Introduction
Differentiated thyroid carcinoma (DTC) is the most
common endocrine malignancy (1).
Standard treatment is surgery and hormone suppression with
levothyroxine, followed by treatment with radioiodine
I−131 (Thyroid carcinoma. NCCN Clinical Practice
Guidelines in Oncology) (2).
Sometimes DTC is classified as ‘bulky’ at
presentation, because of a bulky primary tumor or voluminous local
recurrence or lymph node involvement. In that case, the patient may
have dyspnea and stridor due to compression of the larynx or
trachea, requiring surgical intervention. Tumor size and
infiltration of neighboring organs such as the larynx can mean that
surgery is not always feasible in such situations.
According to the latest American Thyroid Association
(ATA) 2015 guidelines, bulky tumor recurrence is one of the causes
of radioiodine resistance, both in bulky tumor recurrences and
primary tumor presence, mainly because the unresectability of the
primary thyroid gland prevents the administration of radioactive
iodine therapy (2). When radioiodine
resistance occurs, treatment with external beam radiotherapy (EBRT)
for locoregional control and symptomatic palliation is indicated,
as 50% of patients have compromised airways (3,4). External
beam radiation may be ineffective and highly toxic if the tumor is
very large. Therefore, palliative therapy with doxorubicin may be
indicated to improve outcome in some institutions (5–8). In other
tumors, such as non-small cell lung carcinoma, palliative systemic
treatment with multikinase inhibitors has been indicated in cases
of metastatic disease, concomitantly with external radiation
therapy. The combination of multikinase inhibitors and external
radiation therapy was seen to be feasible and tolerance was
acceptable (9). However, the
experience of concomitant therapy (radiotherapy plus targeted
agent) in locally advanced cancers is scant, although synergistic
effects have already been described in some other tumor types
(9,10).
The use of sorafenib in radioiodine-resistant DTC
has been approved since 2014 (11),
although it was previously administered in compassionate use
programs for tumors with rapid progression or poor response to
treatment (12). It remains unclear
whether the association with external radiotherapy is possible, and
if tolerance, toxicity and efficacy are clinically acceptable.
We present the tolerance, toxicity and clinical
activity results of 5 consecutive patients who were seen in the
emergency room (ER) of 2 different institutions for dyspnea and
stridor due to thyroid cancer. All fulfilled criteria that
precluded surgical treatment, and consequently were prescribed
concomitant treatment with local external radiation therapy and
systemic treatment with doxorubicin or sorafenib. Patients were
treated according to best practice protocols, and all procedures
met the ethical principles of the declaration of Helsinki. The main
toxicities for each patient are shown in Table I.
 | Table I.Main toxicities and adverse events by
grade (G) in each patient. |
Table I.
Main toxicities and adverse events by
grade (G) in each patient.
| Toxicities/adverse
events | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|
| Hematological |
|
|
|
|
|
|
Anemia | G2 | G2 |
|
|
|
|
Leukopenia |
| G2 |
|
|
|
|
Lymphopenia |
|
| G3 |
|
|
| Cutaneous-mucous |
|
|
|
|
|
|
Mucositis | G2 |
| G2-3 |
|
|
|
Folliculitis |
|
| G2-3 |
|
|
|
Esophagitis | G2 |
|
|
|
|
|
Alopecia |
| G1 |
|
|
|
|
Epitheliopathy |
| G1 |
|
|
|
| Skin
Rash |
| G2 |
|
|
|
| Metabolic |
|
|
|
|
|
|
Palmoplantar |
|
|
| G1 | G1 |
|
erythrodysesthesia |
|
| Kidney
Infection | G1 |
|
|
|
|
|
Hypocalcemia | G1 |
|
|
|
|
|
Asthenia |
|
|
| G1 | G1 |
| Neurologic |
|
|
|
|
|
|
Migraine |
|
| G3 |
|
|
|
Odynophagia |
|
|
| G2 |
|
| Gastrointestinal |
|
|
|
|
|
|
Vomits |
|
|
| G1 |
|
|
Dysphonia |
|
|
| G1 |
|
Case report
Patient 1
A 50-year-old man presented in the ER for dyspnea
and stridor caused by a bulky left cervical mass. He was in good
health, smoker of 9 packs/year, and was receiving simvastatin for
dyslipidemia. The patient had been diagnosed with papillary thyroid
carcinoma (PTC) T4N0M0 two years previously. Treatment consisted of
total thyroidectomy, revealing an R1 (microscopic margin affected
by the tumor) at the tracheal surgical margin, followed by 100 mCi
radioiodine. The patient was asymptomatic after treatment. The
disease progressed 1 year before the ER consultation in the form of
lung metastases and cervical lymph node recurrence which was
treated with adenectomy followed by radioiodine 100 mCi. Seven
months later he presented cervical relapse and progression of the
pulmonary metastases.
The cervical mass grew rapidly within a few weeks
precipitating the ER consultation; positron emission
tomography/computed tomography (PET-CT) confirmed cervical,
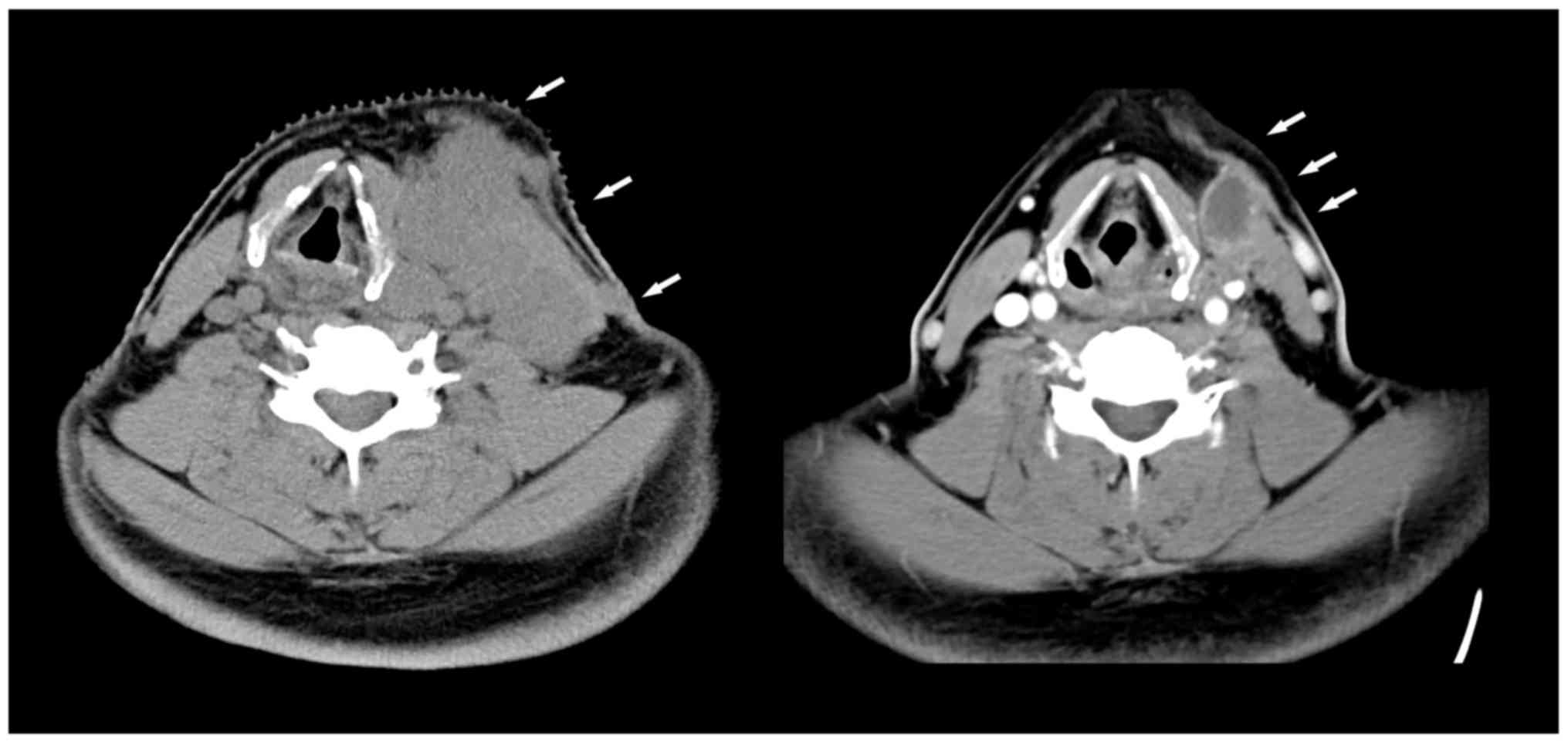
mediastinal and pulmonary progression (Fig. 1, left). Palliative external RT beam
(50 Gy) was administered with a boost of 20 Gy along with
concomitant doxorubicin (20 mg/m2 per week) for 4 weeks
plus sorafenib (400 mg/12 h), achieving partial response (Fig. 1, right) (13). Adverse effects to treatment included
grade 2 anemia, grade 1 renal failure, and hypocalcemia, grade 2
mucositis, and esophagitis that improved within a few weeks of
symptomatic treatment. After 6 months, he presented progression of
pulmonary metastases and was treated in an investigational trial of
lenvatinib. He died 59 months after radiotherapy due to pulmonary
progression.
Patient 2
A 58-year-old man, former smoker with no other
medical history of interest, presented in the ER due to dyspnea and
stridor caused by a fast-growing right cervical mass. The CT scan
showed a 44×47 mm mass at the right thyroid lobe and fine needle
aspiration (FNA) was positive for follicular thyroid carcinoma
(FTC); staging after subtotal thyroidectomy was pT4N3M0. In the
follow-up, PET-CT showed persistent disease with significant
heterogeneous uptake (maximum SUV 37.8), suggesting that the
patient would not benefit from treatment with I-131.
Two months after surgery, he presented rapid growth
of the residual mass, which reached dimensions of 60×94×68 mm with
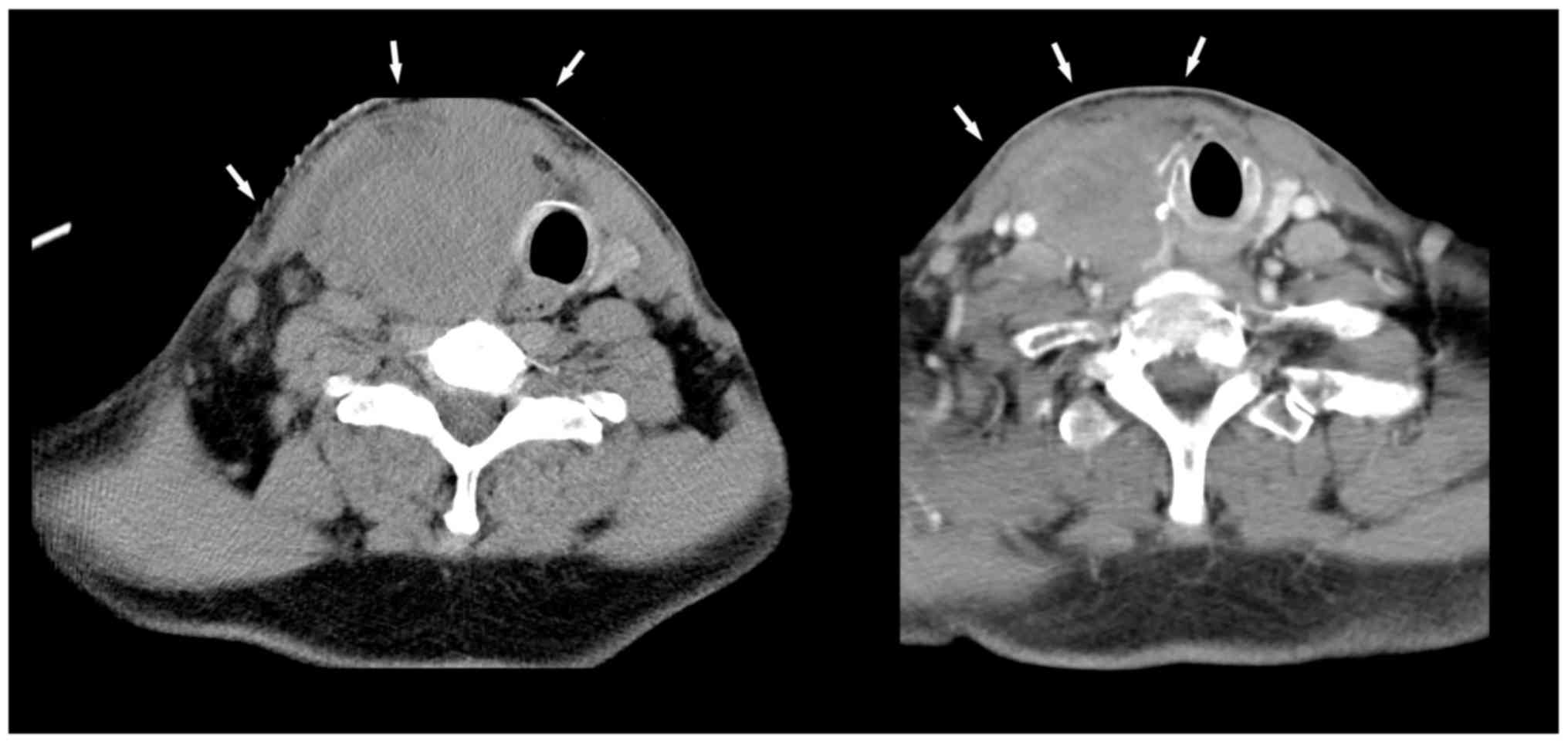
multiple lymph nodes in levels II, III and IV (Fig. 2, left). Because of the rapid
progression and radioiodine resistance, he received external RT at
a total dose (TD) of 50 Gy concomitantly with doxorubicin (20
mg/m2 per week for 6 weeks). Tolerance was good and
adverse effects, including alopecia, grade 1 epithelitis, and grade
2 leukopenia-anemia, were few. Sorafenib treatment was not
authorized as a compassionate use by the patient's health insurance
company (Fig. 2, right).
Six months after treatment, he was evaluated as
partial response, but the reduction in the size of the tumor mass
was insufficient to allow a complete surgical resection. He was
included in a clinical trial with lenvatinib. Despite stabilization
of tumor, the patient died three months later due to
community-acquired pneumonia without leukopenia.
Patient 3
A 37-year-old male presented in the ER because of
dysphonia, dyspnea and stridor due to a left laterocervical mass
that had grown rapidly in 3 months. He was diagnosed with PTC with
unresectable infiltration of the thyroideal cartilage, close to
organs such as the trachea, esophagus, and skin (T4aN1M0). A PET-CT
scan revealed a mass in the left thyroid lobe with intense uptake
(SUV 6.9). A cervical MRI confirmed a bulky left cervical mass with
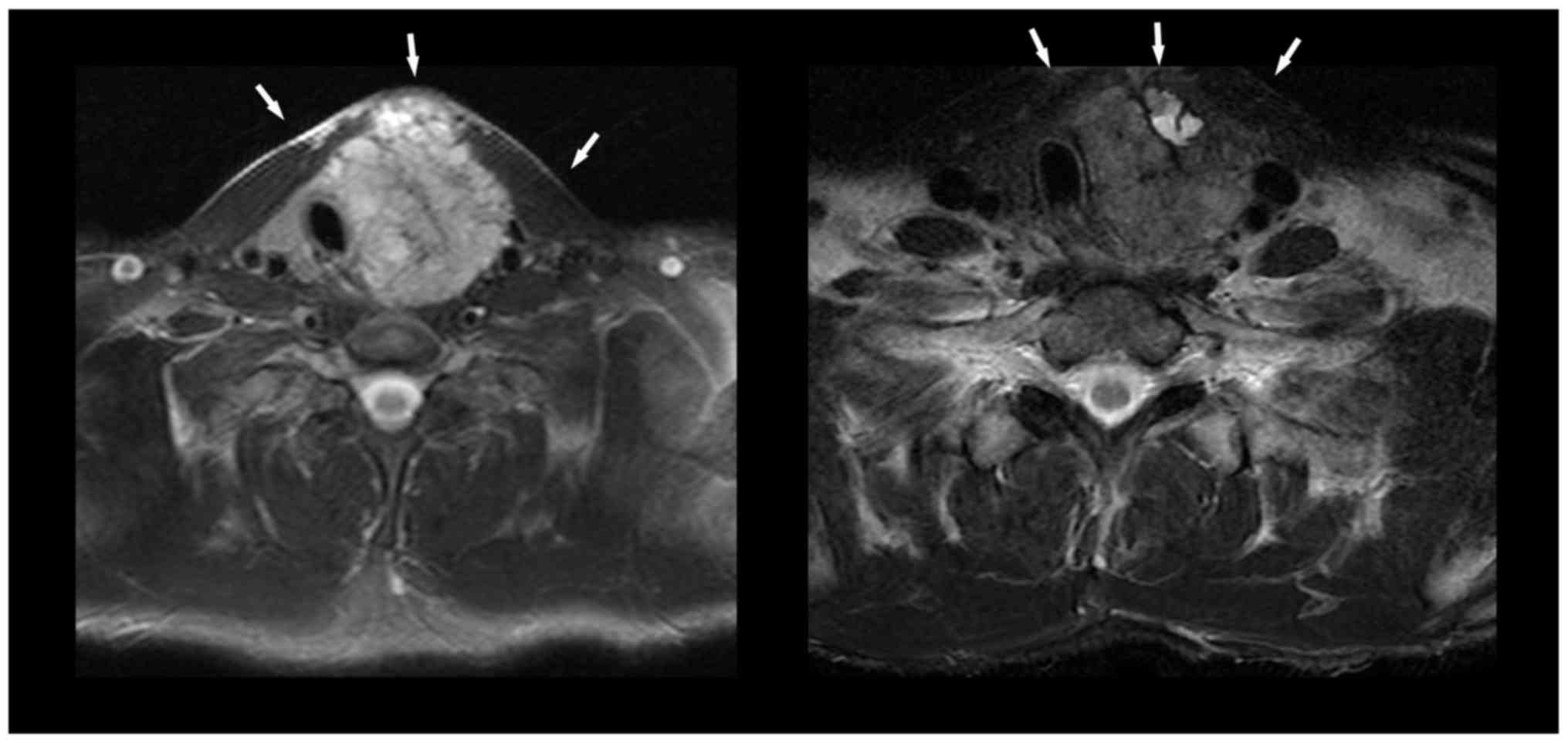
tracheal compression and invasion of the surrounding skin (Fig. 3, left). External radiotherapy (64-Gy)
and weekly doxorubicin (20 mg/m2 IV for 8 weeks) with
sorafenib (400 mg every 12 h p.o.) were administered
concomitantly.
After 13 days receiving sorafenib 800 mg/day, the
patient presented adverse effects with grade 2 erythema and facial
edema requiring dose adjustments (first 600 mg/day for 2 weeks and
subsequently to 400 mg/day), and subsequently grade 3 dermal
toxicity and mucositis/folliculitis and grade 3 headache.
After 8 weeks of doxorubicin and 25 weeks of
sorafenib, response was noted, with reduction in tumor size,
decrease in thyroglobulin (TG) 177 ng/ml (basal serum level 1,090
ng/ml), and radiological partial response (Fig. 3, right). Adverse treatment effects
were grade 3 lymphopenia, grade 2 mucositis/folliculitis and grade
3 headache.
Total thyroidectomy and cervical lymphadenectomy
were subsequently performed. The pathological report revealed a
PTC, 65 mm in size, macrofollicular, with fibroadipose invasion of
extrathyroidal tissue, no vascular-neural invasion, and surgical
margins of 1 mm. Lymphadenectomy was performed showing 2 positive
from 7 resected lymph nodes in area V and 1 positive from 5
resected lymph nodes in area VI.
Nine months after the operation, the patient
remained asymptomatic. His voice was restored and he was pending
radioiodine administration.
Patient 4
A 62-year-old woman with a clinical history of
hypertension and multinodular goiter 3 years previously. In the
last 2 months, the goiter had increased and left cervical
lymphadenopathy appeared. She developed progressive dyspnea and
hemoptysis. Cervicothoracic CT identified thyroid neoplasia with
tracheal infiltration of up to 50% of the lumen in the proximal
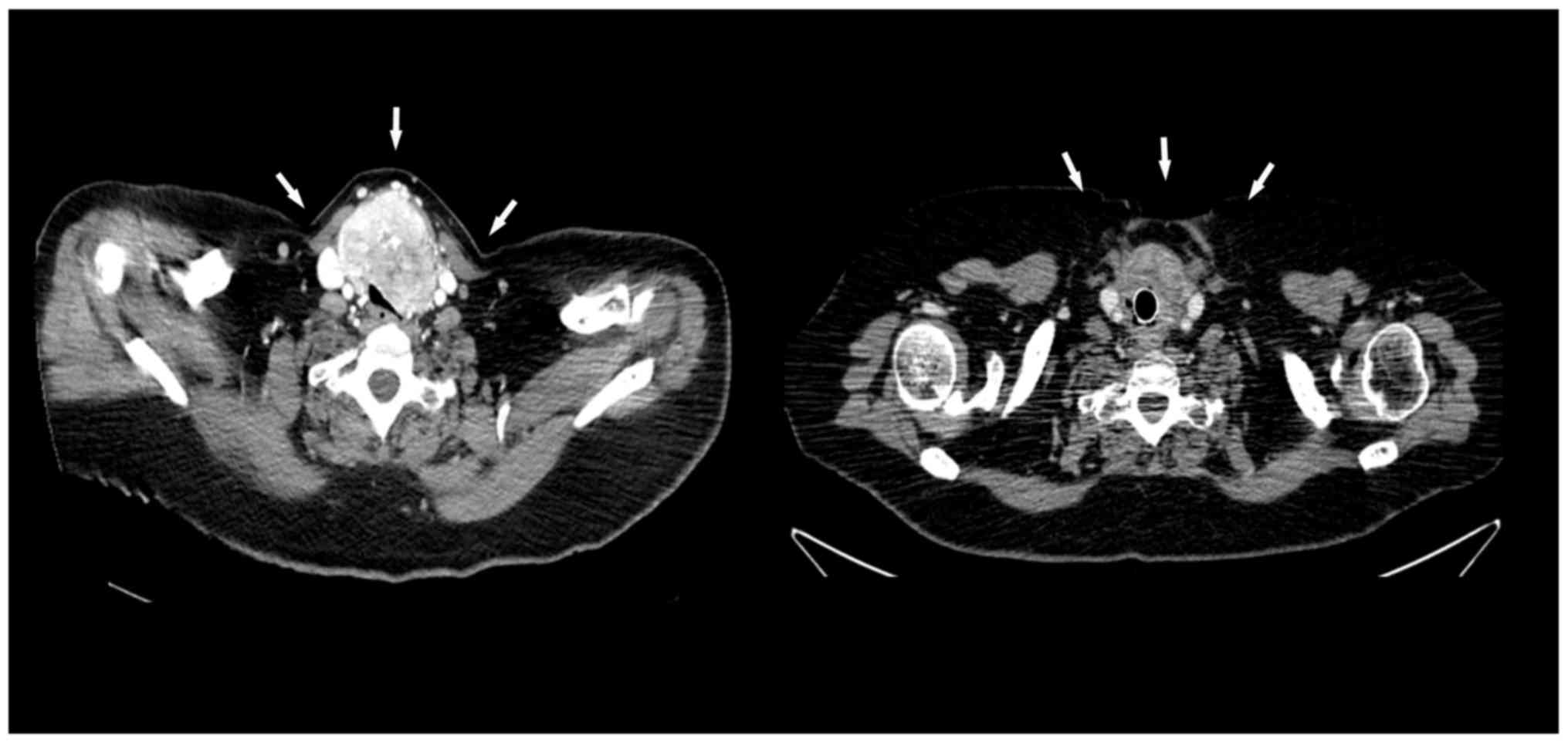
third (Fig. 4, left) and presence of
lung metastases.
FNA reported FTC, and cervicotomy was performed
showing large tumor extension from the cricoid cartilage to the
sternal notch and infiltration of the tracheal rings, so the tumor
was considered unresectable. Tracheal infiltration was confirmed by
bronchoscopy, requiring tracheal prosthesis that was placed and
relocated after rejection; open biopsy of the lesion confirmed
FTC.
Sorafenib 400 mg/12 h p.o. and radiotherapy TD 50 Gy
were administered, showing evidence of clinical and serological
response. TG levels were 230 ng/ml (baseline, 2,153 ng/ml). A
repeat CT showed partial response (Fig.
4, right). Treatment toxicity consisted of grade 1 hand-foot
syndrome, hoarseness, asthenia, and nausea/vomiting and grade 2
sore throat. A significant partial response (>50% of primary
tumor volume) was obtained after sorafenib plus radiotherapy, and
this effect was maintained with sorafenib at standard doses. While
being evaluated for salvage surgery, the patient presented a tumor
infection in the context of significant tumor necrosis, producing
uncontrolled bleeding and finally causing death.
Patient 5
A 61-year-old male, former smoker with history of
acute glomerulonephritis IgA was seen in the outpatient facility
due to asthenia, anorexia and weight loss, and diarrhea. Cervical
ultrasound identified left thyroid nodule and cervical nodes. A
biopsy of the cervical mass was performed and medullary thyroid
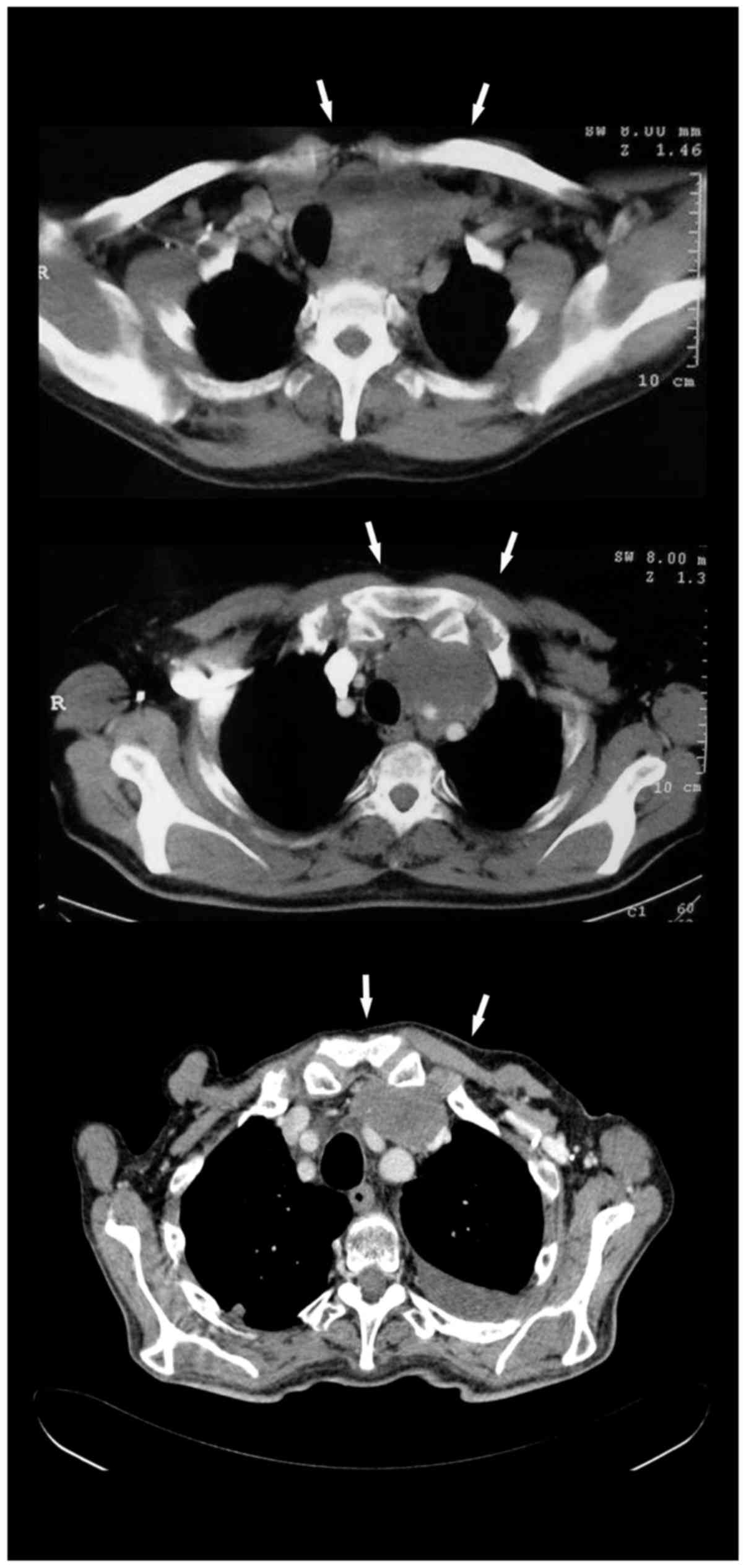
carcinoma was diagnosed. Cervicothoracic CT scan showed a
mediastinal mass (9×7×8 cm) compressing and displacing the thyroid
gland, trachea, esophagus, brachiocephalic vein, internal jugular
vein, associated with enlarged mediastinal lymph nodes (Fig. 5, top); calcitonin was 20,000 IU and
CEA 123 ng/ml. Lanreotide every 28 days was initiated.
After progression 5 months later, the patient
started sorafenib (400 mg/12 h) on a compassionate use basis.
Cervicothoracic CT a month later described a slight increase in
thyroid mass to 9×9×7.5 cm and new mediastinal lymph nodes. Serum
calcitonin level was 48,793 IU.
At that time, external RT, 64.8 Gy TD was
administered concomitantly with oral sorafenib (compassionate use)
400 mg/day p.o. Patient presented serological response with
calcitonin 12,806 IU and CEA 107 ng/ml; reassessment with CT showed
a 16% reduction in the size of the primary and mediastinal lymph
nodes (Fig. 5, middle). Toxicity
consisted of grade 1 palmoplantar erythrodysesthesia and grade 1
asthenia.
Sorafenib at 400 mg/12 h p.o. continued for 8 more
months. At that time, the patient showed serological partial
response and 30–40% reduction of tumor mass according to
mediastinal nodal response on CT (Fig.
5, bottom). This response was maintained for 4 years and 3
months when serological progression, calcitonin 24,000 IU and CEA
83 ng/ml were observed. At that time, he began vandetanib 300
mg/day p.o. After a 3-month period with stable liver disease,
adenopathic, pericardial, pleural, pulmonary and liver progression
was observed causing death a few weeks later due to tumor
progression.
Discussion
This is the first short series of 5 consecutive
patients to appear on the tolerance, toxicity and therapeutic
activity of the association of EBRT with systemic therapy with
doxorubicin or sorafenib in locally advanced thyroid cancer.
The first impression of these clinical results is
that the treatments can be combined with no excessive increase in
toxicity and that the antitumor activity has been acceptably good.
According to the ATA, EBRT in patients with DTC is indicated in
cases of bulky unresectable disease (both primary and local
recurrence) and in other situations, such as high risk of local
recurrence, residual disease post-thyroidectomy, tumor without
I−131 uptake, postoperative serum TG levels ≥1 ng/ml,
and in select patients >45 years old with high likelihood of
microscopic residual disease (2).
Patients with dyspnea and stridor usually present a
serious clinical challenge and they have short life expectancy of a
few weeks. In this situation, EBRT is sometimes the only
therapeutic option (4–6). However to date, the use of EBRT in DTC
has not been supported by randomized studies, probably since
recruitment is limited because of the low incidence of this entity
and the good response to I−131 in most cases (3–5). In the
past, the only palliative systemic drug of choice for treatment of
DCT was doxorubicin (5).
Nevertheless, it is not currently recommended in the clinical
guidelines for use in monotherapy because of its low efficacy and
high toxicity (14). Given the
evidence on the concomitant use of radiation therapy with
doxorubicin in other bulky tumors (such as anaplastic thyroid
carcinoma) (15), we decided to
administer the same treatment regimen with EBRT and weekly
doxorubicin at metronomic doses in patients with DTC and bulky
disease. Treatment was sometimes administered in conjunction with
sorafenib, and the double drug therapy combined with EBRT was not
well tolerated by the patient (Table
I, Patient 3).
In general, tolerance was acceptable, with few side
effects and a low grade of hematological toxicity, occurring mainly
when doxorubicin was associated with radiotherapy. Other authors
also suggest that the combination of sorafenib should not increase
hematological toxicity, and the other side effects described in our
patients were mainly cutaneous (16).
Therapeutic efficacy seems to be acceptable because symptomatic
control of dyspnea and stridor was achieved in all 5 patients.
Furthermore, salvage surgery was performed in one patient,
resulting in local control of the disease. In patient 1, the switch
to lenvatinib after disease progression at 6 months of follow up
may have contributed to his long survival.
These results suggest that the combination of EBRT
with sorafenib has good efficacy with an acceptable toxicity
profile. It is important to take into account the high percentage
of locoregional complications in patients with locally advanced
thyroid cancer when radical combined therapy is applied, as rapid
tumor necrosis can cause fistulae or serious local problems.
These clinical results are limited by the small
sample size of our series. From a mechanistic point of view,
several studies support the notion that the anti-angiogenic effect
of sorafenib might enhance the antitumor activity of radiation
therapy through angiogenesis inhibition, endothelial cell
radiosensitivity, tumor cell apoptosis, or a decrease in the number
of hypoxic cells (improved oxygenation) (17,18).
Nevertheless, these same mechanisms may be responsible for
increased radiation toxicity, mainly causing mucositis and skin
rash (18).
More information must be generated with
retrospective or prospective studies to confirm the good tolerance
and antitumor activity of the combination of external radiotherapy
with new anti-target agents such as sorafenib and doxorubicin in
DTC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JJG and JC collaborated in the conception and design
of the present study. JJG, JC and FR collected and assembled the
data. JJG, KSCM and KH collected and assembled the data. JJG and JC
were involved in data analysis and interpretation. All authors
contributed to writing the manuscript and approved the final
version.
Ethics approval and consent to
participate
Approval was obtained from the Clinical Research
Ethics Committee at Hospital Clínic of Barcelona's (fclinic@clinic.ub.es) Reg.
HCB/2018/00067.
Patient consent for publication
According Spanish law, informed written consent was
obtained from patients still alive for publication of the data in
the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Howlader N, Noone AM, Krapcho M, Miller D,
Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, et
al: SEER Cancer Statistics Review, 1975–2013. National Cancer
Institute; Bethesda, MD: https://seer.cancer.gov/csr/1975_2013/September
12–2016
|
|
2
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American Thyroid Association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American Thyroid Association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwartz DL, Lobo MJ, Ang KK, Morrison WH,
Rosenthal DI, Ahamad A, Evans DB, Clayman G, Sherman SI and Garden
AS: Postoperative external beam radiotherapy for differentiated
thyroid cancer: Outcomes and morbidity with conformal treatment.
Int J Radiat Oncol Biol Phys. 74:1083–1091. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Terezakis SA, Lee KS, Ghossein RA, Rivera
M, Tuttle RM, Wolden SL, Zelefsky MJ, Wong RJ, Patel SG, Pfister
DG, et al: Role of external beam radiotherapy in patients with
advanced or recurrent nonanaplastic thyroid cancer: Memorial
Sloan-kettering cancer center experience. Int J Radiat Oncol Biol
Phys. 73:795–801. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gottlieb JA and Hill CS Jr: Chemotherapy
of thyroid cancer with adriamycin: Experience with 30 patients. N
Engl J Med. 290:193–197. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JH and Leeper RD: Combination
adriamycin and radiation therapy for locally advanced carcinoma of
the thyroid gland. Int J Radiat Oncol Biol Phys. 9:565–567. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim JH and Leeper RD: Treatment of locally
advanced thyroid carcinoma with combination doxorubicin and
radiation therapy. Cancer. 60:2372–2375. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosenthal CJ and Rotman M: Pilot study of
interaction of radiation therapy with doxorubicin by continuous
infusion. NCI Monogr. 285–290. 1988.PubMed/NCBI
|
|
9
|
Welsh JW, Komaki R, Amini A, Munsell MF,
Unger W, Allen PK, Chang JY, Wefel JS, McGovern SL, Garland LL, et
al: Phase II trial of erlotinib plus concurrent whole-brain
radiation therapy for patients with brain metastases from
non-small-cell lung cancer. J Clin Oncol. 31:895–902. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sano D, Matsumoto F, Valdecanas DR, Zhao
M, Molkentine DP, Takahashi Y, Hanna EY, Papadimitrakopoulou V,
Heymach J, Milas L and Myers JN: Vandetanib restores head and neck
squamous cell carcinoma cells' sensitivity to cisplatin and
radiation in vivo and in vitro. Clin Cancer Res.
17:1815–1827. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brose MS, Nutting CM, Jarzab B, Elisei R,
Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R,
Shong YK, et al: Sorafenib in radioactive iodine-refractory,
locally advanced or metastatic differentiated thyroid cancer: A
randomised, double-blind, phase 3 trial. Lancet. 384:319–328. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Capdevila J, Iglesias L, Halperin I,
Segura A, Martínez-Trufero J, Vaz MÁ, Corral J, Obiols G, Grande E,
Grau JJ and Tabernero J: Sorafenib in metastatic thyroid cancer.
Endocr Relat Cancer. 19:209–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pacini F, Castagna MG, Brilli L and
Pentheroudakis G: ESMO Guidelines Working Group: Thyroid cancer:
ESMO clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 23 Suppl 7:vii110–vii119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sherman EJ, Lim SH, Ho AL, Ghossein RA,
Fury MG, Shaha AR, Rivera M, Lin O, Wolden S, Lee NY and Pfister
DG: Concurrent doxorubicin and radiotherapy for anaplastic thyroid
cancer: A critical re-evaluation including uniform pathologic
review. Radiother Oncol. 101:425–430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brierley J and Sherman E: The role of
external beam radiation and targeted therapy in thyroid cancer.
Semin Radiat Oncol. 22:254–262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee CG, Heijn M, de Tomasso E,
Griffon-Etienne G, Ancukiewicz M, Koike C, Park KR, Ferrara N, Jain
RK, Suit HD and Boucher Y: Anti-Vascular endothelial growth factor
treatment augments tumor radiation response under normoxic or
hypoxic conditions. Cancer Res. 60:5565–5570. 2000.PubMed/NCBI
|
|
18
|
Murray L, Longo J, Wan J, Chung C, Wang L,
Dawson L, Milosevic M, Oza A and Brade A: Phase I dose escalation
study of concurrent palliative radiation therapy with sorafenib in
three anatomical cohorts (Thorax, Abdomen, Pelvis): The TAP study.
Radiather Oncol. 124:74–79. 2017. View Article : Google Scholar
|