which are hallmarks of
epithelial-mesenchymal transition (EMT) (21–25),
suggesting that suppression of Hiwi was able to inhibit the EMT
process. The results of Wang et al (18) demonstrated that suppression of Hiwi
was also able to inhibit the migration and invasion of glioma cells
with decreased expression of matrix metalloprotein-2 and matrix
metalloprotein-9 which are associatd with degradation of the
extracellular matrix, thus contributing to the migration and
invasion of cells (26,27). Jiang et al (17) demonstrated that the expression of Hiwi
was associated with metastasis to intrahepatic tissue, local lymph
nodes and remote organs. Raeisossadati et al (15) also demonstrated that Hiwi expression
was associated with tumor invasion depth. Hiwi is also associated
with the angiogenesis of cancer (9).
To conclude, in the present study, the effects of
Hiwi on the growth and epithelial-mesenchymal transition process of
cervical cancer cells was investigated. The results of the present
study indicated that the suppression of Hiwi was able to inhibit
the growth of cervical cancer cells and arrest the cell cycle. The
epithelial-mesenchymal transition was also inhibited by the
downregulation of Hiwi. The present study indicates that Hiwi may
act as an oncogene in cervical cancer cells, and that it may be a
target for novel cervical cancer therapy.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PCR
|
polymerase chain reaction
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
TBST
|
Tris-buffered saline with Tween-20
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Arbyn M, Castellsagué X, de Sanjosé S,
Bruni L, Saraiya M, Bray F and Ferlay J: Worldwide burden of
cervical cancer in 2008. Ann Oncol. 22:2675–2686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu X, Sun Y, Guo J, Ma H, Li J, Dong B,
Jin G, Zhang J, Wu J, Meng L and Shou C: Expression of hiwi gene in
human gastric cancer was associated with proliferation of cancer
cells. Int J Cancer. 118:1922–1929. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seto AG, Kingston RE and Lau NC: The
coming of age for Piwi proteins. Mol Cell. 26:603–609. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hutvagner G and Simard MJ: Argonaute
proteins: Key players in RNA silencing. Nat Rev Mol Cell Biol.
9:22–32. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharma AK, Nelson MC, Brandt JE, Wessman
M, Mahmud N, Weller KP and Hoffman R: Human CD34(+) stem cells
express the hiwi gene, a human homologue of the Drosophila gene
piwi. Blood. 97:426–434. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qiao D, Zeeman AM, Deng W, Looijenga LH
and Lin H: Molecular characterization of hiwi, a human member of
the piwi gene family whose overexpression is correlated to
seminomas. Oncogene. 21:3988–3999. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grochola LF, Greither T, Taubert H, Möller
P, Knippschild U, Udelnow A, Henne-Bruns D and Würl P: The stem
cell-associated Hiwi gene in human adenocarcinoma of the pancreas:
Expression and risk of tumour-related death. Br J Cancer.
99:1083–1088. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taubert H, Greither T, Kaushal D, Würl P,
Bache M, Bartel F, Kehlen A, Lautenschläger C, Harris L, Kraemer K,
et al: Expression of the stem cell self-renewal gene Hiwi and risk
of tumour-related death in patients with soft-tissue sarcoma.
Oncogene. 26:1098–1100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li S, Meng L, Zhu C, Wu L, Bai X, Wei J,
Lu Y, Zhou J and Ma D: The universal overexpression of a cancer
testis antigen hiwi is associated with cancer angiogenesis. Oncol
Rep. 23:1063–1068. 2010.PubMed/NCBI
|
|
10
|
He W, Wang Z, Wang Q, Fan Q, Shou C, Wang
J, Giercksky KE, Nesland JM and Suo Z: Expression of HIWI in human
esophageal squamous cell carcinoma is significantly associated with
poorer prognosis. BMC Cancer. 9:4262009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang D, Fang Z, Dong M, Liang C, Xing C,
Zhao J and Yang Y: Effect of RNA interference-related HiWi gene
expression on the proliferation and apoptosis of lung cancer stem
cells. Oncol Lett. 4:146–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu W, Gao Q, Chen K, Xue X, Li M, Chen Q,
Zhu G and Gao Y: Hiwi facilitates chemoresistance as a cancer stem
cell marker in cervical cancer. Oncol Rep. 32:1853–1860. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu WK, Jiang XY and Zhang ZX: Expression
of PSCA, PIWIL1 and TBX2 and its correlation with HPV16 infection
in formalin-fixed, paraffin-embedded cervical squamous cell
carcinoma specimens. Arch Virol. 155:657–663. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raeisossadati R, Abbaszadegan MR, Moghbeli
M, Tavassoli A, Kihara AH and Forghanifard MM: Aberrant expression
of DPPA2 and HIWI genes in colorectal cancer and their impacts on
poor prognosis. Tumour Biol. 35:5299–5305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng Y, Qu LK, Meng L, Liu CY, Dong B,
Xing XF, Wu J and Shou CC: HIWI expression profile in cancer cells
and its prognostic value for patients with colorectal cancer. Chin
Med J (Engl). 124:2144–2149. 2011.PubMed/NCBI
|
|
17
|
Jiang J, Zhang H, Tang Q, Hao B and Shi R:
Expression of HIWI in human hepatocellular carcinoma. Cell Biochem
Biophys. 61:53–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Tong X, Gao H, Yan X, Xu X, Sun S,
Wang Q and Wang J: Silencing HIWI suppresses the growth, invasion
and migration of glioma cells. Int J Oncol. 45:2385–2392. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taubert H, Würl P, Greither T, Kappler M,
Bache M, Bartel F, Kehlen A, Lautenschläger C, Harris LC, Kaushal
D, et al: Stem cell-associated genes are extremely poor prognostic
factors for soft-tissue sarcoma patients. Oncogene. 26:7170–7174.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lim S and Kaldis P: Cdks, cyclins and
CKIs: Roles beyond cell cycle regulation. Development.
140:3079–3093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin Y, Dong C and Zhou BP: Epigenetic
regulation of EMT: The Snail story. Curr Pharm Des. 20:1698–1705.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmalhofer O, Brabletz S and Brabletz T:
E-cadherin, beta-catenin, and ZEB1 in malignant progression of
cancer. Cancer Metastasis Rev. 28:151–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Radice GL: N-cadherin-mediated adhesion
and signaling from development to disease: Lessons from mice. Prog
Mol Biol Transl Sci. 116:263–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Przybyla L, Muncie JM and Weaver VM:
Mechanical control of epithelial-to-mesenchymal transitions in
development and cancer. Annu Rev Cell Dev Biol. 32:527–554. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kokkinos MI, Wafai R, Wong MK, Newgreen
DF, Thompson EW and Waltham M: Vimentin and epithelial-mesenchymal
transition in human breast cancer-observations in vitro and
in vivo. Cells Tissues Organs. 185:191–203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Webb AH, Gao BT, Goldsmith ZK, Irvine AS,
Saleh N, Lee RP, Lendermon JB, Bheemreddy R, Zhang Q, Brennan RC,
et al: Inhibition of MMP-2 and MMP-9 decreases cellular migration,
and angiogenesis in in vitro models of retinoblastoma. BMC
Cancer. 17:4342017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Itoh Y and Nagase H: Matrix
metalloproteinases in cancer. Essays Biochem. 38:21–36. 2002.
View Article : Google Scholar : PubMed/NCBI
|