Introduction
Pancreatic ductal adenocarcinoma (PDAC) is ahead of
breast cancer as the third leading cause of cancer-related deaths
in the US (1); moreover, PDAC is
predicted to become the second leading cause of cancer-related
deaths by 2020 (2). No effective
screening test for this malignancy exists, and metastatic disease
is commonly present at initial diagnosis. Currently, PDAC has a
distinctive adverse prognosis with an overall 5-year survival rate
of <6% (2). Therefore, novel and
effective therapy approaches for PDAC are urgently needed.
Epithelial-mesenchymal transition (EMT) is a
physiological process that allows epithelial cells to acquire the
motile and invasive characteristics of mesenchymal cells (3). EMT is a well-coordinated process
triggered by many signalling pathways during embryonic development.
A recent study has demonstrated that EMT is involved in the
progression of neoplasia. Cells undergoing EMT progressively lose
the expression of components in the epithelial cell junctions,
produce a mesenchymal vimentin cytoskeleton and acquire both
invasive and chemoresistance properties. Recent studies have also
proposed that metastasis is an early event in the natural history
of PDAC (4). Therefore, improving the
knowledge of the molecular mechanisms of EMT is essential in
designing more effective treatments for this deadly disease.
High-mobility group nucleosome-binding domain 5
(HMGN5) (also named nucleosome-binding protein 1) has been
suggested as an oncogene in some types of cancers. A recent study
has shown that HMGN5 regulates DNA replication, DNA repair, histone
modification and gene transcription in combination with chromatin
regulators (5). An increasing number
of studies have demonstrated that HMGN5 is overexpressed in many
types of cancers, including breast cancer, osteosarcoma, prostate
cancer, glioma, lung and renal cancer (6–14). HMGN5
promotes tumour progression by enhancing proliferation, inhibiting
apoptosis and promoting metastasis. However, the function and
molecular mechanism of HMGN5 in PDAC have not been illustrated.
Nevertheless, HMGN5 may be a potential target for developing cancer
therapies for PDAC.
The aim of the present study was to illustrate the
role and molecular mechanism of HMGN5 in PDAC. Firstly, we detected
the HMGN5 expression in PDAC cell lines and normal pancreatic
ductal cells. Then, we demonstrated that HMGN5 silencing
significantly impaired the PDAC cell viability, proliferation and
metastasis and EMT in vitro and retarded the tumour growth
in vivo. We demonstrated that the HMGN5-mediated
Wnt/β-catenin signalling pathway is one of the critical signal
transduction pathways that link HMGN5 to EMT activation.
Materials and methods
Cell culture
Human PDAC cell lines (Capan-2, PANC-1, SW1990,
COLO357 and MIAPaCa-2) and normal human pancreatic duct epithelial
(HPDE) cell line were obtained from Shanghai Cell Bank (Shanghai,
China). PDAC cell lines were cultured in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS). HPDE cells were maintained in keratinocyte serum-free medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with bovine pituitary extract and epidermal growth
factor. All cells were cultured at 37°C in humidified incubator
with 5% CO2.
Tissue samples
The present study was approved by the Medical Ethics
Committee of LanLing County Hospital. All patients included in this
research were required to provide written informed consent. Fifteen
paired PDAC and adjacent normal tissues were obtained from patients
who underwent surgical resection in LanLing County Hospital. All
tissue samples were snap-frozen in liquid nitrogen immediately
after surgery and stored at −80°C until RNA extraction.
Cell transfection
shRNA plasmids for human HMGN5 were designed against
the HMGN5 gene and constructed in Phblv-u6-puro vectors (Shanghai
GeneChem Co., Ltd., Shanghai, China). The shRNA sequences used in
the present study were as follows: shHMGN5
5′-ATGAGAAAGGAGAAGATGC-3′ and shcontrol: 5′-GAAGAATATCGAAGGAAGA-3′.
To generate stable HMGN5 knockdown cells, PANC-1 cells were grown
in 6-well plates until they reached 60% confluency. The medium was
replaced with 1 ml of fresh culture medium supplemented with 100 µl
viral supernatant (1×108 UT/ml) and 8 µg/ml Polybrene
for 24 h. The PANC-1 cells were further cultured in medium
containing puromycin at 3 µg/ml. Individual puromycin-resistant
colonies were isolated during drug screening. The open reading
frame of β-catenin was inserted into pcDNA3.1 vector to generate
pcDNA3.1/β-catenin overexpression vector. Transient transfection
was performed with a Lipofectamine 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's instructions. Knockdown or overexpression efficiency
was examined via western blot assay.
Colony formation assay
PANC-1 cells transfected with shHMGN5 or shcontrol
and β-catenin plasmid were seeded at 200 cells/well in 6-well
plates. Seven days later, the colonies were stained with crystal
violet, photographed, and then scored.
CCK-8 assay
Cell viability was determined via Cell Counting
Kit-8 (CCK-8) (Beyotime Institute of Biotechnology, Haimen,
China). Briefly, cells (3,000/well) transfected with shHMGN5 or
shcontrol and β-catenin plasmid in 200 µl medium were plated into
96-well plates. Zero, 24, 48 and 72 h later, 10 µl of CCK-8
solution was added into each well. After 2 h, the optical density
values of each well were measured at 450 nm using a microplate
reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA). CCK-8
assay were repeated 3 times.
Transwell assay
Cell metastasis was determined using Boyden chamber
assay. For the invasion assay, the upper sides of the filters were
coated with 50 µl Matrigel (BD Biosciences, Bedford, MA, USA).
Cells (5×104) with 200 µl of serum-free medium were seeded in the
upper chamber. The lower chamber was filled with medium
supplemented with 5% FBS. Following incubation at 37°C with 5%
CO2 for 8 h (migration) or 12 h (invasion), cells on the
lower filter were fixed with methanol, stained with crystal violet,
and then counted under a light microscope.
Western blot analysis
Equal amounts of the protein from lysates of PDAC
cells were subjected to 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and then transferred to
polyvinylidene fluoride (PVDF) membranes. The immunoreactive bands
were firstly incubated with the primary antibodies, including HMGN5
(1:500), wnt1 (1:500), total or phosphorylated β-catenin (1:500),
(all from Cell Signaling Technology, Inc., Danvers, MA, USA)
E-cadherin (1:1,000), N-cadherin (1:1,000), Vimentin (1:1,000) (all
from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), β-actin
(1:2,000; Beyotime Institute of Biotechnology) overnight at 4°C and
horseradish peroxidase-conjugated goat anti-rabbit antibody (Santa
Cruz Biotechnology, Inc.) at room temperature for 2 h. The
intensity of protein bands was detected by Image-Pro Plus 6.0
software. β-actin served as the loading control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from cells was extracted using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and then
reverse-transcribed through a reverse transcription kit (Takara
Biotechnology Co., Ltd., Dalian, China) following the
manufacturer's protocol. RT-qPCR was conducted with
All-in-One™ miRNA RT-qPCR Detection Kit (AOMD-Q020;
GeneCopoeia, Inc., Rockville, MD, USA) on CFX96™
Real-Time PCR Detection System supplied with analytical software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The primers used
for amplification were: HMGN5, forward 5′-CATGGACATGAGCACAATCA-3′
and reverse 5′-CCCTTTTCTGTGGCATCTTC-3′; E-cadherin, forward
5′-GTACTTGTAATGACACATCTC-3′ and reverse 5′-TGCCAGTTTCTGCATCTTGC-3′;
N-cadherin, forward 5′-ATCAAAGACCCATCCACC-3′ and reverse
5′-CCTCCTCACCACCACTA-3′; vimentin, forward 5′-CTTCCGCGCCTACGCCA-3′
and reverse 5′-GCCCAGGCGACCTACTCC-3′; β-actin, forward
5′-GATCATTGCTCCTCCTGAGC-3′ and reverse,
5′-ACTCCTGCTTGCTGATCCAC-3′.
Tumourigenesis assay in nude mice
The protocols for animal experiment study were
approved by the Animal Care and Welfare Committee of LanLing County
Hospital and conducted in strict accordance with the guidelines of
the National Animal Welfare Law of China. Four-week-old male BALB/c
nude mice (n=12) were purchased from the Laboratory Animal Center
of JiLin University (JiLin, China) and maintained in a SPF
environment. Stable PANC-1 shHMGN5 and PANC-1 shcontrol cells
(1×106) were subcutaneously injected into the right
flanks of athymic nude mice. Tumor volume (mm3) was
calculated every 7 days for 35 days using the formula: V = 0.5 ×
length × width2. After 7 weeks, the mice were
euthanized. The tumors were isolated, weighed, photographed.
Immunohistochemical analysis
(IHC)
Paraffin-embedded tissues were cut into 4 µm-thick
consecutive sections and were then dewaxed in xylene and rehydrated
in graded ethanol solutions. Antigen retrieval was performed
following the standard procedure. Sections were cooled and immersed
in a 0.3% hydrogen peroxide solution for 15 min to block endogenous
peroxidase activity, and then rinsed in phosphate-buffered saline
(PBS) for 5 min. Non-specific labeling was blocked by incubation
with 5% bovine serum albumin at room temperature for 30 min.
Sections were then incubated with primary rabbit anti-human
antibody against Ki67 (1:200; Santa Cruz Biotechnology, Inc.) at
4°C overnight, rinsed with PBS with Tween-20 (PBST), incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG secondary
antibody (Santa Cruz Biotechnology, Inc.). The immunohistochemistry
results were scored by the percentage of positive detection of the
staining.
Statistical analysis
All experiments were repeated 3 times. Unless
otherwise indicated, experimental values are expressed as mean ±
standard error mean. Differences between 2 groups were assessed
using Student's t-test (two-tailed). Data of >2 groups were
analyzed using one way analysis of variance with Tukey's post hoc
test. Statistical analyses were performed using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate statistical significance.
Results
Overexpression of HMGN5 was detected
in PDAC cell lines and tissues
To investigate the potential role of HMGN5 in PDAC,
we first examined the expression level of HMGN5 in the PDAC cell
lines Capan-2, PANC-1, SW1990, COLO357 and MIAPaCa-2, as well as in
the normal pancreatic epithelial cell line HPDE by qPCR and western
blot assay. The results showed that the mRNA and protein expression
levels of HMGN5 were significantly higher in PDAC cells compared to
HPDE cells (Fig. 1A and B). The
highest expression levels of HMGN5 were detected in PANC-1 cells.
Furthermore, the expression of HMGN5 was detected in PDAC tissues
and matched normal tissues from 15 patients using qPCR. The results
showed that the expression level of HMGN5 was higher in PDAC
samples than in adjacent normal tissues (Fig. 1C). The increased levels of HMGN5 in
PDAC indicate that HMGN5 may serve as oncogene in PDAC.
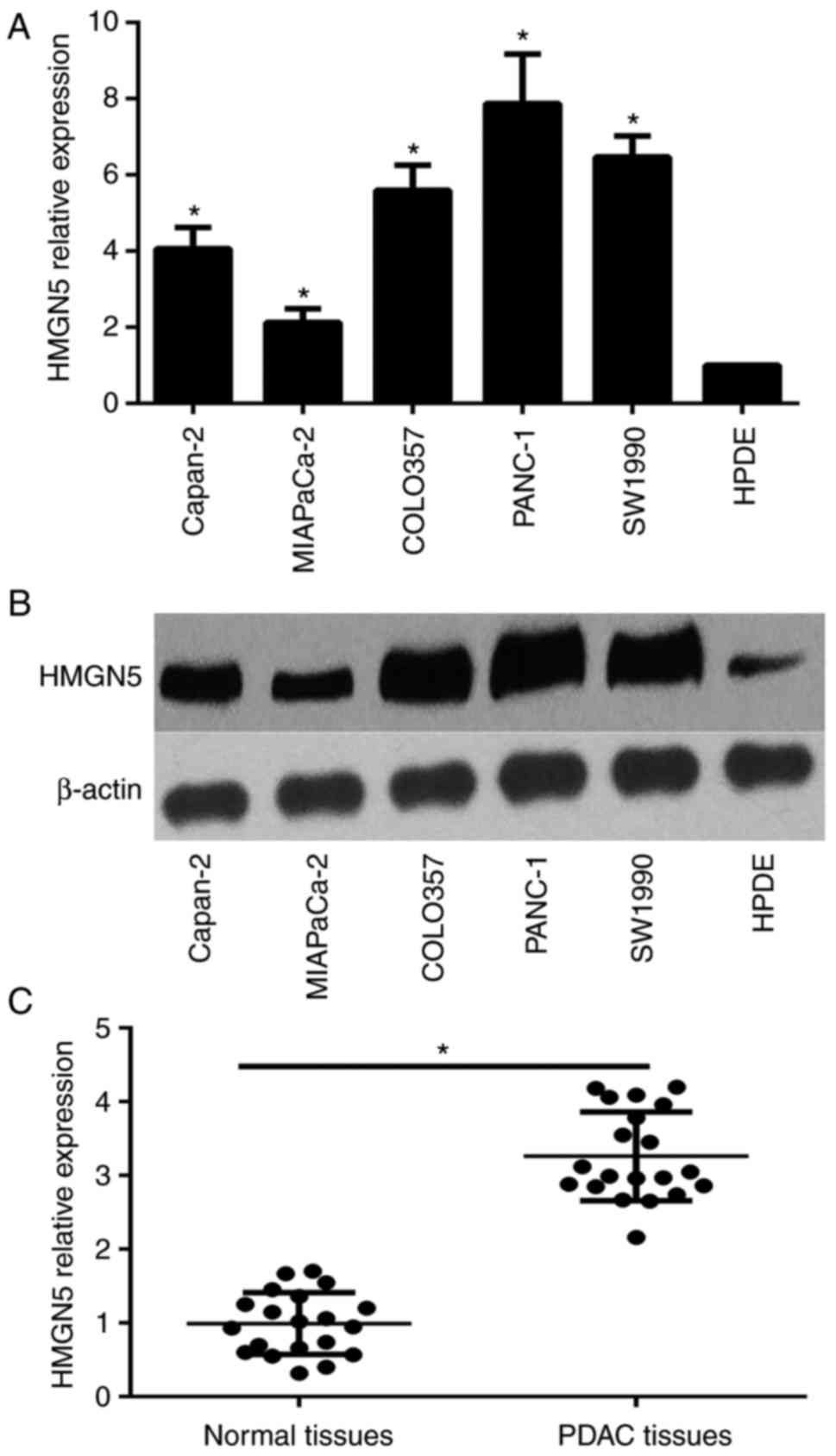 | Figure 1.Overexpression of HMGN5 in PDAC cell
lines and tissues. (A) RT-qPCR analysis revealed the mRNA
expression of HMGN5 in the human PDAC cell lines (PANC-1, Capan-2,
SW1990, COLO357 and MIAPaCa-2) and immortalized pancreatic
epithelial cell line HPDE. *P<0.05 vs. HPDE. (B) Western blot
analysis revealed the HMGN5 protein expression in the human PDAC
cell lines (PANC-1, Capan-2, SW1990, COLO357 and MIAPaCa-2) and
HPDE. (C) The expression level of HMGN5 in the PDAC tissues and
their adjacent normal tissues was detected using RT-qPCR.
*P<0.05, as indicated. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; HMGN5, high
mobility group nucleosome binding domain 5; PDAC, pancreatic ductal
adenocarcinoma. |
HMGN5 silencing inhibited PDAC
progression in vitro
To examine the role of HMGN5 in human PDAC cell
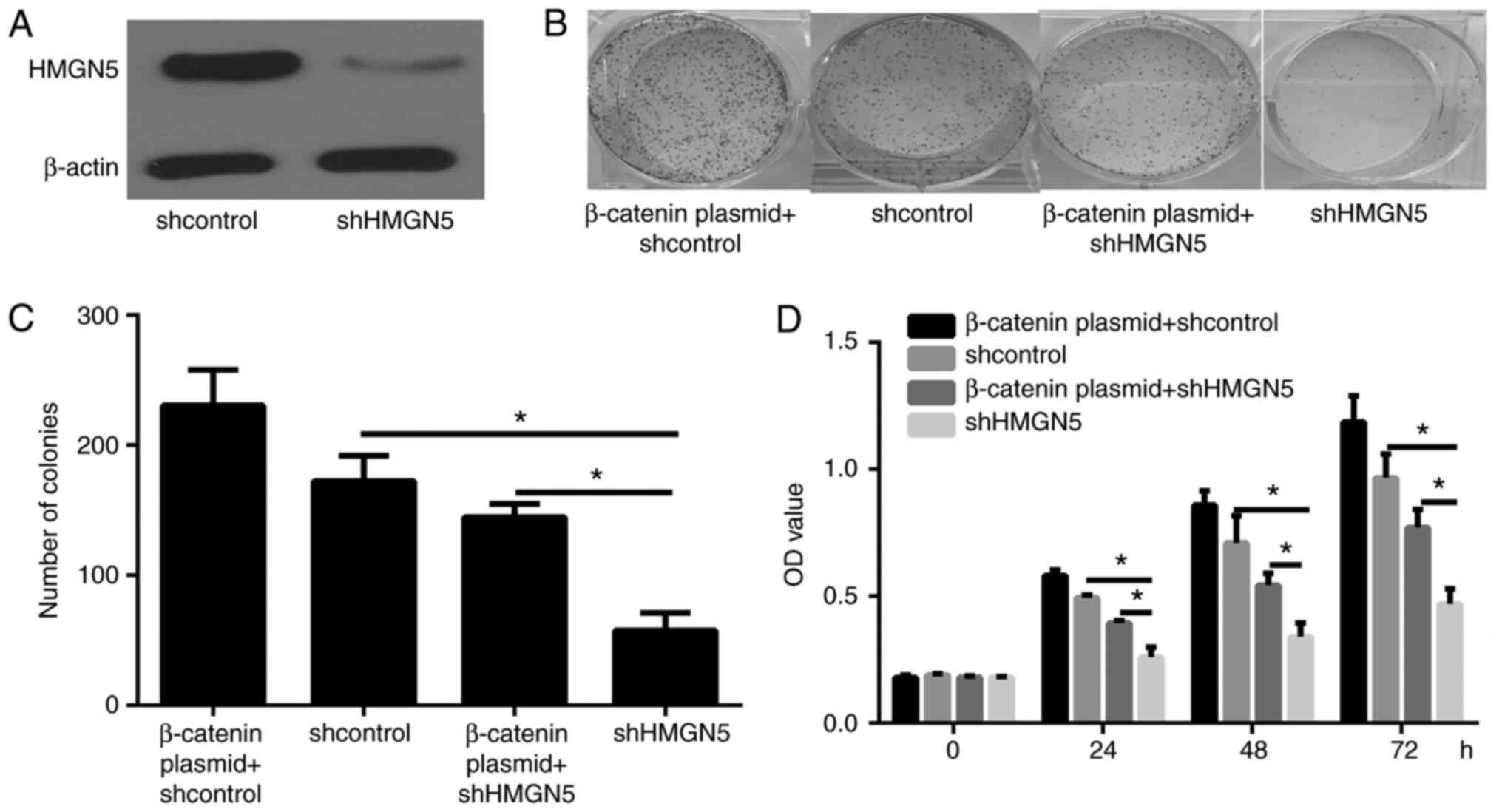
growth, PANC-1 cells were transfected with shcontrol or shHMGN5.
Western blot results showed that the expression level of HMGN5 was
significantly downregulated in cells transfected with shHMGN5
(Fig. 2A). CCK-8 assay showed that
HMGN5 silencing in PANC-1 cells significantly inhibited cell
viability (Fig. 2B). Colony formation
assays illustrated that HMGN5 silencing in PANC-1 cells
significantly inhibited cell proliferation (Fig. 2C and D).
HMGN5 silencing decreased PDAC
metastasis in vitro
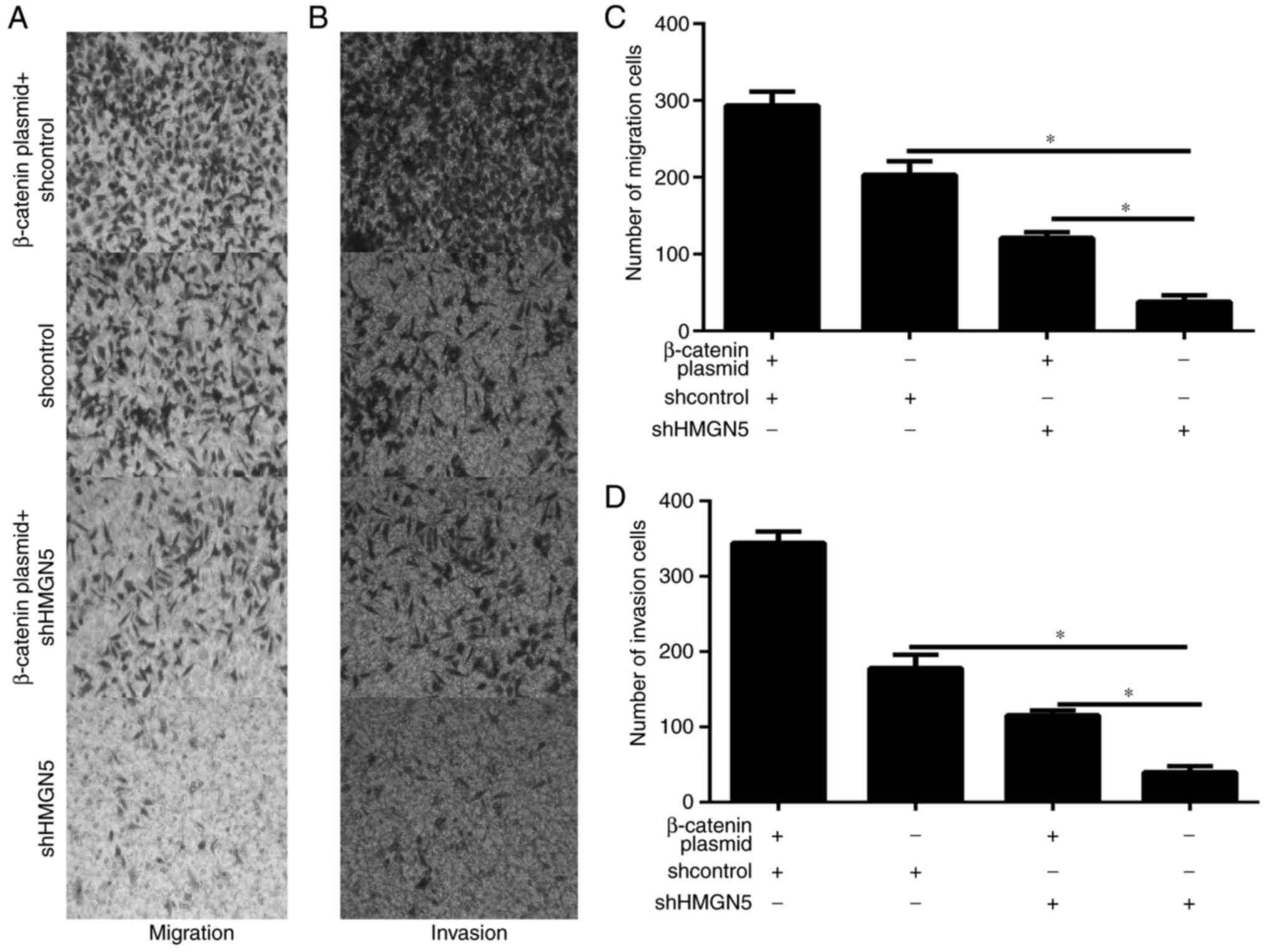
Cell metastasis is critical events in the tumor
progression. Therefore, the effect of HMGN5 on the migration and
invasion of PDAC cell was explored in vitro. PANC-1 cell
migration and invasion were investigated through Boyden chamber
assay. Results showed that HMGN5 silencing significantly decreased
the cell migration and invasion (Fig.
3A-D).
HMGN5 silencing inhibited the tumor
growth in vivo
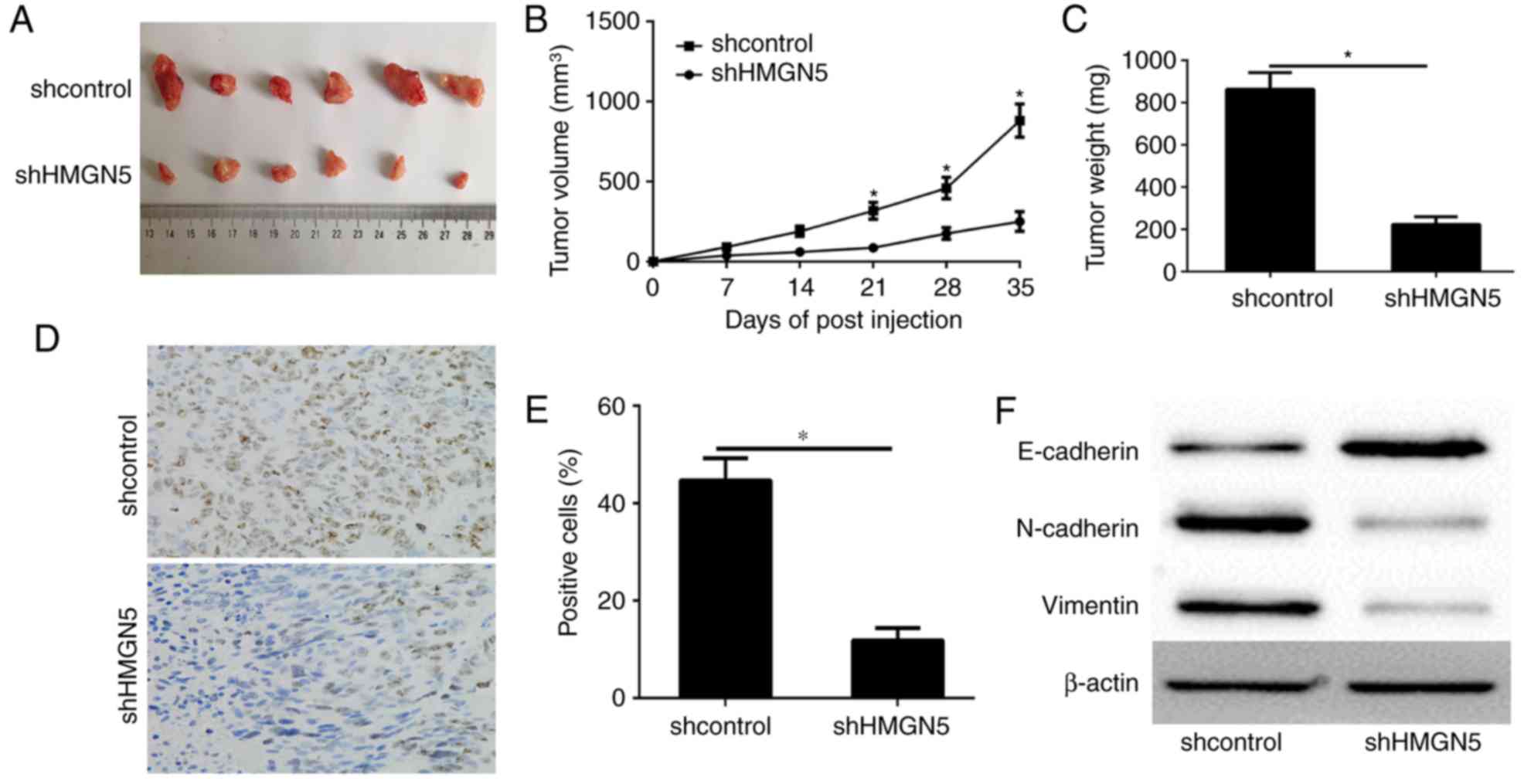
To show the HMGN5 biofunction in promotion of PDAC
cell tumorigenicity in vivo, PANC-1 cells stable expression
shcontrol or shHMGN5 was respectively injected into nude mice. The
tumor volume was measured every 7 days. At 35 days after cell
implantation, the tumors were removed and photographed (Fig. 4A). Tumor volume and the final weight
of excised tumors demonstrated that HMGN5 knockdown had
significantly retarded tumor growth at termination (Fig. 4B and C). Histologic analysis of tumor
proliferation showed that HMGN5 silencing had significantly fewer
Ki-67 positive cells compared to shcontrol tumors (Fig. 4D and E). These data suggested that
HMGN5 may play a critical role in PDAC proliferation and tumor
maintenance in vivo.
HMGN5 enhanced EMT in PDAC
EMT is a vital biological process involved in cell
differentiation in normal embryonic development and is a potential
mechanism for tumour cell metastasis. To address whether HMGN5 has
effects on EMT, The expression of the epithelial marker E-cadherin
and mesenchymal markers N-cadherin and vimentin was determined in
PANC-1 cell transfected with shcontrol and shHMGN5 by western
blotting and qPCR. The results showed that the expression of
E-cadherin was significantly up-regulated compared with the
controls, whereas N-cadherin and vimentin was significantly
down-regulated in PANC-1 cell (Fig.
5B-E).
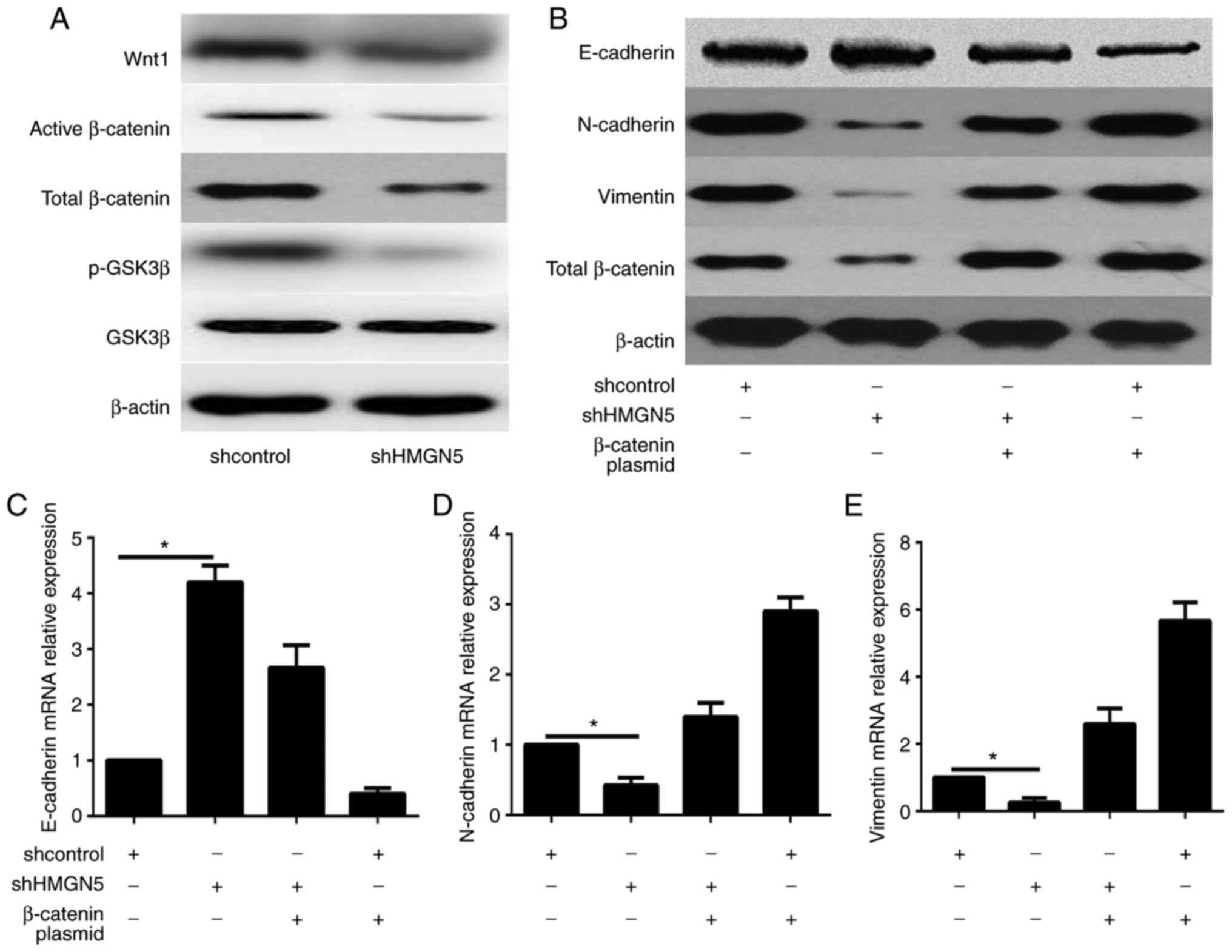 | Figure 5.HMGN5 enhances epithelial-mesenchymal
transition via the Wnt/β-catenin signaling pathway in PDAC cells.
PANC-1 cells were transfected with shcontrol or shHMGN5 and
β-catenin overexpression plasmid. (A) Representative western blots
of the protein expression levels of Wnt1, total and p-GSK3β, and
active and total β-catenin in PANC-1 cells. β-actin served as the
loading control. (B) The protein expression of epithelial and
mesenchymal markers was also detected by western blot assay. The
mRNA expression of the epithelial and mesenchymal markers, (C)
E-cadherin, (D) N-cadherin and (E) vimentin, was detected by
reverse transcription-quantitative polymerase chain reaction.
*P<0.05, as indicated. HMGN5, high mobility group nucleosome
binding domain 5; PDAC, pancreatic ductal adenocarcinoma; sh-,
short hairpin RNA; p-, phosphorylated; GSK3β, glycogen synthase
kinase-3β. |
To confirm the above data in vivo, we
detected the expression of E-cadherin, N-cadherin and vimentin in
the tumour transfected with shcontrol and shHMGN5 by western
blotting. The results showed that the expression of E-cadherin was
significantly up-regulated in shHMGN5 group, whereas N-cadherin and
vimentin was significantly down-regulated in shHMGN5 group
(5F).
HMGN5 positively regulated the
Wnt/β-catenin signaling pathway in PDAC cell
Wnt/β-catenin pathway is one of the major signaling
pathways involved in EMT and plays an important role in metastasis.
Western blot results showed that HMGN5 positively regulated Wnt1
expression in protein (Fig. 5A).
Furthermore, we showed the activation of GSK3β and β-catenin, the
canonical pathway of Wnt signaling pathway. The western blot
results showed that the amount of p-GSK3β (Fig. 5A) and active β-catenin
(dephosphorylated β-catenin, Fig. 5A)
was dramatically reduced by HMGN5 silencing.
HMGN5 promotes PDAC cell viability,
proliferation and metastasis via Wnt/β-catenin signaling pathway
in vitro
We then investigated whether rescuing HMGN5
silencing-mediated-Wnt/β-catenin signaling pathway suppression with
a β-catenin overexpression plasmid attenuates the progression
inhibition effect of HMGN5 silencing on PDAC cells. The expression
of β-catenin was detected by western blot assay (Fig. 5A). The CCK-8 (Fig. 2B) and colony formation assay (Fig. 2C and D) results showed that
restoration of β-catenin expression reversed the inhibition of cell
viability and proliferation by HMGN5 silencing in PANC-1 cell.
Furthermore, the Transwell assay showed that β-catenin
overexpression significantly reversed the inhibition of cell
migration and invasion by HMGN5 knockdown in PANC-1 cell (Fig. 3A-D). Taken together, these results
demonstrated that HMGN5 promoted the PDAC progression via
activation of Wnt/β-catenin signaling pathway.
Overexpression of β-catenin
counteracted the effect of HMGN5 silencing on EMT in PDAC
We further explored the effect of regulatory
relationship of HMGN5 and Wnt/β-catenin signaling pathway on EMT of
PANC-1 cells. qPCR and western blot assay results showed that the
overexpression of β-catenin counteracted the effect of HMGN5 on EMT
as demonstrated by changed expressions of molecular markers
associated with EMT (Fig. 5B-E).
Discussion
In the present study, we discovered that the HMGN5
expression was significantly higher in the PDAC cell lines and PDAC
tissues than in the normal pancreatic ductal cell line and normal
pancreatic tissues. These data suggested that the HMGN5
overexpression may be correlated with PDAC progression.
A previous study has shown that HMGN5 promotes
tumour progression in some types of cancer (6–14).
However, the role of HMGN5 in pancreatic cancer has not been
illustrated. To elucidate the role of HMGN5 in the tumorigenesis of
PDAC, we employed the loss of function approach via the knockdown
of endogenous HMGN5 expression in the PDAC cells. We firstly showed
that HMGN5 silencing decreased the viability of the PANC-1 cells
using the CCK-8 assay. Then, we demonstrated using the colony
formation assay that the proliferation rate of the PANC-1 cells
significantly decreased. All these data were consistent with those
of a previous study that HMGN5 promotes tumour progression.
Metastasis is an important aspect of PDAC. To
illustrate the role of HMGN5 in PDAC metastasis, we employed the
Transwell assay in vitro. Results showed that the HMGN5
silencing significantly inhibited the migration and invasion of the
PANC-1 cells. This result was consistent with that of a previous
study. Tumour metastasis is crucially dependent on EMT in
pancreatic cancer. EMT is characterized by the mutative expression
of an epithelial marker, such as E-cadherin, and mesenchymal
markers, such as N-cadherins and vimentin. The EMT process is
characterized by the loss of E-cadherin. Such loss creates profound
phenotypic alterations that convert the apico-basal polarity of the
epithelial cells into front-rear polarity. As a result, the
epithelial cells gain mesenchymal characteristics and migration and
invasion capacities. Therefore, identifying key molecules involved
in the EMT in pancreatic cancer may provide a new therapeutic
strategy for treating patients with pancreatic cancer. The
correlation of EMT and HMGN5 has not been well illustrated. In the
present study, we showed that HMGN5 enhanced the EMT as
demonstrated by the downregulation of E-cadherin expression and
upregulation of N-cadherins and vimentin in the PANC-1 cells. These
data strongly supported that HMGN5 is an activator of EMT in
pancreatic cancer.
We illustrated that HMGN5 positively regulated the
Wnt signalling pathway. The canonical Wnt signalling pathway is
also known as the Wnt/β-catenin signalling pathway as β-catenin is
a key transducer of the Wnt signal from the cytoplasm to the
nucleus (15). The Wnt/β-catenin
signalling pathway is an established EMT regulatory signalling
pathway due to its maintenance of epithelial integrity and tight
adherent junctions (16). Although
the Wnt/β-catenin signalling pathway is one of the major signalling
pathways involved in the EMT in pancreatic cancer (17–19), its
regulatory mechanisms still remain obscure. The present study
demonstrated for the first time that HMGN5 positively regulated the
Wnt/β-catenin signalling pathway. In the present study, we firstly
demonstrated that HMGN5 promoted the expression of Wnt1, a secreted
ligand that activates the Wnt signalling pathways, which are
strongly correlated with tumorigenesis and progression. Moreover,
we showed that the expression levels of GSK3β, β-catenin and total
β-catenin were downregulated during HMGN5 silencing. All these data
showed that HMGN5 possibly promoted the tumour progression via the
activation of the Wnt/β-catenin signalling pathway. Furthermore,
the reversal of the effect of HMGN5 silencing on tumour growth
inhibition and EMT caused by the overexpression of β-catenin
suggested that the effect was through, at least in part, the
Wnt/β-catenin signalling pathway.
In summary, the present study was the first to
demonstrate that the expression of HMGN5 was upregulated in PDAC.
We also found that HMGN5 promoted the PDAC cell viability,
proliferation, migration, invasion and EMT and suppressed the
tumour growth in vivo via the activation of the
Wnt/β-catenin signalling pathway. These results provided new
insights into the mechanism of PDAC progression and suggested that
HMGN5 is a potential antitumour agent in the treatment of
pancreatic cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during the present
study are included in this published article.
Authors' contributions
XW and JZ conceived and designed the experiments. JZ
and YW conducted all of the experiments, and YW wrote and revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of LanLing County Hospital (Shandong, China). All
patients included in this research were required to provide written
informed consent. The protocols for the animal experiments were
approved by the Animal Care and Welfare Committee of LanLing County
Hospital and were conducted in strict accordance with the
guidelines of the National Animal Welfare Law of China.
Patient consent for publication
Written informed consent was obtained from each
participant.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Melisi D and Budillon A: Pancreatic
cancer: Between bench and bedside. Curr Drug Targets. 13:729–730.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nistico P, Bissell MJ and Radisky DC:
Epithelial-mesenchymal transition: General principles and
pathological relevance with special emphasis on the role of matrix
metalloproteinases. Cold Spring Harb Perspect Biol. 4:pii: a011908.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gaianigo N, Melisi D and Carbone C: EMT
and treatment resistance in pancreatic cancer. Cancers. 9:pii:
E122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rochman M, Malicet C and Bustin M:
HMGN5/NSBP1: A new member of the HMGN protein family that affects
chromatin structure and function. Biochim Biophys Acta. 1799:86–92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji SQ, Yao L, Zhang XY, Li XS and Zhou LQ:
Knockdown of the nucleosome binding protein 1 inhibits the growth
and invasion of clear cell renal cell carcinoma cells in
vitro and in vivo. J Exp Clin Cancer Res. 31:222012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen P, Wang XL, Ma ZS, Xu Z, Jia B, Ren
J, Hu YX, Zhang QH, Ma TG, Yan BD, et al: Knockdown of HMGN5
expression by RNA interference induces cell cycle arrest in human
lung cancer cells. Asian Pac J Cancer Prev. 13:3223–3228. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Su B, Shi B, Tang Y, Guo Z, Yu X, He X, Li
X, Gao X and Zhou L: HMGN5 knockdown sensitizes prostate cancer
cells to ionizing radiation. Prostate. 75:33–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang W, Zheng J, Yu T and Wang J:
Overexpression of microRNA-495 suppresses the proliferation and
invasion and induces the apoptosis of osteosarcoma cells by
targeting high-mobility group nucleosome-binding domain 5. Oncol
Rep. 38:1099–1107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao Y, Zhang L, Wei M, Jiang X and Jia D:
MicroRNA-409-3p represses glioma cell invasion and proliferation by
targeting high-mobility group nucleosome-binding domain 5. Oncol
Res. 25:1097–1107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weng M, Song F, Chen J, Wu J, Qin J, Jin T
and Xu J: The high-mobility group nucleosome-binding domain 5 is
highly expressed in breast cancer and promotes the proliferation
and invasion of breast cancer cells. Tumour Biol. 36:959–966. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gan Y, He L, Yao K, Tan J, Zeng Q, Dai Y,
Liu J and Tang Y: Knockdown of HMGN5 increases the chemosensitivity
of human urothelial bladder cancer cells to cisplatin by targeting
PI3K/Akt signaling. Oncol Lett. 14:6463–6470. 2017.PubMed/NCBI
|
|
13
|
Liu X, Ma W, Yan Y and Wu S: Silencing
HMGN5 suppresses cell growth and promotes chemosensitivity in
esophageal squamous cell carcinoma. J Biochem Mol Toxicol. 31:2017.
View Article : Google Scholar
|
|
14
|
Shi Z, Tang R, Wu D and Sun X: Research
advances in HMGN5 and cancer. Tumour Biol. 37:1531–1539. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang L, Wu RL and Xu AM:
Epithelial-mesenchymal transition in gastric cancer. Am J Transl
Res. 7:2141–2158. 2015.PubMed/NCBI
|
|
17
|
Peng L, Liu Z, Xiao J, Tu Y, Wan Z, Xiong
H, Li Y and Xiao W: MicroRNA-148a suppresses epithelial-mesenchymal
transition and invasion of pancreatic cancer cells by targeting
Wnt10b and inhibiting the Wnt/β-catenin signaling pathway. Oncol
Rep. 38:301–308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bao Z, Xu X, Liu Y, Chao H, Lin C, Li Z,
You Y, Liu N and Ji J: CBX7 negatively regulates migration and
invasion in glioma via Wnt/β-catenin pathway inactivation.
Oncotarget. 8:39048–39063. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamoto M and Hisaoka M:
Clinicopathological Implications of Wingless/int1 (WNT) signaling
pathway in pancreatic ductal adenocarcinoma. J UOEH. 38:1–8. 2016.
View Article : Google Scholar : PubMed/NCBI
|