Introduction
Hepatocellular carcinoma (HCC) is a common tumor
worldwide and its incidence rate is increasing (1). Of the affected patients, 80% have
chronic liver diseases such as hepatitis or cirrhosis; the presence
of these conditions in conjunction with HCC has a major effect on
the prognosis and treatment of this type of cancer (2) Currently, percutaneous ablation
therapies, including percutaneous ethanol injection, microwave
coagulation and radiofrequency ablation, are available and are
important therapeutic modalities for the treatment of HCC (3–8). The
hepatitis B virus (HBV) is responsible for 30% of all cases of
cirrhosis and >50% of HCC cases with an incident rate of 50
million new cases diagnosed annually in endemic countries (9). Irreversible electroporation (IRE) is a
newly developed non-thermal ablation procedure where electrical
pulses are delivered for a few milliseconds to induce nanoscale
defects that increase cell membrane permeability, the procedure
induces apoptosis without harming the extracellular matrix;
therefore, the structural components of the tissues are preserved
(10,11). IRE is a novel, non-thermal form of
tumor ablation that is not affected by heat sink and may result in
less collateral damage based on its mechanism of action (12). IRE relies on short pulses of
high-frequency energy to induce pores in the lipid bilayer of
cells, leading to cell death via apoptosis. In the present study,
hepatic injury in 29 patients following a single IRE session was
retrospectively assessed. The serum transaminases and bilirubin
values were compared between patients who were positive and those
who were negative for hepatitis B and for patients with HCC and
pancreas cancer with liver Metatases, to study the effects of IRE
on liver function and its recovery.
Materials and methods
Ethics
The present study was approved by the Regional
Ethics Committee of Guangzhou Fuda Cancer Hospital (Guangzhou
China). Written informed consent was obtained from all patients,
and the study protocols were in accordance with the tenets of the
Declaration of Helsinki.
Patient selection
Diagnosis of unresectable HCC and liver metastasis
was confirmed by pathological studies. The diagnoses were confirmed
blindly by two pathologists at pathology department affiliated with
Jinan University (Guangzhou, China) and imaging analyses and
measurement of the tumor marker α-fetoprotein. Patients who met the
following criteria were considered to be eligible for the study:
The presence of one or two significant tumors in the liver,
Karnofsky performance status (13)
(KPS) score ≥70, white blood cell count ≥3×109/l,
neutrophil count ≥2×109/l; hemoglobin ≥90 g/l, platelet
count ≥100×109/l, prothrombin time (international
normalized ratio, INR) ≥1.5. Furthermore, the present study
excluded patients who had severe coronary heart disease,
myelosuppression, respiratory disease, acute/chronic infection, or
level 3 hypertension, and had adequate hepatic function [total
bilirubin (T.BIL) <75 µmol/l, direct bilirubin (D.BIL) <39
µmol/l, and a Child-Pugh score (14)
of A or B] and renal function (serum creatinine <130 µm and
serum urea <10 mm). Between July 2015 and December 2016, 29
patients (20 males; age range, 32–75 years; mean age, 55 years; and
9 females; age range, 32–70 years; mean age, 53 years) were
eligible and enrolled from Fuda cancer hospital, Affiliated with
Jinan University (Guangzhou, China).
Irreversible electroporation
procedure
All patients underwent gastric decompression and
endotracheal intubation. General anesthesia was induced intravenous
and inhalation anesthesia by trachea intubation, drugs delivered
via vein and trachea by intravenous infusion, including midazolam
(0.5 mg) and penehyclidine hydrochloride (0.5 mg). For general
anesthesia induction: Etomidate (0.3 mg/kg), benzenesulfonic acid
cisatracurium (0.2 mg/kg) and remifentanil (150 µg) were used, and
for maintenance: Intravenous pumping of diprivan (50–150 mg/h);
remifentanil (320–720 µg/h) and inhalation of sevoflurane (0.6–2%)
benzenesulfonic acid cisatracurium (6 µg/kg). Computed tomography
(CT) was undertaken and the target region was demarcated.
Ultrasound-guided insertion of 2–3 electrodes was performed.
Following anesthesia an additional CT image was taken to confirm
correct placement of the electrodes. IRE was synchronized to
deliver electrical pulses coordinated with cardiac rhythm to
prevent cardiac dysrhythmia. Typically, the distance between
electrodes was 1.5–2 cm. The voltage was set at 1,500–3,000 kV.
Between 70–90 pulses were delivered in 7–9 sets of 10 pulses.
Additional pullbacks were performed if the target region was >2
cm in diameter. The baseline and highest heart rate during IRE were
recorded by electrocardiography. Arrhythmia of any form was
documented. Invasive blood pressure measurement via the femoral
artery was applied for precise systolic blood pressure (SBP)
monitoring. If SBP was >40 mmHg during ablation or >190 mmHg
at any time, electric pulses were suspended for 2–3 min. If no
obvious decrease in SBP was observed after 2–3 min, 2–5 mg of
phentolamine was administered intravenously to lower blood pressure
and prevent hypersensivity.
Assessment of hepatic functional
reserve
Hepatic function was assessed with an automatic
biochemical analyzer (7100; Hitachi Ltd., Tokyo, Japan). The
alanine transaminase (ALT) and aspartate transaminase (AST) levels
were measured with the velocity method using a specialized reagent
kit (AST kit: Aspartate Aminotransferase kit [Aspartate Substrate
Method, cat. no. 1740-2013. ALT kit: Alanine aminotransferase kit.
(Alanine low content method) no. 170781], and the T.BIL and D.BIL
levels were measured using the vanadate method with a commercially
available kit [TBIL: Total Bilirubin kit (Vanadate Oxidation
Method) no. 1737-2013. D.Bil: Direct Bilirubin kit (Vanadate
Oxidation Method) no. 4523-40-201] (BioSino Bio-Technology and
Science Inc., Beijing, China). Blood samples were obtained in the
morning, after overnight fasting. The tests were performed every
1–3 days, until the patients were discharged (on day 20). The
normal ranges for the measured parameters were as follows: ALT,
5–35 U/l; AST, 8–40 U/l; T.BIL, 0–25.5 µmol/l; and D.BIL, 0–13
µmol/l. Values that were above the upper limit of the normal range
were considered to indicate abnormal hepatic function.
Statistical analysis
The revised version of the Response Evaluation
Criteria in Solid Tumors (version 1.1) (15) was used to determine the response of
the hepatic tumors to the treatment. Statistical tests were
performed with commonly used methods, and a nonparametric, one-way
AVONA followed by Bonferroni's multiple comparison post-hoc test
was used for comparisons between days. Test results are expressed
as the mean ± standard error and P<0.05 was considered to
indicate a statistically significant difference. All analyses were
conducted using GraphPad software version 5.0 (GraphPad, Software,
Inc., La Jolla, CA, USA).
Results
Clinical data
Prior to hepatic IRE, detailed data of the patients
were extracted and are presented in Table
I. Of the 29 patients, 22 (75.9%) had HCC, 7 (24.1%) had a
history of hepatitis, 7 (24.1%) had pancreatic cancer with liver
metastases, 10 (34.5%) had undergone initial surgical treatment and
22 (75.86%) had undergone systemic chemotherapy at different
centers.
 | Table I.Detailed data on patients prior to
hepatic irreversible electroporation. |
Table I.
Detailed data on patients prior to
hepatic irreversible electroporation.
| Characteristic | n (%) |
|---|
| Sex |
|
|
Female | 9 (31.03) |
| Male | 20 (69.97) |
| HCC | 22 (75.9) |
| Sex
(male/female) | 15/7 |
| Median
age (years) | 52 |
| AJCC stage (2010)
(37,38) |
|
|
IIIA | 2 (9.09) |
|
IIIB | 1 (4.55) |
|
IIIC | 2 (9.09) |
|
IVA | 17 (77.27) |
| Hepatitis B
positive | 7 (24.1) |
| Child-Pugh stage
(15) |
|
| A | 14 (63.64) |
| B | 8 (36.36) |
| Liver function |
|
| ALT,
U/l | 27±11 |
| AST,
U/l | 44±43 |
| T.BIL,
µmol/l | 18±7 |
| D.BIL,
µmol/l | 7±4 |
| Tumor type |
|
| Single
massive | 17 (77.27) |
|
Multinodular | 5 (22.73) |
| Ascites |
|
|
Yes | 14 (63.64) |
| No | 8 (36.36) |
| Pancreatic cancer
with liver metastasis | 7 (24.1) |
| Sex
(male/female) | 5/2 |
| Median
age (years) | 53 |
| AJCC stage
(24,38) |
|
|
III | 0 |
| IV | 7 |
| Liver function |
|
| ALT,
U/l | 35±11 |
| AST,
U/l | 49±23 |
| T.BIL,
µmol/l | 14±4 |
| D.BIL,
µmol/l | 7±4 |
Perioperative outcomes
All the hepatic lesions were treated with IRE, which
was performed successfully in all patients. Severe complications
(such as rupture or hepatic failure, myoglobinuria and acute renal
failure) did not occur following IRE. There were several mild
adverse effects [7 patients developed fever (38.2–39.8°C), 15
patients had pain for 3–7 days, 1 patient had infection, 2 patients
had abdominal ascites, and 6 patients developed variable degree of
nausea and vomiting]; however the patients recovered without
symptomatic management.
Changes in hepatic functional reserve
after irreversible electroporation
Of the 29 patients, 7 were positive for the
hepatitis B virus, 15 had HCC), and 7 had pancreatic cancer with
liver metastases. A total of 29 IRE procedures were performed. The
transaminase levels were abnormal until the 4th session of
ablation, and the bilirubin level was abnormal until the 2nd
session of ablation (this was the case for all patients). The serum
transaminase levels increased rapidly and reached a peak on day 1
following IRE, after which they gradually decreased. The ALT and
AST levels demonstrated a significant decrease from day 5 and 7,
respectively (P<0.001; Fig. 1A and
B). No significant change in the serum bilirubin level was
observed until 20 days after IRE (Fig. 1C
and D).
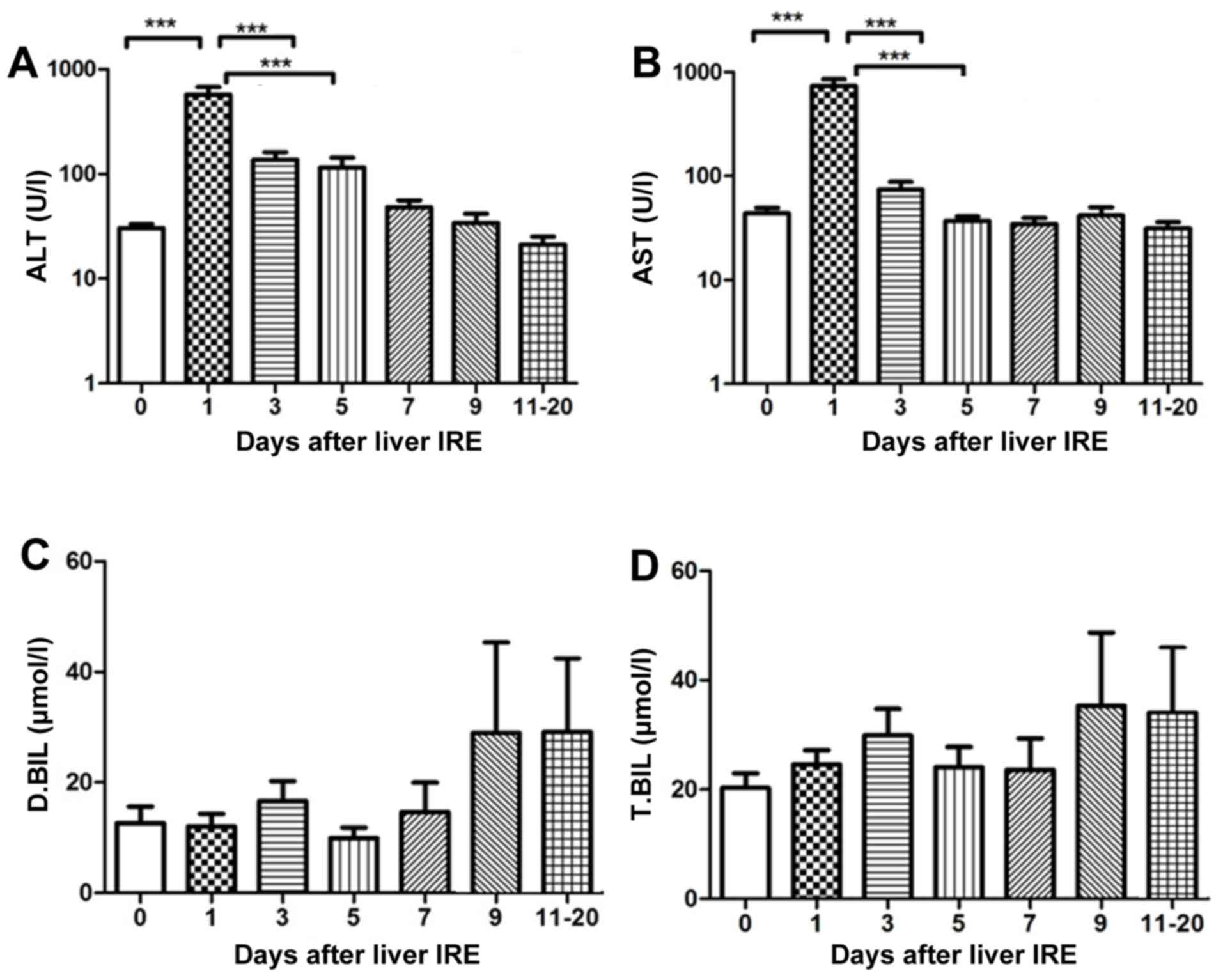 | Figure 1.Variations in hepatic functional
reserve after irreversible electroporation in the 29 patients of
the present study. Bonferroni's multiple comparison was used for
comparison of values between the stated time points. The number of
results varied as follows: 22, 18, 18, 16, 10, 15, 8, 9, 6, 8 and
4: 22 results were obtained on days 0, 1–2, 3–4, 5–6, 7–8, 9–10 and
11–20, respectively. Days on which <3 test results were obtained
were merged with adjacent days. The markers of hepatic functional
reserve were (A) alanine transaminase, (B) aspartate transaminase,
(C) direct bilirubin and (D) total bilirubin. ***P<0.001 vs.
IRE, irreversible electroporation; ALT, alanine transaminase; ALT,
aspartate transaminase; D.BIL, direct bilirubin; T.BIL, total
bilirubin. |
Changes in hepatic functional reserve
in seven patients with hepatitis B
Each of the 7 patients who were positive for
hepatitis B underwent IRE. Prior to the 2nd and 3rd ablation
sessions, the transaminase and bilirubin levels were abnormal (data
not shown). Additionally, 1 day after IRE was performed, the serum
transaminase levels increased rapidly until they reached a peak
(P<0.001), after which they gradually decreased. The ALT and AST
levels showed a significant decrease from day 5 (P<0.01) and day
3, respectively (P<0.001; Fig. 2A and
B). There was no obvious variation in the serum bilirubin level
until 20 days following IRE (Fig. 2C and
D).
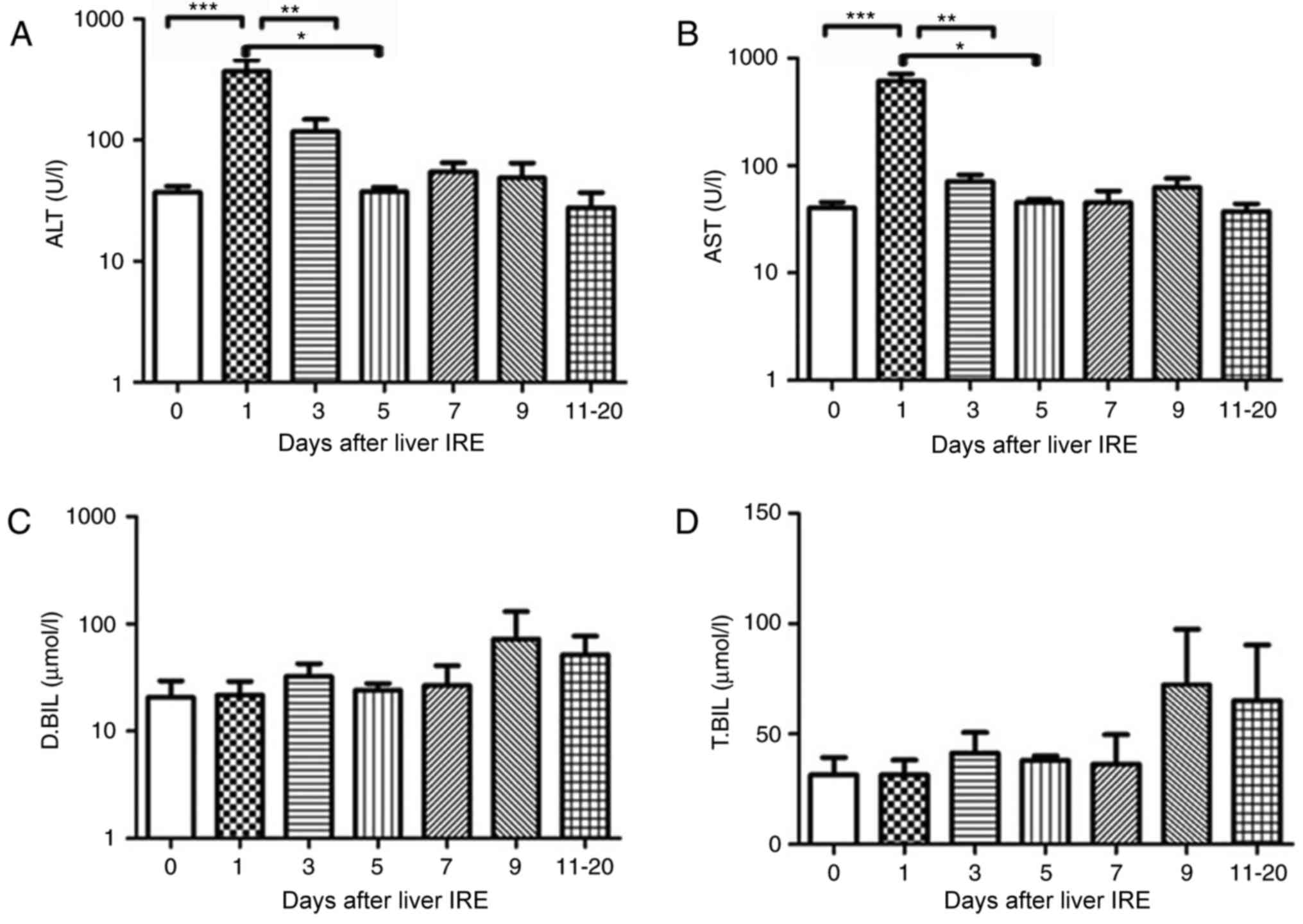 | Figure 2.Variations in hepatic functional
reserve in seven patients with hepatitis B. Bonferroni's multiple
comparison post-hoc test was used to analyze differences in values
between time points. The number of results obtained varied as
follows: 18, 8, 6, 4, 4, 5 and 6: 6 results were obtained on days
0, 1–2, 3–4, 5–6, 7–8, 9–10 and 11–20, respectively. Days on which
<3 test results were obtained were merged with adjacent days.
The following markers of hepatic functional reserve were used: (A)
alanine transaminase, (B) aspartate transaminase, (C) direct
bilirubin and (D) total bilirubin. ***P<0.001, **P<0.01 and
*P<0.05 vs. Day 0, 1, 3 or 5. IRE, irreversible electroporation;
ALT, aspartate transaminase; D.BIL, direct bilirubin; T.BIL, total
bilirubin. |
Changes in hepatic functional reserve
in 22 patients without hepatitis B
All the 22 patients who were negative for hepatitis
B underwent liver IRE. None of the patients had normal transaminase
levels; in addition, the bilirubin level was abnormal before the
8th ablation session. The serum ALT and AST level increased within
2 days after IRE (P<0.01; Fig. 3A and
B) and then decreased gradually, a markedly significant
decrease was noted after 8–10 days. No significant change in the
serum bilirubin level was observed (Fig.
3C and D).
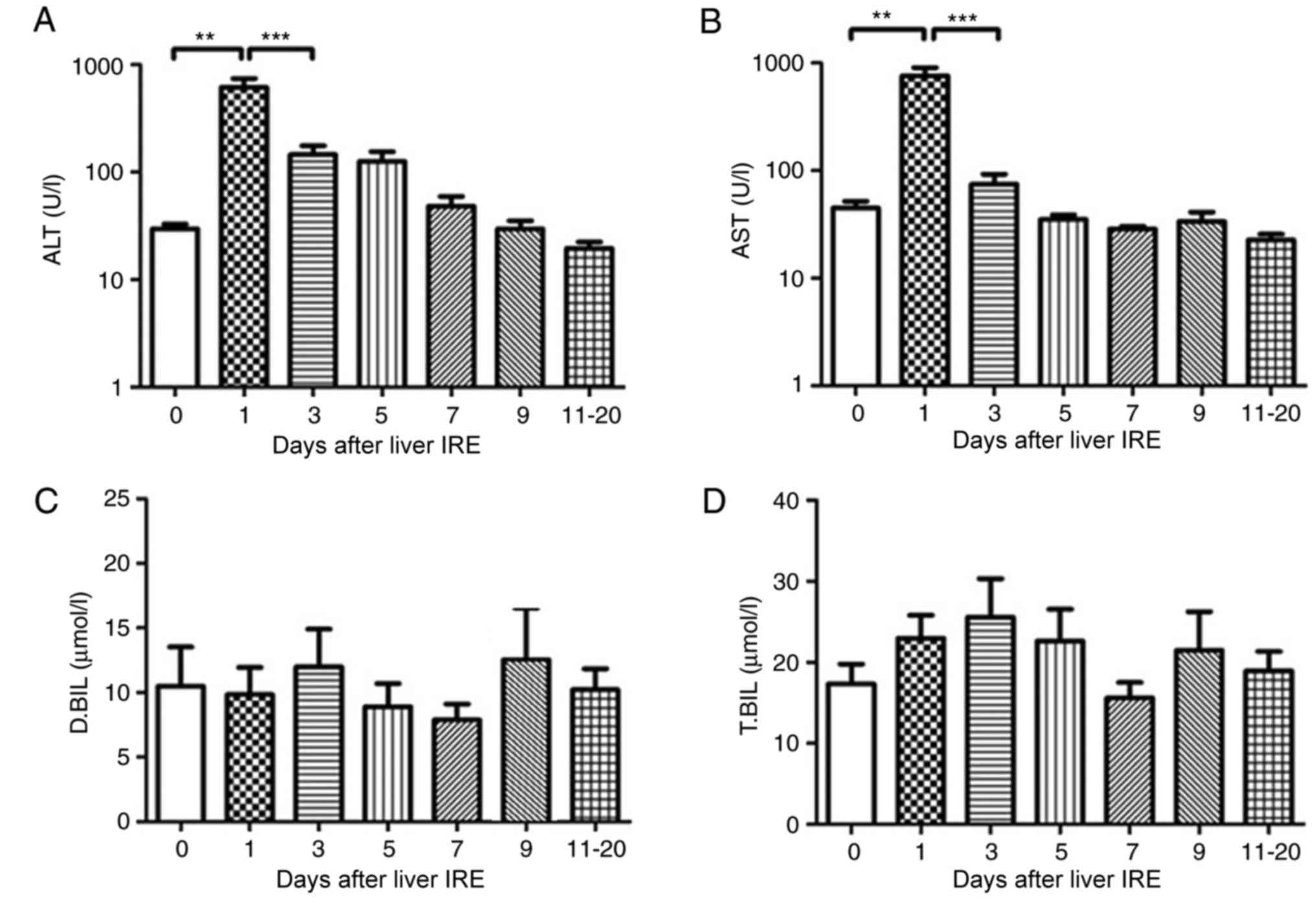 | Figure 3.Variations in hepatic functional
reserve in 22 patients without hepatitis B. Bonferroni's multiple
comparison was used to compare values across time points. The
number of results varied between days: 48, 16, 12, 10, 6, 11, 10
and 12: 18 results were obtained on days 0, 1, 2, 3, 4, 5, 6–7,
8–10 and 11–20, respectively. Days on which <3 test results were
obtained were merged with adjacent days. The hepatic functional
reserve markers were (A) alanine transaminase, (B) aspartate
transaminase, (C) direct bilirubin and (D) total bilirubin.
**P<0.01 and ***P<0.001 vs. Day 0, 1 or 3. IRE, irreversible
electroporation; ALT, aspartate transaminase; D.BIL, direct
bilirubin; T.BIL, total bilirubin. |
Variations in hepatic functional
reserve patients with HCC
In the 15 patients with HCC who underwent IRE, the
serum transaminase levels increased rapidly till they reached a
peak the day after IRE, following which they decreased gradually.
The serum ALT and AST levels showed a significant decrease from day
0, 1, 3 and 5, respectively (P<0.01; Fig. 4A and B). However, no significant
change was observed in the serum bilirubin levels until 20 days
following IRE (Fig. 4C and D).
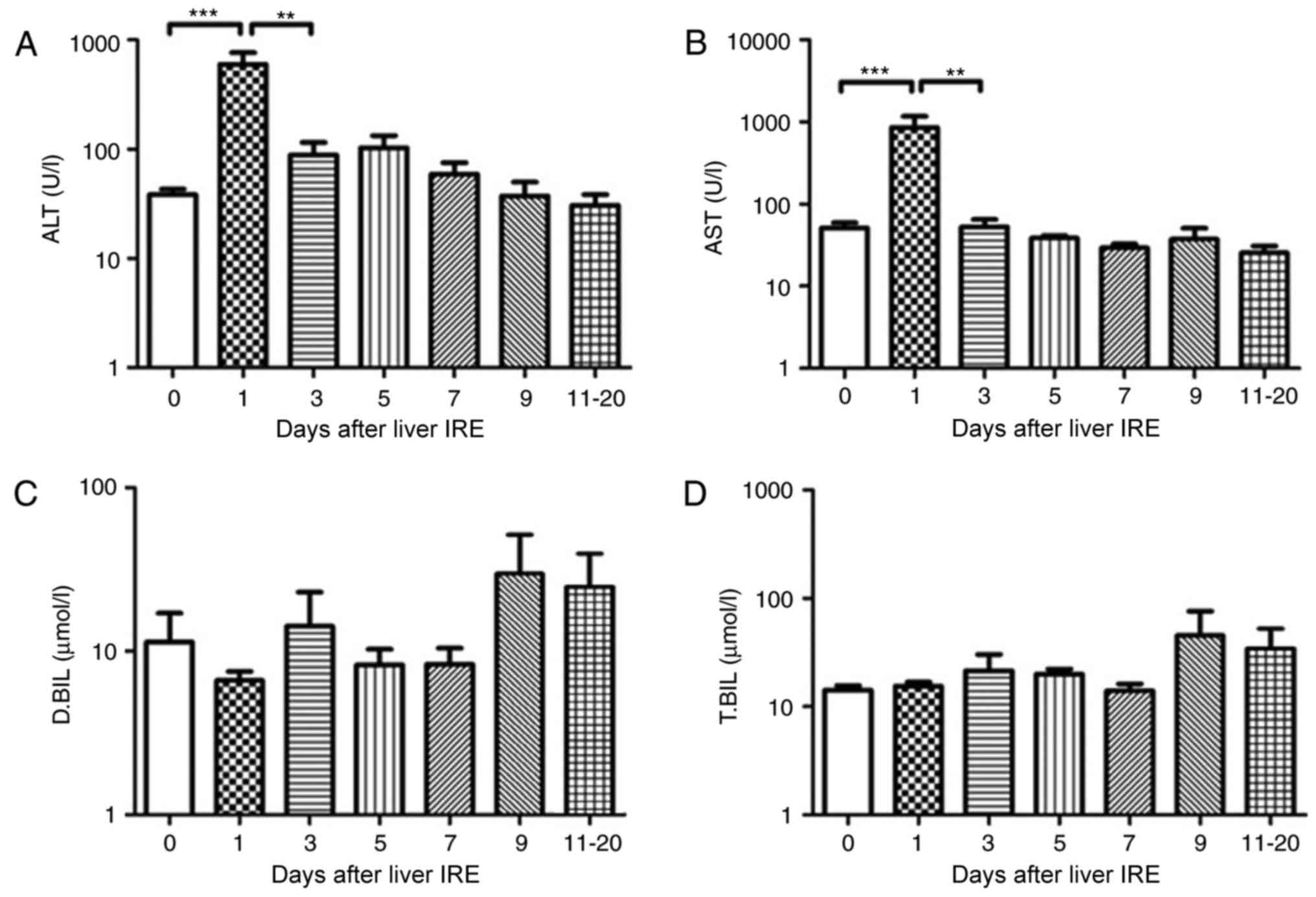 | Figure 4.Variations in hepatic functional
reserve after irreversible electroporation in 15 patients with
hepatocellular carcinoma. Bonferroni's multiple comparison was used
to compare values between different time points. The number of
results obtained varied across time points as follows: 20, 16 16,
16, 15, 8, 9, 6 and 4: 22 results were obtained on Days 0, 1–2,
3–4, 5–6, 7–8, 9–10 and 11–20, respectively. Days on which <3
test results were obtained were merged with adjacent days. The
hepatic functional reserve markers used were (A) alanine
transaminase, (B) aspartate transaminase, (C) direct bilirubin and
(D) total bilirubin. ***P<0.001 and **P<0.01 vs. Day 0, 1 or
3 IRE, irreversible electroporation; ALT, aspartate transaminase;
D.BIL, direct bilirubin; T.BIL, total bilirubin. |
Changes in hepatic functional reserve
in patients with pancreatic cancer with liver metastases
The 7 patients with pancreatic cancer with liver
metastases also underwent liver IRE. The serum ALT and AST value
increased within 2 days following IRE (P<0.01) and then fell
gradually, with a significant decrease observed after day 10
(Fig. 5A and B; P<0.05). The serum
bilirubin levels increased gradually until they reached a peak at
10–12 days after IRE (Fig. 5C and D;
P<0.05), following which it decreased gradually.
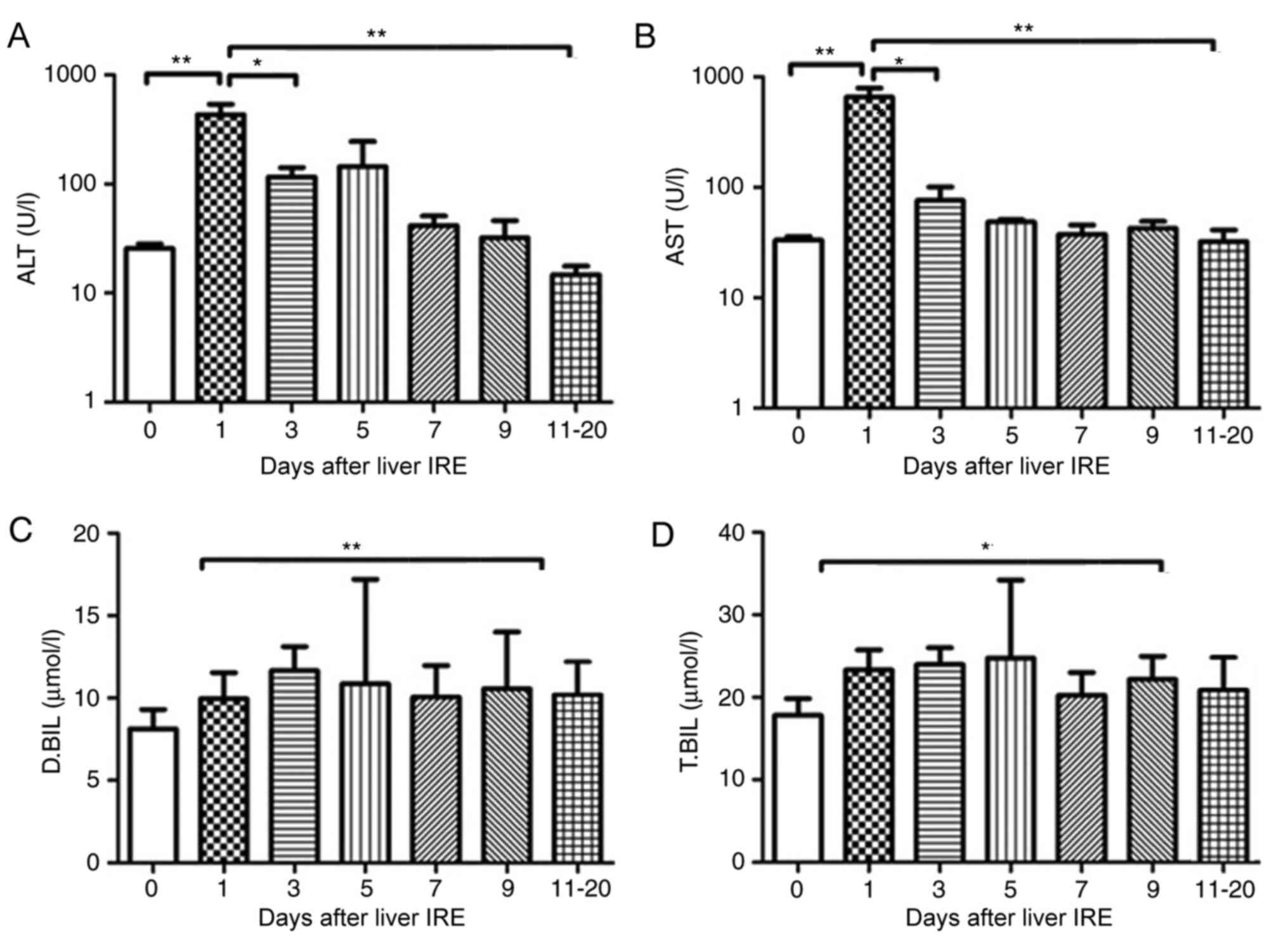 | Figure 5.Variations in hepatic functional
reserve in 7 patients with pancreatic cancer with liver metastases.
Bonferroni's multiple comparison was used to compare values between
different time points. The number of test results varied between
different measurement days as follows: 18, 8, 6, 4, 4, 5 and 6: 6
results were obtained on days 0, 1–2, 3, 4, 5, 6, 7–9 and 10–20,
respectively. Days on which <3 test results were obtained were
merged with adjacent days. The following markers of hepatic
functional reserve were used: (A) alanine transaminase, (B)
aspartate transaminase, (C) direct bilirubin and (D) total
bilirubin. *P<0.05, **P<0.01 vs. Day 0, 1, 3 or 10. IRE,
irreversible electroporation; ALT, aspartate transaminase; D.BIL,
direct bilirubin; T.BIL, total bilirubin. |
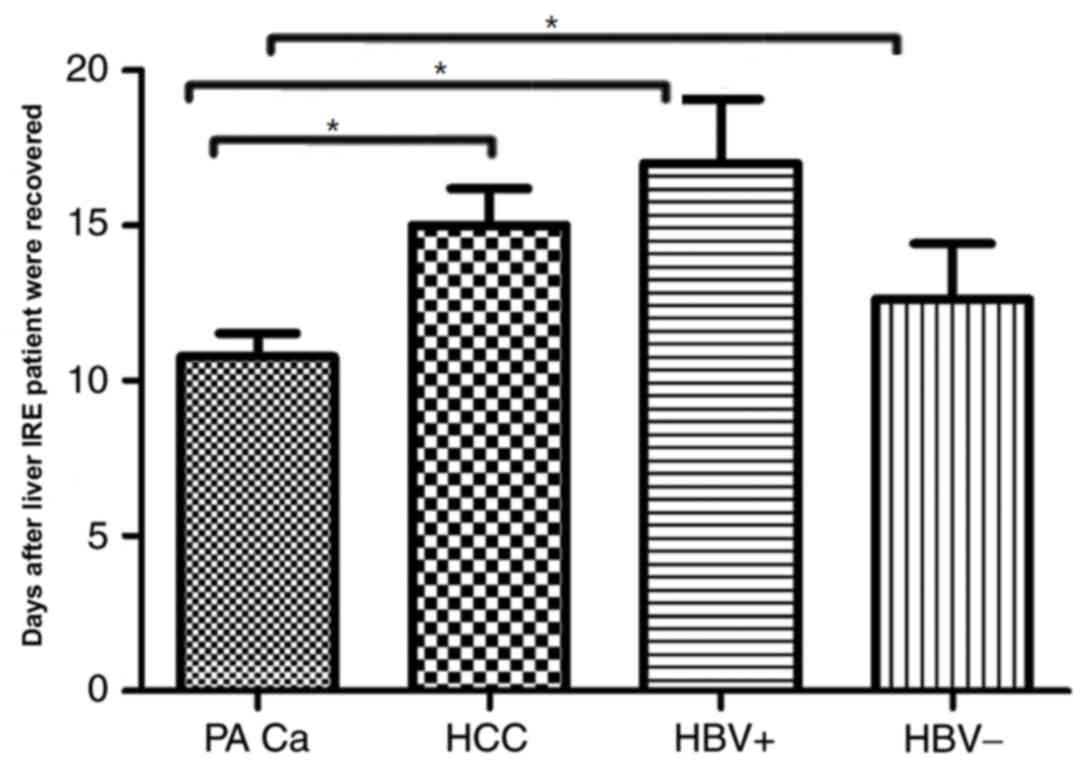
Changes in hepatic functional reserve
in the 4 patient subgroups
Each result of the 4 subgroups of patients with
cancer demonstrated a variation between different measurement days
and recovery with patients positive for the hepatitis B virus
taking the longest duration to recover (recovery was considered to
be when liver functions returned to normal) i.e., 17±3 days,
meanwhile, the patients negative for the hepatitis B viruses and
patients with HCC whose recovery is observed in 15±2.9 and
12.62±4.3 days respectively, by contrast patients with pancreatic
cancer with liver metastases took the shortest time to achieve
recovery 10.78±2 days. The result was statistically significant
between pancreatic cancer with liver metastases and the other
groups (HCC, HBV+ and HBV−; P<0.05;
Fig. 6). Overall patients suffering
from primary hepatic diseases took longer duration to recover when
compared with patients with metastatic disease.
Discussion
IRE is a promising procedure for unresectable
hepatocellular carcinoma (16). The
complications of IRE when used to treat malignant hepatic tumors
(such as fever, local pain, abdominal distension, ascites, nausea
and vomiting) are acceptable and there is evidence to indicate that
the therapy may improve survival. Thus, it is an effective liver
tumor ablative therapy that results in only mild and transient
adverse effects (17–20). IRE is a novel ablation technique
wherein electroporation or electro-permeabilization is used to
generate electric pulses that create nanoscale defects in the cell
membrane and alter its permeability (21). Ablative treatment has been identified
to be safe and effective in patients with primary and secondary
liver cancer, and those who are not suitable for surgery (22). In the present study, one session of
IRE was performed for primary liver tumors and secondary metastases
to avoid serious adverse effects. Follow-up liver function tests
and routine blood tests revealed immediate liver function damage
followed by recovery, consistent with the findings of a study by
Chen et al (23). IRE causes
significant abnormalities in liver function; however, in the
majority of patients these are self-limiting, do not preclude
treatment and resemble the changes observed following
radiofrequency ablation or cryoablation of the liver (24).
IRE for the treatment of liver cancer is associated
with hepatocyte destruction and the subsequent release of
transaminases and bilirubin, which are considered as important
markers of hepatic functional reserve (25). Similarly, a recent study has reported
marked elevations in AST, ALT, T.BIL, and D.BIL (26), which are usually transient and
normalize within a few days. It was identified that 1 day after
ablation, the ALT level was 3.3-fold higher (mean peak level, 241
U/l) and the AST level was 5-fold higher (mean peak level, 427
U/l); however, they had almost reverted to the preoperative levels
within 5 days following the procedure (26). A similar study (22,27)
identified 15 bile duct injuries (narrowing, n=8; dilation, n=7) in
subacute follow-up magnetic resonance images examinations of 3
patients demonstrated transient abnormalities of laboratory values
of bilirubin, 1.6–5.2 mg/dl. Short-term laboratory values were
abnormal in 1 patient (increased alkaline phosphatase of 533 U/l
vs. baseline) as a result of local tumor recurrence.
Recently, Silk et al (28) reported where IRE was used to treat 22
hepatic metastases in 11 patients; the laboratory values were
abnormal after 4 treatment sessions in 3 patients (bilirubin,
2.6–17.6 mg/dl; alkaline phosphatase, 130–1,035 U/l). These
abnormal values were transient after 2 sessions, with 2 patients
exhibiting prolonged elevation of these abnormal values and 1
requiring stent placement. In the former 2 patients, these adverse
effects appeared to be secondary to tumor progression rather than
bile duct injury (28). Conversely,
the present study did not identify a prolonged significant increase
in serum bilirubin. The participants were classified into the
hepatitis B virus-positive and hepatitis B-negative groups, and an
increase in T.BIL by >3 times [74 µmol/l following IRE vs. 23
µmol/l prior to IRE (3.2 times greater)] and a similar increase in
D.BIL [45 µmol/l after IRE vs. 12 µmol/l prior to IRE (3.8 times
greater)] were only observed in the hepatitis B-positive group at
3–5 days following treatment. The increase in AST and ALT levels
observed in the present study was consistent with previous studies;
however the range of increase varied between studies (18,29–31). The
minimal increase that was observed in the present study may be
explained by the time points of the measurements. Overall, the
results indicate the importance of ALT and AST as markers of
post-IRE liver function.
In patients with hepatitis B, serum bilirubin
increased >3 times compared with the preoperative level 7–9 days
following IRE. The reason for this increase remains unclear and
warrants further research. In the present study, all the patients
tolerated the treatment and there were no records of severe
complications following IRE. Compared with radiofrequency ablation,
IRE resulted in faster liver regeneration, which demonstrated the
safety and efficacy of the technique (32). A recent prospective study of IRE
treatment of 44 patients with HCC, colorectal, and other lesions
reported the following local, recurrence-free survival rates: 97.4%
at 3 months, 94.6% at 6 months and 59.5% at 12 months, the
recurrence rates tended to be higher in lesions with diameter >4
cm (32). In a smaller cohort of 28
patients treated with IRE, portal vein thrombosis was reported in
only 1 case in which the tumor was located within 1 cm of a major
portal pedicle (33). Based on the
findings, the authors of the study concluded that IRE was safe even
for the treatment of liver lesions present near major vascular
structures, and their findings are consistent with the reported
theoretical benefits of non-thermal ablation (34–36).
Cannon et al (32) reported
that in a cohort of 44 patients, who underwent IRE for primary and
secondary liver cancer, the incidence of complications was low.
Only 5 patients were observed to have developed adverse events, but
the complications were resolved within 30 days of treatment
(32). Thus, of previously published
studies, many have demonstrated that IRE is a safe and efficient
therapeutic method for the treatment of patients with pancreatic
and colorectal liver metastases (36).
The limitations of the present study are as follows:
No biochemical, hematologic or immunologic factors other than ALT,
AST and serum bilirubin were investigated. Although the long-term
results are as yet unknown, the future of IRE is promising;
however, significant concerns remain regarding its safety over an
extended period of time.
To conclude, IRE for primary and metastatic liver
cancer may be considered safe in terms of its short-term effects on
liver function; however, further study is required to confirm the
findings of the present study.
Acknowledgements
Not applicable.
Funding
The present study was supported by Scientific and
Technological Plan, Guangdong province, China (grant no.
2016A020216018) and International Foundation for Sciences of
Guangzhou Fuda Cancer Hospital (grant no. Y2016-ZD-008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LN, MA and AMQ were responsible for study conception
and design. MA and AMQ contributed to the acquisition and
interpretation of data; JC drafted the work and revised the
manuscript. JC and KX acquired and interpreted the data. All the
authors approved the article for publication and KX agreed to be
accountable for all the aspects of the work including its
integrity.
Ethics approval and consent to
participate
The present study was approved by the Regional
Ethics Committee of Guangzhou Fuda Cancer Hospital, China. Written
informed consent was obtained from all the patients, and the study
protocols were in accordance with the tenets of the Declaration of
Helsinki.
Patient consent for publication
Participants gave informed consent for data
sharing.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ali AM, Lizhi N, Jialiang L, Fei Y, Yuan
W, Jianying Z, Jin Y, Jibing C, Feng M and Kecheng X:
Cryoprotective therapy for hepatocellular carcinoma: Study of 51
patients with a single lesion. Cryobiology. 69:61–67. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alnaggar M, Niu L, Li J, Yao F, Wang Y,
Zeng J, Ye J, Chen J, Mu F and Xu K: Cryoprotective therapy for
huge hepatocellular carcinoma: A study of 14 patients with a single
lesion. Cryobiology. 69:457–461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pearson AS, Izzo F, Fleming RY, Ellis LM,
Delrio P, Roh MS, Granchi J and Curley SA: Intraoperative
radiofrequency ablation or cryoablation for hepatic malignancies.
Am J Surg. 178:592–599. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chung CD, Lau LL, Ko KL, Wong AC, Wong S,
Chan AC, Poon RT, Lo CM and Fan ST: Laparoscopic liver resection
for hepatocellular carcinoma. Asian J Surg. 33:168–172. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ravikumar TS, Kane R, Cady B, Jenkins R,
Clouse M and Steele G Jr: A 5-year study of cryosurgery in the
treatment of liver tumors. Arch Surg. 126:1520–1524. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sarantou T, Bilchik A and Ramming KP:
Complications of hepatic cryosurgery. Semin Surg Oncol. 14:156–162.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seifert JK and Morris DL: World survey on
the complications of hepatic and prostate cryotherapy. World J
Surg. 23:109–114. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shafir M, Shapiro R, Sung M, Warner R,
Sicular A and Klipfel A: Cryoablation of unresectable malignant
liver tumors. Am J Surg. 171:27–31. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schweitzer A, Horn J, Mikolajczyk RT,
Krause G and Ott JJ: Estimations of worldwide prevalence of chronic
hepatitis B virus infection: A systematic review of data published
between 1965 and 2013. Lancet. 386:1546–1555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Al-Sakere B, André F, Bernat C, Connault
E, Opolon P, Davalos RV, Rubinsky B and Mir LM: Tumor ablation with
irreversible electroporation. PloS One. 2:e11352007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Edd JF, Horowitz L, Davalos RV, Mir LM and
Rubinsky B: In vivo results of a new focal tissue ablation
technique: Irreversible electroporation. IEEE Trans Biomed Eng.
53:1409–1415. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Charpentier KP, Wolf F, Noble L, Winn B,
Resnick M and Dupuy DE: Irreversible electroporation of the liver
and liver hilum in swine. HPB (Oxford). 13:168–173. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Péus D, Newcomb N and Hofer S: Appraisal
of the karnofsky performance status and proposal of a simple
algorithmic system for its evaluation. BMC Med Inform Decis Mak.
13:722013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cholongitas E, Papatheodoridis GV, Vangeli
M, Terreni N, Patch D and Burroughs AK: Systematic review: The
model for end-stage liver disease-should it replace Child-Pugh's
classification for assessing prognosis in cirrhosis? Aliment
Pharmacol Ther. 22:1079–1089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu HM, Chen Y, Xu J and Zhou Q:
Drug-induced liver injury in hospitalized patients with notably
elevated alanine aminotransferase. World J Gastroenterol.
18:5972–5978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kos B, Voigt P, Miklavcic D and Moche M:
Careful treatment planning enables safe ablation of liver tumors
adjacent to major blood vessels by percutaneous irreversible
electroporation (IRE). Radiol Oncol. 49:234–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dollinger M, Muller-Wille R, Zeman F,
Haimerl M, Niessen C, Beyer LP, Lang SA, Teufel A, Stroszczynski C
and Wiggermann P: Irreversible electroporation of malignant hepatic
tumors-alterations in venous structures at subacute follow-up and
evolution at mid-term follow-up. PloS One. 10:e01357732015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dollinger M, Zeman F, Niessen C, Lang SA,
Beyer LP, Müller M, Stroszczynski C and Wiggermann P: Bile duct
injury after irreversible electroporation of hepatic malignancies:
Evaluation of MR imaging findings and laboratory values. J Vasc
Interv Radiol. 27:96–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dollinger M, Beyer LP, Haimerl M, Niessen
C, Jung EM, Zeman F, Stroszczynski C and Wiggermann P: Adverse
effects of irreversible electroporation of malignant liver tumors
under CT fluoroscopic guidance: A single-center experience. Diagn
Interv Radiol. 21:471–475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scheffer HJ, Nielsen K, de Jong MC, van
Tilborg AA, Vieveen JM, Bouwman AR, Meijer S, van Kuijk C, van den
Tol PM and Meijerink MR: Irreversible electroporation for
nonthermal tumor ablation in the clinical setting: A systematic
review of safety and efficacy. J Vasc Interv Radiol. 25:997–1011;
quiz 1011. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cohen EI, Field D, Lynskey GE and Kim AY:
Technology of irreversible electroporation and review of its
clinical data on liver cancers. Expert Rev Med Devices. 15:99–106.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ryan MJ, Willatt J, Majdalany BS, Kielar
AZ, Chong S, Ruma JA and Pandya A: Ablation techniques for primary
and metastatic liver tumors. World J Hepatol. 8:191–199. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Ren Z, Zhu T, Zhang X, Peng Z, Xie
H, Zhou L, Yin S, Sun J and Zheng S: Electric ablation with
irreversible electroporation (IRE) in vital hepatic structures and
follow-up investigation. Sci Rep. 5:162332015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Min JH, Waters P, Vincent A, Lee S, Shin Y
H, Lee H K and Kim BJ: Reduced serum uric acid levels in
neuromyelitis optica: Serum uric acid levels are reduced during
relapses in NMO. Acta Neurol Scand. 126:287–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lord J, Willis S, Eatock J, Tappenden P,
Trapero-Bertran M, Miners A, Crossan C, Westby M, Anagnostou A,
Taylor S, et al: Economic modelling of diagnostic and treatment
pathways in National Institute for Health and Care Excellence
clinical guidelines: The modelling algorithm pathways in guidelines
(MAPGuide) project. Health Technol Assess. 17(v-vi): 1–192.
2013.
|
|
26
|
Wichtowski M, Nowaczyk P, Kocur J and
Murawa D: Irreversible electroporation in the treatment of locally
advanced pancreas and liver metastases of colorectal carcinoma.
Contemp Oncol (Pozn). 20:39–44. 2016.PubMed/NCBI
|
|
27
|
Froud T, Venkat SR, Barbery KJ, Gunjan A
and Narayanan G: Liver function tests following irreversible
electroporation of liver tumors: Experience in 174 procedures.
Techn Vasc Interv Radiol. 18:140–146. 2015. View Article : Google Scholar
|
|
28
|
Silk MT, Wimmer T, Lee KS,
Srimathveeravalli G, Brown KT, Kingham PT, Fong Y, Durack JC,
Sofocleous CT and Solomon SB: Percutaneous ablation of peribiliary
tumors with irreversible electroporation. J Vasc Interv Radiol.
25:112–118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kingham TP, Karkar AM, D'Angelica MI,
Allen PJ, Dematteo RP, Getrajdman GI, Sofocleous CT, Solomon SB,
Jarnagin WR and Fong Y: Ablation of perivascular hepatic malignant
tumors with irreversible electroporation. J Am Coll Surg.
215:379–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thomson KR, Cheung W, Ellis SJ, Federman
D, Kavnoudias H, Loader-Oliver D, Roberts S, Evans P, Ball C and
Haydon A: Investigation of the safety of irreversible
electroporation in humans. J Vasc Interv Radiol. 22:611–621. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sugimoto K, Moriyasu F, Kobayashi Y, Saito
K, Takeuchi H, Ogawa S, Ando M, Sano T, Mori T, Furuichi Y and
Nakamura I: Irreversible electroporation for nonthermal tumor
ablation in patients with hepatocellular carcinoma: Initial
clinical experience in Japan. Jpn J Radiol. 33:424–432. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cannon R, Ellis S, Hayes D, Narayanan G
and Martin RC II: Safety and early efficacy of irreversible
electroporation for hepatic tumors in proximity to vital
structures. J Surg Oncol. 107:544–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hosein PJ, Echenique A, Loaiza-Bonilla A,
Froud T, Barbery K, Lima Rocha CM, Yrizarry JM and Narayanan G:
Percutaneous irreversible electroporation for the treatment of
colorectal cancer liver metastases with a proposal for a new
response evaluation system. J Vasc Interv Radiol. 25(1233–1239):
e22014.
|
|
34
|
Distelmaier M, Barabasch A, Heil P,
Kraemer NA, Isfort P, Keil S, Kuhl CK and Bruners P: Midterm safety
and efficacy of irreversible electroporation of malignant liver
tumors located close to major portal or hepatic veins. Radiology.
285:1023–1031. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Q and Teng G: Interventional therapy
of colorectal liver metastasis. Zhonghua Wei Chang Wai Ke Za Zhi.
20:621–624. 2017.(In Chinese). PubMed/NCBI
|
|
36
|
Lyu T, Wang X, Su Z, Shangguan J, Sun C,
Figini M, Wang J, Yaghmai V, Larson AC and Zhang Z: Irreversible
electroporation in primary and metastatic hepatic malignancies: A
review. Medicine (Baltimore). 96:e63862017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|