Introduction
The human ether-a-go-go-related gene (hERG) encodes
the pore-forming subunits of the rapid delayed rectifier potassium
channel (1). Studies have reported
that a wide variety of therapeutic compounds could induce acquired
long QT syndrome (acLQTS) by inhibiting hERG channels, including
antiarrhythmic drugs and antitumor agents (2–4). By
contrast, the association between the enhanced activation of hERG
channels and tumorigenesis remain unclear. Therefore, it is
essential to examine novel compounds or therapies for restoring the
physiological state of ion channel balance.
Arsenic trioxide (As2O3), a
traditional Chinese medicine, is an effective treatment for acute
pro-myelocytic leukemia (APL) (5,6). Previous
studies have demonstrated that As2O3 has an
inhibitory effect on the proliferation of various types of cancer,
including APL, gastric carcinoma and breast cancer (7,8). On the
other hand, the carcinogenic effects of As2O3
have been concurrently reported. It has been demonstrated that
low-dose As2O3 can promote the occurrence and
development of tumors (9–11). However, the underlying mechanism of
this effect remains unclear. In addition, increasing evidence has
indicated that plasma membrane hERG channels are aberrantly
expressed in various types of cancer and serve essential roles in
numerous crucial cellular events (12,13).
Inhibition of the hERG channel has indicated a significant
therapeutic effect in various types of tumors (12,14). Based
on previous findings that As2O3 could
regulate the protein expression level of hERG through multiple
signal pathways (15), we hypothesize
that a low dose of As2O3 exerts ahormesis
effect by affecting the expression and/or function of hERG
channels.
In the present study, the possible role of hERG in
tumor progression was investigated when tumor cells were exposed to
low-dose As2O3 (0.1 µM). Notably, the data
demonstrate that a low dose of As2O3 has a
hormesis effect on tumor cells, possibly due to the involvement of
hERG channels. To the best of our knowledge, we are the first to
hypothesize that the hERG channel participates in the dual effects
of As2O3 in tumor cells. Prior to this,
As2O3 was considered to be an hERG channel
inhibitor (15,16) and that a low dose of
As2O3, compared with a high dose of
As2O3, could exert an inverse effect.
Furthermore, As2O3 has been demonstrated to
have a dual effect on hERG channels according to different
concentrations, providing a theoretical basis for the potential
clinical use of As2O3. Further research
examining the crucial dosages As2O3 for
apoptosis and hormesis processes is required.
Materials and methods
Cell culture
The following cell lines were purchased from Chinese
Peking Union Medical College (Peking, China): 293 cells that stably
expressed the wild-type hERG gene (hERG-293), the breast cancer
cell line, MCF-7, and the lung cancer cell line A549. hERG-293
cells were cultured in DMEM medium containing 10% (v/v) fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and 400 mg/ml of geneticin (G-418; Invitrogen; Thermo
Fisher Scientific, Inc.). MCF-7 cells and A549 cells were grown in
DMEM and Kaighn's Modification of Ham's F-12 (F-12K) medium (both
Gibco; Thermo Fisher Scientific, Inc.) respectively, with 10% (v/v)
FBS. All cells were cultured at 37°C in 5% CO2. The
present study was approved by the Ethical Committee of the Harbin
Medical University (Heilongjiang, China).
Whole cell patch-clamp recordings
hERG currents were recorded in the whole cell
voltage-clamp (VC) mode, and action potential durations (APDs) were
recorded in the current-clamp mode with an Axopatch 200B amplifier
(Molecular Devices, LLC, Sunnyvale, CA, USA) in hERG-HEK293 cells.
Heat-polished patch pipettes had tip resistances of 1–3 MΩ when
filled with the internal pipette solution. The pipette solution for
hERG current recording contained 130 mM KCl, 1 mM
MgCl2·6H2O, 10 mM HEPES, 5 mM Mg-ATP, 5 mM
EGTA, and 0.1 mM GTP (pH 7.3 with KOH). The extracellular solution
contained 136 mM NaCl, 5.4 mM KCl, 5 mM HEPES, 1 mM
MgCl2·6H2O, 1 mM CaCl2 and 10 mM
glucose (pH 7.4 with NaOH). hERG currents were evoked through a
4-sec repolarizing step to −50 mV and followed by a 2-sec
depolarization step with a 10 mV stepwise increase from −60 to 40
mV, with the primary holding potential being −80 mV. For the
activation curves of hERG channels, cells were evoked through a
2.5-sec repolarizing step to an +40 mV inactivation step, following
a 10 mV stepwise increase from −120 to +20 mV, while the onset of
inactivation curves was recorded with a 10 mV stepwise increase
from −120 to +30 mV. The time interval of onset of inactivation was
investigated by fitting a single exponential function to current
traces in the third pulse of the protocol. The recovery of hERG
channels from inactivation curves was recorded with a 10 mV
stepwise increase from −120 to +30 mV. The time constant of
recovery from inactivation was calculated by fitting a single
exponential function to the peak of tail current traces between −60
and −30 mV. Experiments were recorded and analyzed on one cell per
recording. Currents were fitted to a Boltzmann function using
Clampfit 9.2 (Axon Instruments; Molecular Devices, LLC, Sunnyvale,
CA, USA), where V1/2 is the half-maximum activation
voltage.
Western blot analysis
The protein expression level of hERG was monitored
using western blot analysis. As2O3 was
diluted (0.1, 1 and 10 µM) and added to 1×106 hERG-293
cells for 24 h at 37°C prior to analysis by western blotting. The
cells were washed using ice-cold PBS. Subsequently, protein was
harvested with radio immunoprecipitation assay buffer containing 1%
protease inhibitor (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Protein concentration was assessed using the bicinchoninic acid
assay (Beyotime Institute of Biotechnology, Haimen, China). A total
of 150 µg protein was loaded per lane and separated using 8%
SDS-PAGE. Proteins were then transferred to nitrocellulose
membranes (Agilent Technologies, Inc., Santa Clara, CA, USA) and
incubated with 5% non-fat milk (BD Biosciences, Franklin Lakes, NJ
or San Jose, CA, USA) for 2 h at room temperature. The membranes
were subsequently incubated with primary antibodies against hERG
(dilution, 1:1,000; cat. no., sc-20130; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and β-actin (dilution, 1:500; cat. no.,
TA-09; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing,
China) overnight at 4°C with agitation. The membranes were washed
with 0.05% Tris-buffered saline (TBST) three times and incubated
with IRDye 800CW-labelled, goat anti-rabbit IgG secondary
antibodies (1:8,000; cat. no. 926–32211; Li-Cor Inc., Lincoln, NE,
USA) for 1 h in the dark at room temperature. Subsequent to being
washed three times with 0.05% TBST, bands were detected and
analyzed with the Odyssey CLx Infrared imaging system (LI-COR
Biosciences, Lincoln, NE, USA). To quantify the western blotting
data, the intensities of proteins of interest in each gel were
firstly normalized to their respective actin intensities, then the
normalized intensities were compared with the intensity of the
control group and expressed as relative values to their
controls.
MTT assay
Proliferation was assessed using an MTT assay.
Briefly, 5×103 cells were treated with various
concentrations (0.1, 1 and 10 µM) of As2O3
for 24 and 72 h. Next, a mixture of 180 µl culture medium and 20 µl
MTT reagent (Sigma-Aldrich; Merck KGaA) was added to each well.
Subsequently, 100 µl DMSO was added to each well and mixed to
solubilize the colored crystals. The absorbance was measured at 490
nm after 4 h of incubation at 37°C.
Flow cytometry (FCM) analysis of
apoptosis
Quantitative assessment of apoptosis was conducted
using an Annexin V Assay kit (Beyotime Institute of Biotechnology).
Cells were centrifuged at 1,000 × g for 5 min at 4°C following
trypsinization, then washed with ice-cold PBS twice. The pellet was
resuspended in the ice-cold binding buffer provided with the kit.
Subsequently, 10 µl Annexin V-FITC and 5 µl propidium iodide (PI)
were added to the cell suspension, which was maintained on ice in
the dark for 15 min. The samples were then assessed for viable
(Annexin V−/PI−), early apoptotic (Annexin
V+/PI−), late apoptotic (Annexin
V+/PI+) and necrotic (Annexin
V−/PI+) cells using a flow cytometer
(Fc500MDL) and analyzed by CXP Analysis Software (version 2.0)
(both Beckman Coulter, Inc., Brea, CA, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. The Student's t-test was used for comparisons between two
groups, and analysis of variance was used for the comparison of
multiple groups with the Student-Newman-Keuls post hoc test.
P<0.05 (two-tailed) was considered to indicate a statistically
significant difference. GraphPad Prism 5.0 software was used for
statistical analysis (GraphPad Software, Inc., La Jolla, CA,
USA).
Results
Effects of hormesis on tumor cells
induced by As2O3
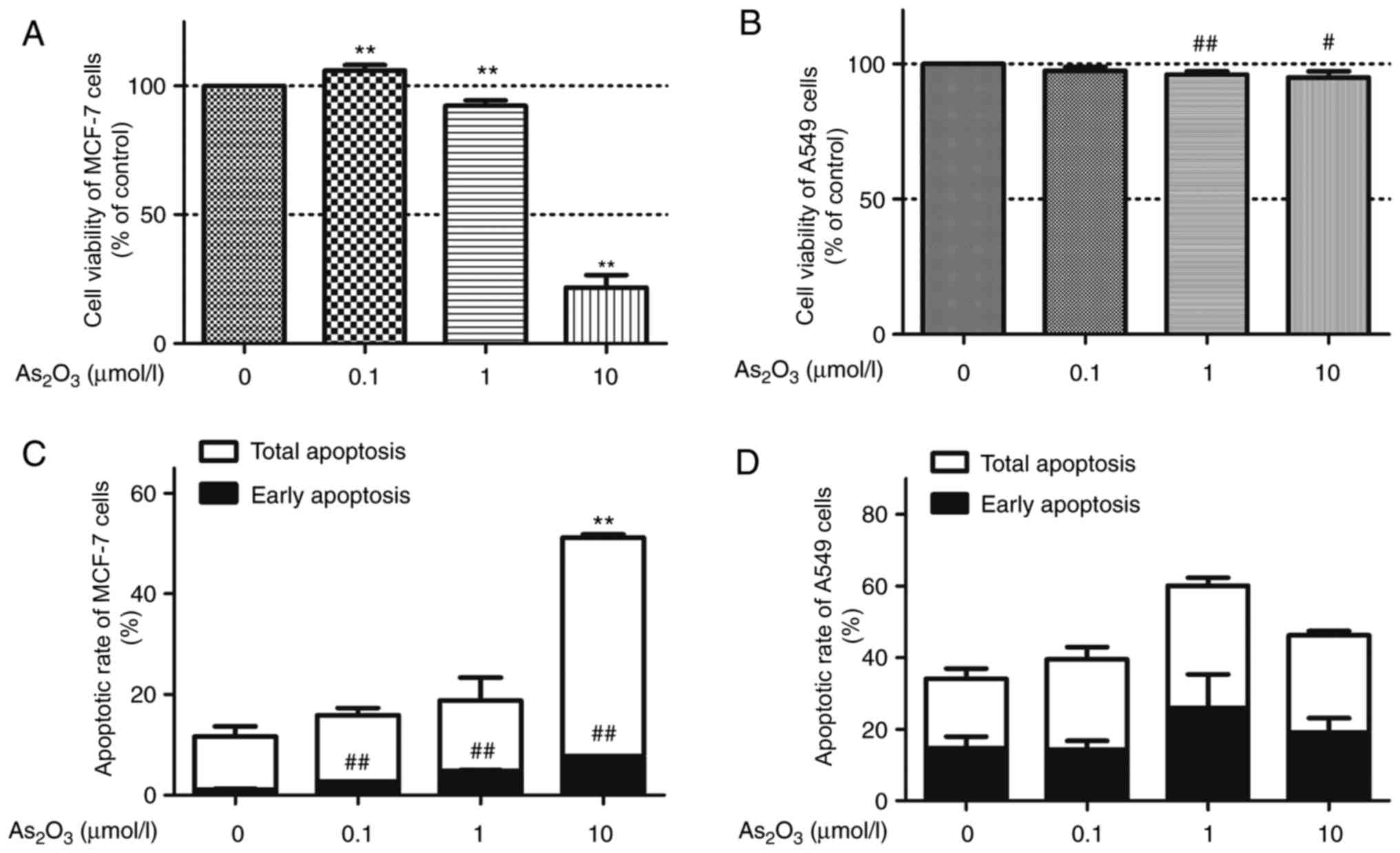
The viability in response to
As2O3 stimulation was compared in two types
of cancer cells: MCF-7 and A549 cells. In our previous screening
experiments, it was indicated that MCF-7 cells overexpressed hERG
channels, whereas A549 cells rarely expressed hERG channels. In
contrast with the A549 cells, the MCF-7 cells expressed endogenous
hERG channels (17). An MTT assay was
performed to assess viability following treating the cells with
0.1, 1 and 10 µM of As2O3 for 72 h (Fig. 1A). Application of 1 or 10 µM
As2O3 produced a suppressive effect on MCF-7
cell proliferation, resulting in a 7.69 and 78.30% reduction in
MCF-7 cell viability at 1 and 10 µM, respectively. Notably, a dual
effect of As2O3 was observed, since MCF-7
cell viability increased by ~6% in the 0.1 µM
As2O3 group compared with the control group.
However, a low concentration (0.1 µM) of
As2O3 did not express the hormesis effect on
the proliferation of A549 cells. A high dose of
As2O3 (1 and 10 µM) reduced the viability of
A549 cells in a concentration-dependent manner, while 0.1 µM
As2O3 did not significantly affect A549
proliferation compared with the control group (Fig. 1B).
Considering that numerous antitumor drugs suppress
proliferation and simultaneously promote apoptosis, a
quantification of As2O3-induced apoptotic
cells was conducted via flow cytometry with Annexin V/PI analysis.
MCF-7 cells and A549 cells were treated with 0.1, 1 and 10 µM of
As2O3 for 72 h. As2O3
treatment resulted in a dose-dependent increase in apoptotic rate
in MCF-7 cells compared with control (Fig. 1C). Subsequent to treatment with 0.1, 1
and 10 µM As2O3 for 72 h, apoptotic rate of
15.9, 18.8 and 51.1% in MCF-7 cells were observed, respectively.
However, As2O3 did not indicate significant
cytotoxic effects in A549 cells (Fig.
1D; P>0.05).
In the apoptosis experiments, all cells were
cultured without FBS for 72 h, therefore only apoptosis occurred.
However, in the proliferation experiments, all the cells were
cultured with FBS for 72 h, resulting in proliferation and
apoptosis. Therefore, the data primarily demonstrated that a low
dose of As2O3 could stimulate MCF-7
proliferation, whereas no significant effect on A549 cells was
exhibited.
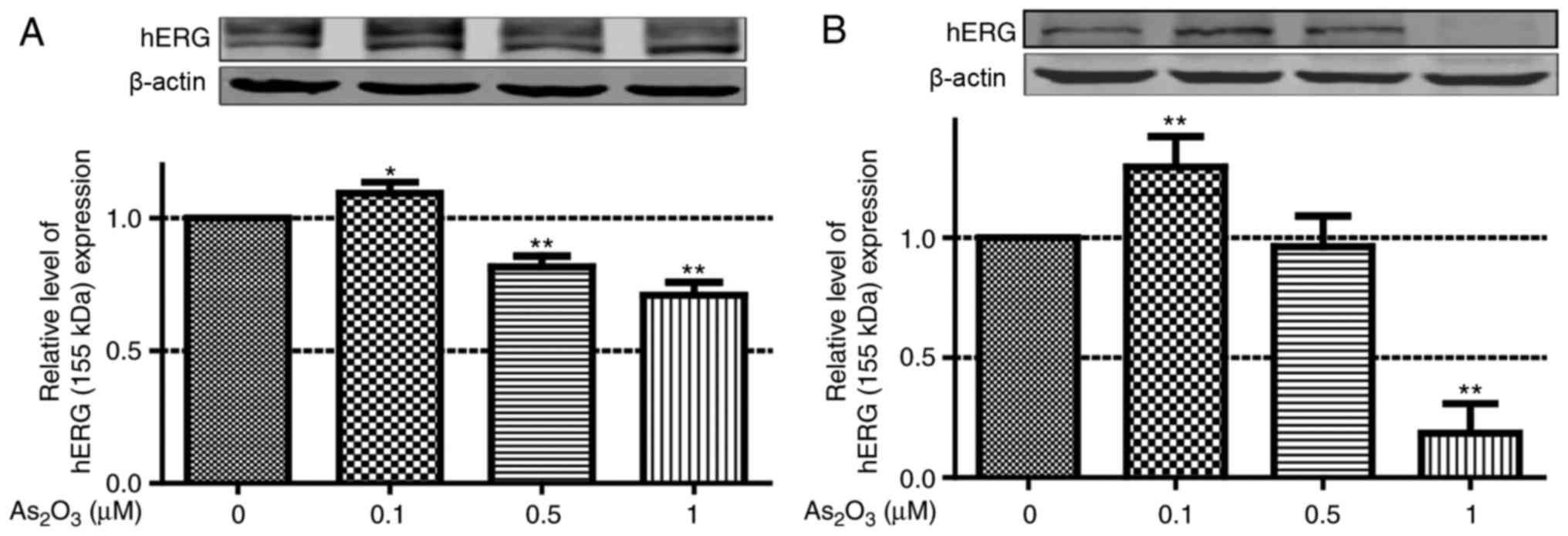
Dual effects of
As2O3 on the expression of hERG channels
In our previous study, it was demonstrated that 3 µM
As2O3 could damage the hERG current and
downregulate the expression of hERG channels by disturbing the
trafficking process of hERG protein to the cellular membrane
(18). However, the effect of
low-dose As2O3 on hERG channels requires
further investigation. Therefore, in the present study, western
blot analysis was used to identify the effect of low-dose
As2O3 on the protein expression level of
hERG. Following incubation with low-dose
As2O3 (0.1, 0.5 and 1 µM) for 24 h, hERG
protein expression level in hERG-293 cells was examined. Doses of
0.5 and 1 µM As2O3 significantly reduced hERG
protein expression. By contrast, 0.1 µM As2O3
increased hERG protein expression (Fig.
2A). Subsequently, the endogenous hERG levels were also
determined in MCF-7 cells, as well as following treatment with 0.1,
1 and 10 µM As2O3 for 24 h (Fig. 2B). The protein expression level of
hERG in the 0.1 µM As2O3 group increased by
35.17% compared with the control group.
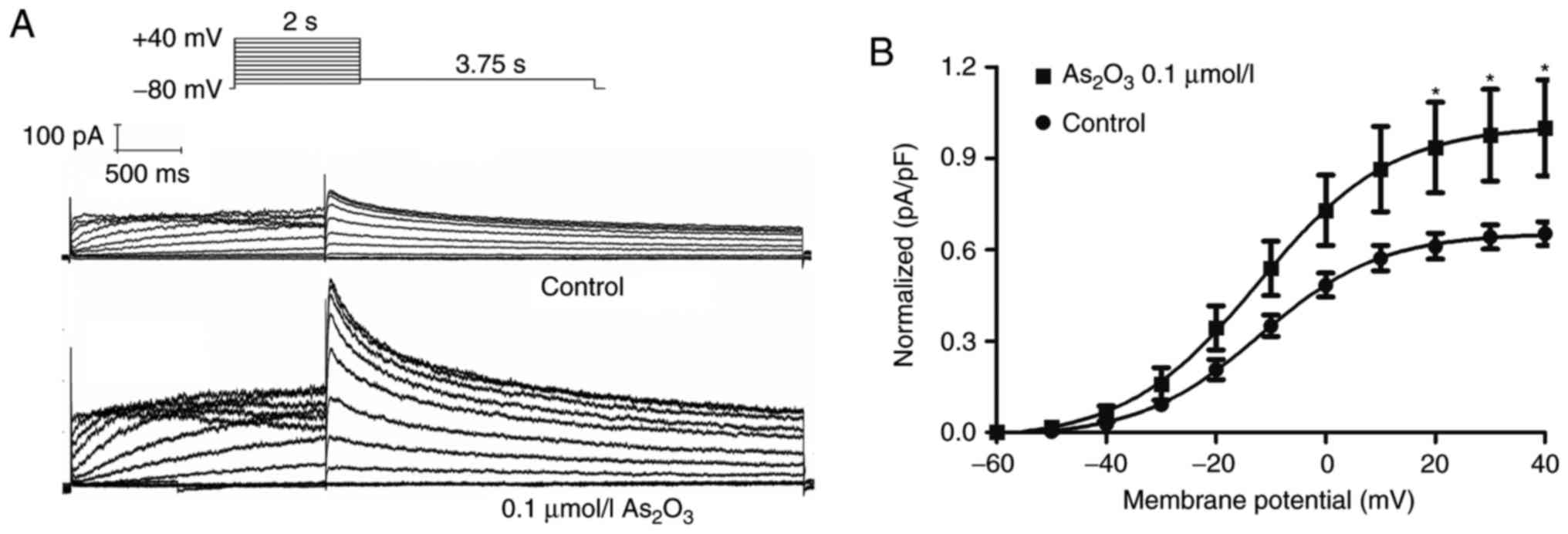
Low-dose As2O3
enhances the expression of functional hERG channels
Since the transport speed of ions through the
membrane depends on the number of functional ion channel proteins,
it is necessary to detect the current (Ikr or
IhERG) to verify whether 0.1 µM
As2O3 could enhance the expression of
functional hERG channels. Patch clamp recordings were conducted to
detect the effect of 0.1 µM As2O3 on hERG-293
cells after 24 h of incubation. IhERG was elicited
during 2-sec depolarizations (ranging from −60 to +40 mV) from a
holding potential of −80 mV, then a repolarization to −50 mV was
used to elicit the tail current (Fig.
3A). It was demonstrated that 0.1 µM
As2O3 enhanced the hERG tail current
amplitude by 53.14% (Fig. 3B). These
results suggest that 0.1 µM As2O3
significantly upregulated the expression of functional hERG
channels.
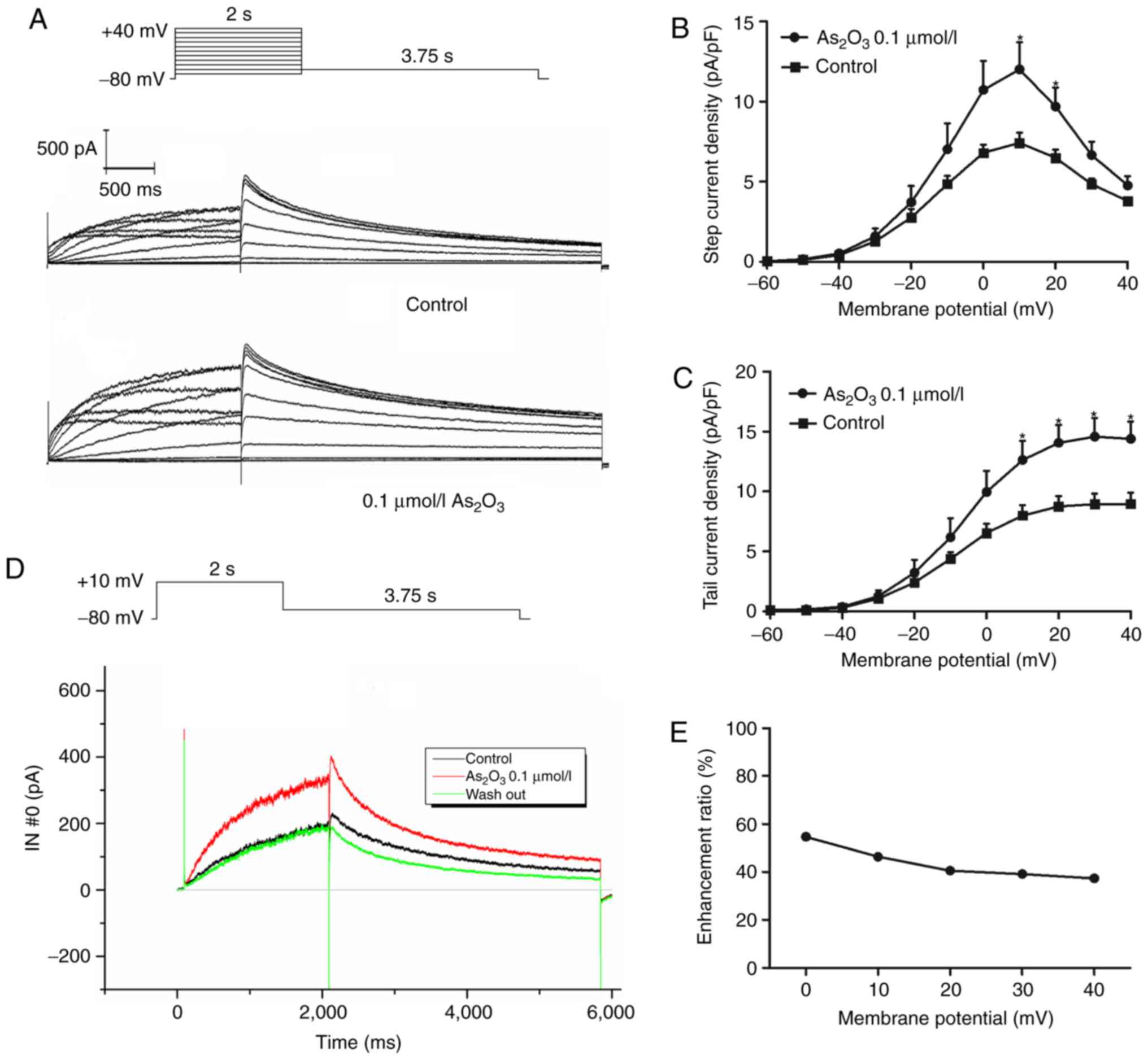
Low-dose As2O3
accelerates hERG channel activation
The properties and/or kinetics of potassium channels
are often altered by drugs. Therefore, the activation, steady-state
inactivation, and the time courses of the hERG channel were
determined in the control and the
As2O3-treated groups. The transient effect of
0.1 µM As2O3 on the hERG current was
measured. To investigate the effect of low-dose
As2O3 (0.1 µM) on hERG potassium channels,
patch-clamp experiments were performed on 293 cells stably
expressing the wild-type hERG gene. The current-voltage (I–V)
association is indicated in Fig. 4.
Whole-cell current was first recorded under control conditions,
then 0.1 µM As2O3 was added through perfusion
equipment at 1.5 ml/min for 5 min prior to the next recording. In
the presence of 0.1 µM As2O3, the hERG
current was notably increased and could be subsequently reversed
subsequent to washing out As2O3. The
activation current and tail current reached peak amplitudes at 10
and 20 mV, respectively. The ratio of enhancement was calculated
using the activation current from 0 to 20 mV and tail current from
0 to 40 mV with the function:
(Iato-Ictl)/Ictl. These
electrophysiological results suggested that 0.1 µM
As2O3 had a significant effect (P<0.05) on
the activation of hERG channels.
Low-dose As2O3
treatment affects hERG channel kinetics
In a previous study, it was reported that various
drugs can influence ion channels by altering the voltage dependence
or kinetics of channel gating (19).
To investigate the underlying mechanism of the enhanced hERG
currents induced by low dose As2O3 with
transient administration, its effects on the voltage dependence of
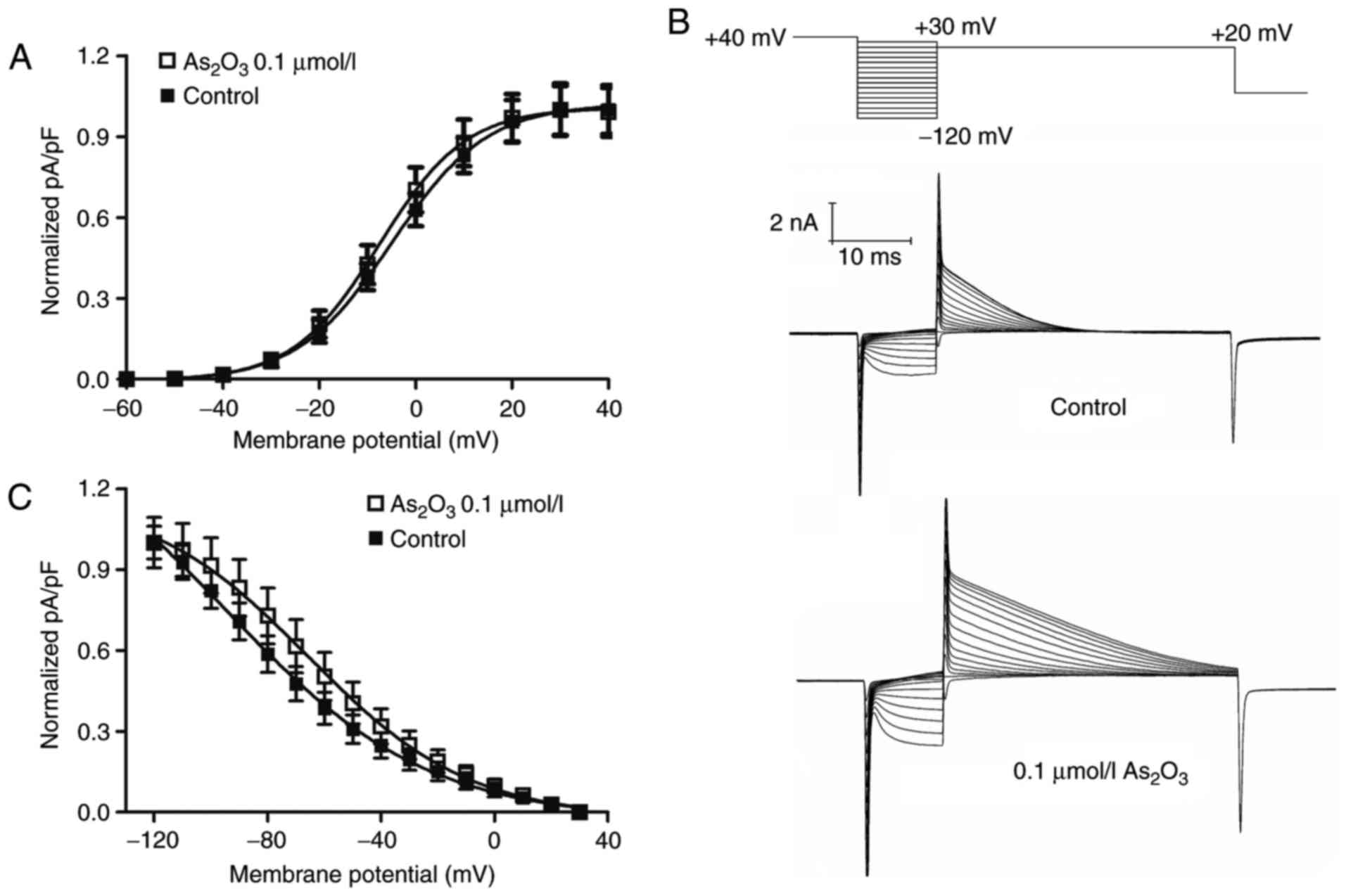
activation and inactivation were examined (Fig. 5). To obtain the half maximal voltage,
a Boltzmann function was fitted to the data. The results indicated
that As2O3 shifted the half maximal
activation voltage of hERG channels slightly towards negative
potentials, from −4.95±0.26 mV (in the control group) to −7.50±0.25
mV (Fig. 5A) and half maximal
inactivation voltage to more positive potentials, from −94.64±5.25
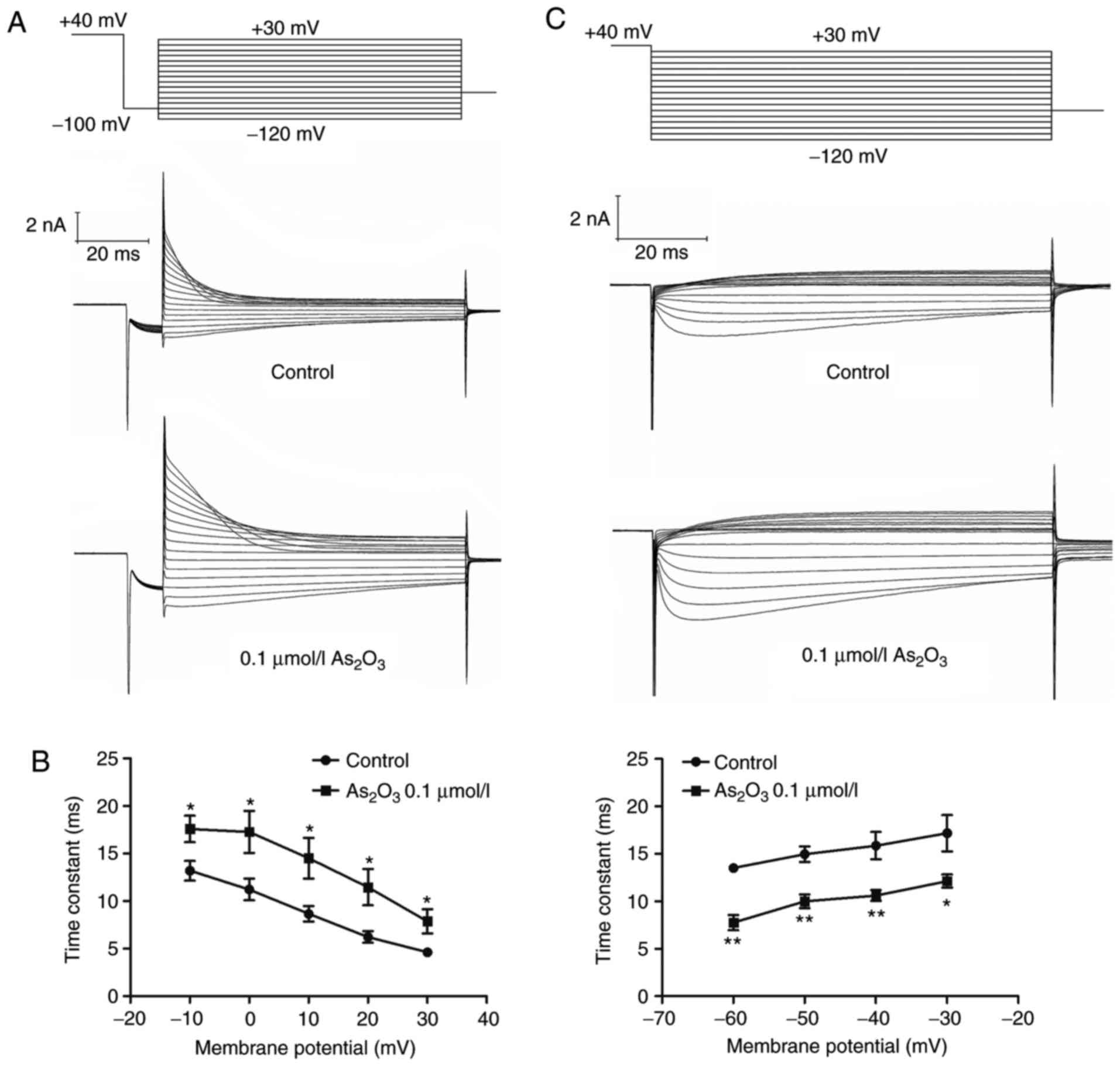
mV (in the control group) to −65.58±1.71 mV (Fig. 5C). The time interval of onset of
inactivation was investigated by fitting a single exponential
function to current traces in the third pulse of the protocol
(Fig. 6A). The statistical data
(Fig. 6B) indicated that inactivation
was slower in the presence of As2O3. The
result indicated that low-dose As2O3
significantly altered the hERG channel kinetics by decreasing the
time period of recovery from inactivation (Fig. 6C).
Discussion
As2O3 is a popular therapeutic
agent for various types of cancer and has been demonstrated to have
a high therapeutic effect in APL. However, the adverse effects of
arsenic trioxide, including cardiotoxicity and carcinogenicity,
remain a clinical problem (20). The
adverse effects of chronic arsenic toxicity in humans are referred
to as arsenicosis (21,22). Although researchers have reported
significant associations between the occurrence of tumors and
arsenicosis, the underlying mechanism remains unclear.
The most common oxidation states of arsenic include
arsenite and arsenate (23). Among
these, As2O3 is the most prevalent inorganic
arsenical found in the air or in underground water
(AsO2−) (24).
Therefore, the present study was mainly based on
As2O3 and not arsenate. In our previous
studies, As2O3 was demonstrated to regulate
the expression of hERG channels (18). In addition, it has been demonstrated
that the proliferation-facilitating effect of
As2O3 is significantly more pronounced in
hERG-expressing cells than in hERG-lacking tumor cells (25), which indicates that hERG channels
serve an important role in the regulation of tumor progression.
Accordingly, we hypothesized that hERG channels may be involved in
the process of arsenicosis. To investigate this, viability and
apoptotic rate was compared in response to
As2O3 stimulation in MCF-7 and A549 cells.
The results indicate that a medium or high dose of
As2O3 inhibited proliferation and promoted
apoptosis. However, a low dose of As2O3
treatment simultaneously promoted proliferation and increased
apoptosis in MCF-7 cells compared with control group. This hormesis
phenomenon is similar to that of the tumor necrosis factor (TNF)
superfamily. TNF has been demonstrated to be an endogenous cancer
promoter that stimulates cancer cell invasion and proliferation
(22,23), but by contrast, TNF has also been
proven to be an endogenous cancer killer (26). This hormesis phenomenon was not
observed in A549 cells. This suggests that MCF-7 cells were more
sensitive than A549 cells to As2O3.
The hormesis of As2O3 has also
been demonstrated by other research groups (10,25). It
was reported that a low dose of As2O3 could
stimulate osteoblast and MTLn3 cell proliferation (10). Wang et al (27) demonstrated that hERG protein
expression is associated with tumor-cell proliferation and cancer
development. To address whether hERG channels were involved in this
process, 293 cells overexpressing hERG channels were used in the
present study. hERG current and electrophysiological
characteristics are relatively easy to detect in this cell model
(28–30). hERG-293 cells were incubated with 0.1,
0.5 and 1 µM As2O3 for 24 h. The results
demonstrated that 0.1 µM As2O3 was able to
increase the expression of hERG channels compared with the control
group. In addition, 0.1 µM As2O3 increased
endogenous hERG expression in MCF-7 cells. This is notable since it
was previously accepted that As2O3 was a hERG
channel inhibitor. This is the first study, to the best of our
knowledge, to demonstrate that a low dose of
As2O3 could exert an inverse effect compared
with high-dose administration.
To verify the results, the influence of a low dose
of As2O3 on hERG current and channel kinetics
was analysed. The results indicated that a low dose of
As2O3 not only increased the hERG current,
but also accelerated hERG channel activation. In addition, low-dose
As2O3 treatment markedly affected hERG
channel kinetics by causing a slower rate of channel inactivation
and faster recovery time, compared with the untreated cells.
The hERG channel has previously been demonstrated
to positively regulate tumor proliferation (31). The data of the present study revealed
that a low dose of As2O3 promoted hERG
protein expression and provided a plausible explanation for
arsenicosis-induced tumorigenesis. However, the present study did
not investigate the involvement of any other molecules or signaling
pathways, which could affect this process. Therefore, further
research is required.
It should be noted that the research of the present
study also has certain limitations. Due to the specificity of tumor
cells and limitations in experimental techniques, particularly the
patch-clamp technique, tracking and recording the characteristics
of ion channels on tumor cells was not possible. Therefore,
hERG-293 cells were used as a mature cell model for the
investigation of electrophysiological characteristics of hERG
potassium channels, as previously described (18,32).
In summary, the present study provides evidence
that a low dose of As2O3 promotes
tumorigenesis through upregulating hERG channel expression and
affecting hERG channel kinetics. Although
As2O3 was previously demonstrated to be an
hERG channel inhibitor, it was demonstrated that a low dose of
As2O3 exhibits the opposite effect, promoting
hERG channel expression and enhancing hERG current. These findings
may help to identify therapeutic strategies for patients with
arsenicosis, and provide a novel understanding of the association
between As2O3 and hERG channels.
Acknowledgements
The authors would like to thank Dr Yuanqi Shi
(Department of Cardiology, The First Affiliated Hospital of Harbin
Medical University, Harbin, China) for providing assistance with
the patch-clamp technology.
Funding
This study was supported by grants from the Major
Program of the National Natural Science Foundation of Heilongjiang
(grant no. ZD 2015015), and the National Natural Science Foundation
of China (grant nos. 81673636 and 81173050).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
XZ is responsible for the writing and design of the
manuscript. DY is responsible for performance of the western blot
assay. HG is responsible for performance of the western blot assay.
FL is responsible for performance of the Patch clamp. LL is
responsible for performance of Flow Cytometry. LZ is responsible
for performance of the Patch clamp. CY is responsible for the
design of the subject and writing of the manuscript. BL is the
provider of the funding and is responsible for the study
design.
Ethics approval and consent to
participate
There is no clinical, patient, tissue or animal
experimentation involved in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Curran ME, Splawski I, Timothy KW, Vincent
GM, Green ED and Keating MT: A molecular basis for cardiac
arrhythmia: HERG mutations cause long QT syndrome. Cell.
80:795–803. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haverkamp W, Breithardt G, Camm AJ, Janse
MJ, Rosen MR, Antzelevitch C, Escande D, Franz M, Malik M, Moss A
and Shah R: The potential for QT prolongation and proarrhythmia by
non-antiarrhythmic drugs: Clinical and regulatory implications.
Report on a policy conference of the European Society of
Cardiology. Eur Heart J. 21:1216–1231. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farkas AS and Nattel S: Minimizing
repolarization-related proarrhythmic risk in drug development and
clinical practice. Drugs. 70:573–603. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kannankeril P, Roden DM and Darbar D:
Drug-induced long QT syndrome. Pharmacol Rev. 62:760–781. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bi LL, Ma Q and Wang SQ: Treatment of 32
cases with recurring acute promyelocytic leukemia with tablets of
composite natural indigo. Zhonghua Er Ke Za Zhi. 43:702–703.
2005.(In Chinese). PubMed/NCBI
|
|
6
|
Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J,
Cai X, Han ZG, Ni JH, Shi GY, Jia PM, et al: Use of arsenic
trioxide (As2O3) in the treatment of acute promyelocytic leukemia
(APL): I. As2O3 exerts dose-dependent dual effects on APL cells.
Blood. 89:3345–3353. 1997.PubMed/NCBI
|
|
7
|
Zhang TC, Cao EH, Li JF, Ma W and Qin JF:
Induction of apoptosis and inhibition of human gastric cancer
MGC-803 cell growth by arsenic trioxide. Eur J Cancer.
35:1258–1263. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ye J, Li A, Liu Q, Wang X and Zhou J:
Inhibition of mitogen-activated protein kinase kinase enhances
apoptosis induced by arsenic trioxide in human breast cancer MCF-7
cells. Clin Exp Pharmacol Physiol. 32:1042–1048. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang C, Chen X, Wu J, Liu H, Ji Z, Shi H,
Gao C, Han D, Wang L, Liu Y, et al: Low-dose arsenic trioxide
enhances 5-aminolevulinic acid-induced PpIX accumulation and
efficacy of photodynamic therapy in human glioma. J Photochem
Photobiol B. 127:61–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raja WK, Satti J, Liu G and Castracane J:
Dose response of MTLn3 cells to serial dilutions of arsenic
trioxide and ionizing radiation. Dose Response. 11:29–40. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ganapathy S, Li P, Fagman J, Yu T,
Lafontant J, Zhang G and Chen C: Low doses of arsenic, via
perturbing p53, promotes tumorigenesis. Toxicol Appl Pharmacol.
306:98–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Becchetti A, Crescioli S, Zanieri F,
Petroni G, Mercatelli R, Coppola S, Gasparoli L, D'Amico M,
Pillozzi S, Crociani O, et al: The conformational state of hERG1
channels determines integrin association, downstream signaling, and
cancer progression. Sci Signal. 10:pii: eaaf3236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Crociani O, Lastraioli E, Boni L, Pillozzi
S, Romoli MR, D'Amico M, Stefanini M, Crescioli S, Masi A, Taddei
A, et al: hERG1 channels regulate VEGF-A secretion in human gastric
cancer: Clinicopathological correlations and therapeutical
implications. Clin Cancer Res. 20:1502–1512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fortunato A: The role of hERG1 ion
channels in epithelial-mesenchymal transition and the capacity of
riluzole to reduce cisplatin resistance in colorectal cancer cells.
Cell Oncol (Dordr). 40:367–378. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ficker E, Kuryshev YA, Dennis AT,
Obejero-Paz C, Wang L, Hawryluk P, Wible BA and Brown AM:
Mechanisms of arsenic-induced prolongation of cardiac
repolarization. Mol Pharmacol. 66:33–44. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wan X, Dennis AT, Obejero-Paz C, Overholt
JL, Heredia-Moya J, Kirk KL and Ficker E: Oxidative inactivation of
the lipid phosphatase phosphatase and tensin homolog on chromosome
ten (PTEN) as a novel mechanism of acquired long QT syndrome. J
Biol Chem. 286:2843–2852. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen SZ, Jiang M and Zhen YS: Correlation
of HERG K+ channel protein expression to chemosensitivity of tumor
cells to doxorubicin and its modulation by erythromycin. Ai Zheng.
24:924–929. 2005.(In Chinese). PubMed/NCBI
|
|
18
|
Zhang Y, Dong Z, Jin L, Zhang K, Zhao X,
Fu J, Gong Y, Sun M, Yang B and Li B: Arsenic trioxide-induced hERG
K(+) channel deficiency can be rescued by matrine and oxymatrine
through up-regulating transcription factor Sp1 expression. Biochem
Pharmacol. 85:59–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nicholson GM, Blanche T, Mansfield K and
Tran Y: Differential blockade of neuronal voltage-gated Na(+) and
K(+) channels by antidepressant drugs. Eur J Pharmacol. 452:35–48.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pace C, Dagda R and Angermann J:
Antioxidants protect against arsenic induced mitochondrial
Cardio-toxicity. Toxics. 5:pii: E38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sarkar A and Paul B: The global menace of
arsenic and its conventional remediation-A critical review.
Chemosphere. 158:37–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oremland RS and Stolz JF: Arsenic,
microbes and contaminated aquifers. Trends Microbiol. 13:45–49.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Orloff K, Mistry K and Metcalf S:
Biomonitoring for environmental exposures to arsenic. J Toxicol
Environ Health B Crit Rev. 12:509–524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reeve BB, Burke LB, Chiang YP, Clauser SB,
Colpe LJ, Elias JW, Fleishman J, Hohmann AA, Johnson-Taylor WL,
Lawrence W, et al: Enhancing measurement in health outcomes
research supported by Agencies within the US Department of Health
and Human Services. Qual Life Res. 16 Suppl 1:S175–S186. 2007.
View Article : Google Scholar
|
|
25
|
Xu WX, Liu Y, Liu SZ, Zhang Y, Qiao GF and
Yan J: Arsenic trioxide exerts a double effect on osteoblast growth
in vitro. Environ Toxicol Pharmacol. 38:412–419. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin X, Chen Q, Huang C and Xu X: CYLD
promotes TNF-α-induced cell necrosis mediated by RIP-1 in human
lung cancer cells. Mediators Inflamm. 2016:15427862016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang H, Zhang Y, Cao L, Han H, Wang J,
Yang B, Nattel S and Wang Z: HERG K+ channel, a regulator of tumor
cell apoptosis and proliferation. Cancer Res. 62:4843–4848.
2002.PubMed/NCBI
|
|
28
|
Li P, Ninomiya H, Kurata Y, Kato M, Miake
J, Yamamoto Y, Igawa O, Nakai A, Higaki K, Toyoda F, et al:
Reciprocal control of hERG stability by Hsp70 and Hsc70 with
implication for restoration of LQT2 mutant stability. Circ Res.
108:458–468. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dong ZX, Zhao X, Gu DF, Shi YQ, Zhang J,
Hu XX, Hu MQ, Yang BF and Li BX: Comparative effects of liensinine
and neferine on the human ether-a-go-go-related gene potassium
channel and pharmacological activity analysis. Cell Physiol
Biochem. 29:431–442. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Z, Prinsen JK, Bersell KR, Shen W,
Yermalitskaya L, Sidorova T, Luis PB, Hall L, Zhang W, Du L, et al:
Azithromycin causes a novel proarrhythmic syndrome. Circ Arrhythm
Electrophysiol. 10:pii: e003560. 2017. View Article : Google Scholar
|
|
31
|
Staudacher I, Jehle J, Staudacher K, Pledl
HW, Lemke D, Schweizer PA, Becker R, Katus HA and Thomas D: HERG K+
channel-dependent apoptosis and cell cycle arrest in human
glioblastoma cells. PLoS One. 9:e881642014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi YQ, Yan M, Liu LR, Zhang X, Wang X,
Geng HZ, Zhao X and Li BX: High glucose represses hERG K+ channel
expression through trafficking inhibition. Cell Physiol Biochem.
37:284–296. 2015. View Article : Google Scholar : PubMed/NCBI
|