Introduction
Prostate cancer is a type of malignant tumor
developed in the prostate tissue, a gland serving pivotal roles in
male reproductive system (1). The
common characterization of the majority of prostate cancer cases is
a slow growth without obvious symptoms (2,3). However,
in certain cases, prostate cancer tumor grew relatively faster and
migrated to other parts of the body, including bone tissues, lymph
nodes and soft tissues, if proper treatment is not applied in time
(4,5).
With the development of cancer, clinical manifestations, including
bloody urine, pelvic pain and difficulty urinating, will occur
(6). In Europe, the mortality rate of
the patients diagnosed with prostate cancer is the highest of the
first among all types of cancer (7).
In China, despite the tremendous progress of corresponding
therapies to control the development of prostate cancer, the
incidence rate of this disease is gradually increasing due to
lifestyle changes (8). In addition,
the application of traditional therapies (chemotherapy or radiation
theory) is confronted with challenges, including drug tolerance and
adverse side effects (9).
Astragaloside (As) is the active ingredient of the
Traditional Chinese Medicine Astragalus membranaceus
(10). With the proven biological
activities in different physiological pathways and signaling
transductions, As has been widely used in the treatment of a
variety of human diseases, including breast cancer (10), glioma (11), lung cancer (12), liver fibrosis (13) and allergic inflammation (14). In addition, numerous studies have
demonstrated that As may regulate ER response through multiple
pathways, including the phosphorylated protein kinase R-like ER
kinase (PERK)/iron-regulated transcriptional activator Aft 4
(ATF4)/C/EBP homologous protein (CHOP) pathway and
thioredoxin-interacting protein/NACHT, LRR and PYD
domains-containing protein 3 signaling (15–17). In
view of the biological activities of as and the pathogenesis of
prostate cancer, we hypothesized that As may also have
pharmacological values in the treatment of prostate cancer.
Endoplasmic reticulum (ER) is an organelle
responsible for the folding and trafficking of cellular proteins
(18), which is very sensitive to
intracellular chemical, physiological changes and extracellular
stimuli. The aforementioned negative effects on ER may usually lead
to protein misfolding, which in turn affects a variety of cellular
signaling transductions in order to further induce ER stress
(19). ER stress is involved in
different types of cellular processes and the development of
various types of human disease. Previous studies have demonstrated
that the accumulation of ER mutant proteins is associated with
autophagy, indicating that autophagy may be mediated by ER stress
(20). Recent studies have
demonstrated that ER stress may be induced by quercetin and that
the induced ER stress in prostate cancer PC-3 cells contributed to
the anticancer activity of quercetin (21), indicating that ER stress may
potentially be a target for the treatment of prostate cancer. Wang
et al (17) have reported that
intravenous as attenuates proteinuria in streptozotocin-induced
diabetic nephropathy via the inhibition of ER stress (17). Therefore, we hypothesized that As may
protect prostate cancer DU-145 cells through the ER stress pathway.
The protective effect of As on the apoptosis and proliferation of
DU-145 cells was investigated. In addition, the changes in the
expression of ER stress-associated factors were also detected
through reverse transcription-quantitative polymerase chain
reaction and western blot analysis.
Materials and methods
Cell grouping and treatments
Human prostate cancer DU-145 cells were provided by
the Shanghai Institute of Cellular Biology of Chinese Academy of
Sciences (Shanghai, China) and were cultured in modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
containing 10% fetal bovine serum, 2 mM L-glutamine and 1%
penicillin-streptomycin at 37°C in a 5% CO2 incubator.
Logarithmic-growth-phase DU-145 cells were collected to be applied
in the experimental analysis. The cells were divided into 5 groups:
Control group treated with 10 µl dimethyl sulfoxide (DMSO) into the
medium for follow-up incubation; low-dose As (L-As) group,
moderate-dose As (M-As) group and high-dose As (H-As) group treated
with 10 µl As-DMSO solution at a concentration of 20, 50 and 100
nmol/l, respectively; and tauroursodeoxycholic acid (TUDCA) group
treated with 10 µl TUDCA-DMSO solution (EMD Millipore, Billerica,
MA, USA).
Flow cytometry analysis
Flow cytometry was used to analyze the apoptotic
percentage of DU-145 cells. In brief, 1×106 DU-145 cells
were harvested, centrifuged at 60 × g, 4°C for 5 min and
resuspended in phosphate-buffered saline. Subsequently, 5 µl
Annexin V (1 µg/ml; Beckman Coulter, Inc., Brea, CA, USA) was added
into cells and incubated at room temperature for 15 min.
Subsequently, propidium iodide (1 µg/ml) was added and incubated
for 5 min at room temperature. All staining incubation steps were
performed in the dark. Next, cell apoptosis was detected using a
FC500 series flow cytometer and MXP software (version 2.2), and the
data were analyzed by CXP_9.3 version software (both from Beckman
Coulter, Inc. Brea, CA, USA).
Cell proliferation assay
Cells were seeded onto 96-well plates at a density
of 1.0×104 cells/well. The culture medium was replaced with DMSO
medium solution, H-As-DMSO medium solution (100 nmol/l) and
TUDCA-DMSO medium solution for 24 h. Subsequently, 10 µl Cell
Counting kit-8 solution (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was added at 12, 24, 48 and 72 h, respectively.
DU-145 cells were incubated at 37°C in 5% CO2 for
another 4 h. The optical density value of each well was measured at
450 nm using a microplate reader (HBS-1096A; http://www.detielab.com/).
RT-qPCR
Cells were collected from each group and the total
RNA was extracted using TRIzol kit (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols. The
purity and integrity of total RNA were checked and reverse
transcription was performed using an Easy Script First-Strand cDNA
Synthesis Super Mix kit (TransGen Biotech, Co., Ltd., Beijing,
China). The conditions of cDNA synthesis were as follows: 25°C, 10
min followed by 42°C, 30 min according to the manufacturer's
protocol. A SYBR-Green master kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) and 1 µl cDNA were used to prepare the PCR
reaction system. All mRNA quantification data were normalized to
β-actin using the 2-∆∆Cq method (22).
The sequences of the primers used were as follows:
BiP forward, 5′-GGTATTGAAACTGTGGGAGGTG-3′ and reverse,
5′-GATTGTCTTTTGTCAGGGGTCT-3′; CHOP forward,
5′-CTGAGTCATTGCCTTTCTCCTT-3′ and reverse,
5′-CCACTTTCCTTTCATTCTCCTG-3′; caspase-12 forward,
5′-GAAGGAATCTGTGGGGTGAA-3′ and reverse,
5′-TCCCTTTGCTTGTGGGATACC-3′; and β-actin forward,
5′-TGGCACCCAGCACAATGAA-3′ and reverse,
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′. The reaction conditions were as
follows: 94°C for 5 min, followed by 35, 37 or 40 cycles of 94°C
for 30 sec, 53°C for 30 sec and 72°C for 30 sec. The reaction was
performed using a RT-qPCR instrument (Biometra GmbH, Göttingen,
Germany).
Western blot analysis
The total protein was extracted using Protein
Extraction Buffer (Bioo Scientific Corporation, Austin, TX, USA)
and the concentration was measured using bicinchoninic acid protein
quantification kit (Pierce; Thermo Fisher Scientific, Inc.). The
protein (40 µg) from the samples of each group was subjected to 10%
SDS-PAGE, prior to being transferred onto polyvinylidene difluoride
membranes. Next, the membranes were blocked with 5% skimmed milk
for 1 h and incubated overnight at 4°C with the following primary
antibodies diluted in bovine serum albumin solution (50 g/l):
Rabbit anti-human IRE1 polyclonal antibody (dilution, 1:1,000; no.
ab37073, Abcam, Cambridge, UK), rabbit anti-human p-PERK and PERK
polyclonal antibodies (dilution, 1:500; no. sc-32577 and sc-13073,
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and rabbit
anti-human AFT4 and AFT6 antibodies (dilution, 1:200; no. 11815 and
65880, Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C
overnight. Following washing with TBST (TBS, with 1 ml/l Tween-20)
3 times (for 5 min each time), membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (dilution, 1:10,000; ab6721; Abcam) for 1 h at room
temperature. Protein expression was detected using an enhanced
chemiluminescent agent (Boster Biological Technology, Pleasanton,
CA, USA). Protein expression levels were normalized to GAPDH and
ImageJ 2.1 software (National Institutes of Health, Bethesda, MD,
USA) was used to scan and quantify the gray values.
Statistical analyses
Data are presented as the mean ± standard deviation
and were analyzed using SPSS 19.0 statistical software (IBM Corp.,
Armonk, NY, USA). Differences among multiple groups were assessed
using one-way analysis of variance, followed by Dunnett's method.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Application of as promotes the
apoptosis of prostate cancer cells in a dose-dependent manner
Analysis of cell apoptosis using a flow cytometry
assay demonstrated that As accelerated the apoptosis of DU-145
cells in a dose-dependent manner, with a significant increase in
the rate of apoptosis at concentrations of 20, 50 and 100 nmol/l
(Fig. 1; P<0.05). This result
indicated that As administration significantly increased the
percentage of apoptosis (P<0.05) in As-treated groups,
particularly in the H-As group, compared with that in the control
group. However, treatment with TUDCA exhibited a weaker effect on
the promotion of apoptosis. The results of the present study
suggested that as was able to promote the apoptosis of prostate
cancer cells in a dose-dependent manner.
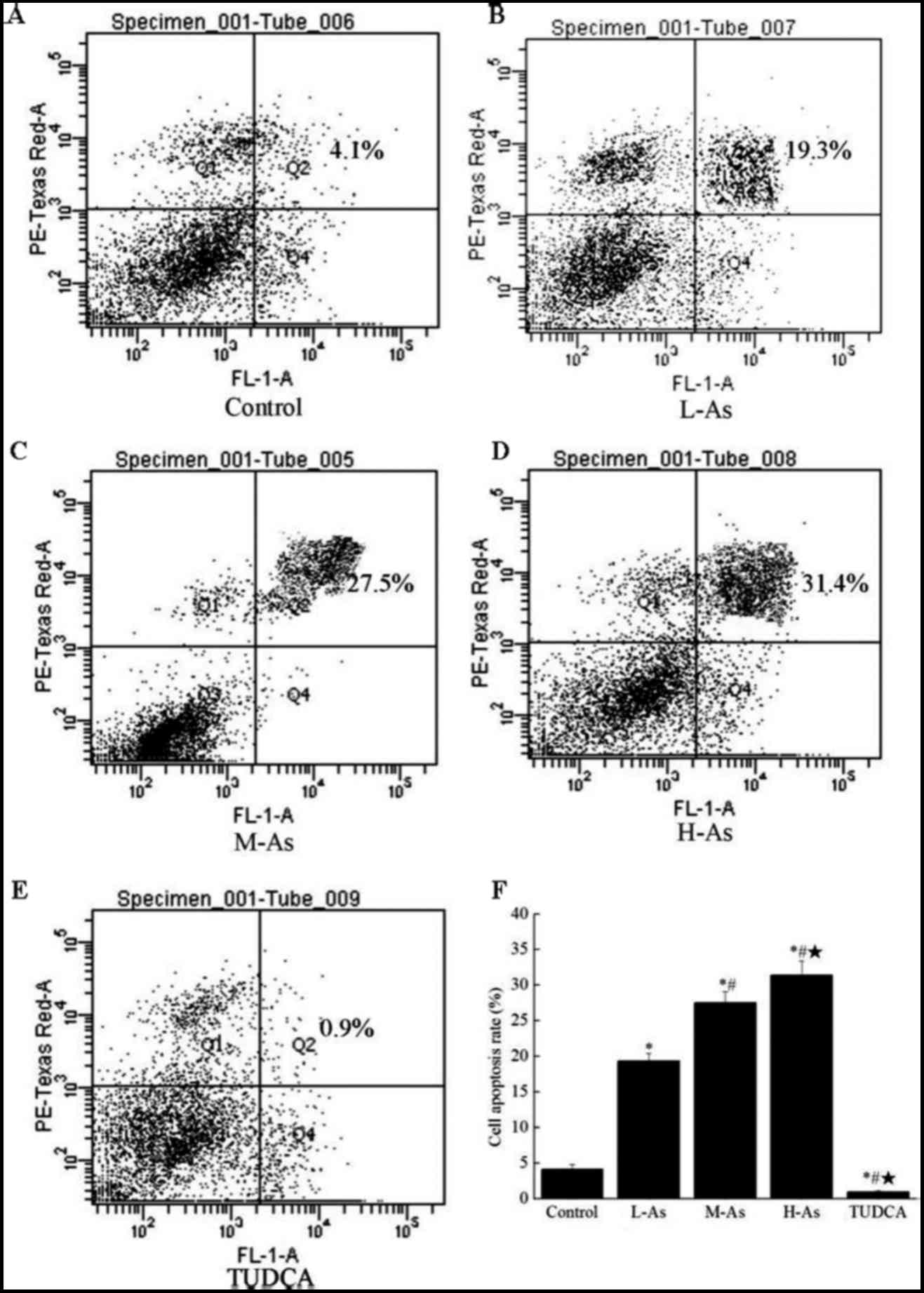 | Figure 1.The cell apoptosis rate analyzed by
flow cytometry. (A) Control group, (B) L-As group, (C) M-As group,
(D) H-As group and (E) TUDCA group, (F) quantitative bar graph
illustrating the cell apoptosis rate. DU-145 cells were treated
with 10 µl DMSO, As-DMSO solution with a concentration of 20, 50
and 100 nmol/l, respectively, and TUDCA-DMSO solution. Data are
presented as the mean ± standard deviation (n=5). *P<0.05,
compared with the control group; #P<0.05, compared
with the L-As group; *P<0.05, compared with the M-As group.
L-As, low-dose astragaloside; M-As, moderate-dose astragaloside;
H-As, high-dose astragaloside; TUDCA, tauroursodeoxycholic acid;
DMSO, dimethyl sulfoxide. |
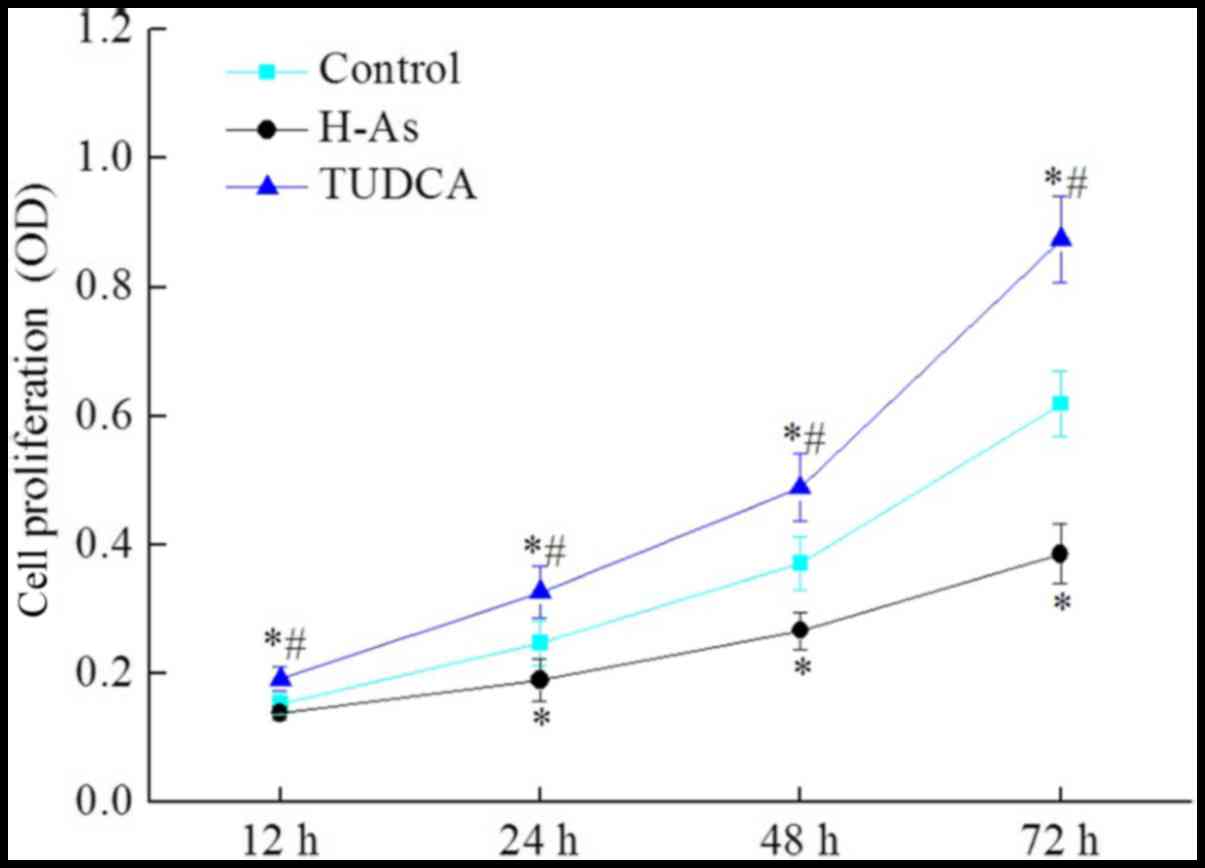
Application of as inhibits the
proliferation of prostate cancer cells
Next, the cell proliferation of DU-145 cells in the
control group, H-As group and TUDCA group was measured at different
time point. As demonstrated in Fig.
2, TUDCA induced a slight inhibitory effect on the viability of
DU-145 cells (0.874%), consistent with the result of the flow
cytometric analysis. However, compared with the control group, as
treatment significantly inhibited cell proliferation (0.368% at 72
h; P<0.05) in a time-dependent manner. These results suggested
that as may inhibit the proliferation of prostate cancer cells,
there by inhibiting tumor development.
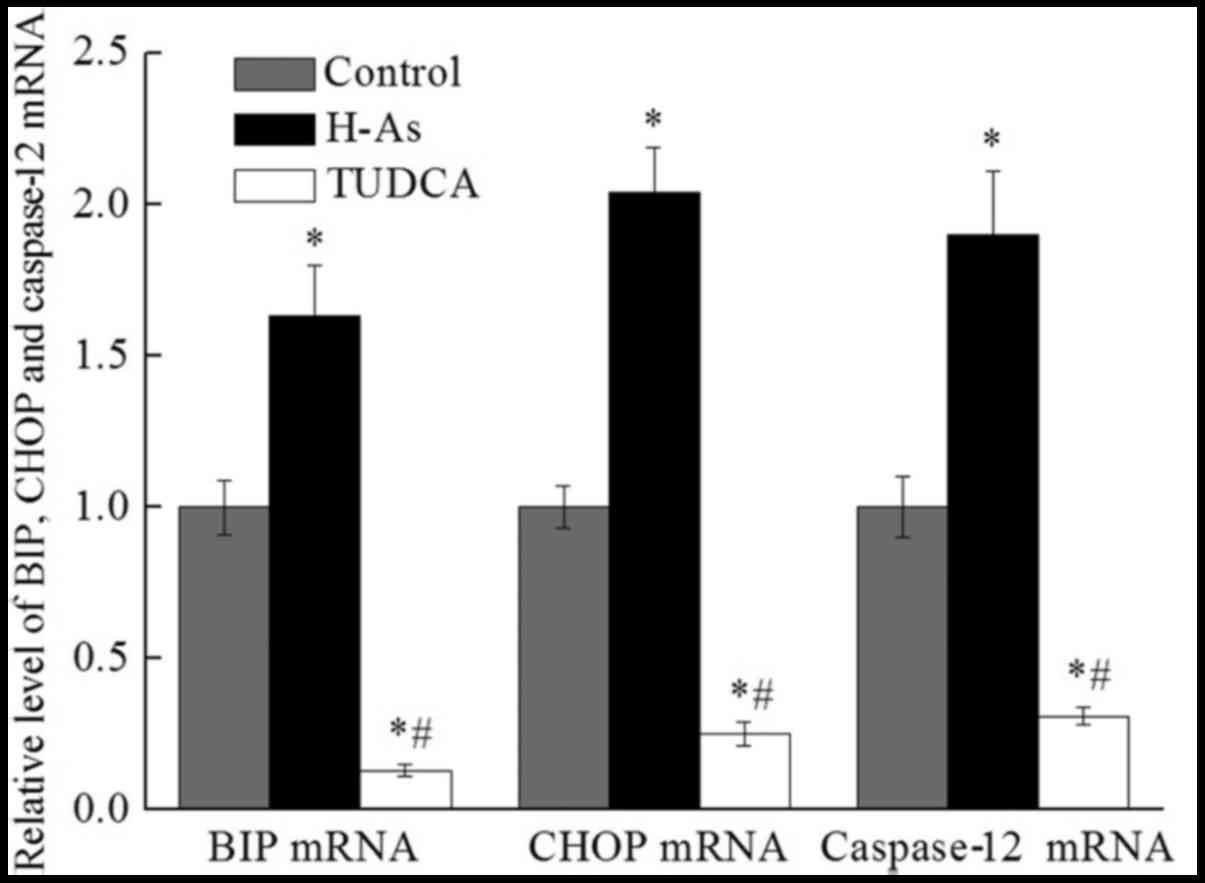
Application of as increases the
expression of BiP, CHOP and caspase-12 mRNA in prostate cancer
cells
In order to elucidate the underlying mechanism of As
activity through ER stress pathway in prostate cancer cells, the
mRNA levels of BiP, CHOP and caspase-12 following treatment with
100 nmol/l As were assessed (Fig. 3).
Compared with the control group, the mRNA levels of BiP, CHOP and
caspase-12 were significantly increased (P<0.05) in H-As group
by 1.63-fold, 2.04-fold and 1.90-fold, respectively. Furthermore,
the expression of these mRNA in the TUDCA group was significantly
decreased. Taken together, these results suggested that as
increased the expression of BiP, CHOP and caspase-12 mRNA through
the ER stress pathway.
Application of as increased the levels
of IRE1, p-PERK, AFT4 and AFT6 protein in prostate cancer
cells
The expression levels of IRE1, p-PERK, AFT4 and
AFT6, were detected in DU-145 cells through western blot analysis
(Fig. 4). As demonstrated in Fig. 4A, the protein bands indicated
expression of IRE1, p-PERK, AFT4 and AFT6 in the three groups.
Additionally, the quantified results are displayed in Fig. 4B. The results indicated that the
expression of IRE1, AFT4 and AFT6 protein were significantly
increased following treatment with As (Fig. 4A and B; P<0.05). However, the level
of these proteins in the TUDCA group was extremely lower than that
in the control group and the TUDCA group. Additionally, the ratio
of p-PERK/PERK (Fig. 4C) was also
calculated, indicating that the ratio of p-PERK/PERK in the H-As
group was significantly (P<0.05) higher than that in control
group. These results indicated that as application may increase the
expression of IRE1, AFT4 and AFT6, which are important proteins in
the ER stress pathway, and that As is also able to increase the
phosphorylation of PERK without affecting the level of PERK, which
in turn increases the p-PERK/PERK ratio.
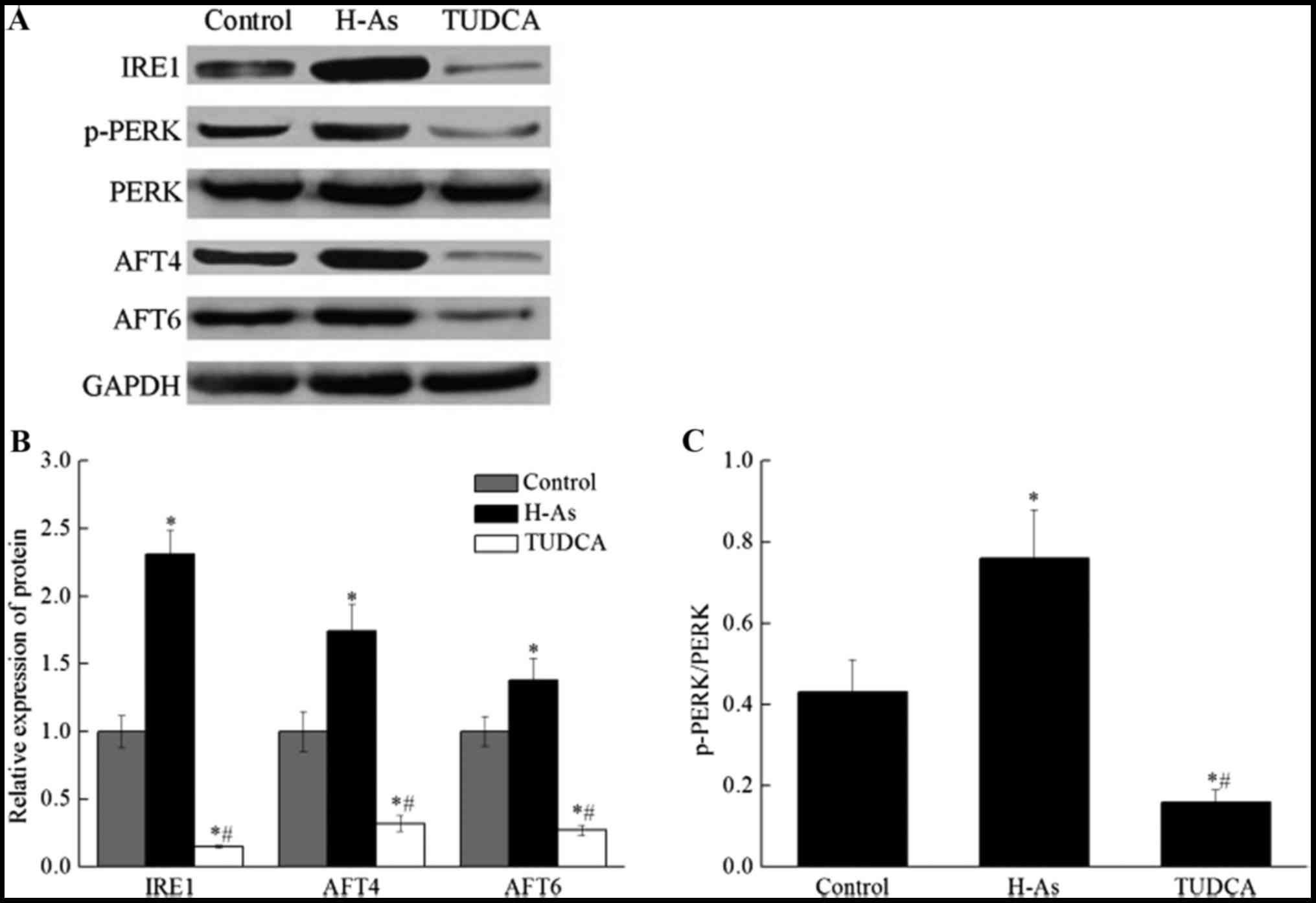 | Figure 4.The levels of IRE1, PERK, p-PERK, AFT4
and AFT6 protein in the control, H-As and TUDCA groups. (A) Western
blot and (B) densitometric analyses were performed to detect the
protein expression of IRE1, p-PERK, PERK, AFT4 and AFT6. (C) The
ratio of p-PERK/PERK in the control, H-As and TUDCA groups. All
protein quantification data were normalized to GAPDH. Data are
presented as the mean ± standard deviation. *P<0.05, compared
with the control group; #P<0.05, compared with the
H-As group. IRE1, inositol-requiring enzyme 1; PERK, protein kinase
R-like endoplasmic reticulum kinase; p-PERK, phosphorylated PERK;
AFT, iron-regulated transcriptional activator Aft; H-As, high-dose
astragaloside; TUDCA, tauroursodeoxycholic acid. |
Discussion
Appropriate responses to ER stress are fundamentally
important in order for cells to maintain normal physiological and
biochemical functions (23). However,
abnormal ER stress may also cause cell apoptosis, thereby reducing
bodily functions and inducing the development of various diseases,
including diabetes, neurodegenerative diseases, renal disease and
atherosclerosis (24). Despite the
adverse effects of ER stress on the health of the body, ER
stress-based therapies are widely used in the treatment of cancer
due to their ability to induce cancer cell apoptosis. Nawrocki
et al (25) reported that
Bortezomib was able to inhibit the function of protein kinase
R-like ER kinase and to promote cell apoptosis in human pancreatic
cancer cells by increasing ER stress. In addition, in the treatment
of gastric cancer, the anticancer effects of WZ35 was also revealed
to be correlated with its ability to induce Jun N-terminal kinase
signaling transduction and ER stress-related apoptotic pathways
(26). Recently, Liu et al
(21) revealed that the anticancer
activity of quercetin was also positively associated with the
enhanced ER stress in prostate cancer PC-3 cells (21). From the aforementioned studies, we
hypothesized that induction of ER stress in prostate cancer cells
may potentially be used in the treatment of prostate cancer.
As has been widely used in the treatment of
different types of cancer. In the treatment of breast cancer, As
III was revealed to be able to effectively decrease the survival
rate of cancer cells in vitro and to reduce the growth rate
of tumors in vivo by inducing cell apoptosis-related
signaling pathways (10). A previous
study also revealed that As IV may inhibit the invasion and
migration of the human lung cancer A549 cell line by regulating the
protein kinase C-α/ERK1/2/nuclear factor-κB pathway (27). However, to the best of our knowledge,
the application of As in the treatment of prostate cancer has yet
to be reported. Therefore, the present study primarily investigated
the roles of the ER stress signaling pathway in the As-mediated
cell death of human prostate cancer DU-145 cells. It was revealed
that as (20–100 nmol/l) promoted the apoptosis of prostate cancer
cells in a dose-dependent manner. In the present study, a
concentration of 100 nmol/l was selected for further study. In
addition to the activity of As in inducing cell death, As may also
inhibit cell proliferation. The results of the present study
suggested that As may be used to treat prostate cancer due to its
effects in the apoptosis and proliferation of cancer cells.
Furthermore, there were a number of studies
reporting the association between as and ER stress-induced cell
apoptosis in various models and physiological pathways, for example
Chen et al (15) and Bouman
et al (28) reported the
downregulation of PERK/ATF4/CHOP pathway by As IV treatment,
further inhibiting the ER stress-induced podocyte apoptosis in
diabetic rats. Cycloastragenol and As IV, which are active
ingredients of Astragalus membranaceus, were revealed to
have almost equal efficiency in inhibiting ROS-associated ER
stress, which in turn regulates the activity of AMPK and inhibits
the TXNIP/NLRP3 inflammasome (receptors and sensors of the innate
immune system that may induce inflammation molecules derived from
host proteins and infectious microbes, and may regulate caspase-1
activation) activation (29). Despite
the wide application of As in the treatment of various types of
cancer, the association between the anticancer activity of As and
ER stress has not been reported in prostate cancer. Certain studies
have demonstrated that IRE1, PERK and ATF4 are the key players in
ER stress (30). Under normal
conditions, ER stress factor BiP is able to bind to IRE1, PERK and
ATF6 in order to stay in an inactive state, while the external
stimuli lead to overexpression of BiP, which in turn affects the ER
stress-related apoptotic pathways, including ATF4/CHOP, caspase-12,
autophagy and RIDD (31). In the
present study, as was revealed to be able to increase the protein
expression of IRE1, p-PERK (the active form of PERK), AFT4 and AFT6
in prostate cancer cells without affecting the total level of PERK.
In addition, as also increased the expression levels of BiP, CHOP
and caspase-12 mRNA in prostate cancer cells. These results
indicated that as may serve a role through the ER stress pathway in
prostate cancer cells and may activate the ER stress-related
apoptotic pathways.
In conclusion, the results of the present study
demonstrated that prostate cancer was sensitive to As through
activation of the ER stress pathway. These observations indicated
that As upregulated the expression levels of related proteins and
genes, providing a novel therapeutic approach for the treatment of
prostate cancer.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BT and RJ conceived and designed the experiments;
BT, RJ and GW performed the experiments; JY analyzed the data; GW
and JY wrote the paper and substantively revised it. All authors
read and approved the final manuscript.
Ethics statement and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest to declare.
References
|
1
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nyquist MD and Dehm SM: Interplay between
genomic alterations and androgen receptor signaling during prostate
cancer development and progression. Horm Cancer. 4:61–69. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamid AR, Hoogland AM, Smit F, Jannink S,
van Rijt-van de Westerlo C, Jansen CF, van Leenders GJ and Verhaegh
GW: The role of HOXC6 in prostate cancer development. Prostate.
75:1868–1876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crawford ED, Stone NN, Evan YY, Koo PJ,
Freedland SJ, Slovin SF, Gomella LG, Berger ER and Keane TE:
Challenges and recommendations for early identification of
metastatic disease in prostate cancer. Urology. 83:664–669. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rustin GJ, Bast RC Jr, Kelloff GJ, Barrett
JC, Carter SK, Nisen PD, Sigman CC, Parkinson DR and Ruddon RW: Use
of CA-125 in clinical trial evaluation of new therapeutic drugs for
ovarian cancer. Clin Cancer Res. 10:3919–3926. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Victorson DE, Brucker PS, Bode RK, Eton
DT, Talcott JA, Clark JA, Knight SJ, Litwin MS, Moinpour CM, Reeve
BB, et al: Ensuring comprehensive assessment of urinary problems in
prostate cancer through patient-physician concordance. Urol Oncol.
32(26): e25–e31. 2014.
|
|
7
|
Schröder FH, Hugosson J, Roobol MJ,
Tammela TL, Zappa M, Nelen V, Kwiatkowski M, Lujan M, Määttänen L,
Lilja H, et al: Screening and prostate cancer mortality: results of
the European Randomised Study of Screening for Prostate Cancer
(ERSPC) at 13 years of follow-up. Lancet. 384:2027–2035. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Freytag SO, Stricker H, Lu M, Elshaikh M,
Aref I, Pradhan D, Levin K, Kim JH, Peabody J, Siddiqui F, et al:
Prospective randomized phase 2 trial of intensity modulated
radiation therapy with or without oncolytic adenovirus-mediated
cytotoxic gene therapy in intermediate-risk prostate cancer. Int J
Radiat Oncol Biol Phys. 89:268–276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S, Tang L and Chen F: Astragaloside
III from Astragalus membranaceus antagonizes breast cancer
growth. AJTCAM. 12:183–186. 2015. View Article : Google Scholar
|
|
11
|
Li B, Wang F, Liu N, Shen W and Huang T:
Astragaloside IV inhibits progression of glioma via blocking
MAPK/ERK signaling pathway. Biochem Biophys Res Commun. 491:98–103.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai PC, Liu DL, Zhang L, Ye J, Wang Q,
Zhang HW, Lin XH and Lai GX: Astragaloside IV sensitizes non-small
cell lung cancer cells to gefitinib potentially via regulation of
SIRT6. Tumour Biol. 39:10104283176975552017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yongping M, Zhang X, Xuewei L, Fan W, Chen
J, Zhang H, Chen G, Liu C and Liu P: Astragaloside prevents
BDL-induced liver fibrosis through inhibition of notch signaling
activation. J Ethnopharmacol. 169:200–209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang X, Tang L, Wang F and Song G:
Astragaloside IV attenuates allergic inflammation by regulation
Th1/Th2 cytokine and enhancement CD4+ CD25+ Foxp3 T cells in
ovalbumin-induced asthma. Immunobiology. 219:565–571. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Gui D, Chen J, He D, Luo Y and
Wang N: Down-regulation of PERK-ATF4-CHOP pathway by Astragaloside
IV is associated with the inhibition of endoplasmic reticulum
stress-induced podocyte apoptosis in diabetic rats. Cell Physiol
Biochem. 33:1975–1987. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Y, Li Q, Zhao W, Li J, Sun Y, Liu K,
Liu B and Zhang N: Astragaloside IV and cycloastragenol are equally
effective in inhibition of endoplasmic reticulum stress-associated
TXNIP/NLRP3 inflammasome activation in the endothelium. J
Ethnopharmacology. 169:210–218. 2015. View Article : Google Scholar
|
|
17
|
Wang ZS, Xiong F, Xie XH, Chen D, Pan JH
and Cheng L: Astragaloside IV attenuates proteinuria in
streptozotocin-induced diabetic nephropathy via the inhibition of
endoplasmic reticulum stress. BMC Nephrol. 16:442015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao SS and Kaufman RJ: Endoplasmic
reticulum stress and oxidative stress in cell fate decision and
human disease. Antioxid Redox Sign. 21:396–413. 2014. View Article : Google Scholar
|
|
19
|
Gardner BM, Pincus D, Gotthardt K,
Gallagher CM and Walter P: Endoplasmic reticulum stress sensing in
the unfolded protein response. Cold Spring Harb Perspect Biol.
5:a0131692013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deegan S, Saveljeva S, Gorman AM and
Samali A: Stress-induced self-cannibalism: On the regulation of
autophagy by endoplasmic reticulum stress. Cell Mol Life Sci.
70:2425–2441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu KC, Yen CY, Wu RS, Yang JS, Lu HF, Lu
KW, Lo C, Chen HY, Tang NY, Wu CC and Chung JG: The roles of
endoplasmic reticulum stress and mitochondrial apoptotic signaling
pathway in quercetin-mediated cell death of human prostate cancer
PC-3 cell. Environ Toxicol. 29:428–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nawrocki ST, Carew JS, Dunner K Jr, Boise
LH, Chiao PJ, Huang P, Abbruzzese JL and McConkey DJ: Bortezomib
inhibits PKR-like endoplasmic reticulum (ER) kinase and induces
apoptosis via ER stress in human pancreatic cancer cells. Cancer
Res. 65:11510–11519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou P, Zhang J, Xia Y, Kanchana K, Guo G,
Chen W, Huang Y, Wang Z, Yang S and Liang G: ROS generation
mediates the anti-cancer effects of WZ35 via activating JNK and ER
stress apoptotic pathways in gastric cancer. Oncotarget.
6:5860–5876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng X, Gu J, Zhang M, Yuan J, Zhao B,
Jiang J and Jia X: Astragaloside IV inhibits migration and invasion
in human lung cancer A549 cells via regulating PKC-α-ERK1/2-NF-κB
pathway. Int Immunopharmacol. 23:304–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bouman L, Schlierf A, Lutz AK, Shan J,
Deinlein A, Kast J, Galehdar Z, Palmisano V, Patenge N, Berg D, et
al: Parkin is transcriptionally regulated by ATF4: Evidence for an
interconnection between mitochondrial stress and ER stress. Cell
Death Differ. 18:769–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao Y, Li Q, Zhao W, Li J, Sun Y, Liu K,
Liu B and Zhang N: Astragaloside IV and cycloastragenol are equally
effective in inhibition of endoplasmic reticulum stress-associated
TXNIP/NLRP3 inflammasome activation in the endothelium. J
Ethnopharmacol. 169:210–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu ZW, Zhu HT, Chen KL, Dong X, Wei J,
Qiu C and Xue JH: Protein kinase RNA-like endoplasmic reticulum
kinase (PERK) signaling pathway plays a major role in reactive
oxygen species (ROS)-mediated endoplasmic reticulum stress-induced
apoptosis in diabetic cardiomyopathy. Cardiovasc Diabetol.
12:1582013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jheng JR, Ho JY and Horng JT: ER stress,
autophagy and RNA viruses. Front Microbiol. 5:3882014. View Article : Google Scholar : PubMed/NCBI
|