Introduction
Endometrial carcinoma is the most common type of
gynecological malignant tumor, ranking fourth in female cancers
(1,2).
The detection rate of endometrial carcinoma is on the rise among
young women who have a demand for childbearing. The epidemiologic
investigation has confirmed that approximately 1.6% of patients
with endometrial carcinoma are aged 20–34 years, and 6.1% are aged
35–44 years (3). Furthermore, the
prognosis of patients with early endometrial carcinoma is quite
good, after surgical treatments and adjunctive therapies. However,
patients usually get late diagnosis due to lack of effective and
early diagnostic methods (4). There
are multiple factors affecting therapeutic treatment of endometrial
carcinoma, such as clinical factors (surgical-pathologic staging,
depth of myometrial invasion and adjunctive therapy) and biological
factors (steroid receptors, growth factors, oncogenes and
suppressor genes) (5,6). Recently, screening of biological indexes
and gene therapies for endometrial carcinoma is becoming an active
research field.
E74-like factor 5 (ELF5) (also known as ESE2), is a
member of the E-twenty-six (ETS) transcription factor family which
regulates cell proliferation, differentiation and apoptosis.
Further, it has epithelial specificity and plays an important
regulatory role in placenta, lung airway and mammary gland
(7–10). A recent study by Chakrabarti et
al (11) showed that ELF5 has
potential effects of suppressor genes in normal mammary gland. Loss
of ELF5 expression might lead to proliferation of adult mammary
stem cells, increasing the risk of breast cancer. As for ovarian
cancer, earlier studies of the above research group discovered that
the expression of ELF5 mRNA showed decrease in epithelial ovarian
cancer tissues. The loss of expression affected the occurrence and
development of the disease. It might inhibit the invasion and
metastasis of ovarian cancer cells by regulation of matrix
metalloproteinase-2 (MMP-2) and MMP-9. In this way, it accelerates
cell cycle and apoptosis by increasing the expressions of P21 and
caspase-3 in the human ovarian cancer tissues (12–14).
However, in endometrial carcinoma, the effects of ELF5 have not
been fully revealed. Therefore, the present study was planned to
explore the expression of ELF5 in endometrial carcinoma and its
clinical significance. The study would provide new targets and
ideas for diagnosis, prognosis and treatment of endometrial
carcinoma.
Materials and methods
Study subjects
All the specimens were excised from the patients who
received surgical treatments in the Department of Obstetrics and
Gynecology, The Affiliated Hospital of Xuzhou Medical University
(Jiangsu, China) from January 2010 to December 2014. The Ethics
Committee of the hospital approved the study, and signed informed
consent was obtained from all the patients. Inclusion criteria for
endometrial carcinoma group were as follows: Patients with
endometrial carcinoma having complete clinical and pathological
data along with postoperative follow-up. The exclusion criteria
were: patients complicated with other systemic malignant tumors or
metastatic carcinomas from tumors of other organs in the
reproductive system (including synchronous primary carcinomas) as
well as patients who received chemotherapy, radiotherapy or
endocrine therapy as the first line of treatment were excluded.
Study design
The present study was divided into three groups
viz. endometrial carcinoma group, atypical hyperplasia group
and a normal endometrial tissue group.
i) Endometrial carcinoma group: this group included
paraffin blocks of tissues excised from 84 patients, which were
confirmed surgically and pathologically for endometrial carcinoma.
The patients were aged 38–70 years, with an average age of 54.61
years. According to the surgical-pathologic staging [International
Federation of Gynecology and Obstetrics (FIGO), 2009] the subjects
were classified into different stages. There were 54 cases in stage
I, 10 cases in stage II and 20 cases in stage III+IV. Further,
there were 52 cases with the depths of myometrial invasion ≤1/2 and
32 cases >1/2. Forty-one cases were accompanied with lymph node
metastasis and 26 cases without lymph node metastasis (with a total
of 67 cases undergoing lymphadenectomy). The pathological typing
was composed of 68 cases with endometrioid adenocarcinoma and 16
cases with non-endometrioid adenocarcinoma. The pathological
grading of endometrial adenocarcinoma showed 50 cases of G1, 8
cases of G2 and 10 cases of G3; 15 patients had menstruation and 69
patients had no menstruation.
ii) Atypical hyperplasia group: this group included
paraffin blocks of 30 patients who received surgical treatments due
to dysfunctional uterine bleeding. These patients were confirmed
pathologically for atypical hyperplasia and archived in the same
period. The patients were aged 40–74 years, with an average age of
45.93 years.
iii) Normal endometrial tissue group: this group
included paraffin blocks of 30 patients who received surgical
treatments due to hysteromyoma, but were pathologically confirmed
as normal endometrial tissues. Subsequently they were also archived
in the same period. The patients were aged 37–51 years, with an
average age of 49.73 years.
Detection of ELF5 protein expression
in tissues
All the specimens were fixed with 4% neutral
formalin, embedded in paraffin and serially sliced to thickness of
4 µm. The slices were dipped and were dewaxed in xylene and
gradient ethanol and H2O2 (3%) was then added
and were incubated at room temperature for 10 min, in order to
eliminate the activity of endogenous peroxidase. This was followed
by execution of antigen retrieval with citrate buffer under high
pressure. ELF5 antibody was dripped and incubated at 4°C overnight.
The hypersensitive two-step rabbit detection kit and DAB kit were
utilized for color development. The specimens were counterstained,
conventionally dehydrated, cleared, mounted with neutral balsam and
then observed under a microscope for the staining results. The ELF5
antibody (bs-14564R) was procured from Beijing Bioss Biological
Technology Co., Ltd. (Beijing, China), and the hypersensitive
two-step rabbit detection kit (PV-9001) and DAB kit (ZLI9018) were
purchased from Beijing Zhongshan Goldenbridge Biotechnology Co.,
Ltd. (Beijing, China).
Staining analyses
The staining results were analyzed via double-blind
reviewing method. Two experienced physicians reviewed the images.
Ten high-power fields with 100 cells each were randomly selected
using semi-quantitative method. The cells were given points
according to the percentage of positive cells in each high-power
field: percentage of positive cells <5%, 0 point; 5–25%, 1
point; 26–50%, 2 points; 51–75%, 3 points; >75%, 4 points.
Furthermore, scores were recorded according to the color of the
nuclei: no coloring, 0 point; light yellow, 1 point; yellow, 2
points; and brownish yellow, 3 points. The product of the scores of
the two scoring systems was regarded as the total score: 0 point,
negative; 1–4 points, weakly positive (+); 5–8 points,
intermediately positive (++); and 9 points and above, strongly
positive (+++). + to +++ were marked as positive. Total positive
rate = (case of weakly positive + case of intermediately positive +
case of strongly positive)/total case.
Postoperative follow-up of patients
with endometrial carcinoma
Postoperative follow-up was conducted in all the
patients with endometrial carcinoma. This included mainly
outpatient follow-up and telephone interview. The follow-up started
from the day when the patient underwent the surgery and ended June
30, 2017.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
19.0 software (IBM Corp., Armonk, NY, USA) was utilized for
statistical processing; χ2 test was used to examine the
expression of ELF5 in different endometrial tissues. Kaplan-Meier
method for survival analysis was applied for univariate analysis
for evaluation of the effects of ELF5 and clinicopathologic
parameters on the postoperative survival time of patients with
endometrial carcinoma. Cox's proportional hazards regression model
was utilized for univariate and multivariate analyses. The log-rank
test was performed to examine the difference in survival rates
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparisons of ELF5 expressions in
different endometrial tissues (Table
I)
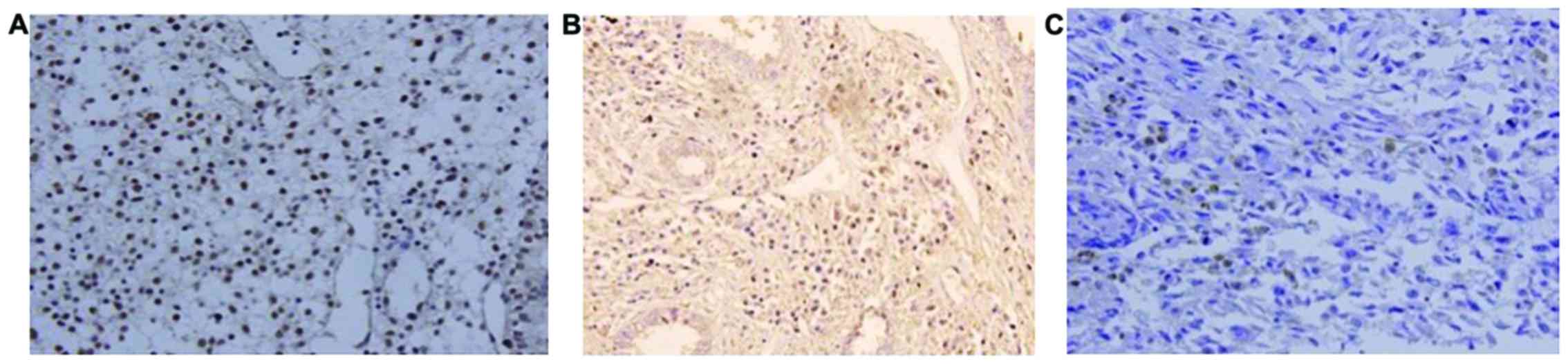
ELF5 was mainly expressed in nuclei and appeared as
brownish yellow granules (Fig. 1). In
normal endometrial tissue, atypical hyperplasia and endometrial
carcinoma tissues, the positive expression rates of ELF5 were
93.33% (28/30), 83.33% (25/30) and 69.05% (58/84), respectively.
The above observation showed a decreasing tendency
(χ2=8.218, P=0.016). However, the statistically
significant difference was observed only between normal group and
endometrial carcinoma group (χ2=5.787, P=0.016).
Furthermore, pairwise revealed non-significant results.
Relationship between ELF5 expressions
and clinicopathologic parameters in endometrial carcinoma tissues
(Table II)
Relationship between ELF5 expressions and age. In
the group aged ≤55 years and the group aged >55 years, the
positive expression rates of ELF5 were 70.73% (29/41) and 67.41%
(29/43), respectively, but the difference was not statistically
significant (χ2=0.016, P=0.744). These age groups were
selected to cover overall age of the patients affected with this
disorder.
Relationship between ELF5 expressions
and menstrual status
In premenopausal group and postmenopausal group, the
positive expression rates of ELF5 were 66.67% (10/15) and 69.57%
(48/69), respectively, but the difference was not statistically
significant (χ2=0.048, P=0.826).
Relationship between ELF5 expression
and FIGO staging
In phases I, II and III+IV, the positive expression
rates of ELF5 were 94.44% (51/54), 40.00% (4/10) and 15.00% (3/20),
respectively, and the differences among the three phases were
statistically significant (χ2=47.757, P<0.05).
Relationship between ELF5 expression
and pathological grading
According to the pathological grading and grouping,
there were 68 cases of endometrial adenocarcinoma in total,
including 50 cases of G1, 8 cases of G2 and 10 cases of G3; the
positive expression rates of ELF5 were 94.00% (47/50), 37.5% (3/8)
and 30% (3/10). All of the above differences among the three grades
were statistically significant (χ2=25.831,
P<0.05).
Relationship between ELF5 expression
and lymph node metastasis
A total of 67 out of 84 patients with endometrial
carcinoma underwent lymph node biopsy or lymphadenectomy. The
positive expression rates of ELF5 in non-lymph node metastasis
group and lymph node metastasis group were 92.68% (38/41) and
19.23% (5/26), respectively. The differences between the two groups
were statistically significant (χ2=34.212,
P<0.05).
Relationship between ELF5 expression
and myometrial invasion
The positive expression rates of ELF5 in group with
no myometrial invasion or with depth of myometrial invasion ≤1/2
and group with depth of myometrial invasion >1/2 were 98.08%
(51/52) and 21.88% (7/32), respectively. The differences between
the two groups were statistically significant
(χ2=51.414, P<0.05).
Relationship between ELF5 expressions
and pathological types
The positive expression rates of ELF5 in
endometrioid adenocarcinoma group and group with other pathological
types were 77.94% (53/68) and 31.25% (5/16), respectively. The
differences between the two groups were statistically significant
(χ2 =11.118, P=0.001).
Analysis on factors influencing prognosis
of patients with endometrial carcinoma
Kaplan-Meier method for survival analysis
(Table III)
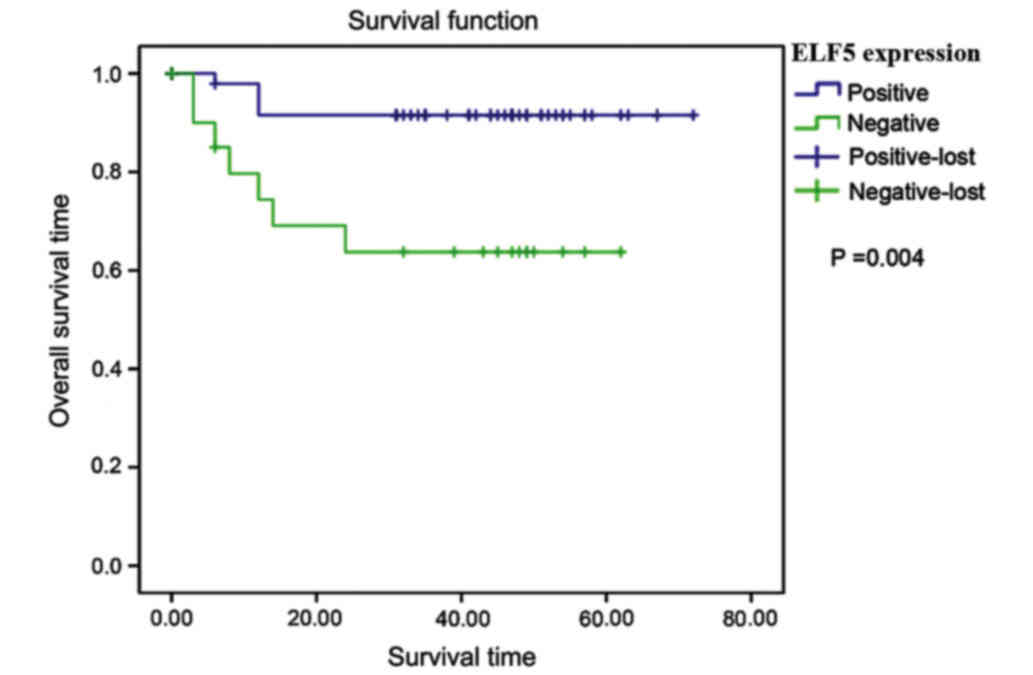
Relationship between ELF5 expression
and patient prognosis
A long-term follow-up for 6–67 months was conducted
in 84 patients with endometrial carcinoma after operation, of which
18 patients were lost to follow-up and 11 patients died. The
follow-up rate was 78.57%. The mean survival time of patients was
66.766 months in the negative ELF5 expression group, and it was
42.842 months in the positive ELF5 expression group. The overall
survival time in the positive ELF5 expression group was higher
(χ2=8.508, P=0.004) (Fig.
2).
Relationship between
clinicopathological parameters and patients' prognosis
i) Age (χ2=0.196, P=0.658), menstrual
status (χ2=0.001, P=0.975) or pathological type
(χ2=0.921, P=0.337) revealed non-significant
results.
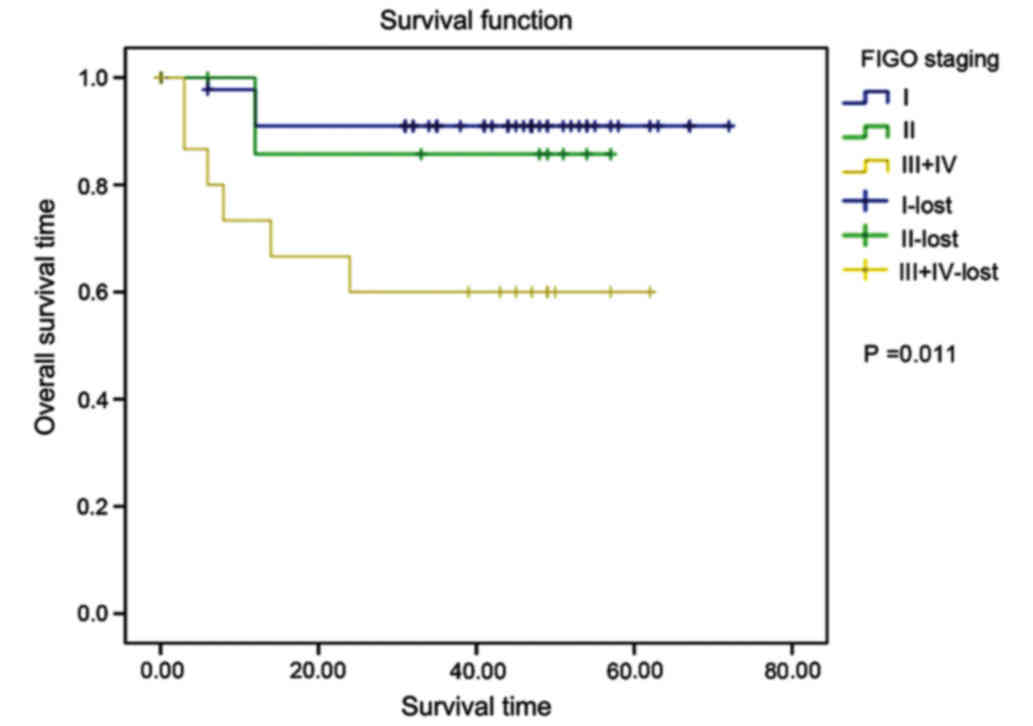
ii) FIGO staging: the mean survival time in phases
I, II and III+IV was 66.409, 50.571 and 41.067 months,
respectively; the later the FIGO staging was, the poorer the
prognosis would be; the differences among the three phases were
statistically significant (χ2=8.619, P=0.011) (Fig. 3).
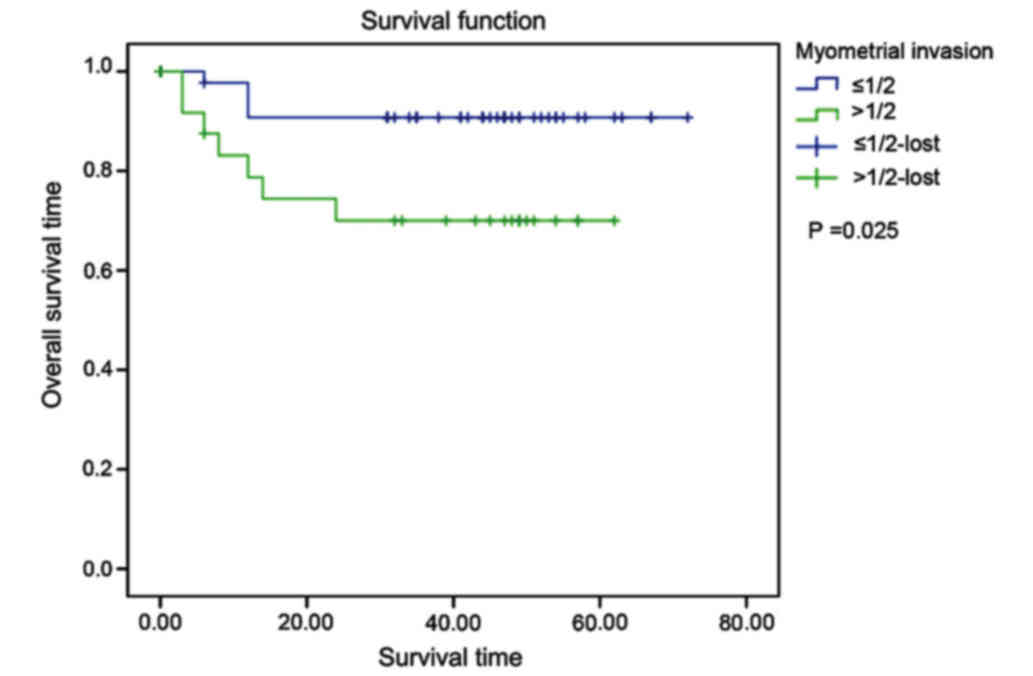
iii) Degree of myometrial invasion: the mean
survival time in group with no myometrial invasion or with depth of
myometrial invasion ≤1/2 was 66.545 months, and it was 46.174
months in group with depth of myometrial invasion >1/2. The
differences between the two groups were statistically significant
(χ2=5.109, P=0.025) (Fig.
4).
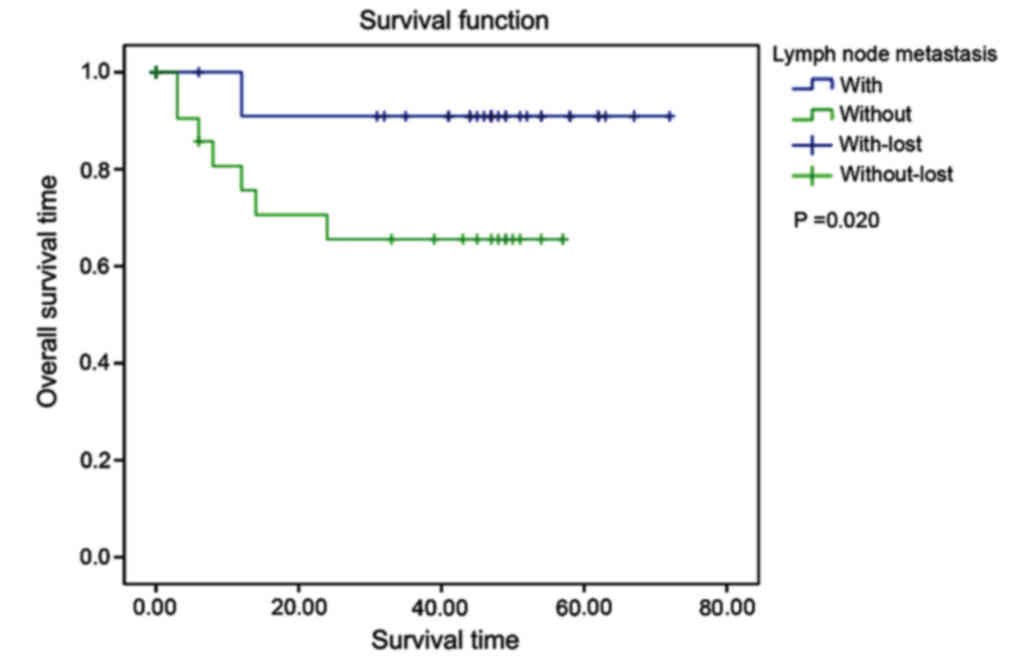
iv) State of lymph node metastasis: the mean
survival time in non-lymph node metastasis group was 66.545 months,
and it was 40.550 months in lymph node metastasis group. The
differences between the two groups were statistically significant
(χ2=5.380, P=0.020) (Fig.
5).
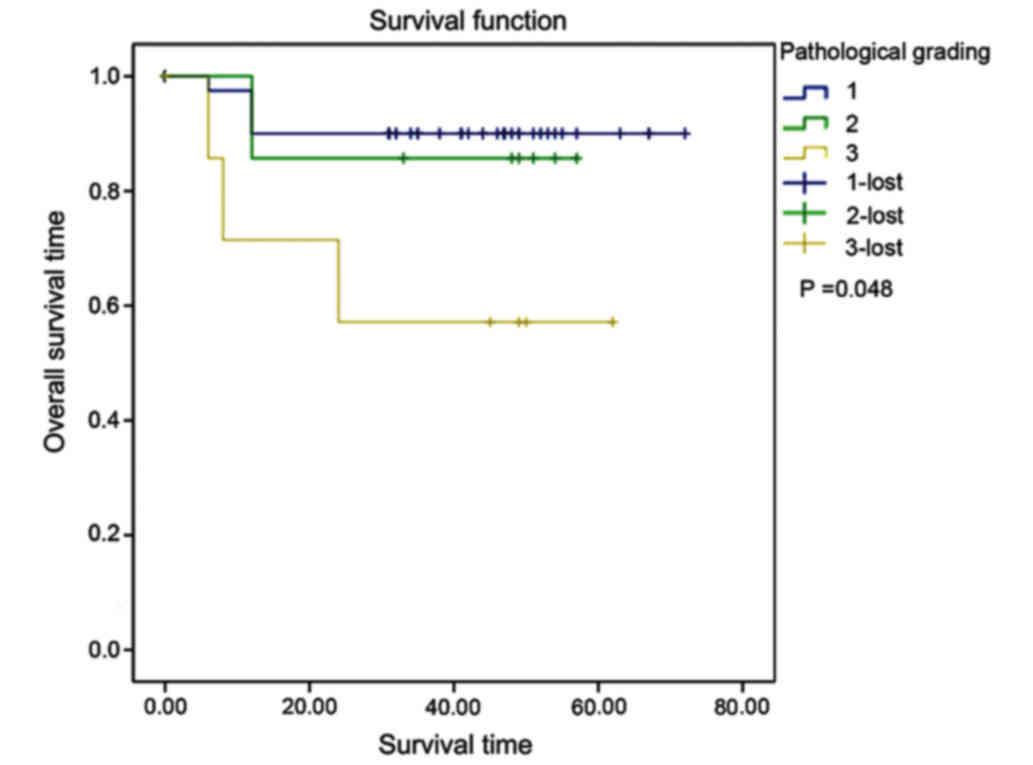
v) Pathological grading: the mean survival time of
patients with endometrial adenocarcinoma at G1, G2 and G3 was
65.850, 50.571 and 40.857 months, respectively. The higher the
pathological grading was, the poorer the prognosis would be. The
differences among the three grades were statistically significant
(χ2=6.093, P=0.048) (Fig.
6).
Cox's regression model analyses. i) The univariate
Cox's model analysis showed that age (χ2=0.190,
P=0.663), pathological type (χ2=0.870, P=0.351) and
menstrual status (χ2=0.001, P=0.975) were not associated
with prognosis. On the other hand, FIGO staging
(χ2=6.619, P=0.010), depth of myometrial invasion
(χ2=4.337, P=0.037), lymph node metastasis
(χ2=4.788, P=0.029), pathological grading
(χ2=4.225, P=0.040) and ELF5 (χ2=6.706,
P=0.010) were related to prognosis (Table IV).
 | Table IV.Analysis results of Cox's
proportional hazards regression model in patients with endometrial
carcinoma. |
Table IV.
Analysis results of Cox's
proportional hazards regression model in patients with endometrial
carcinoma.
|
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|
|---|
| Variables | Regression
coefficient | Standard error | Wald
χ2 | P-value | Exp (B) | Lower limit | Upper limit |
|---|
| FIGO staging | 0.848 | 0.330 | 6.619 | 0.010 | 2.335 | 1.224 | 4.456 |
| Myometrial
invasion | 1.306 | 0.627 | 4.337 | 0.037 | 3.691 | 1.080 | 12.617 |
| Lymph node
metastasis | 1.511 | 0.691 | 4.788 | 0.029 | 4.532 | 1.171 | 17.545 |
| ELF5
expression | −1.625 | 0.627 | 6.706 | 0.010 | 0.197 | 0.058 | 0.674 |
| Age | −0.137 | 0.313 | 0.190 | 0.663 | 0.872 | 0.472 | 1.612 |
| Pathological
type | −0.316 | 0.339 | 0.870 | 0.351 | 0.729 | 0.375 | 1.416 |
| Menstruation | −0.012 | 0.391 | 0.001 | 0.975 | 0.988 | 0.459 | 2.125 |
| Pathological
grading | 0.815 | 0.397 | 4.225 | 0.040 | 2.269 | 1.039 | 1.039 |
ii) Multivariate Cox's model analysis: the
multivariate Cox's model analysis was performed on FIGO staging,
depth of myometrial invasion, lymph node metastasis, pathological
grading and ELF5 expression. The final screening results confirmed
that ELF5 is an independent factor influencing the prognosis of
patients with endometrial carcinoma [χ2=4.424, P=0.035;
hazard ratio (HR) = 0.226, 95% CI: 0.056–0.904].
Discussion
Members of the ETS family play a vital regulatory
role in normal physiological activities, such as development,
differentiation, proliferation and apoptosis of cells (7). An earlier study reported that the
expression of ETS-related proteins was upregulated in renal cell
carcinoma in comparison to normal kidney (15). ELF5, also known as ESE2, is a member
of the ETS transcription factor family. It has two isomers, namely
ESE2a and ESE2b that exist in human body as a result of alternate
transcriptional start sites localized in distinctive exon 1s.
Moreover, ELF5 has epithelial specificity and is expressed in
placenta, lung airway, mammary gland, kidney and other organs
(7,10).
In research related to ELF5 and breast cancer,
researchers have observed loss of ELF5 expression in tissues and
cell lines of human breast cancer as a potential suppressor gene
(10). In the present study, ELF5
expression in normal endometrial tissue, atypical hyperplasia of
endometrium and endometrial carcinoma tissues, showed a decreasing
tendency. Further, the group wise comparison revealed
non-significant results due to the small sample size of the present
study. Further, the immunohistochemical and statistical results
showed that reduced or absent expression of ELF5 might have
promoted the occurrence of endometrial carcinoma. Yet the relevant
mechanism has not been determined. Moreover, it is quite possible
that, like that of other ETS factors, the abnormal expression in
cancers leads to dysregulation of target genes, thus causing cell
transformation.
An earlier study confirmed that the expression of
ELF5 mRNA had a negative correlation with staging and lymph node
metastasis, but it was not associated with age, pathological
grading, tissue type and ascites (12). Similarly in the present study, the
positive expression rate of ELF5 in endometrial carcinoma tissue
showed decrease. The study also showed the increase of FIGO
staging, pathological grading, depth of myometrial invasion and
progression of lymph node metastasis. However, it was not related
to age and menopause. This confirmed that the changes in ELF5
expression have a correlation with tumor development. The ELF5
showed a close association with the staging of endometrial
carcinoma in an earlier study (16).
This might be related to the fact that ELF5 could inhibit the
occurrence of epithelial-mesenchymal transition (EMT). Some studies
showed that ELF5 not only acts as a crucial regulator of cell
lineage, but also suppresses the occurrence of EMT by inhibition of
the transcription of Snail2 (17).
EMT affects cell adhesion, polarity and migration by means of
changing the state of epithelial cells to mesenchymal stem cells.
In tumor cells, EMT and its reverse program were activated so that
the tumor cells could acquire higher-level of invasiveness and
features related to relapse (18). It
could be concluded that the decrease or loss of ELF5 expression in
endometrial carcinoma could weaken the inhibition to EMT, thereby,
accelerated the development of endometrial carcinoma.
The expression of ELF5 is closely associated with
hormones. In mammary gland, prolactin and progesterone can promote
the expression of ELF5, which could accelerate the development of
embryonic precursor cells into estrogen receptor α and progesterone
receptors (19). Type I endometrial
carcinoma is estrogen-dependent, and its mechanism is a result of
long-term effect of estrogen without antagonism of progesterone
(20). The results of this research
showed that the positive expression rate of ELF5 in endometrioid
adenocarcinoma was higher than that of other pathological types
(such as serous carcinoma and squamous cell carcinoma). The
association of ELF5 with estrogen, progesterone and their
receptors, as well as its molecular mechanism, needs to be
determined by further studies.
There are many influencing factors for the prognosis
of patients with endometrial carcinoma. Zhang et al
(21) revealed by univariate survival
analysis that age, menopause, vaginal bleeding, pathological type,
histological grading, surgical-pathologic staging, degree of
myometrial invasion, lymph node metastasis, positive peritoneal
cytology and postoperative adjunctive therapy have effects on the
prognosis of endometrial carcinoma. Biological factors related to
prognosis of cancers have attracted the attention of the
researchers (22); for instance,
multiple studies have indicated that the expression levels of ER
and progesterone receptors (PR) are important factors for judging
the prognosis of patients with endometrial carcinoma. In the
present study, the univariate survival analysis revealed that
surgical-pathologic staging, histological grading, depth of
myometrial invasion, lymph node metastasis and ELF5 were the
factors affecting the prognosis of endometrial carcinoma.
Multivariate Cox regression model analysis showed that ELF5 was an
independent factor influencing endometrial carcinoma. The mortality
risk of patients with positive ELF5 in endometrial carcinoma was
decreased in comparison with the patients with negative ELF5 (HR =
0.226, 95% CI: 0.056–0.904). Moreover, the mortality risk of
patients with positive ELF5 was 0.226 times as high as that of
patients with negative ELF5. So, ELF5 could be utilized as an ideal
biological parameter for judging the prognosis of endometrial
carcinoma. The number of follow-up cases was small, of which 12
cases had a follow-up time longer than 5 years (including 5 years)
in the present study. Therefore, it would be more persuasive if the
number of follow-up cases was more and the follow-up time was
extended.
In conclusion, ELF5 could be used as an independent
impact factor for evaluating the prognosis of endometrial
carcinoma. It has good prospects for clinical application in early
screening and prognosis evaluation of endometrial carcinoma but
future studies with large sample sizes are essential for concrete
conclusion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HY collected and analyzed the general information of
patients. HY and HCY were responsible for IHC and follow-up
analysis. Both authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Affiliated Hospital of
Xuzhou Medical University (Jiangsu, China) approved the study and
written informed consents were signed by the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morice P, Leary A, Creutzberg C,
Abu-Rustum N and Darai E: Endometrial cancer. Lancet.
387:1094–1108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stewart CJ, Doherty DA, Havlat M, Koay MH,
Leung YC, Naran A, O'Brien D, Ruba S, Salfinger S and Tan J:
Transtubal spread of endometrial carcinoma: Correlation of
intra-luminal tumour cells with tumour grade, peritoneal fluid
cytology, and extra-uterine metastasis. Pathology. 45:382–387.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maritschnegg E, Wang Y, Pecha N, Horvat R,
Van Nieuwenhuysen E, Vergote I, Heitz F, Sehouli J, Kinde I, Diaz
LA Jr, et al: Lavage of the uterine cavity for molecular detection
of Müllerian duct carcinomas: A Proof-of-Concept Study. J Clin
Oncol. 33:4293–4300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Panici Benedetti P, Basile S, Salerno MG,
Di Donato V, Marchetti C, Perniola G, Palagiano A, Perutelli A,
Maneschi F, Lissoni AA, et al: Secondary analyses from a randomized
clinical trial: age as the key prognostic factor in endometrial
carcinoma. Am J Obstet Gynecol. 210:363.e1–363.e10. 2014.
View Article : Google Scholar
|
|
6
|
Abu-Rustum NR1, Iasonos A, Zhou Q, Oke E,
Soslow RA, Alektiar KM, Chi DS and Barakat RR: Is there a
therapeutic impact to regional lymphadenectomy in the surgical
treatment of endometrial carcinoma? Am J Obstet Gynecol. 198(457):
e1–e6. 2008.
|
|
7
|
Lee HJ and Ormandy CJ: Elf5, hormones and
cell fate. Trends Endocrinol Metab. 23:292–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee HJ, Gallego-Ortega D, Ledger A,
Schramek D, Joshi P, Szwarc MM, Cho C, Lydon JP, Khokha R,
Penninger JM, et al: Progesterone drives mammary secretory
differentiation via RankL-mediated induction of Elf5 in luminal
progenitor cells. Development. 140:1397–1401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalyuga M, Gallego-Ortega D, Lee HJ, Roden
DL, Cowley MJ, Caldon CE, Stone A, Allerdice SL, Valdes-Mora F,
Launchbury R, et al: ELF5 suppresses estrogen sensitivity and
underpins the acquisition of antiestrogen resistance in luminal
breast cancer. PLoS Biol. 10:e10014612012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pearton DJ, Smith CS, Redgate E, van
Leeuwen J, Donnison M and Pfeffer PL: Elf5 counteracts precocious
trophoblast differentiation by maintaining Sox2 and 3 and
inhibiting Hand1 expression. Dev Biol. 392:344–357. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chakrabarti R, Wei Y, Romano RA, DeCoste
C, Kang Y and Sinha S: Elf5 regulates mammary gland stem/progenitor
cell fate by influencing notch signaling. Stem Cells. 30:1496–1508.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan H, Qiu L, Xie X, Yang H, Liu Y, Lin X
and Huang H: ELF5 in epithelial ovarian carcinoma tissues and
biological behavior in ovarian carcinoma cells. Oncol Rep.
37:1412–1418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Endo A, Tomizawa D, Aoki Y, Morio T,
Mizutani S and Takagi M: EWSR1/ELF5 induces acute myeloid leukemia
by inhibiting p53/p21 pathway. Cancer Sci. 107:1745–1754. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan H, Qiu L, Xie X, Yang H, Liu Y, Lin X
and Huang H: ELF5 in epithelial ovarian carcinoma tissues and
biological behavior in ovarian carcinoma cells. Oncol Rep.
37:1412–1418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grassmeyer J, Mukherjee M, deRiso J,
Hettinger C, Bailey M, Sinha S, Visvader JE, Zhao H, Fogarty E and
Surendran K: Elf5 is a principal cell lineage specific
transcription factor in the kidney that contributes to Aqp2 and
Avpr2 gene expression. Dev Biol. 424:77–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Risinger JI, Maxwell GL, Chandramouli GV,
Jazaeri A, Aprelikova O, Patterson T, Berchuck A and Barrett JC:
Microarray analysis reveals distinct gene expression profiles among
different histologic types of endometrial cancer. Cancer Res.
63:6–11. 2003.PubMed/NCBI
|
|
17
|
Chakrabarti R, Hwang J, Blanco Andres M,
Wei Y, Lukačišin M, Romano RA, Smalley K, Liu S, Yang Q, Ibrahim T,
et al: Elf5 inhibits the epithelial-mesenchymal transition in
mammary gland development and breast cancer metastasis by
transcriptionally repressing Snail2. Nat Cell Biol. 14:1212–1222.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou ZJ, Dai Z, Zhou SL, Hu ZQ, Chen Q,
Zhao YM, Shi YH, Gao Q, Wu WZ, Qiu SJ, et al: HNRNPAB induces
epithelial-mesenchymal transition and promotes metastasis of
hepatocellular carcinoma by transcriptionally activating SNAIL.
Cancer Res. 74:2750–2762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oakes SR, Naylor MJ, Asselin-Labat ML,
Blazek KD, Gardiner-Garden M, Hilton HN, Kazlauskas M, Pritchard
MA, Chodosh LA, Pfeffer PL, et al: The Ets transcription factor
Elf5 specifies mammary alveolar cell fate. Genes Dev. 22:581–586.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kreizman-Shefer H, Pricop J, Goldman S,
Elmalah I and Shalev E: Distribution of estrogen and progesterone
receptors isoforms in endometrial cancer. Diagn Pathol. 9:772014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boks DE, Trujillo AP, Voogd AC, Morreau H,
Kenter GG and Vasen HF: Survival analysis of endometrial carcinoma
associated with hereditary nonpolyposis colorectal cancer. Int J
Cancer. 102:198–200. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong P, Kaneuchi M, Konno Y, Watari H,
Sudo S and Sakuragi N: Emerging therapeutic biomarkers in
endometrial cancer. BioMed Res Int. 2013:1303622013. View Article : Google Scholar : PubMed/NCBI
|