Introduction
Giant cell tumor of the bone (GCTB) is generally a
benign, but often locally aggressive osteolytic tumor which easily
causes severe bone destruction at the meta-epiphyseal region of the
long bones and more than half of GCTBs occur around the knee
(1,2).
With modern surgical techniques, more aggressive curettage may aid
in avoiding higher recurrence rates (3). However, it has been reported that
~10–60% of GCTBs exhibit local postoperative recurrence (4–6), 10% of
GCTBs undergo malignant transformation and up to 5% of GCTBs
exhibit pulmonary metastases (7).
GCTBs have diverse histological subtypes (8). Characterized by high proliferative
abilities, GCT stromal cells are the major type in GCTBs (9). Although numerous studies have focused on
cell proliferation and cell cycle regulation of GCTBs, which serve
a key role in the decreased recurrence rates and improved clinical
outcomes, there is little evidence of certain regulators or
signaling pathways, which could be regarded as predictive markers
for recurrence or metastasis. In this regard, it is necessary to
investigate the biological and clinical function of certain
molecules involved in the development and metastasis of GCTBs,
which may be used as novel biomarkers for the diagnosis and
prognosis of patients with giant cell tumors.
Programmed cell death 4 (PDCD4) is a novel tumor
suppressor and a promising candidate for a targeted molecular
therapy for tumors based on regulating different cellular signal
transduction pathways. PDCD4 could restrain the growth, malignant
transformation and metastasis of tumor cells at mRNA, protein and
cellular levels (10). It has been
reported that nuclear PDCD4 inhibits the activity of transcription
factor activator protein-1 (AP-1) and controls gene transcription
in mouse epidermal JB6 cells (11,12). PDCD4
could also suppress cap-dependent translation of mRNAs with highly
structured 5′-regions through interaction with the eukaryotic
translation initiation factor 4A helicase (13). Furthermore, PDCD4 gene knockout mice
developed spontaneous tumors of lymphoid origin (14). However, Jansen et al (15) observed a significant reduction in the
carcinoma incidence and papilloma-to-carcinoma conversion frequency
in PDCD4 transgenic mice compared with wild-type mice.
Recently, numerous studies have identified a
decreased expression of PDCD4 in multiple types of human cancer
cell lines and primary tumors, including cervical cancer (16), gastric cancer (17), glioma (18), hepatocellular carcinoma (19), gastrointestinal stromal tumors
(20) and nasal inverted papilloma
(21). Certain studies have
demonstrated that PDCD4 served a role in the progression of
osteocarcinoma. Nevertheless, the precise regulation of PDCD4 in
GCTBs remains largely unknown. In the present study, expression of
PDCD4 was decreased in GCTBs compared with adjacent non-tumor
tissues. In addition, it was demonstrated that abnormal PDCD4
expression level was associated with clinicopathological features,
including the Campanacci grade and recurrence.
Materials and methods
Clinical specimens
A total of 83 GCTB samples, including 27 frozen and
56 paraffin-embedded tissues, were collected from patients (median
age, 40 years old), who underwent surgery at the Department of
Orthopedics, Shandong Provincial Hospital Affiliated to Shandong
University from September 2015 to March 2017. The specimens were
immediately frozen in liquid N after surgery and stored at −80°C.
Written informed consent was obtained from all participants. The
present study was approved by the ethics guidelines of Chinese
Medical Association. The protocol was completely approved by the
Shandong Provincial Hospital Institutional Review Board (IRB). None
of the patients had received immunotherapy, radiotherapy or
chemotherapy prior to surgery. GCTBs were staged using the
Campanacci grading system (22).
RNA Extraction and quantitative
reverse transcription-PCR (qRT-PCR)
Total RNA was extracted from frozen tissues of
primary GCTBs using a modified TRIzol one-step extraction method
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
(23,24). First-strand cDNA was synthesized from
3 µg total RNA using the Revert Aid First Strand c-DNA Synthesis
kit (Promega Corporation, Madison, WI, USA) according to the
manufacturer's protocol. The PCR primer pairs specific for PDCD4
were as follows: Forward, 5′-CCAAAGAAAGGTGGTGCA-3′ and reverse,
5′-TGAGGTACTTCCAGTTCC-3′. The following thermocycling conditions
were used for the PCR: Initial denaturation at 94°C for 2 min; 35
cycles of denaturation at 95°C for 90 sec, annealing at 66°C for 90
sec and extension at 72°C for 90 sec. Human β-actin was used as an
internal control. The primers for β-actin were forward,
5′-CATGTACGTTGCTATCCAGGC-3′, and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′. Each sample was obtained from three
independent experiments and used for analysis of relative
normalized mRNA expression.
Western blot analysis
Protein lysates were separated by SDS-PAGE. The
concentration of protein was determined using BCA Protein Assay
(Beyotime Institute of Biotechnology, Haimen, China). Then, the
protein were transferred onto polyvinylidene difluoride membranes
and were blocked with 5% skim milk in TBST containing 0.1% Tween-20
for 1 h. The filters were incubated with primary antibodies against
PDCD4 (1:5,000, cat. no. 9535; Cell Signaling Technology, Inc.,
Danvers, MA, USA) and β-actin (1:1,000, sc-47778; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), followed by secondary
antibody (1:2,000, Anti-rabbit IgG, HRP-linked Antibody cat. no.
7074; Cell Signaling Technology, Inc.) conjugated with peroxidase
for 1 h at room temperature. The immune complexes were visualized
by the enhanced chemiluminescence reagent (SuperSignal West Pico
Chemiluminescent Substrate; Pierce; Thermo Fisher Scientific,
Inc.). Western blot analysis was performed at least 3 times for
each sample.
Immunohistochemistry
Tissue sections (4–6 µm) from frozen and paraffin
blocks were dewaxed in xylene and rehydrated in alcohol. Antigen
retrieval of sections was achieved by microwaving in citric saline
and treatment with 3% hydrogen peroxide. Immunohistochemical
staining using PDCD4 (1:100, PAB10308; Abnova, Taipei, Taiwan) and
anti-Ki-67 antibodies (1:500, M724029, Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA) was performed to delineate PDCD4
expression and cell proliferation in tumor samples. Sections were
also stained with hematoxylin. The intensity and percentage area of
PDCD4 staining was categorized into five grades: Score 0 (−), score
1 (+), score 2 (++), score 3 (+++), score 4 (++++) and score 5
(+++++). Scores of 4 or 5 indicated relatively high expression of
PDCD4, while scores of 0–3 indicated relatively low expression. All
staining experiments were performed in duplicate. Slides were
evaluated by two independent pathologists.
Statistical analysis
All statistical analyses were performed using SPSS
v.22.0 statistical software package (IBM Corp., Armonk, NY, USA).
Two-way analysis of variance with Student-Newman-Keuls post hoc
test or Student's t-test was performed to determine statistical
significance. The significance of differences between groups was
estimated by χ2 and Pearson's coefficient tests. The
associations were analyzed by Spearman's correlation and
multivariate regression analyses. All statistical analyses were
two-sided and values are presented as the mean ± standard error of
the mean. P<0.05 was considered to indicate a statistically
significant difference. Statistical significance was evaluated with
data from at least three independent experiments.
Results
Decreased expression of PDCD4 is
observed in GCTBs at the mRNA level
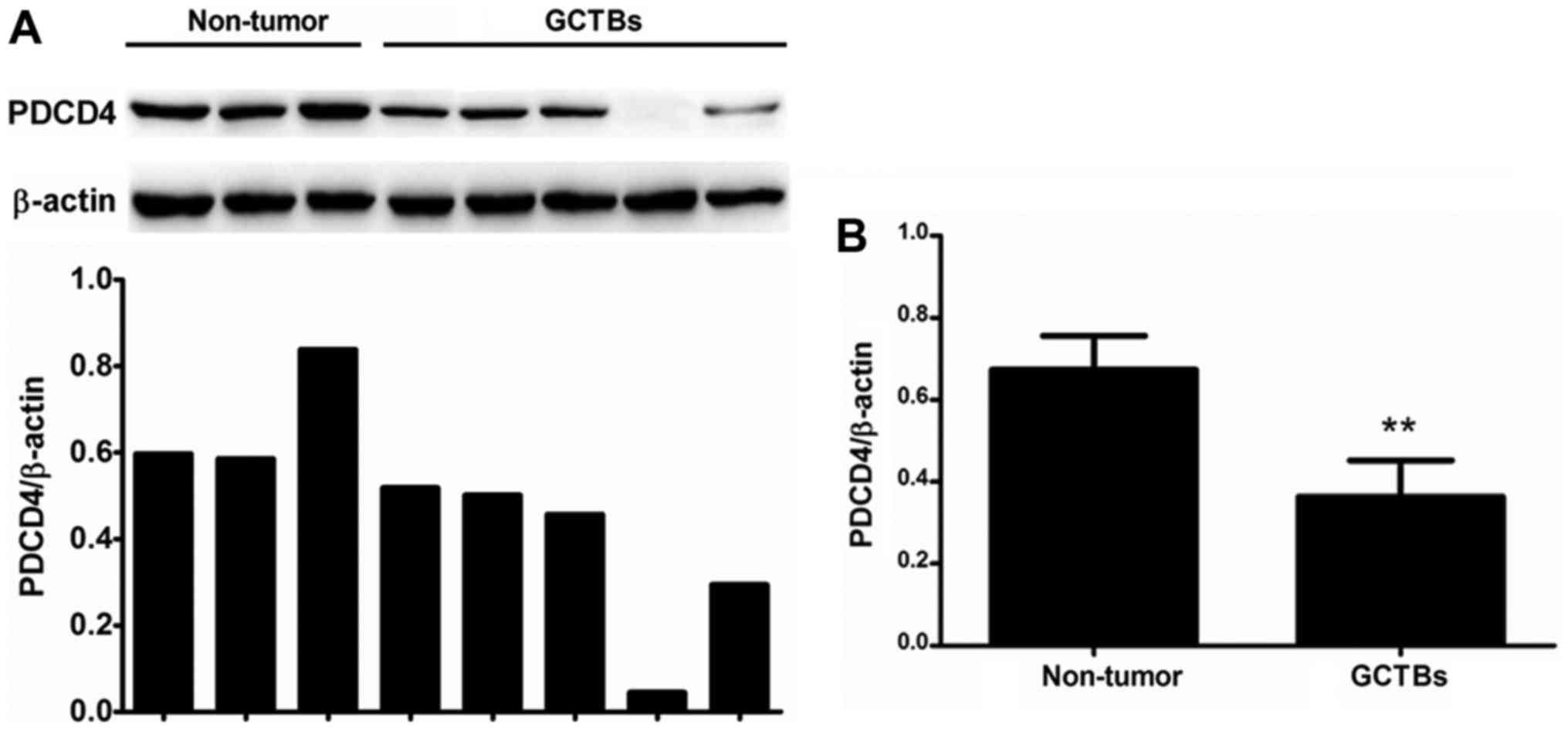
The present study first quantified the expression of
PDCD4 mRNA in primary GCTBs by qRT-PCR. The mRNA level of PDCD4 was
markedly decreased or absent in 63% (17/27) of the frozen GCTB
samples compared with adjacent non-tumorous tissues (Fig. 1A). The results demonstrated that there
was a significantly differential expression of PDCD4 between
primary GCTBs and non-tumorous tissues at the mRNA level (Fig. 1B).
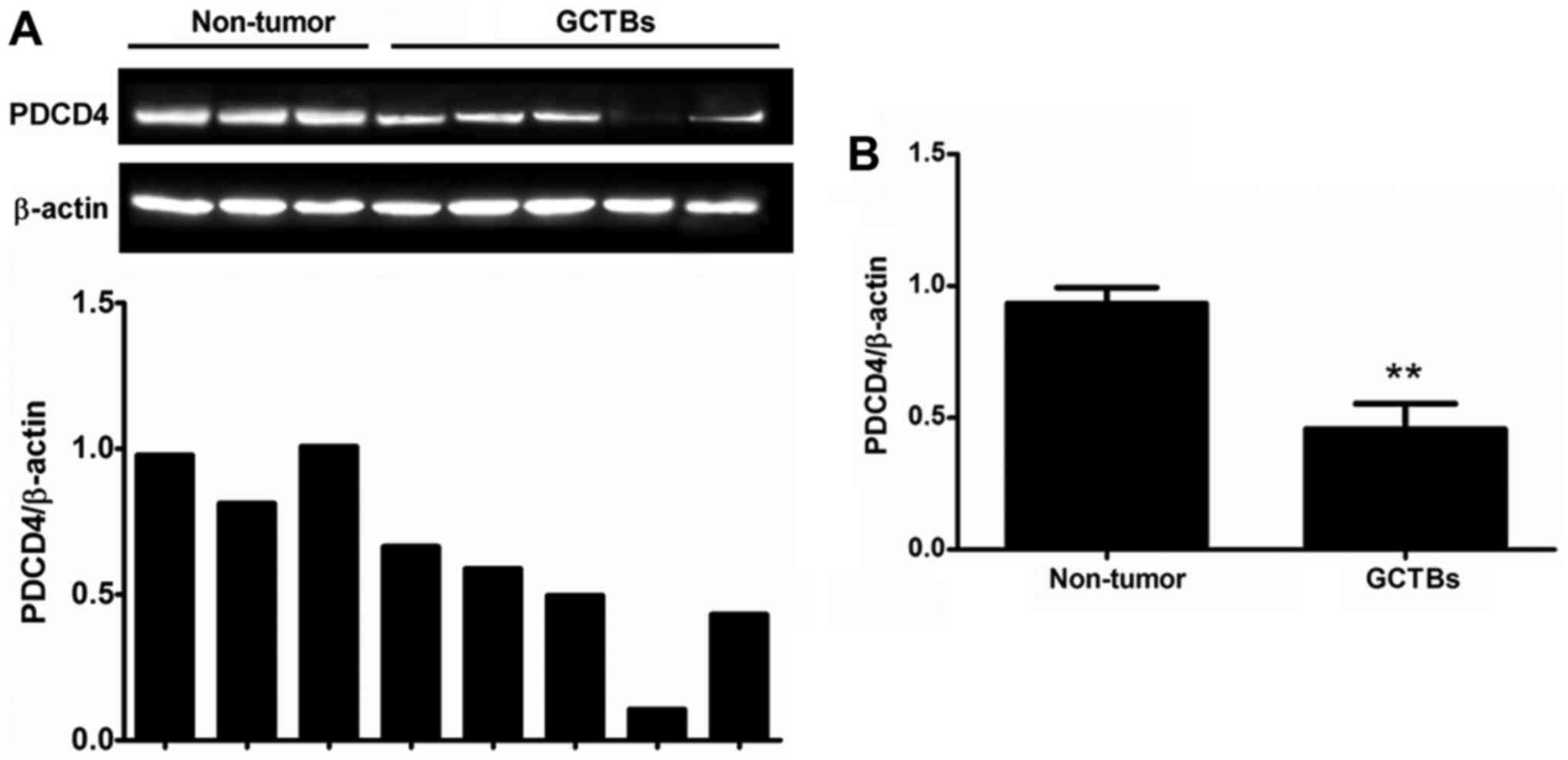
PDCD4 protein expression is decreased
in GCTBs
To further determine the PDCD4 expression, western
blot analysis was performed to identify the protein level of PDCD4.
The results demonstrated that the protein level of PDCD4 was
significantly decreased in 63% of frozen GCTB samples (17/27)
compared with adjacent non-tumorous tissues and this observation
was in accordance with experimental results at the mRNA level
(Fig. 2). Furthermore, the protein
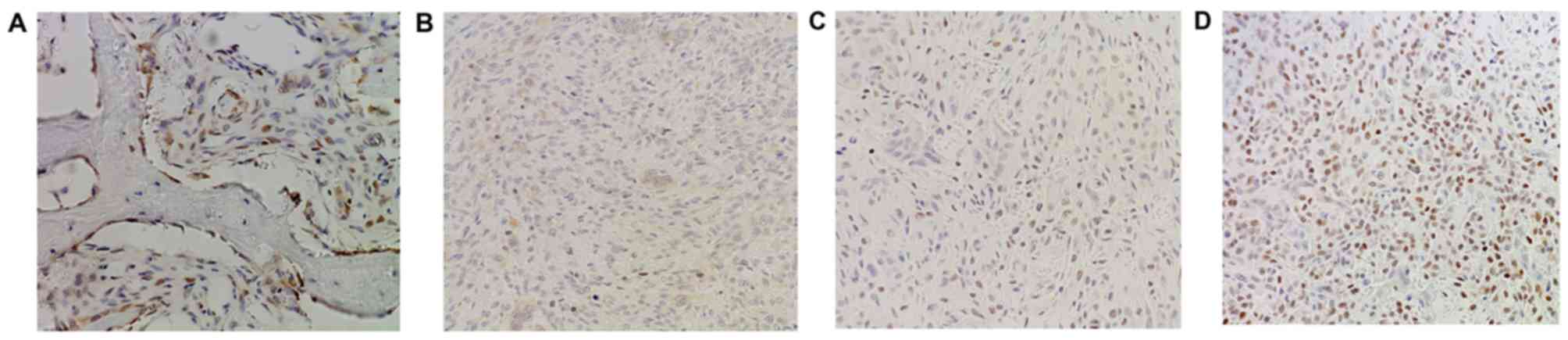
level of PDCD4 was also detected in GCTB samples by
immunohistochemistry using frozen and paraffin-embedded tissues. It
was demonstrated that normal tissues adjacent to tumors exhibited
strong PDCD4 staining (Fig. 3A),
while diminished or no staining of PDCD4 was observed in the tumor
tissues (Fig. 3B-D). Altogether, the
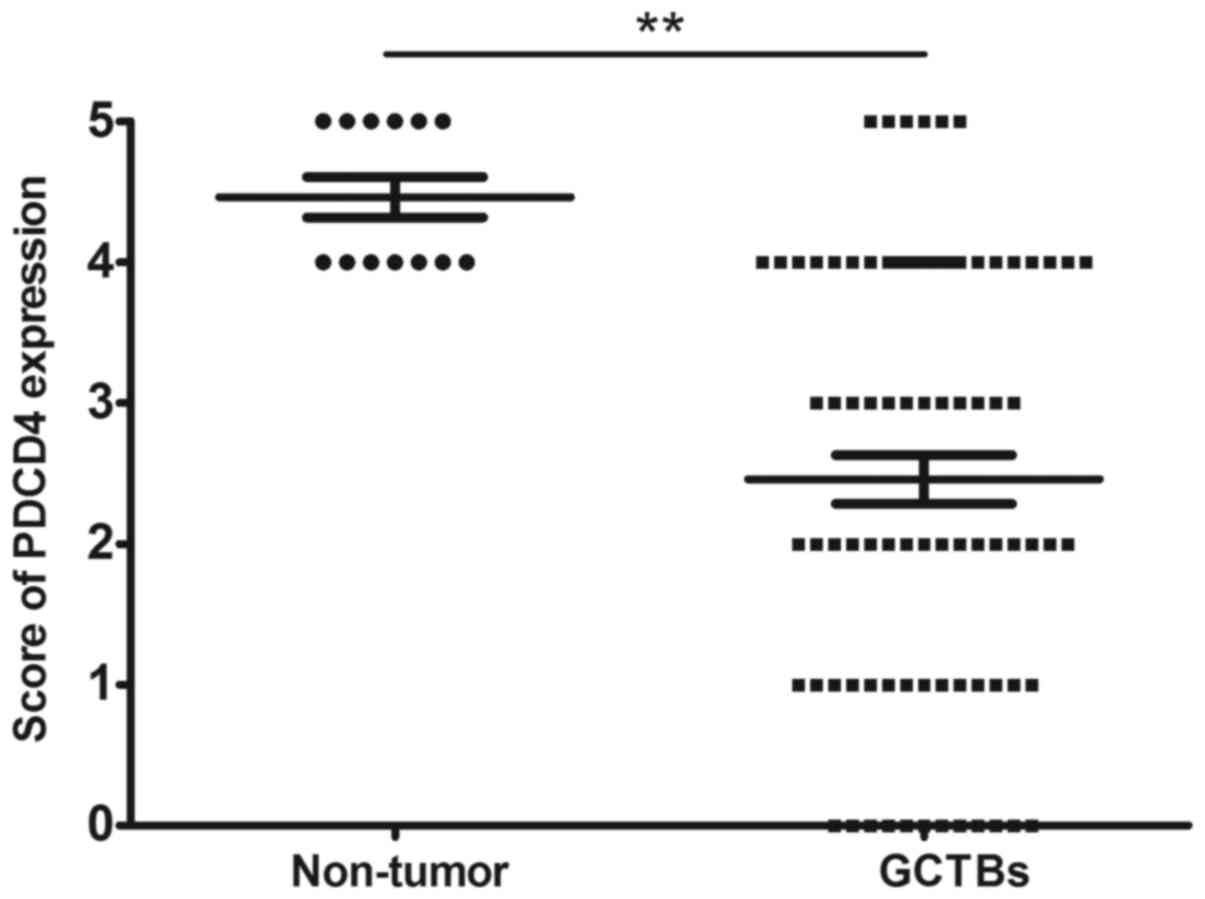
expression of PDCD4 at the protein level was decreased in 65%
(54/83) of tumor specimens, with a marked differential expression
of PDCD4 between GCTBs and normal tissues at the protein level
(Fig. 4).
PDCD4 expression is associated with
clinicopathological characteristics in primary GCTBs
The clinical role of PDCD4 in primary GCTBs was
subsequently analyzed and the association between the PDCD4
expression and clinicopathological characteristics of GCTBs was
studied. No significant correlation was determined between PDCD4
expression and patients' sex, age, tumor location and tumor size.
However, PDCD4 expression was significantly associated with the
Campanacci grade and tumor recurrence (P<0.05; Table I), which indicated that decreased
PDCD4 expression may promote the progression of GCTBs.
 | Table I.Association between PDCD4 expression
and clinicopathological characteristics in patients with giant cell
tumor of the bone. |
Table I.
Association between PDCD4 expression
and clinicopathological characteristics in patients with giant cell
tumor of the bone.
| Parameters | PDCD4 low
expression | PDCD4 high
expression | P-value |
|---|
| Total no. of
patients | 54 | 29 |
|
| Sex |
|
| 0.0952 |
| Male | 23 | 7 |
|
|
Female | 31 | 22 |
|
| Age (years) |
|
| 0.8942 |
| ≥40 | 16 | 9 |
|
|
<40 | 38 | 20 |
|
| Tumor location |
|
| 0.9941 |
| Proximal
tibia | 17 | 9 |
|
| Distal
femur | 15 | 8 |
|
| Proximal
humerus | 10 | 5 |
|
| Distal
radius | 8 | 4 |
|
| Other
position | 4 | 3 |
|
| Tumor size |
|
| 0.0787 |
| ≥5
cm | 19 | 16 |
|
|
<5cm | 35 | 13 |
|
| Campanacci
grade |
|
| 0.0234a |
| I | 12 | 15 |
|
| II | 31 | 10 |
|
|
III | 11 | 4 |
|
| Recurrence |
|
| 0.0426a |
|
Yes | 21 | 5 |
|
| No | 33 | 24 |
|
| Ki-67 labeling
index (%) | 13.28±5.06 | 5.83±3.84 |
<0.0001b |
PDCD4 inhibits malignant proliferation
of GCTBs
It was previously demonstrated that Ki-67 protein is
closely associated with the malignant proliferation of GCTBs
(25). Therefore, the present study
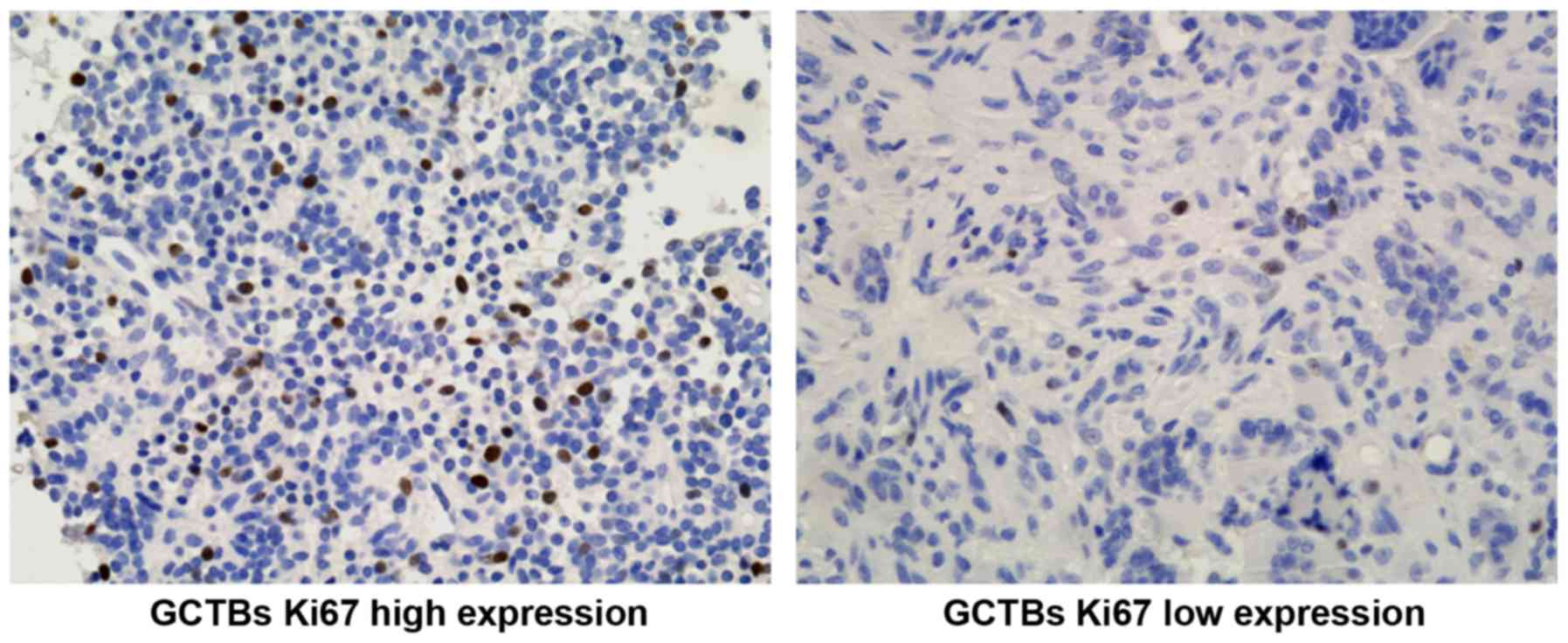
further examined the Ki-67 expression at the protein level in GCTBs
(Fig. 5) and confirmed the
association between the Ki-67 LI (Ki-67 labeling index) and the
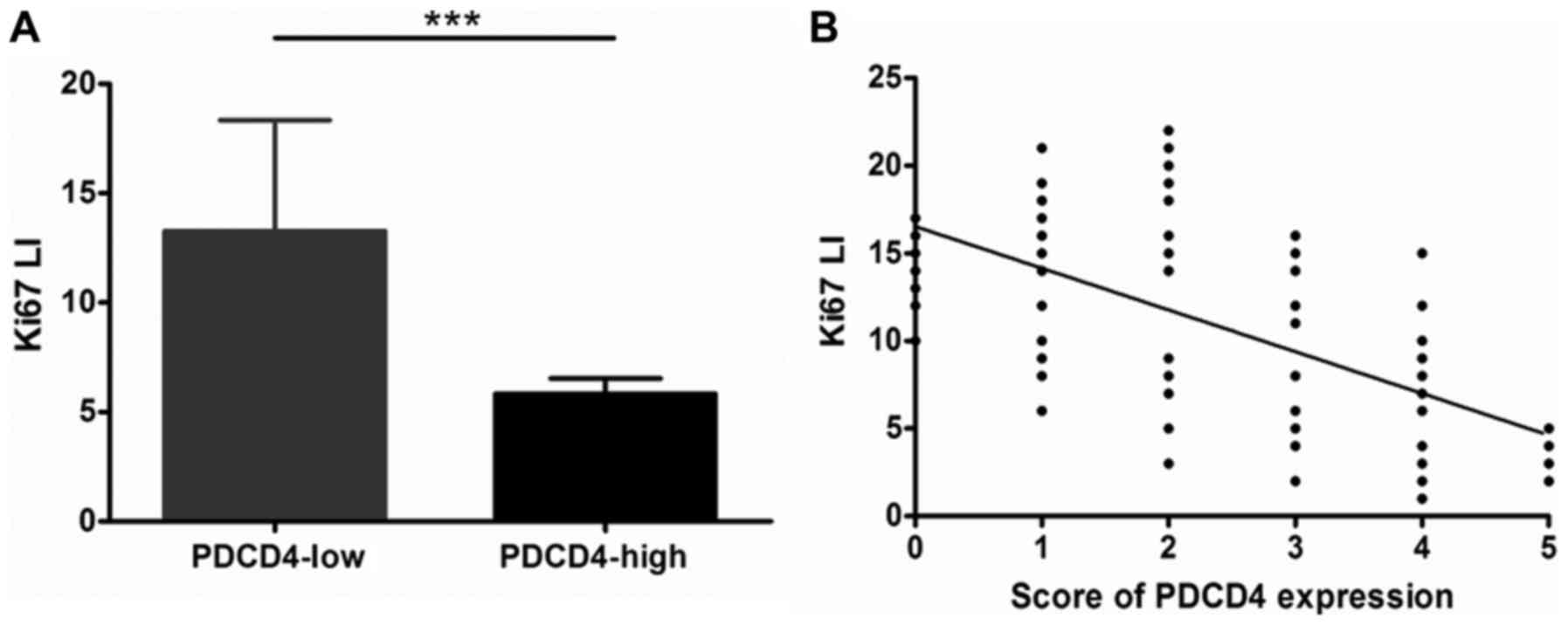
expression of PDCD4. The Ki-67 LI of GCTBs with low expression of
PDCD4 was markedly increased compared with GCTBs exhibiting high
expression of PDCD4 (P<0.001; Fig.
6A). The results demonstrated that PDCD4 may have a negative
association with the Ki-67 LI (r=−0.6392; P<0.001; Fig. 6B). All data suggested that PDCD4 may
have an important effect on inhibition of the malignant
proliferation of GCTBs.
Discussion
Multiple studies using animal models and human
tumors have confirmed that PDCD4 is a novel tumor suppressor gene
that can inhibit tumor progression and neoplastic transformation.
Decreased or absent PDCD4 expression is associated with the
development and prognosis of various types of cancer. The present
study analyzed PDCD4 expression in GCTBs samples and adjacent
non-tumorous tissues and revealed that PDCD4 expression at the mRNA
level was reduced in 63% of frozen samples. The protein level was
diminished in more than half of all samples compared with the
control, the adjacent non-tumor tissues with high expression of
PDCD4 at the mRNA and protein level. In addition, there was an
association between the PDCD4 expression and the Campanacci grade
or recurrence. Furthermore, PDCD4 exhibited a negative association
with the Ki-67 LI, which indicated the increased proliferation of
tumor cells. PDCD4 expression may serve a role in tumor malignant
progression and may aid the prognosis prediction of human
GCTBs.
Surgery is a standard treatment for localized
primary GCTBs. Although en bloc excision of a primary tumor can
reduce the recurrence rate to <20%, intralesional curettage has
been reported to result in high recurrence rates of up to 40–50% in
certain series (26). The evaluation
of expression levels of prognostic marker proteins at the time of
diagnosis may be taken into consideration for the classification of
GCTBs into categories characterized by a different risk of relapse
(27). Therefore, it is particularly
important to identify an increased number of novel target molecules
for early diagnosis and prediction of clinical behavior for
decreasing recurrence and improving the survival of patients with
GCTBs.
Previous studies have identified PDCD4 as a novel
tumor suppressor gene. Recently, numerous studies of various tumors
have demonstrated that PDCD4 is a target for anticancer therapies
and may be a potential diagnostic and prognostic marker (28,29). PDCD4
can inhibit the malignant growth of tumor cells and induce an
effect on more important cancer-associated genes at different
transcriptional and/or translational levels (30). However, it was not previously known
that there may be an association between the PDCD4 expression and
certain clinical, and pathological features in human primary GCTBs
tissues, including tumor progression and prognosis.
In the present study, decreased PDCD4 expression at
mRNA and protein levels was determined in primary GCTBs. It was
previously reported that the expression of PDCD4 could be regulated
in various ways at different transcriptional and translational
levels (10). Certain studies have
suggested that epigenetic mechanisms served a key role in the
regulation of expression of PDCD4. We confirmed that PDCD4 5′-CpG
island methylation was associated with reduced PDCD4 expression at
the mRNA level in human glioma cell lines and tissues. Furthermore,
PDCD4 expression could be restored with the inhibition of
methylation in glioma cells (31).
Therefore, the molecular mechanisms responsible for decreased PDCD4
expression in GCTBs remain to be further elucidated.
The present study demonstrated that abnormal PDCD4
expression was markedly associated with certain clinicopathological
features of GCTBs, including the Campanacci grade and tumor
recurrence. It has been previously demonstrated that PDCD4 protein
expression was associated with clinical and pathological
characteristics of diverse types of tumors (10). Our previous study indicated that
enhanced PDCD4 expression could improve the prognosis of patients
with high-grade glioma (31). Other
studies have demonstrated that the expression of PDCD4 protein was
associated with a high grade of lung adenocarcinoma. In the present
study, lower PDCD4 expression appeared in high Campanacci grade
GCTB. PDCD4 negatively regulated Ki-67 expression and inhibited
GCTB proliferation. These results suggested that PDCD4 may be an
indicator of malignant progression. Based on the correlation
between PDCD4 and tumor recurrence, it was concluded that PDCD4 may
be a key prognostic molecule in the development of GCTB. Positive
and effective therapeutic strategies to upregulate PDCD4 expression
may shed light on the treatment of GCTBs (32). PDCD4 was initially identified as a new
cell-apoptosis related gene. It may exhibit a marked inhibitory
effect on GCTSCs, the primary neoplastic cells of GCTB, but this
hypothesis requires further investigation in the future.
In conclusion, the present study suggested that
PDCD4 expression may serve an essential role in the malignant
progression and proliferation of GCTBs, and may contribute to an
improvement in the prognosis of GCTBs. However, the precise
molecular mechanism by which PDCD4 expression affects the malignant
development of GCTBs requires further investigation.
Acknowledgements
The authors would like to thank Dr Meng Zhang and Dr
Rong Wang for their technical support and Dr Xiao Wang for their
helpful comments associated with the present study (all Shandong
University Qilu Hospital, Jinan, China).
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81470403 and
81701404), the Key Research and Development Plan of Shandong
Province (grant nos. 2016GGE27013-2016GSF201091 and 2016GSF201141)
and the China Postdoctoral Science Foundation (grant no.
2017M610431).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FG designed research. WZ and LD performed cases
collection and molecular biology assays. Immunohistochemistry
analysis was performed by MZ, SH and ZM. FG wrote the manuscript,
and the final version was read and approved by all authors.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participants prior to their inclusion within the study. The present
study was conducted in accordance with the Ethics guidelines of the
Chinese Medical Association and the study protocol was approved by
the Shandong Provincial Hospital Institutional Review Board.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Turcotte RE: Giant cell tumor of bone.
Orthop Clin North Am. 37:35–51. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu M, Song ZG, Xu CX, Rong GH, Fan KX,
Chen JY, Zhang W, Jia JP, Han G, Wang W, et al: IL-17A stimulates
the progression of giant cell tumors of bone. Clin Cancer Res.
19:4697–4705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Müller DA, Beltrami G, Scoccianti G,
Campanacci DA, Franchi A and Capanna R: Risks and benefits of
combining denosumab and surgery in giant cell tumor of bone-a case
series. World J Surg Oncol. 14:2812016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karpik M: Giant cell tumor (tumor
gigantocellularis, osteoclastoma)-epidemiology, diagnosis,
treatment. Ortop Traumatol Rehabil. 12:207–215. 2010.(In English,
Polish). PubMed/NCBI
|
|
5
|
Muramatsu K, Ihara K and Taguchi T:
Treatment of giant cell tumor of long bones: Clinical outcome and
reconstructive strategy for lower and upper limbs. Orthopedics.
32:4912009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balke M, Schremper L, Gebert C, Ahrens H,
Streitbuerger A, Koehler G, Hardes J and Gosheger G: Giant cell
tumor of bone: Treatment and outcome of 214 cases. J Cancer Res
Clin Oncol. 134:969–978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dominkus M, Ruggieri P, Bertoni F,
Briccoli A, Picci P, Rocca M and Mercuri M: Histologically verified
lung metastases in benign giant cell tumours-14 eases from a single
institution. Int Orthop. 30:499–504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van der Heijden L, Dijkstra PDS, Blay JY
and Gelderblom H: Giant cell tumour of bone in the denosumab era.
Eur J Cancer. 77:75–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamamoto H, Iwasaki T, Yamada Y, Matsumoto
Y, Otsuka H, Yoshimoto M, Kohashi K, Taguchi K, Yokoyama R,
Nakashima Y and Oda Y: Diagnostic utility of histone H3.3 G34W,
G34R, and G34V mutant-specific antibodies for giant cell tumors of
bone. Hum Pathol. 73:41–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lankat-Buttgereit B and Göke R: The tumour
suppressor Pdcd4: Recent advances in the elucidation of function
and regulation. Biol Cell. 101:309–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yasuda M, Nishizawa T, Ohigashi H, Tanaka
T, Hou DX, Colburn NH and Murakami A: Linoleic acid metabolite
suppresses skin inflammation and tumor promotion in mice: Possible
roles of programmed cell death 4 induction. Carcinogenesis.
30:1209–1216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu TC, Young MR, Cmarik J and Colburn NH:
Activator protein 1 (AP-1)- and nuclear factor kappaB
(NF-kappaB)-dependent transcriptional events in carcinogenesis.
Free Radic Biol Med. 28:1338–1348. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Loh PG, Yang HS, Walsh MA, Wang Q, Wang X,
Cheng Z, Liu D and Song H: Structural basis for translational
inhibition by the tumour suppressor Pdcd4. EMBO J. 28:274–285.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hilliard A, Hilliard B, Zheng SJ, Sun H,
Miwa T, Song W, Göke R and Chen YH: Translational regulation of
autoimmune inflammation and lymphoma genesis by programmed cell
death 4. J Immunol. 177:8095–8102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jansen AP, Camalier CE and Colburn NH:
Epidermal expression of the translation inhibitor programmed cell
death 4 suppresses tumorigenesis. Cancer Res. 65:6034–6041. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z, Wang J, Li J, Wang X and Song W:
MicroRNA-150 promotes cell proliferation, migration, and invasion
of cervical cancer through targeting PDCD4. Biomed Pharmacother.
97:511–517. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu X, Wang Y, Liang H, Fan Q, Zhu R, Cui
J, Zhang W, Zen K, Zhang CY, Hou D, et al: miR-23a/b promote tumor
growth and suppress apoptosis by targeting PDCD4 in gastric cancer.
Cell Death Dis. 8:e30592017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao F, Zhang P, Zhou C, Li J, Wang Q, Zhu
F, Ma C, Sun W and Zhang L: Frequent loss of PDCD4 expression in
human glioma: Possible role in the tumorigenesis of glioma. Oncol
Rep. 17:123–128. 2007.PubMed/NCBI
|
|
19
|
Huang H, Wang X, Wang C, Zhuo L, Luo S and
Han S: The miR-93 promotes proliferation by directly targeting
PDCD4 in hepatocellular carcinoma. Neoplasma. 64:770–777. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding L, Zhang X, Zhao M, Qu Z, Huang S,
Dong M and Gao F: An essential role of PDCD4 in progression and
malignant proliferation of gastrointestinal stromal tumors. Med
Oncol. 29:1758–1764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Ding L, Zhang X, Zhao M, Qu Z,
Huang S, Zhang Y, Li Y and Gao F: Clinical significance of
programmed cell death 4 expression in malignant progression of
human nasal inverted papillomas. Med Oncol. 29:2505–2011. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Campanacci M, Baldini N, Boriani S and
Sudanese A: Giant-cell tumor of bone. J Bone Joint Surg Am.
69:106–114. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chadderton T, Wilson C, Bewick M and Glück
S: Evaluation of three rapid RNA extraction reagents: Relevance for
use in RT-PCR's and measurement of low level gene expression in
clinical samples. Cell Mol Biol (Noisy-le-grand). 43:1227–1234.
1997.PubMed/NCBI
|
|
24
|
Culley DE, Kovacik WP Jr, Brockman FJ and
Zhang W: Optimization of RNA isolation from the archaebacterium
Methanosarcina barkeri and validation for oligonucleotide
microarray analysis. J Microbiol Methods. 67:36–43. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Antal I, Sápi Z and Szendröi M: The
prognostic significance of DNA cytophotometry and proliferation
index (Ki-67) in giant cell tumors of bone. Int Orthop. 23:315–319.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Raskin KA, Schwab JH, Mankin HJ,
Springfield DS and Hornicek FJ: Giant cell tumor of bone. J Am Acad
Orthop Surg. 21:118–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gamberi G, Serra M, Ragazzini P, Magagnoli
G, Pazzaglia L, Ponticelli F, Ferrari C, Zanasi M, Bertoni F, Picci
P and Benassi MS: Identification of markers of possible prognostic
value in 57 giant cell tumors of bone. Oncol Rep. 10:351–356.
2003.PubMed/NCBI
|
|
28
|
Pennelli G, Galuppini F, Barollo S,
Cavedon E, Bertazza L, Fassan M, Guzzardo V, Pelizzo MR, Rugge M
and Mian C: The PDCD4/miR-21 pathway in medullary thyroid
carcinoma. Hum Pathol. 46:50–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fassan M, Pizzi M, Battaglia G, Giacomelli
L, Parente P, Bocus P, Ancona E and Rugge M: Programmed cell death
4 (PDCD4) expression during multistep Barrett's carcinogenesis. J
Clin Pathol. 63:692–696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang WQ, Zhang H, Wang HB, Sun YG, Peng
ZH, Zhou G, Yang SM, Wang RQ and Fang DC: Programmed cell death 4
(PDCD4) enhances the sensitivity of gastric cancer cells to
TRAIL-induced apoptosis by inhibiting the PI3K/Akt signaling
pathway. Mol Diagn Ther. 14:155–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao F, Wang X, Zhu F, Wang Q, Zhang X, Guo
C, Zhou C, Ma C, Sun W, Zhang Y, et al: PDCD4 gene silencing in
gliomas is associated with 5′CpG island methylation and
unfavourable prognosis. J Cell Mol Med. 13:4257–4267. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Briest F, Berndt A, Clement J, Junker K,
Eggeling Fv, Grimm S and Friedrich K: Tumor-stroma interactions in
tumorigenesis: Lessons from stem cell biology. Front Biosci (Elite
Ed). 4:1871–1887. 2012. View
Article : Google Scholar : PubMed/NCBI
|