Introduction
The incidence rate of gastric cancer has declined
worldwide (10–20% per decade), from being the most common cancer in
1980 to the fourth most common cancer in 2017 (1). Nevertheless, gastric cancer mortality
still accounts for a significant proportion of all cancer
mortalities (1).
Itraconazole is a Food and Drug
Administration-approved antifungal drug belonging to the azole
family. Itraconazole kills fungi by inhibiting lanosterol
14a-demethylase, which is essential for the conversions of
lanosterol to ergosterol in fungi and lanosterol to cholesterol in
humans (2). However, an increasing
number of reports have revealed that itraconazole has a potential
antitumor function (2–6). A number of these reports have concluded
that itraconazole has potent and selective inhibitory activity on
the proliferation of endothelial cells and multiple key aspects of
tumor angiogenesis in vitro and in vivo (7,8).
5-fluorouracil (5-FU) is widely used as a potent
drug for the treatment of gastric cancer. 5-FUis a type of
pyrimidine antagonist with cytotoxic mechanisms; it has the ability
to incorporate its metabolites as false precursors into DNA to
cause DNA instability (9,10).
The purpose of the present article is to assess the
effects of itraconazole on gastric cancer. In vitro
experiments were performed to determine the effects of itraconazole
and 5-FU alone or in combination in SGC-7901 cells. Whether
itraconazole is able to affect the survival of gastric cancer
patients was also assessed.
Materials and methods
Cell lines and culture
Human SGC-7901 cell lines were purchased from The
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China) and grown in standard RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT,
USA), 100 mg/ml streptomycin and 100 U/ml penicillin (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C in a 5% CO2
incubator.
Cell viability assay
SGC-7901 cells were seeded into Nunclon-96-well flat
bottom plates at a density of 5,000 cells per well containing 100
µl growth medium per well and incubated for 24 h. Then, the medium
was replaced with 100 µl fresh medium containing various
concentrations of itraconazole (Xi'an Janssen Pharmaceutical,
Shanxi, China) and 5-FU (Shanghai Xudong Haipu Pharmaceutical Co.
Ltd, Pudong, China), alone and in combination. After 72 h
treatment, cell viability was assessed by Cell Counting Kit-8
(CCK-8) assay (Beyotime Institute of Biotechnology, Haimen, China).
Briefly, CCK-8 solution was added (10 µl/well), and the culture
plates were stirred gently followed by incubation in CO2
incubator at 37°C for 2 h. Then, the plates were measured at 450 nm
(Multiskan FC; Thermo Fisher Scientific, Inc.). All experiments
were repeated at least three times. Dose-response curves were
mapped. The values were expressed as the percentage of control,
medium. The IC50 values were obtained using GraphPad
Prism (version 6.00; GraphPad Software, Inc., La Jolla, CA,
USA).
Analysis of combined drug effects
Drug effects were assessed using the isobologram
method (11–13), which is based on the median effect
principle of Chou and Talalay (14),
and the CalcuSyn software (version 2.1; Biosoft, Cambridge, UK).
The isobologram method is a graphic description of pharmacological
interactions, which is constructed by choosing a desired fractional
affected cell apoptosis (Fa). An isobologram was generated by
drawing a straight line to connect Fa points that are plotted
against experimentally used non-constant-ratio combinations of drug
1 (5-FU) and drug 2 (itraconazole) on x- and y-axes to. Combined
data points that are on the line are represented as an additive
interaction, while points that were below or above the line
represented synergism or antagonism, respectively.
Cell-cycle distribution assay
SGC-7901 cells (5×105/2 ml) were seeded
in 6-well plates and treated the following day with itraconazole
(15 µM), 5-FU (4.25 µM) or itraconazole combined with 5-FU
(traconazole 15 µM: 5-FU 4.25 µM). Following incubation for an
additional 72 h, non-adherent cells were removed. The cells were
trypsinized and collected. Subsequently, the cells were washed
twice with PBS then resuspended in 0.5 ml PBS and fixed in 4.5 ml
70% ethanol overnight. The cells were collected by centrifugation
and were resuspended in 0.2 mg/ml of propidium iodide (PI)
containing 0.1% Triton X-100 and 0.1 mg/ml RNase A. Subsequently,
the cell suspension was incubated for 30 min in the dark at room
temperature and analyzed using a flow cytometer. The percentages of
cells in different phases were sorted using the ModFit 5.2 program
(Verity Software House, Inc., Topsham, ME, USA). The percentage of
cells at each phase was obtained from three independent
experiments.
Apoptosis assay by Annexin
V-fluorescein isothiocyanate (FITC)/PI staining
The cells (5×105/2 ml) were seeded in
6-well plates and treated on the following day with itraconazole
(15 µM), 5-FU (4.25 µM), or itraconazole combined with 5-FU
(traconazole 15 µM: 5-FU 4.25 µM). Following incubation for an
additional 72 h (36.5°C), the adherent and floating cells were
harvested, washed with PBS and stained using an Annexin V-FITC/PI
kit according to the manufacturer's instructions. Apoptosis was
then analyzed using the FACScan flow cytometer with 20,000 cells in
each group. Data analysis was performed using the Cell Quest
software (5.1, BD Biosciences, Franklin Lakes, NJ, USA).
Reproducibility was checked in three independent experiments.
Single-cell gel electrophoresis assay
(SCGE)
DNA damage was assessed using the SCGE assay as
described by Singh et al (15) and
Gao et al (16) with slight
modifications (electrophoresis condition: 25 V, 300 mA, 25 min).
The cells were cultured as aforementioned. A total of 72 h after
treatment, the cells were harvested and resuspended with PBS. Then,
20 µl cell suspension was mixed with 160 µl 0.7% LMA (low melting
point agarose) and spread onto frosted slides that were coated with
1% NMA (normal melting point agarose). After solidification at 4°C,
the slides were immersed in lysis buffer (2.5 M NaCl, 100 mM EDTA,
10 mM Tris, pH 10, 1% Triton X-100 and 1% sodium sarcosinate) at
4°C for 180 min. The slides were subsequently placed in alkaline
solution (1 mM Na2EDTA and 300 mM NaOH, pH 13.0) for 20
min, and the DNA was allowed to unwind prior to electrophoresis (25
V, 300 mA) for 25 min. Subsequently, the cells were neutralized
three times with 0.4 M Tris (pH 7.5) and stained for three times
with 50 µl Gelview (20°C, 5 min Genestar Company, Shanghai, China,
http://www.bioon.com.cn/show/index_88950.html). The
images were captured using a fluorescence microscope with an
excitation filter of 549 nm and a barrier filter of 590 nm. All
steps were carried out in the dark to prevent additional DNA
damage. A total of 100 cells from each sample were selected
randomly. Parameters (tail length, percentage of DNA in tail and
tail moment) were analyzed by using CASP software. (CasP
1.2.3beta1, Krzysztof Końca, CaspLab.com) The genotoxicity of
biomaterials was quantified.
Patients and methods
The medical records of 60 patients with histological
diagnoses of advanced gastric cancer and accepted treatment at the
Oncology Department of The First Affiliated Hospital of Xi'an
Jiaotong University (Shaanxi, China) between January 2010 and June
2016. A total of 12 were retrospectively reviewed [18 women and 42
men, mean age: 54 (39–73) years]. The patients were included if
they had received 5-FU-based chemotherapy with or without
itraconazole. The clinical and pathological features of the
patients were reviewed, and survival rate was calculated.
The Response Evaluation Criteria in Solid Tumors
(RECIST; version 1.1) (17) was
employed. The present study was approved by the Committee for the
Conduct of Human Ethics Committee of the First Affiliated Hospital
of Xi'an Jiaotong University, and all patients signed written
informed consent forms.
Statistical analysis
Statistical comparisons between the experimental
groups and the control group was performed using the chi-square
test and Cox's proportional hazards regression model. The SCGE
assay data was analyzed with an unpaired Student's test. Version
11.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Inhibition of proliferation of
SGC-7901 cells
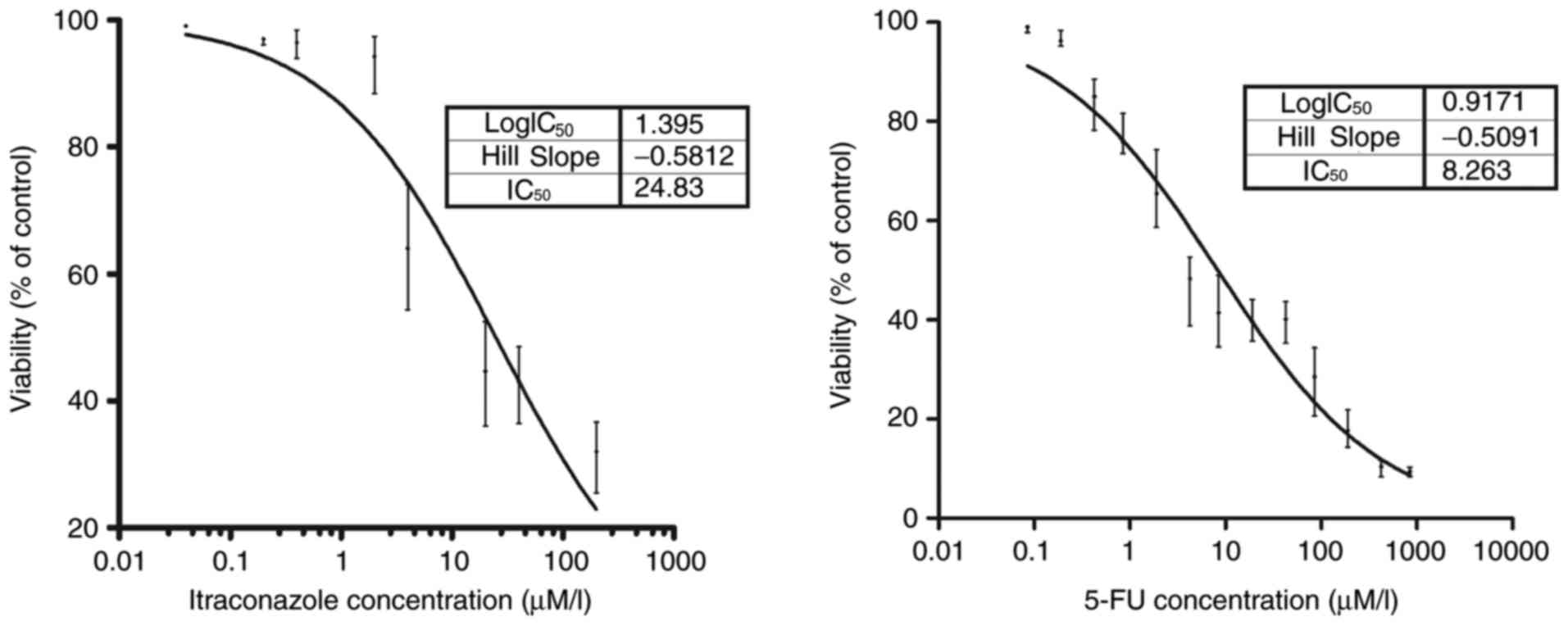
The inhibitory concentrations of itraconazole and
5-FU on the growth of SGC-7901 cells are indicated in Fig. 1. A potent inhibitory effect on the
SGC-7901 gastric cancer cell line compared with the control was
detected following treatment with 0.4 µM itraconazole (P<0.05).
Similarly, a potent inhibitory effect of 5-FU was observed compared
with the control following the treatment of 0.19 µM 5-FU
(P<0.05). The IC50 values of itraconazole and 5-FU
were obtained. The values were 24.83 µM for itraconazole and 8.26
µM for 5-FU (goodness of fit, itraconazole, R2=0.919;
5-FU, R2=0.961).
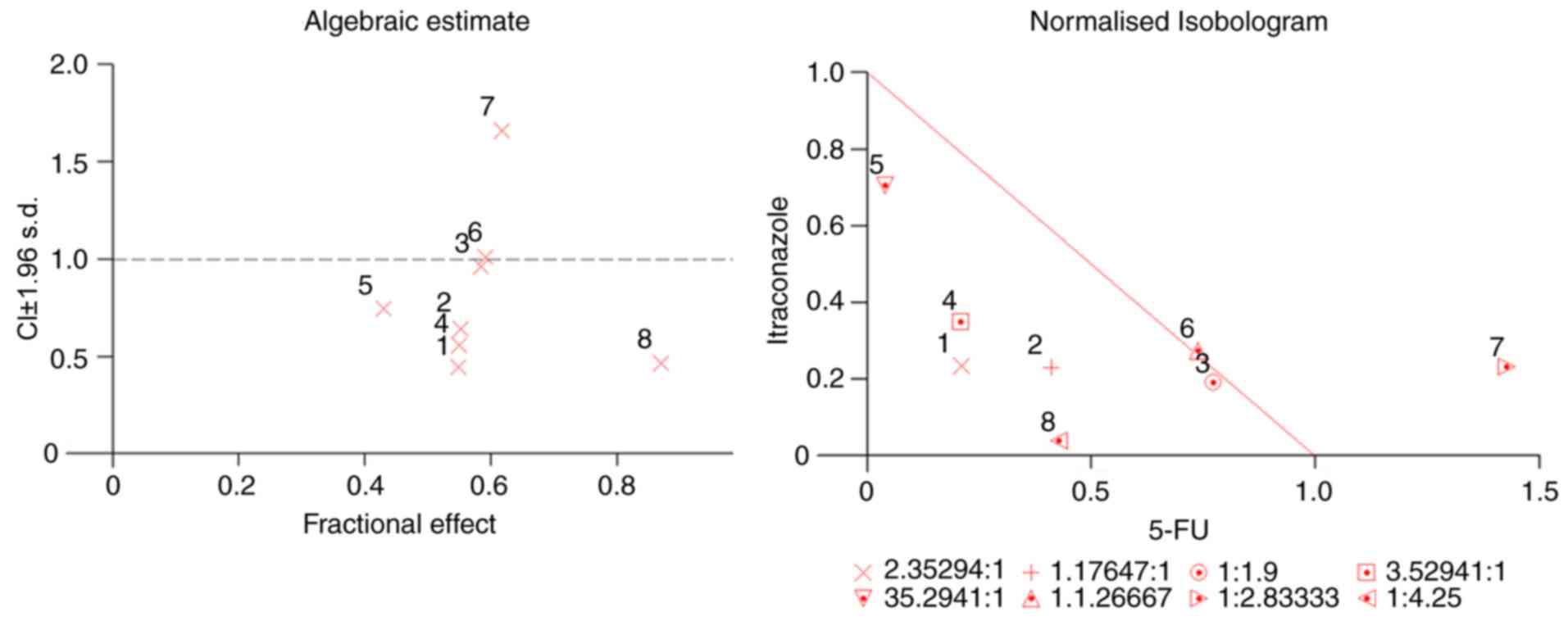
In vitro study of itraconazole in
combination with 5-FU
Based on the IC50 values as
aforementioned, different ratios of itraconazole and 5-FU were
selected (itraconazole: 5-FU), and different concentrations were
chosen for subsequent investigation of the effect of itraconazole
and 5-FU combination treatment. The CI values that provided
quantitative data for the degree of drug interaction were
calculated by CalcuSyn software, at Fa values of 0.50. Isobologram
and median effect analysis plots were constructed (Fig. 2) for values that represent 50% growth
inhibition. In the median effect analysis, the points that were
below the line indicated synergism. The synergistic effect of the
two drugs was more prominent when the concentrations of both drugs
were below the IC50 value.
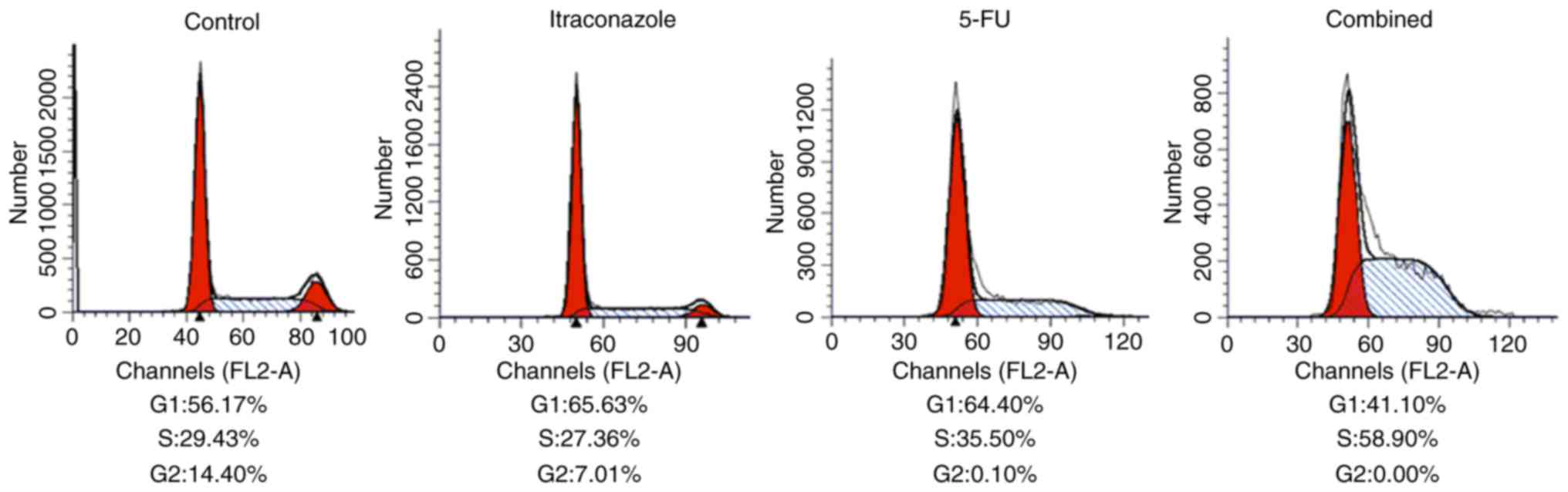
Changes in cell cycle of SGC-7901
cells
As indicated by flow cytometric analysis with
propidium iodide staining, the overall proportion of cells in the
G1 plus S phases increased compared with the control following the
treatment with 15 µM itraconazole and 4.25 µM 5-FU, alone or in
combination (Fig. 3). However, at the
same time, the proportion of cells in G2 decreased following
itraconazole and 5-FU treatments (alone or in combination) compared
with the control. When compared with treatment itraconazole or 5-FU
alone, treating the cells with a combination of itraconazole and
5-FU was able to increase the proportion of cells in the S phase
(Fig. 3). These findings indicate
that separate treatments of itraconazole and 5-FU were able to
inhibit cell cycle distribution, and the effects of the agents were
increased when they are used in combination.
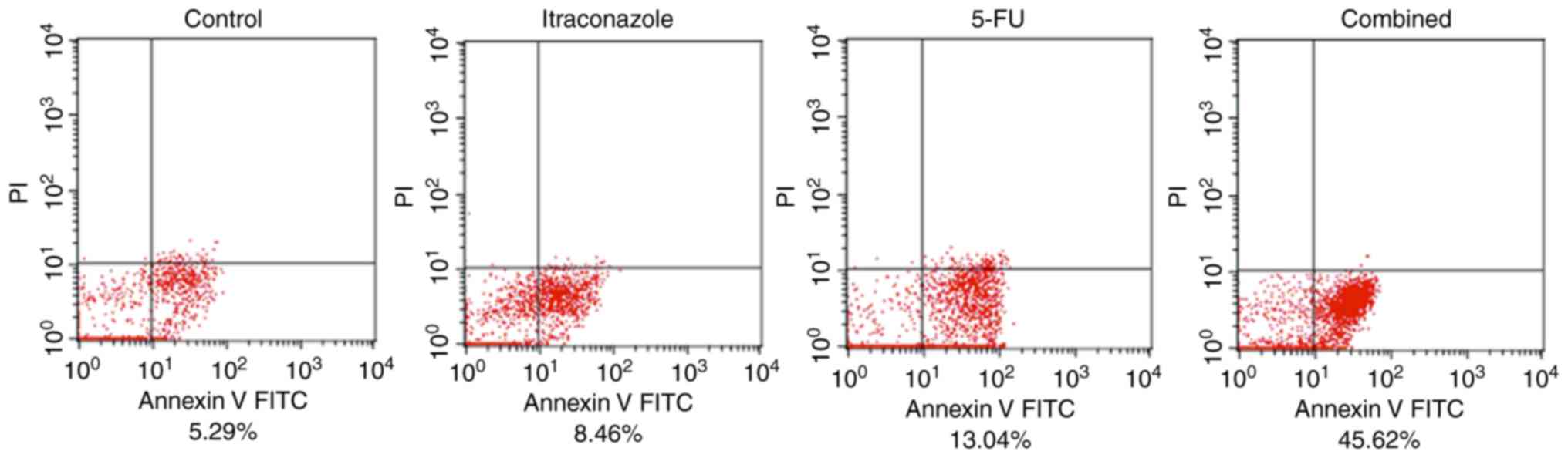
Apoptosis is induced in SGC-7901 cells
by itraconazole and 5-FU
Flow cytometric analysis with Annexin V/PI double
staining of SGC-7901 cells indicated that the percentage of early
apoptotic cells was increased by treatment with 15 µM itraconazole
alone for 72 h compared with the control (Fig. 4). Treatment with 4.25 µM 5-FU alone
also increased the percentage of early apoptotic cells.
Furthermore, when SGC-7901 cells were treated with a combination of
5-FU and itraconazole, the percentage of early apoptotic cells was
increased compared with the control or treatment with 5-FU or
itraconazole alone. This finding indicates that treatment of
SGC-7901 cells with itraconazole and 5-FU is able to lead to early
apoptosis; the effects of these agents are markedly increased when
they are used in combination (Fig.
4).
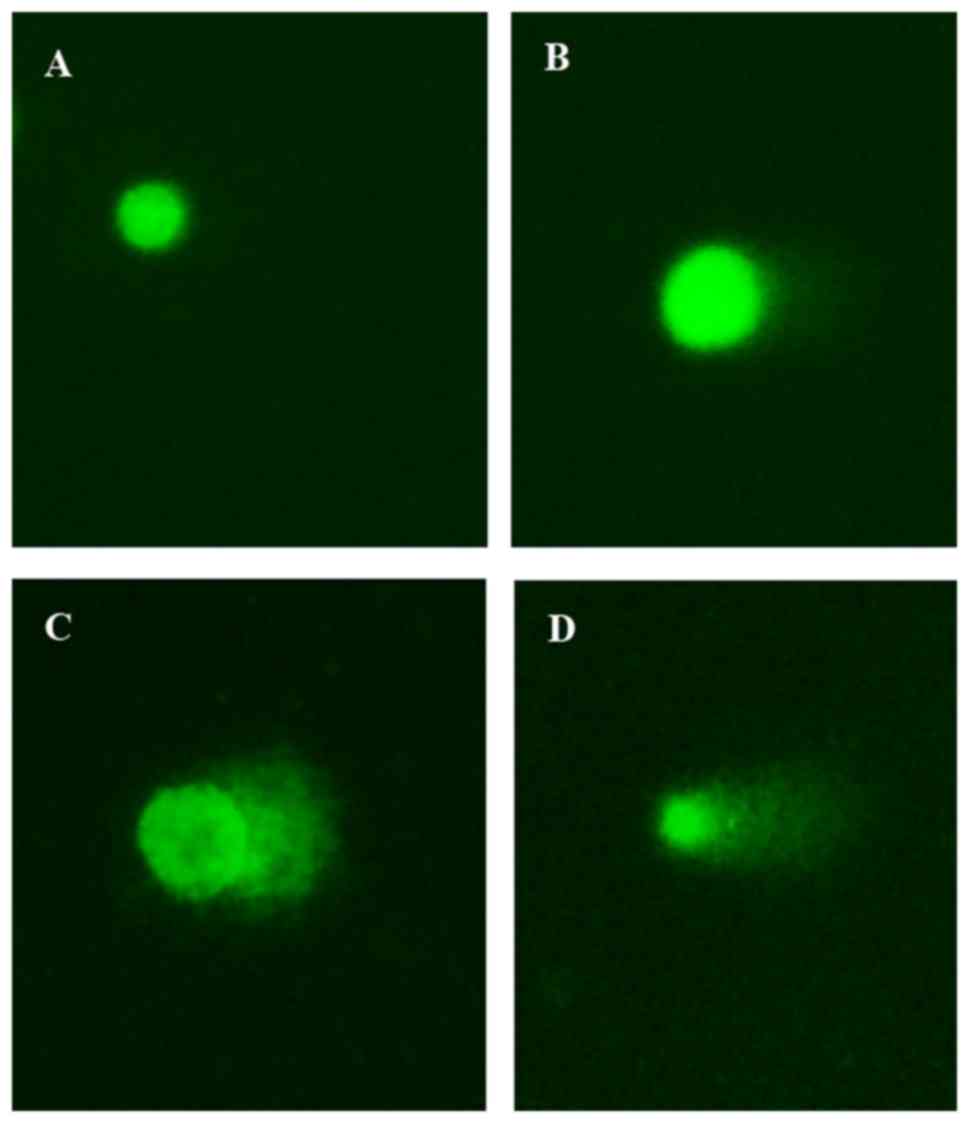
Effect on DNA damage
In the Comet assay, SGC-7901 cells treated
separately or with a combination of itraconazole (15 µM) and 5-FU
(4.25 µM) were green when stained with Gelview. Normal DNA strands
were observed in cells with complete nuclei, whereas fragmented DNA
strands were detected in cells with marked comet tail. The length
of the comet tail increased following treatment with itraconazole
or 5-FU alone when compared with the control (P=0.045 and P=0.022;
Fig. 5). Furthermore, when a
combination of itraconazole and 5-FU was used, there was a
significant increase in the length of the comet tail compared with
treatment with itraconazole or 5-FU alone (both P<0.012;
Fig. 5).
Itraconazole is associated with the
clinical outcome of patients with advanced gastric cancer
Between January 2010 and June 2016, 60 patients were
eligible for inclusion in the final analyses. A summary of patient
demographics is shown in Table I. All
chemotherapies were 5-FU-based, with or without itraconazole. Among
the patients, 22 received itraconazole therapy. Itraconazole was
administered intravenously at a daily dose of 200–400 mg for 4–5
days.
 | Table I.Demographics of patients. |
Table I.
Demographics of patients.
| Groups | Itraconazole,
n=22 | Control, n=38 |
|---|
| Median age
(range) | 53 (43–71) | 56 (39–73) |
| Women, n (%) | 6 (27.2) | 12 (31.6) |
| Men, n (%) | 16 (72.8) | 26 (68.4) |
| ECOG PS, n (%) |
|
|
| 0 | 2 (9.0) | 3 (7.9) |
| 1 | 17 (77.3) | 30 (79) |
| 2 | 3 (13.6) | 5 (13.1) |
| Metastasis positive,
n (%) | 4 (18.2) | 8 (21.1) |
| Histological type, n
(%) |
|
|
|
Adenocarcinoma | 22 (100.0) | 37 (97.3) |
|
Other | 0 (0.0) | 1 (2.7) |
Efficacy of 5-FU-based chemotherapy
with itraconazole
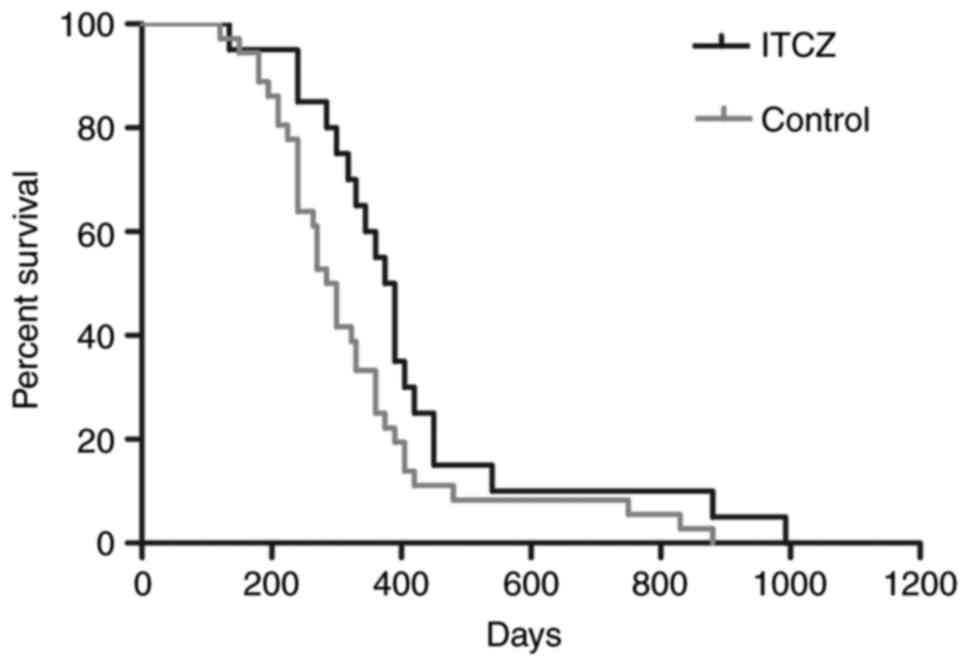
A total of 13 patients (59%, total=22) responded to
chemotherapy with itraconazole, whereas 17 (45%, total=38)
responded to regimens without itraconazole (P=0.284). The median
PFS for patients with and without itraconazole was 204 and 153
days, respectively (P=0.015), and the corresponding median overall
survival was 382 and 301 days, respectively (P=0.045; Fig. 6 and Table
II).
 | Table II.Factors affecting progression-free
survival and overall survival. |
Table II.
Factors affecting progression-free
survival and overall survival.
| A, FPS |
|---|
|
|---|
|
| P-value | HR | 95% CI |
|---|
| Age | 0.336 | 0.984 | 0.951–1.017 |
| Sex | 0.696 | 1.136 | 0.598–2.158 |
| Histological
type | 0.088 | 0.489 | 0.215–1.112 |
| ECOG PS 1 | 0.058 | 0.291 | 0.081–1.044 |
| ECOG PS 2 | 0.329 | 0.669 | 0.299–1.498 |
| w/wo ITCZ | 0.015a | 0.483 | 0.268–0.870 |
|
| B, OS |
|
|
| P-value | HR | 95% CI |
|
| Age | 0.526 | 0.989 | 0.957–1.022 |
| Sex | 0.951 | 1.022 | 0.545–1.912 |
| Histological | 0.526 | 0.774 | 0.351–1.709 |
| ECOG PS 1 | 0.231 | 0.484 | 0.148–1.588 |
| ECOG PS 2 | 0.759 | 0.882 | 0.394–1.971 |
| w/wo ITCZ | 0.045a | 0.547 | 0.304–0.987 |
Discussion
While itraconazole is a traditional antifungal drug,
it has been reported to inhibit angiogenesis and to have anticancer
effects (3,6). Although the effects of itraconazole has
been demonstrated on a number of types of cancer (including,
prostate cancer and non-small cell lung cancer (7,8), whether
itraconazole has an effect on gastric cancer remains unknown.
5-FU is a chemotherapeutic agent, which was created
~50 years ago, but it remains in wide usage as a treatment for a
variety of cancer types, either alone or in combination with other
drugs (9). As such, the anticancer
effects of itraconazole with 5-FU on gastric cancer in vitro
and vivo are of interest for evaluation. In the present
study, itraconazole exhibited marked anticancer effect on gastric
cancer cells, both alone and in combination with 5-FU. Furthermore,
treatment with a combination of itraconazole and FU-based
chemotherapy was indicated to improve the outcome of patients with
gastric cancer.
The results of the present study were consistent
with the finding of previous studies on other types of cancer
(18–22). In the present study, it was
demonstrated that itraconazole is able to inhibit proliferation,
and DNA damage was induced by itraconazole in gastric cancer cells.
Furthermore, a synergetic effect was observed when SGC-7901 gastric
cancer cells were treated with a combination of itraconazole and
5-FU.
In Fig. 4, the
apoptotic cells are mostly located in the forth quadrant. These
cells were identified to be early apoptotic cells, as they did not
take up PI. The concentration of itraconazole and 5-FU used and
incubation time were selected following cell viability assay and
analysis of a combination of the two agents. However, the drug
concentration and incubation time may have not been sufficient to
induce late apoptosis and potentially a longer incubation time
would lead to apoptosis, which should be examined in future
studies.
The treatment of SGC-7901 cells with a combination
of itraconazole and 5-FU was able to markedly increase the number
of cells in the S phase compared with treatment with itraconazole
or 5-FU alone. This may be due to the cells being inhibited and
arrested at the S phase, which consistent with the findings of
previous studies (18–22).
According to American Joint Committee on Cancer
(AJCC) staging system (23), all the
patients in the present study were diagnosed with stage IV gastric
cancer. A combination of 5-Fu and irinotecan was the most commonly
used regimen for advanced gastric cancer according to the National
Comprehensive Cancer Network guidelines (23). However, only 60 patients were enrolled
during the 7-year period, as there were not many patients that were
treated with itraconazole for mycotic infection during
chemotherapy. This limited number of patients might have affected
statistical accuracy, but it did not alter the outcomes of the
study.
All 60 enrolled patients were diagnosed with
advanced gastric cancer, and the final AJCC stage was stage IV. The
stage IV patients are usually treated with a combination of 5-FU
and irinotecan. Therefore, the majority of the patients were
treated with a combination of 5-FU and irinotecan.
In the present study, the 5-year survival rate was
very low (0%), which is due total 60 patients presented with stage
IV gastric cancer. These patients usually withdrew from treatments
due to financial reasons.
Although it was demonstrated by the present study
that itraconazole was able to inhibit proliferation and alter the
cell cycle of gastric cancer cells, there is limited knowledge on
the mechanism of action of itraconazole. Previous studies have
reported that itraconazole was able to inhibit the angiogenesis of
cancer by suppressing a number of signaling pathways such as
inhibiting the binding of vascular endothelial growth factor (VEGF)
to VEGF receptor 2 (VEGFR2) (5). In
the present study, it was demonstrated that itraconazole was able
to directly inhibit the proliferation of gastric cells.
The present study has a number of limitations. Due
to funding and time constraints, it was not possible to perform the
in vitro experiments using an additional gastric carcinoma
cell line. However, the present study forms a preliminary analysis
of the effects of itraconazole in conjunction with 5′FU. The
authors will conduct further experiments as well as a phase I study
to elucidate the underlying mechanisms of itraconazole and to
confirm the conclusions in the present study.
In addition, the inhibitory concentrations of
itraconazole and 5′FU might not be accurate, as the goodness of fit
(itraconazole, R2=0.919; 5-FU, R2=0.961) was
not perfect (24). However, it was
observed that the addition of itraconazole to 5-FU-based
chemotherapy was able to improve the survival of patients with
gastric cancer. The present study is a case-control study, and the
number of cases treated with itraconazole was limited. Therefore, a
randomized controlled trial is also required for further study.
In summary, treatment with itraconazole alone and in
combination with 5-FU was able to inhibit the growth of gastric
cancer cells in vitro and prolong the survival of patients
with gastric cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KeL and KZ performed the experimental procedures. WL
and ZX collected the data. KeL, KZ, RY, CD and KaL interpreted the
data and wrote the paper. KaL directed the research. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Committee for
the Conduct of Human Ethics Committee of the First Affiliated
Hospital of Xi'an Jiaotong University, and all patients signed
written informed consent forms.
Patient consent for publication
The patients have provided consent for the
publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bevan R, Young C, Holmes P, Fortunato L,
Slack R and Rushton L: British Occupational Cancer Burden Study
Group: Occupational cancer in Britain. Gastrointestinal cancers:
Liver, oesophagus, pancreas and stomach. Br J Cancer. 107 Suppl
1:S33–S40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim J, Tang JY, Gong R, Kim J, Lee JJ,
Clemons KV, Chong CR, Chang KS, Fereshteh M, Gardner D, et al:
Itraconazole, a commonly used antifungal that inhibits Hedgehog
pathway activity and cancer growth. Cancer Cell. 17:388–399. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim J, Aftab BT, Tang JY, Kim D, Lee AH,
Rezaee M, Kim J, Chen B, King EM, Borodovsky A, et al: Itraconazole
and arsenic trioxide inhibit Hedgehog pathway activation and tumor
growth associated with acquired resistance to smoothened
antagonists. Cancer Cell. 23:23–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sano T, Ozaki K, Kodama Y, Matsuura T and
Narama I: Effects of the antifungal agent itraconazole on
proliferative changes of the forestomach mucosa in alloxan-induced
diabetic rats. Toxicol Pathol. 37:790–798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nacev BA, Grassi P, Dell A, Haslam SM and
Liu JO: The antifungal drug itraconazole inhibits vascular
endothelial growth factor receptor 2 (VEGFR2) glycosylation,
trafficking, and signaling in endothelial cells. J Biol Chem.
286:44045–44056. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu R, Li J, Zhang T, Zou L, Chen Y, Wang
K, Lei Y, Yuan K, Li Y, Lan J, et al: Itraconazole suppresses the
growth of glioblastoma through induction of autophagy: Involvement
of abnormal cholesterol trafficking. Autophagy. 10:1241–1255. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzman DL and Antonarakis ES: High-dose
itraconazole as a noncastrating therapy for a patient with
biochemically recurrent prostate cancer. Clin Genitourin Cancer.
12:e51–e53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aftab BT, Dobromilskaya I, Liu JO and
Rudin CM: Itraconazole inhibits angiogenesis and tumor growth in
non-small cell lung cancer. Cancer Res. 71:6764–6772. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blaschke M, Cameron S, Goeschen C and
Ramadori G: 5-FU schedules, serum 5-FU levels and their
relationship to therapy response and toxicity in patients with
gastrointestinal cancer. Int J Clin Pharmacol Ther. 51:56–58. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai J, Chen S, Zhang W, Wei Y, Lu J, Xing
J and Dong Y: Proteomic analysis of differentially expressed
proteins in 5-fluorouracil-treated human breast cancer MCF-7 cells.
Clin Transl Oncol. 16:650–659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao L, Wientjes MG and Au JL: Evaluation
of combination chemotherapy: Integration of nonlinear regression,
curve shift, isobologram, and combination index analyses. Clin
Cancer Res. 10:7994–8004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pandita A, Kumar B, Manvati S, Vaishnavi
S, Singh SK and Bamezai RN: Synergistic combination of gemcitabine
and dietary molecule induces apoptosis in pancreatic cancer cells
and down regulates PKM2 expression. PLoS One. 9:e1071542014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scarano W, de Souza P and Stenzel MH:
Dual-drug delivery of curcumin and platinum drugs in polymeric
micelles enhances the synergistic effects: A double act for the
treatment of multidrug-resistant cancer. Biomater Sci. 3:163–174.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh NP and Stephens RE: Microgel
electrophoresis: Sensitivity, mechanisms, and DNA
electrostretching. Mutat Res. 383:167–175. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao W, Jiang L, Ge L, Chen M, Geng C, Yang
G, Li Q, Ji F, Yan Q, Zou Y, et al: Sterigmatocystin-induced
oxidative DNA damage in human liver-derived cell line through
lysosomal damage. Toxicol In Vitro. 29:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nacev BA and Liu JO: Synergistic
inhibition of endothelial cell proliferation, tube formation, and
sprouting by cyclosporin A and itraconazole. PLoS One.
6:e247932011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Antonarakis ES, Heath EI, Smith DC,
Rathkopf D, Blackford AL, Danila DC, King S, Frost A, Ajiboye AS,
Zhao M, et al: Repurposing itraconazole as a treatment for advanced
prostate cancer: A noncomparative randomized phase II trial in men
with metastatic castration-resistant prostate cancer. Oncologist.
18:163–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rudin CM, Brahmer JR, Juergens RA, Hann
CL, Ettinger DS, Sebree R, Smith R, Aftab BT, Huang P and Liu JO:
Phase 2 study of pemetrexed and itraconazole as second-line therapy
for metastatic nonsquamous non-small-cell lung cancer. J Thorac
Oncol. 8:619–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim DJ, Kim J, Spaunhurst K, Montoya J,
Khodosh R, Chandra K, Fu T, Gilliam A, Molgo M, Beachy PA and Tang
JY: Open-label, exploratory phase II trial of oral itraconazole for
the treatment of basal cell carcinoma. J Clin Oncol. 32:745–751.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsubamoto H, Sonoda T, Yamasaki M and
Inoue K: Impact of combination chemotherapy with itraconazole on
survival of patients with refractory ovarian cancer. Anticancer
Res. 34:2481–2487. 2014.PubMed/NCBI
|
|
23
|
Ajani JA, D'Amico TA, Almhanna K, Bentrem
DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, et
al: Gastric Cancer, Version 3.2016, NCCN Clinical Practice
Guidelines in Oncology. J Natl Compr Canc Netw. 14:1286–1312. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
What is a good value for R-squared?
https://people.duke.edu/~rnau/rsquared.htmMay
7–2016
|