Introduction
Breast cancer is one of the most common types of
gynecological malignant tumors, which has become one of the major
disease types that seriously affect female's physical and
psychological health (1). The
pathogenesis of breast cancer has long been the focus of clinical
and basic studies. With the continuous development of molecular
biology, more and more genes related to the incidence of breast
cancer have been found. Heat shock factor 1 (HSF1) is a
transcription factor for heat shock proteins (HSPs). It can
specifically bind to heat shock original proteins that are
necessary for the upstream transcription of the HSP gene promoter,
thus activating the transcription of HSPs as well as unknown
proteins (2,3). Current studies have shown that breast
cancer, lung cancer and other malignant tumors, and it is closed
related to clinicopathologic features and clinical prognosis
(4,5).
HSF1 is highly expressed in esophagus cancer, which can be used as
a marker for clinical diagnosis of oral cancer (6). Another study showed that the expression
of HSF1 was significantly increased in esophageal carcinoma tissues
and correlated with the prognosis of patients (7). Studies on cell level have shown that
HSF1 plays an important role in tumorigenesis, apoptosis and
proliferation (8,9). Reducing HSF1 can significantly inhibit
the growth of tumor cells and promote apoptosis of tumor cells
(10). However, what role it plays in
tumors is still unclear. In this study, the effects of HSF1 on
biological functions of breast cancer cells were analyzed, and the
possible molecular mechanism was explored. HSF1 is expected to
provide certain targets for the treatment of breast cancer.
Materials and methods
Experimental materials
Balb/c nude mice, weighing 18–20 g were purchased
from Shanghai SLAC Laboratory Animal Co., Ltd.; Michigan Cancer
Foundation-7 (MCF-7) breast cancer cell lines were purchased from
American Type Culture Collection (ATCC); MCF-7 cells are estrogen
dependent breast cancer cells. Embedding estrogen can promote the
formation of breast cancer solid tumor. Dulbecco's modified Eagle's
medium (DMEM), fetal bovine serum (FBS), trypsin and streptomycin
were purchased from Gibco; Thermo Fisher Scientific, Inc.,
(Waltham, MA, USA);
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was purchased from Sigma-Aldrich; Merck KGaA, (Darmstadt, Germany);
24 mm Transwell® with 3 µm Pore was purchased from
Corning Incorporated, Corning, NY, USA (cat. no. 3452); HSF1
lentiviruses and no-load control viruses were packaged by Shandong
Vigene Biosciences Co., Ltd. (Shandong, China); Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit was
purchased from Beyotime Institute of Biotechnology, (Haimen,
China); 70 kilodalton HSP (HSP70), HSP90, B-cell lymphoma 2
(Bcl-2), Bcl-2-associated X (Bax) and macrophage migration
inhibitory factor (MIF) monoclonal antibodies were purchased from
Santa Cruz Biotechnology, Inc., (Dallas, TX, USA). Other reagents
were analyzed and purified by companies in China. The present study
was approved by the Ethics Committee of Affiliated Zhongshan
Hospital of Dalian University (Dalian, China).
Construction and verification of
stable HSF1-knockdown cell lines
MCF-7 cells cultured to the logarithmic growth phase
were inoculated into 6-well plates. After the cells were adherent
to the wall, 100 µl HSF1 short hairpin ribonucleic acid (shRNA)
viruses and control viruses were added to the medium in two wells.
Cells were further cultured for 24 h, and then the medium was
replaced. At 48 h after infection, the infection of viruses was
observed under a fluorescence microscope (IX70; Olympus, Tokyo,
Japan), and puromycin was added to cells for killing the uninfected
cells. The survived cells were successfully infected cells. Some
cells were collected, and the expression level of HSF1 proteins was
analyzed by western blotting.
Analysis of the effect of HSF1 on the
proliferation of MCF-7 cells by MTT
The cells in the control and experimental groups
were cultured to the logarithmic growth phase. After the digestion
with trypsin, the cell density was adjusted to 1×105/ml,
and 100 µl cells were inoculated in 48-well plates. After the cells
were adherent to the wall, 20 µl (5 mg/ml) MTT was added to cells
at 12, 24, 36 and 48 h after incubation, respectively. After that,
cells were further cultured in an incubator for 3 h. Then the
medium was removed, and cells in the two groups were cultured for 6
h, respectively. After that, the medium was discarded, and each
well was added with 100 µl dimethyl sulfoxide (DMSO) and shaken
evenly. The optical density (OD) value of cells in each well at the
wavelength of 570 nm was detected using a microplate reader
(Bio-Rad, Hercules, CA, USA), the survival rate of cells was
calculated, and the corresponding concentration-survival curve was
drawn.
Analysis of the effect of HSF1 on the
apoptosis of MCF-7 cells by flow cytometry
Cells in the control and experimental groups were
digested with trypsin and centrifuged, followed by the washing with
phosphate-buffered saline (PBS) after centrifugation at 1,000 × g
for 5 min. Then the cells were gently resuspended with PBS and
counted. A total of 5–10×106 were taken to be
centrifuged at 1,000 × g for 5 min, and after the supernatant was
discarded, 195 µl Annexin V-FITC solution was added to gently
resuspend the cells. A total of 5 µl Annexin V-FITC and 10 µl
propyl iodide were added and gently mixed with the solution.
Afterwards, the cells were incubated at room temperature (20–25°C)
for 10–20 min in a dark place and placed in an ice bath, and then
they were analyzed by flow cytometry (FACSCalibur; BDBiosciences,
Detroit, MI, USA). Annexin V-FITC showed green fluorescence, and
propidium iodide (PI) showed red fluorescence (11–13).
Analysis of the effects of HSF1 on the
invasion of MCF-1 cells by Transwell assay
The chamber was placed in culture plates. A total of
300 µl pre-warmed serum-free medium was added to the upper chamber
and placed for standing at room temperature for 15–30 min so as to
rehydrate the matrix gel. Then the remaining culture medium was
extracted. After cells in the control and experimental groups grew
to the logarithmic growth phase, they were digested with trypsin.
At the end of digestion, the culture medium was discarded by
centrifugation at 1,500 × g for 5 min at 4°C, and the cells were
washed twice with PBS and resuspended in the serum-free medium
containing bovine serum albumin (BSA). The cell density was
adjusted to 1×105/well. A total of 100 µl cell
suspension was taken and placed into the Transwell chamber.
Generally, 500 µl medium containing FBS was added into the lower
chamber, and the routine culture was conducted for 48 h. The cells
were then stained with crystal violet for calculating the number of
invasive cells (11–13).
Analysis and verification of
proteomics of cells both in the control and experimental groups by
mass spectrometry
The cells in the control and experimental groups
were cultured to the logarithmic growth phase. After the digestion
with trypsin, the cells were fully lysed with the cell lysate and
centrifuged at 10,500 × g for 20 min. The total protein was
quantitatively analyzed. The content of the total protein in the
two groups was adjusted to 1 mg/ml, and then proteomics analysis
was conducted to explore the difference in the expression of cell
proteins between the two groups. The results of mass spectrometry
were then analyzed by western blotting. Operations: Cell lysates in
the two groups were treated with sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE), after which target
proteins were transferred to the nitrocellulose (NC) membrane and
blocked with 5% skimmed milk powder for 1 h. After that, target
proteins were diluted with rabbit monoclonal HSF1 antibody
(dilution, 1/500; cat. no. ab52757; Abcam, Cambridge, MA, USA) and
incubated overnight at 4°C. After washing with PBS with Tween 20
(PBST) 3 times the next day, HRP-labeled secondary goat anti-rabbit
(HRP) IgG antibody (dilution, 1/2,000; cat. no. ab6721, Abcam) was
used for incubation at room temperature for 1 h. At the end of
that, proteins were washed with PBST 3 times for 5 min, and then
enhanced chemiluminescence (ECL) (Merck Millipore, Billerica, MA,
USA) was used for color development with β-tubulin as an internal
reference.
Effects of HSF1 on tumorigenesis
ability of MCF-7 cells in vivo
The nude mice were embedded with estrogenic
sustained release tablets (0.36 mg/tablet; Innovative Research,
Novi, MI, USA) subcutaneously before inoculation of the tumor.
After 3 days of inoculation, 1×107 MCF-7 control cells
and HSF1 knockout MCF-7 cells were inoculated in the subaxillary
fat pad of nude mice respectively. The tumor was observed every day
and the volume of the tumor was measured. The longest diameter was
1.5 cm. The volume of the tumor was=1/2 length ×
width2.
Statistical analysis
All the data were analyzed using Statistical Product
and Service Solutions (SPSS, Inc., Chicago, IL, USA) v.13.0
statistical software. Measurement data were expressed as mean ±
standard deviation and compared using the t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Construction of stable HSF1-knockdown
cell lines
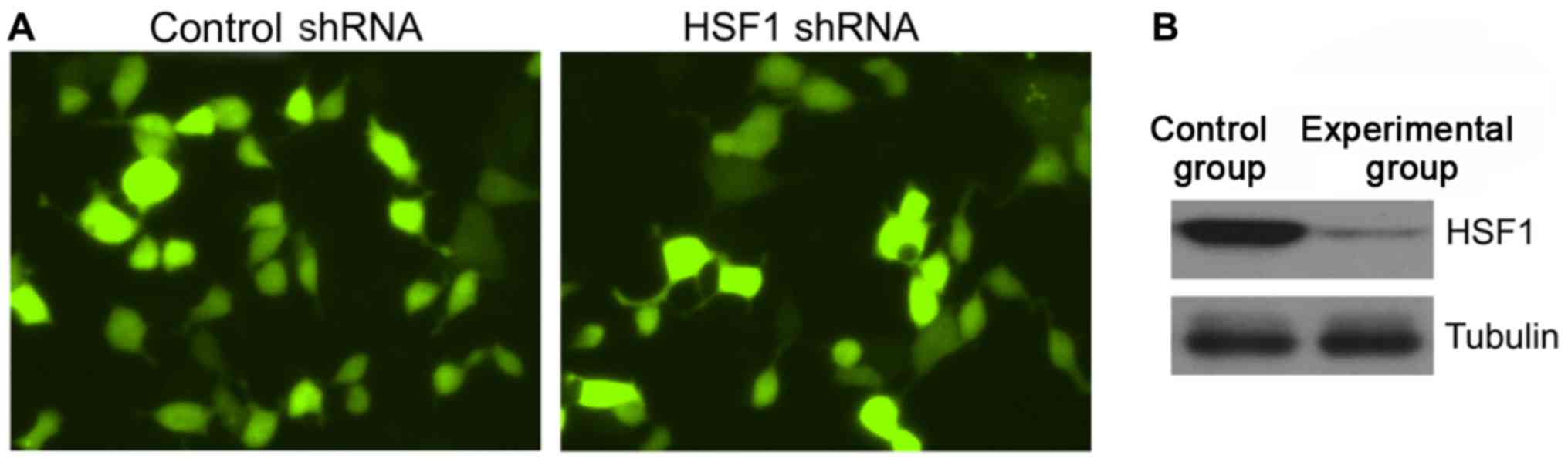
As shown in Fig. 1,
lentivirus-mediated shRNA was used to construct stable
HSF1-knockdown cell lines in this study. After the puromycin
screening for 72 h, green fluorescent proteins (GFPs) could be seen
in approximately 85% of the cells, suggesting that
lentivirus-mediated shRNA is successfully infected in MCF-7 cells.
Western blotting further verified in this study that the
HSF1/Tubulin ratio (0.24±0.10) of the experimental group was
significantly decreased compared with that (2.13±0.25) of the
control group, and the difference was statistically significant
(P<0.05).
Effects of HSF1 on cell proliferation
and tumorigenesis ability
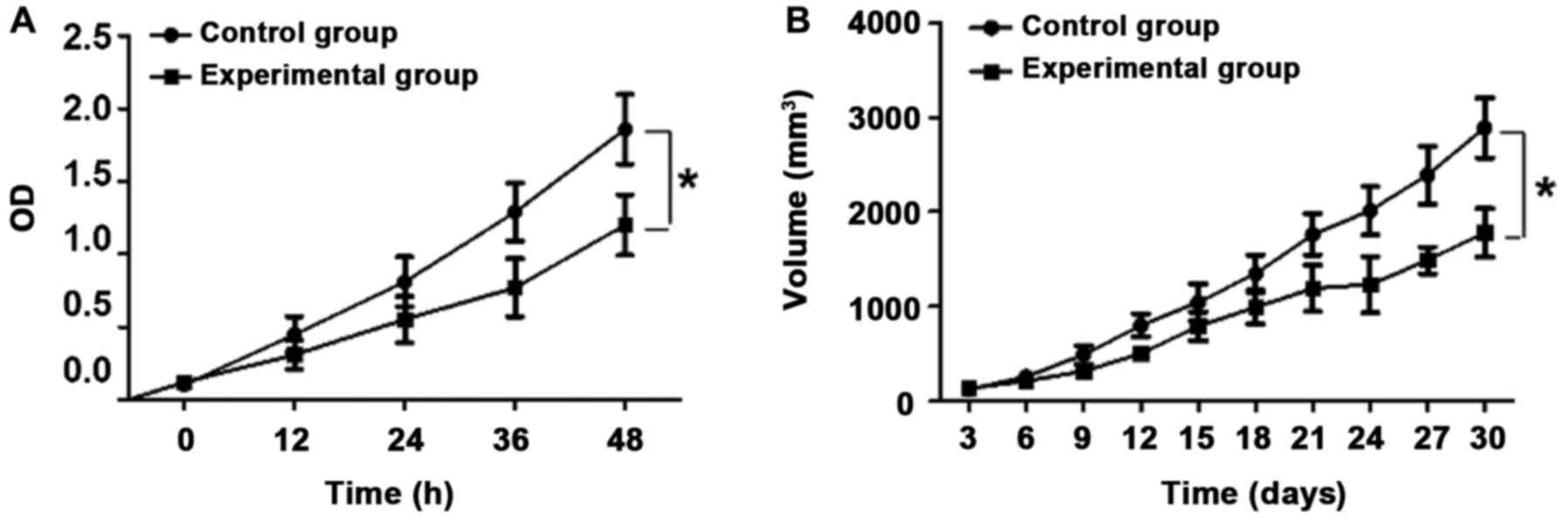
As shown in Fig. 2,
the cell growth rate of the experimental group significantly became
slower compared with that of the control group, and the difference
was statistically significant (P<0.05). In vivo tumor
formation assay confirmed that the tumor formation rate and growth
rate of cells in the control group were significantly increased
compared with those of cells in the experimental group, and the
differences were statistically significant (P<0.05).
The effects of HSF1 on the apoptosis
level of cells
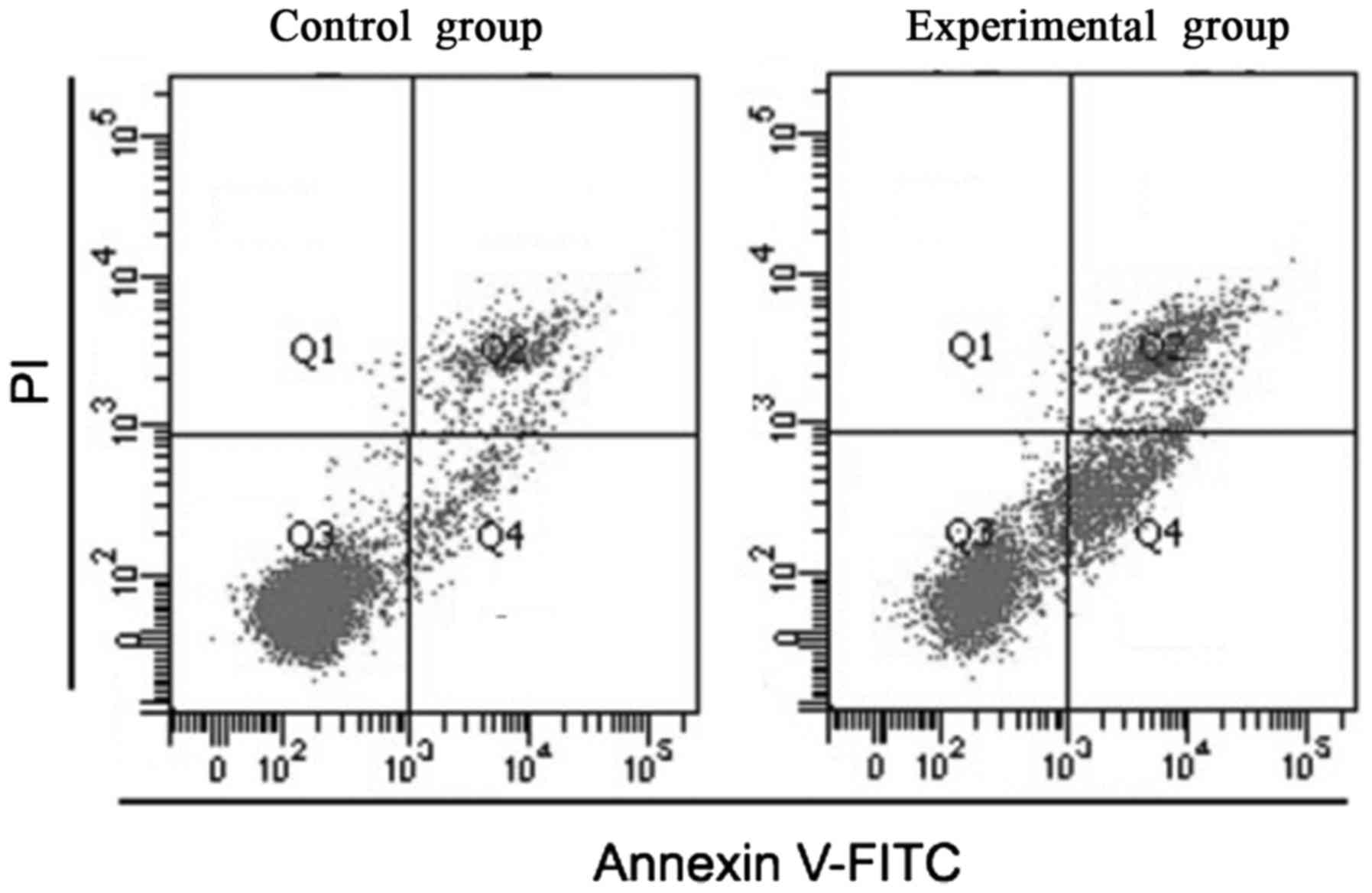
As shown in Fig. 3,
the proportion of apoptotic cells in the control group was
(2.38±0.58), and that in the experimental group was (12.35±2.35).
The difference was statistically significant (P<0.05).
The effects of HSF1 on the invasion
ability of cells
The invasion ability of cells (372.29±53.21)/well in
the experimental group was significantly decreased compared with
that of cells in the control group (143.29 exper)/well, and the
difference was statistically significant (P<0.05).
The effect of HSF1 on the expression
of downstream target proteins
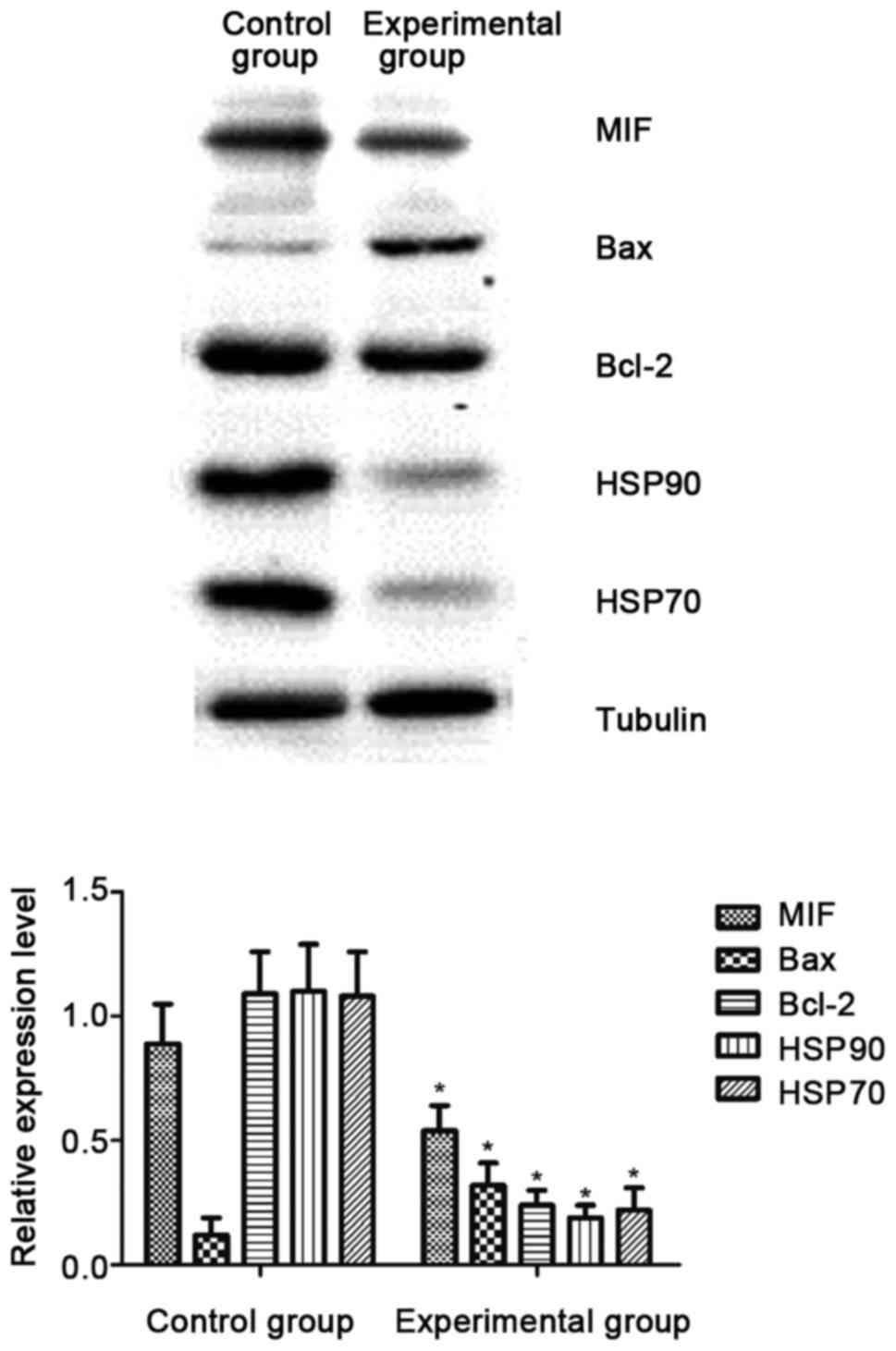
In this experiment, the proteomics analysis was
conducted for cells both in the control and experimental groups.
The results revealed that there were differences in the expression
of HSP70, HSP90, anti-apoptotic Bcl-2 proteins, Bax proteins and
MIFs. Western blotting further verified in this experiment that the
levels of HSP70, HSP90, anti-apoptotic Bcl-2 proteins and MIFs in
the experimental group were significantly downregulated compared
with those in the control group, and the differences were
statistically significant (P<0.05; Fig. 4).
Discussion
HSF1, as a transcription factor, can promote the
transcription and expression of HSP genes. These HPSs, as molecular
chaperones, play an important role in maintaining the toxicity of
proteins in the body and the body's metabolism, growth and
development. However, tumor cells are more dependent on the
function of HSF1 than normal cells, as studies have shown that HSF1
is necessary in regulating tumor cell abnormal signals, inhibiting
mitosis so as to increase genomic aneuploidy, inhibiting tumor cell
apoptosis and promoting tumor cell metastasis and metabolism. HSF1
can only play its role in the form of trimerization, and then
proteins shift into the nucleus and highly phosphorylated, thus
binding to heat shock elements (HSEs) and activating the activation
of downstream genes (14,15). Although it is highly expressed in many
tumor tissues, its effects on the cell biological function and how
it affects the cell biological function are not clear.
The present study showed that the proliferation rate
and tumor growth rate of HSF1-knockdown cells were significantly
decreased, indicating that HSF1 may be involved in the
proliferation of breast cancer cells. From the perspective of
apoptosis, it was found that the apoptosis rate of HSF1-knockdown
MCF-7 breast cancer cells was significantly higher than that of the
control cells, indicating that the overexpression of HSF1
significantly reduces cell apoptosis and promotes cell
proliferation in another aspect. In addition, the invasion ability
of cells reflected the features of the metastasis and infiltration
of tumor cells at a particular level. It was also found that the
invasion ability of HSF1-knockdown MCF-7 cells was significantly
decreased, manifesting that HSF1 is related to the invasion ability
of MCF-7 cells.
Recent studies have shown that the AKT-mTOR
signaling pathway regulates the activation of HSF1 protein. The
activation of HSF1 significantly upregulated the transcription and
translation of the oncogene, resulting in abnormal cell
proliferation or migration and invasion, and the deterioration of
cell properties (16–18). In order to further analyze how HSF1
knockdown affects the above biological behaviors of MCF-7 cells,
proteomics techniques were used to analyze the difference in
protein expression between control cells and HSF1-knockdown cells.
The results revealed that the expression of HSP70, HSP90,
anti-apoptotic Bcl-2 proteins, pro-apoptotic Bax proteins and MIFs
in the two groups were significantly different. HSP70 and HSP90 are
two important HSPs and one of the target proteins of HSF1, which
are currently found to be highly expressed in many tumor tissues
for promoting tumor development and metastasis (19,20).
Anti-apoptotic Bcl-2 proteins and pro-apoptotic Bax proteins are
the key proteins determining the fate of cells, and Bcl-2/Bax ratio
variation determines the cells' tendency to apoptosis or normality
(21). MIF is a unique cytokine that
promotes the development of malignant tumors. A previous study has
shown that the expression level of MIF is inseparable from tumor
progression and tumor angiogenesis (22). This study revealed that the levels of
HSP70, HSP90, anti-apoptotic Bcl-2 proteins and MIFs were
significantly downregulated, and the level of Bax was significantly
increased after HSF1 knockdown, and this result was consistent with
the above-mentioned variation trend of cell biological
behaviors.
In summary, HSF1 may promote cell proliferation,
inhibit cell apoptosis and increase cell invasion so as to promote
disease progression by acting on its downstream signaling
molecules, and it is expected to be used as a target for the
treatment of tumors.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW, DZ and CN conceived and designed the study. MC,
JB, BW and TL collected, analyzed and interpreted the data. XW
drafted and revised the manuscript critically for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Affiliated Zhongshan Hospital of Dalian University
(Dalian, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen YF, Wang SY, Yang YH, Zheng J, Liu T
and Wang L: Targeting HSF1 leads to an antitumor effect in human
epithelial ovarian cancer. Int J Mol Med. 39:1564–1570. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou Z, Li Y, Jia Q, Wang Z, Wang X, Hu J
and Xiao J: Heat shock transcription factor 1 promotes the
proliferation, migration and invasion of osteosarcoma cells. Cell
Prolif. 50:2017. View Article : Google Scholar
|
|
4
|
Wang B, Lee CW, Witt A, Thakkar A and Ince
TA: Heat shock factor 1 induces cancer stem cell phenotype in
breast cancer cell lines. Breast Cancer Res Treat. 153:57–66. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cui J, Tian H and Chen G: Upregulation of
nuclear heat shock factor 1 contributes to tumor angiogenesis and
poor survival in patients with non-small cell lung cancer. Ann
Thorac Surg. 100:465–472. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsukao Y, Yamasaki M, Miyazaki Y, Makino
T, Takahashi T, Kurokawa Y, Miyata H, Nakajima K, Takiguchi S,
Mimori K, et al: Overexpression of heat-shock factor 1 is
associated with a poor prognosis in esophageal squamous cell
carcinoma. Oncol Lett. 13:1819–1825. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SA, Kwon SM, Yoon JH and Ahn SG: The
antitumor effect of PLK1 and HSF1 double knockdown on human oral
carcinoma cells. Int J Oncol. 36:867–872. 2010.PubMed/NCBI
|
|
8
|
Ishiwata J, Kasamatsu A, Sakuma K, Iyoda
M, Yamatoji M, Usukura K, Ishige S, Shimizu T, Yamano Y, Ogawara K,
et al: State of heat shock factor 1 expression as a putative
diagnostic marker for oral squamous cell carcinoma. Int J Oncol.
40:47–52. 2012.PubMed/NCBI
|
|
9
|
Nguyen HA and Kim SA:
2′-Hydroxycinnamaldehyde induces apoptosis through HSF1-mediated
BAG3 expression. Int J Oncol. 50:283–289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cigliano A, Wang C, Pilo MG, Szydlowska M,
Brozzetti S, Latte G, Pes GM, Pascale RM, Seddaiu MA, Vidili G, et
al: Inhibition of HSF1 suppresses the growth of hepatocarcinoma
cell lines in vitro and AKT-driven hepatocarcinogenesis in
mice. Oncotarget. 8:54149–54159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong C, Zhang L, Sun R, Liu J, Yin H, Li
X, Zheng X and Zeng H: Role of thioredoxin reductase 1 in
dysplastic transformation of human breast epithelial cells
triggered by chronic oxidative stress. Sci Rep. 6:368602016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan X, Chen X, Liang H, Deng T, Chen W,
Zhang S, Liu M, Gao X, Liu Y, Zhao C, et al: miR-143 and miR-145
synergistically regulate ERBB3 to suppress cell proliferation and
invasion in breast cancer. Mol Cancer. 13:2202014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tao WY, Wang CY, Sun YH, Su YH, Pang D and
Zhang GQ: MicroRNA-34c suppresses breast cancer migration and
invasion by targeting GIT1. J Cancer. 7:1653–1662. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Antonietti P, Linder B, Hehlgans S,
Mildenberger IC, Burger MC, Fulda S, Steinbach JP, Gessler F, Rödel
F, Mittelbronn M and Kögel D: Interference with the HSF1/HSP70/BAG3
pathway primes glioma cells to matrix detachment and BH3
mimetic-induced apoptosis. Mol Cancer Ther. 16:156–168. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sawai M, Ishikawa Y, Ota A and Sakurai H:
The proto-oncogene JUN is a target of the heat shock transcription
factor HSF1. FEBS J. 280:6672–6680. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou SD, Prince T, Gong J and Calderwood
SK: mTOR is essential for the proteotoxic stress response, HSF1
activation and heat shock protein synthesis. PLoS One.
7:e396792012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nicoletti F, Fagone P, Meroni P, McCubrey
J and Bendtzen K: mTOR as a multifunctional therapeutic target in
HIV infection. Drug Discov Today. 16:715–721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sokolosky ML, Stadelman KM, Chappell WH,
Abrams SL, Martelli AM, Stivala F, Libra M, Nicoletti F, Drobot LB,
Franklin RA, et al: Involvement of Akt-1 and mTOR in sensitivity of
breast cancer to targeted therapy. Oncotarget. 2:538–550. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horibe T, Torisawa A, Kohno M and Kawakami
K: Synergetic cytotoxic activity toward breast cancer cells
enhanced by the combination of Antp-TPR hybrid peptide targeting
Hsp90 and Hsp70-targeted peptide. BMC Cancer. 14:6152014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Proia DA and Kaufmann GF: Targeting
heat-shock protein 90 (HSP90) as a complementary strategy to immune
checkpoint blockade for cancer therapy. Cancer Immunol Res.
3:583–589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teymournejad O, Mobarez AM, Hassan ZM and
Talebi Bezmin Abadi A: Binding of the Helicobacter pylori OipA
causes apoptosis of host cells via modulation of Bax/Bcl-2 levels.
Sci Rep. 7:80362017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nobre CC, de Araújo JM, Fernandes TA,
Cobucci RN, Lanza DC, Andrade VS and Fernandes JV: Macrophage
migration inhibitory factor (MIF): Biological activities and
relation with cancer. Pathol Oncol Res. 23:235–244. 2017.
View Article : Google Scholar : PubMed/NCBI
|