Introduction
Oral tongue squamous cell carcinoma (OTSCC) is one
of the most common types of malignancy in the head and neck region
and specifically in the oral cavity, with a global incidence
estimated at 275,000 novel cases/year in 2002 (1). Although treatments have progressed over
the past two decades, 5-year survival rates have remained at a low
level of 50% (2). The high death rate
is caused by frequent invasion and metastasis, and the
identification of the associated target molecules are necessary
prerequisites for the early detection of OTSCC and the
identification of treatment strategies (3).
Urokinase-type plasminogen activator receptor (uPAR)
is involved in tissue reorganization events, including mammary
gland involution and wound healing. uPAR focuses uPA proteolytic
activity on the cell membrane, mediates cell adhesion to
vitronectin and activates cell signaling pathways by associating
with cell surface molecules (4).
Previous studies have demonstrated that uPAR has an increased
expression in numerous malignant tumor types, including oral
squamous cell, breast and pancreatic carcinomas (5–7), and it
has been indicated to regulate a number of events, including
angiogenesis, immune suppression and cell migration (8). Therefore, it has been considered that
activation of uPAR serves a notable role in cancer cell invasion
and is correlated with a poor long-term prognosis (9). However, the mechanism underlying the
role of uPAR in OTSCC invasion and migration is not completely
understood.
Ts cells, established and characterized at the
Department of Oral Biology, College of Stomatology, Fourth Military
Medical University Laboratory, exhibited a higher metastatic
potential than the parental Tca-8113 cells in vitro and
in vivo (10). In our previous
study, the Ts cells transfected with short hairpin RNA (shRNA)
targeting uPAR were successfully constructed and identified
(11). On this basis, the aim of the
present study was to investigate the effects of uPAR inhibition on
tumor cell invasion and metastasis in the OTSCC Ts cell line via
RNA interference. The results of the present study indicated that
blocking uPAR in the OTSCC cells decreased the progression and
invasion in vitro and decreased the number of lung
metastases in orthotopic models; therefore, combination therapies
targeting uPAR may represent a novel therapeutic approach that
synergistically decreases the invasion and metastasis of OTSCC in
the future.
Materials and methods
Cell culture and transfection
The Ts cell line was obtained from the Department of
Oral Biology, College of Stomatology, Fourth Military Medical
University. This cell line was established from cells obtained from
the brain metastases of nude mice that had been injected with
TCA8113 cells. Plasmid pWH1 was designed by Dr. Wu Yuan-Ming
(Department of Pathology and Pathophysiology of the Fourth Military
Medical University) (12).
Lipofectamine® 2000 Transfection reagent (catalog no.
11668019) were purchased from Invitrogen (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Bgl II, EcoL I and Hind III were purchased
from Takara Bio Inc. (Otsu, Japan). The Ts cells transfected with
pWH1-upar (0.8 µg/50 µl; obtained from Dr Wu Yuan-Ming, Department
of Pathology and Pathophysiology of the Fourth Military Medical
University) expression vector exhibited a lower mRNA and protein
expression of Upar for 48 h. In control group (shRNA-C),
transfection was performed by transfecting Ts cells with pWH1 (0.8
µg/50 µl; obtained from Dr. Wu Yuan-Ming, Department of Pathology
and Pathophysiology of the Fourth Military Medical University) for
48 h. Control cells were incubated with Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.; catalog no:
12491-015) alone without shRNA. Cells were grown in DMEM
supplemented with 10% fetal calf serum (Gibco; Thermo Fisher
Scientific, Inc.; catalog no: 10099141) in a humidified atmosphere
containing 5% CO2 at 37°C for 24 h.
Immunohistochemistry
Immunohistochemical studies were conducted on
sections of paraffin-embedded tissues of clinical OTSCC samples.
All the patients (n=65; 42 male, 23 female; age range, 40–85 years;
mean age, 64 years) were examined and treated at The First Hospital
of Lanzhou University Lanzhou, China or Lanzhou University Second
Hospital, Lanzhou, China between January 2010 to January 2013.
Eligibility criteria included that patients had not received
postoperative adjuvant therapy, including chemotherapy or
radiotherapy, or any other treatment prior to surgery. Tissues were
fixed in 4% paraformaldehyde at 4°C for 24 h and were embedded in
paraffin. For immunohistochemistry, surgically-resected OTSCC
samples, including adjacent tissues, were cut to a thickness of 4
µm. The sections were sequentially dewaxed in xylene, rehydrated
with a descending alcohol series (100, 95, 90, 80, 70%) and
distilled water and then subjected to antigen retrieval for 30 min
at 95°C. Normal goat serum (catalog no. 5425; Cell Signaling
Technology, Inc., Danvers, MA, USA) in PBS was used as blocking
buffer for 1 h at 37°C. The slides were subsequently incubated
overnight at 4°C with a primary rabbit polyclonal antibody specific
against uPAR (1:100; catalog no. 12713; Cell Signaling Technology,
Inc., Danvers, MA, USA). Slides were then treated with an
biotin-conjugated goat anti-rabbit secondary antibody (catalog no.
TA130016; OriGene Technologies, Inc., Beijing, China) diluted in
0.01M PBS (1:100) at room temperature for 1 h and developed using
avidin-conjugated horseradish peroxidase with 3,3′-diaminobenzidine
as a substrate (OriGene Technologies, Inc.), followed by
hematoxylin counterstaining for 10 min at room temperature. The
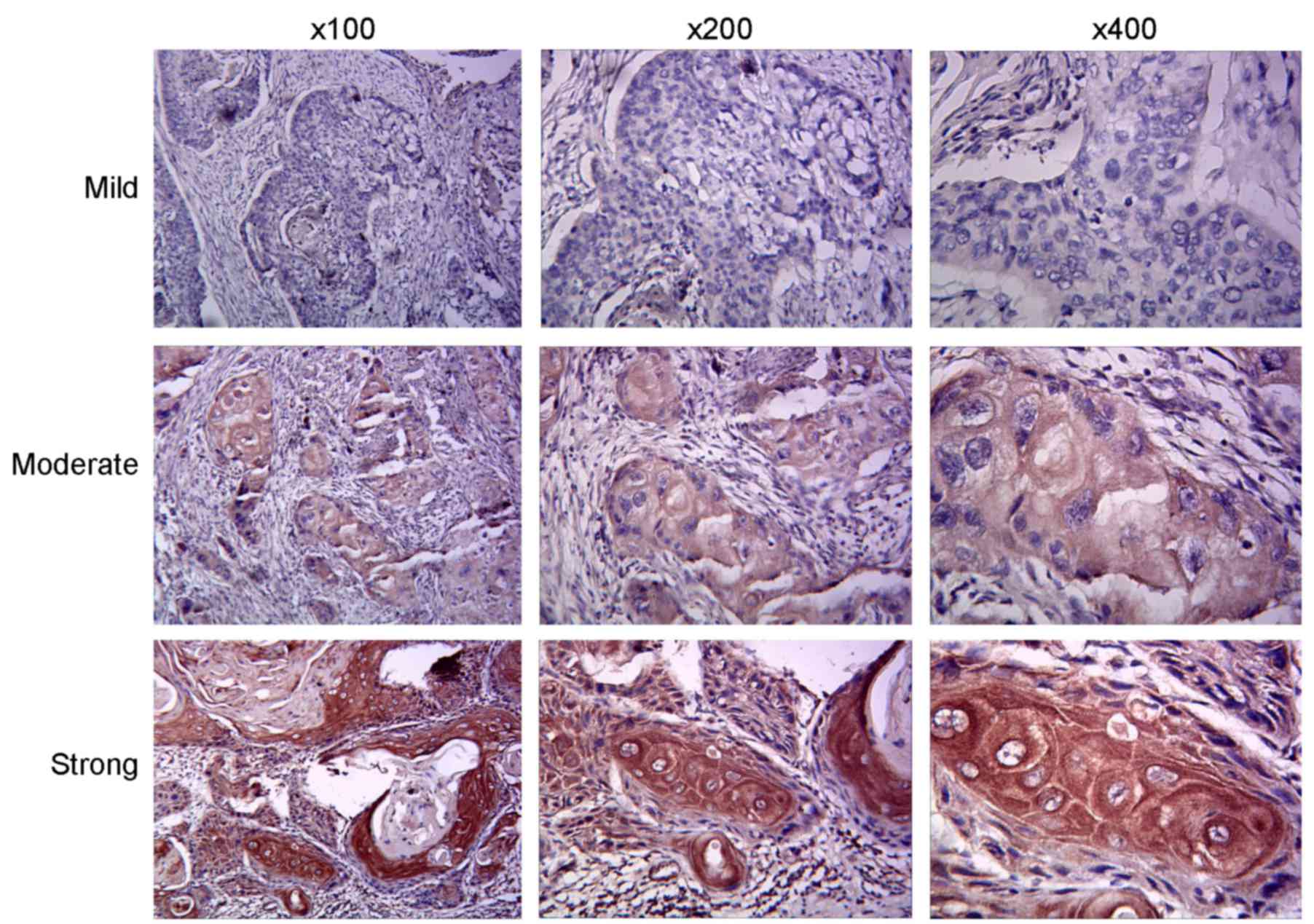
assessment of the uPAR expression level was classified according to
semi-quantitative immunohistochemistry (13). uPAR immunoreactivity was scored
separately in cancerous or adjacent non-cancerous sections, as
described previously (14). The
slides were reviewed with a light microscope (×100, ×200 and ×400)
by two investigators blind to the clinical diagnosis of OTSCC.
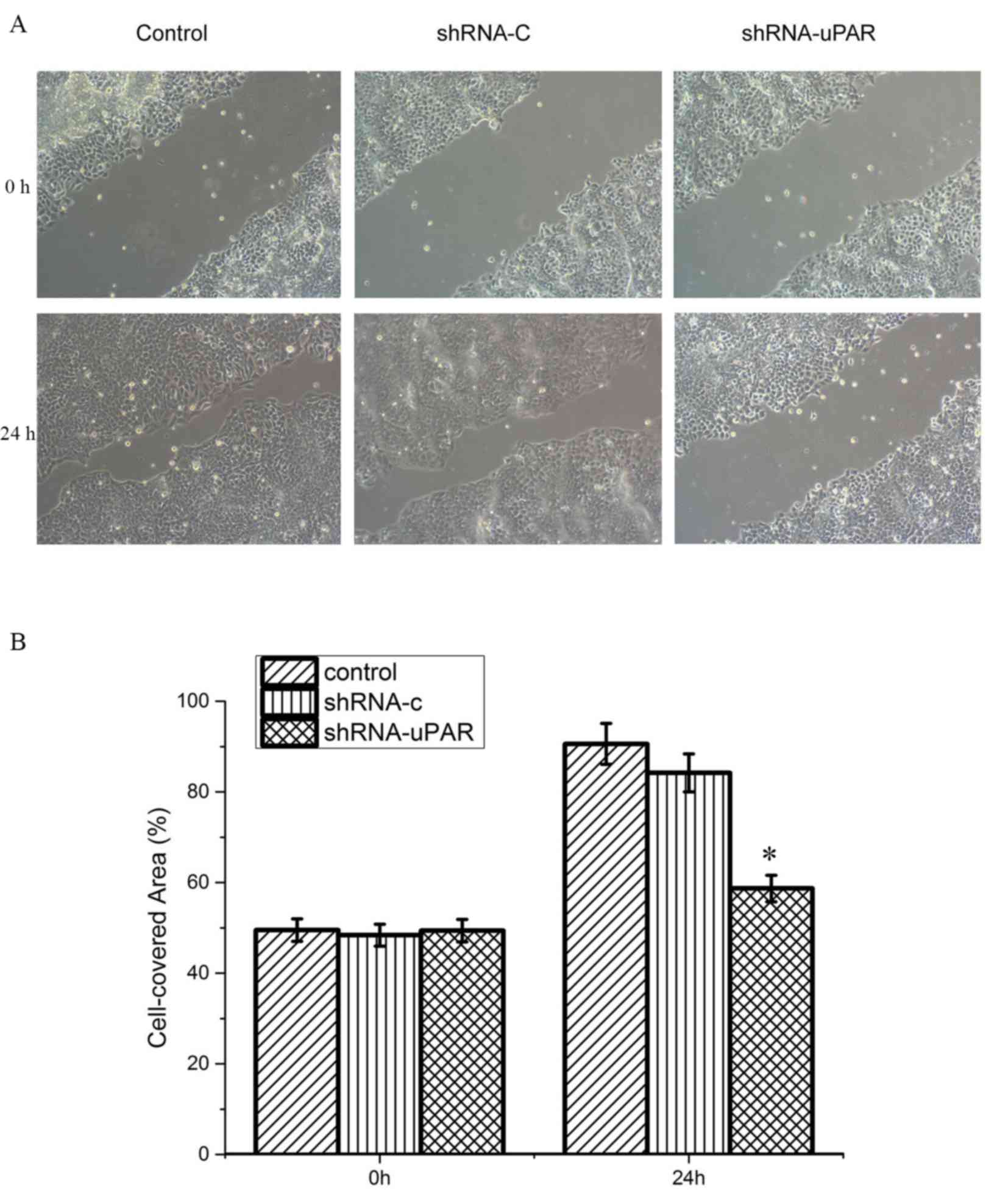
Wound-healing assay
To determine the effects of uPAR shRNA transfection
on the motility of the Ts cell line, cells were plated at
1×105/well in a 6-well plate in DMEM supplemented with
10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific,
Inc.). Once the cells reached 90% confluency, sterile pipette tips
were used to scratch a wound ~600-µm wide uniformly. Following
this, the cells were washed with PBS, and DMEM was added with 10
g/l bovine serum albumin. After 24 h of incubation at 37°C, the
medium was replaced with fresh DMEM supplemented with 10% FBS. The
scratched area was imaged with a ×100 magnification light
microscope at 0 and 24 h. Cell migration was analyzed using ImageJ
(version 1.48) software (National Institutes of Health, Bethesda,
MD, USA) by counting the number of cells in the scratched areas.
Each experiment was conducted in triplicate.
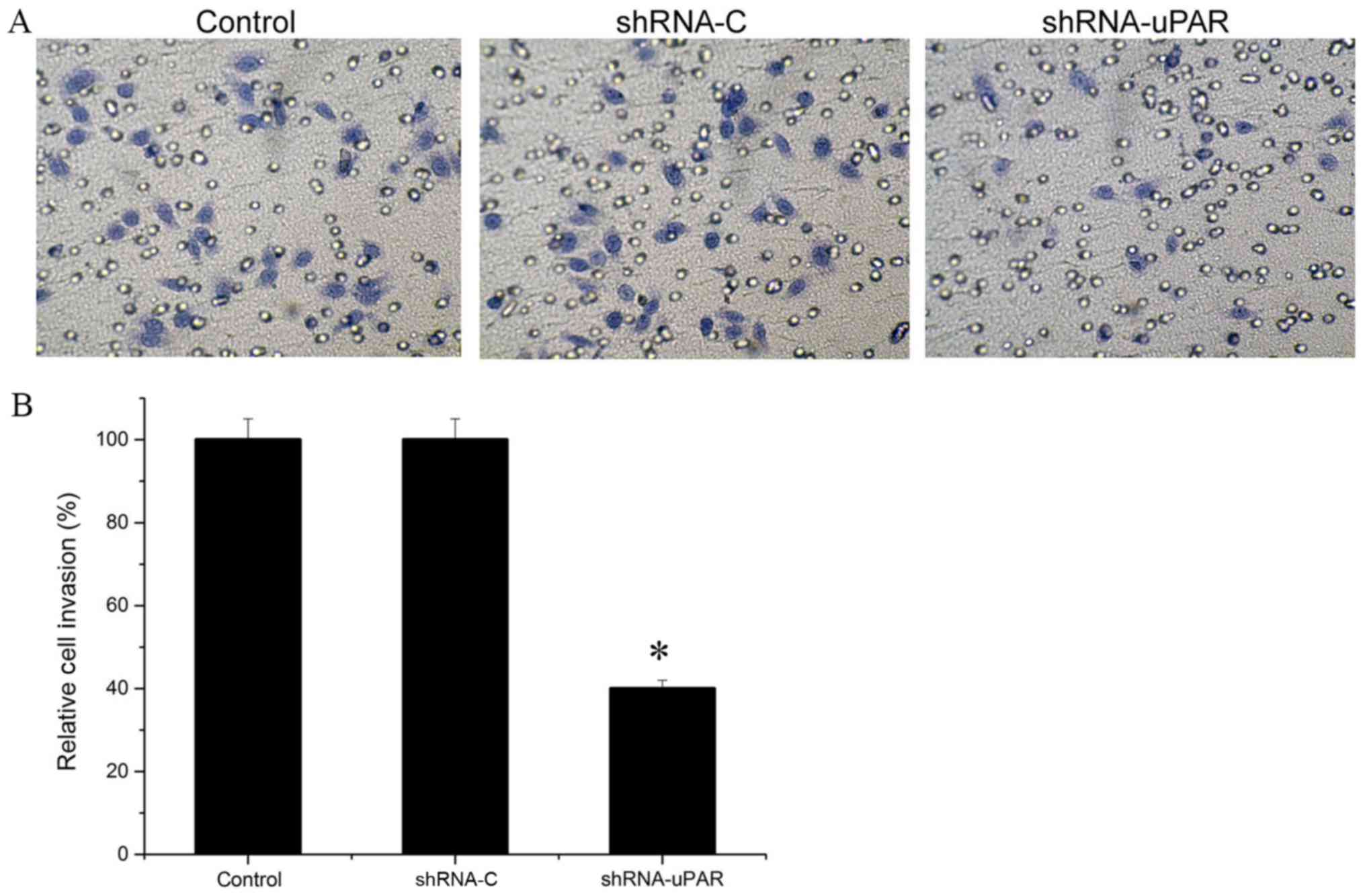
Tumor cell invasion assay
The invasion assay was performed with
Matrigel-coated Transwell inserts (8-µm pore size; EMD Millipore,
Billerica, MA, USA). Isolated cells at a concentration of
1×105cells/well resuspended in DMEM were placed into the
upper chamber. DMEM with 20% FBS was placed into the lower chamber.
Cells were allowed to migrate through the Matrigel for 48 h. After
48 h, non-invading cells were removed from the upper chamber using
a cotton swab. Invading cells that adhered to the outer surface of
the Transwell insert or that had invaded through the Matrigel were
fixed in methanol and stained with crystal violet for 0.5 h at
37°C. The invasiveness was determined by counting the penetrated
cells under a light microscope at ×200 magnification in 10 randomly
selected fields in each filter. Each experiment was performed in
triplicate.
Western blot analysis
Western blot analysis was performed using standard
techniques (15). Briefly, the three
groups of harvested cells were washed with PBS and lysed with RIPA
buffer (catalog no. 9806; Cell Signal Technology, Inc.). The
protein concentration was determined using a bicinchoninic acid
protein assay kit (Thermo Fisher Scientific, Inc.). Protein samples
(20 µg) were separated in 10% SDS-PAGE by electrophoresis and
subsequently transferred onto a polyvinylidene fluoride (PVDF)
membranes by electroblotting. Following electrophoresis and
transfer to PVDF membranes, then blocked with 5% non-fat dried milk
for 1 h at room temperature and detection of specific proteins was
conducted using antibodies) against matrix metalloprotease (MMP)-9
(catalog no. 13667; 1:1,000), MMP-2 (catalog no. 87809; 1:1,000),
phosphorylated-extracellular signal-regulated kinase (p-ERK)
(catalog no. 4370; 1:1,000), ERK (catalog no. 4695; 1:1,000),
p-protein kinase B (p-Akt; catalog no. 9271; 1:2,000), Akt (catalog
no. 4691; 1:1,000) and GAPDH (catalog no. 5174, 1:1,000) (Cell
Signaling Technology, Inc.) overnight at 4°C. Following this, the
immunoreactive bands were incubated with horseradish
peroxidase-conjugated immunoglobulin G anti-rabbit secondary
antibody (catalog no. 7074; dilution, 1:10,000; Cell Signaling
Technology, Inc.) at room temperature for 2 h. Subsequently, the
signals were detected using enhanced chemiluminescence reagents
X-ray films. Images were analyzed by ImageJ (version 1.48; National
Institutes of Health, Bethesda, MD, USA).
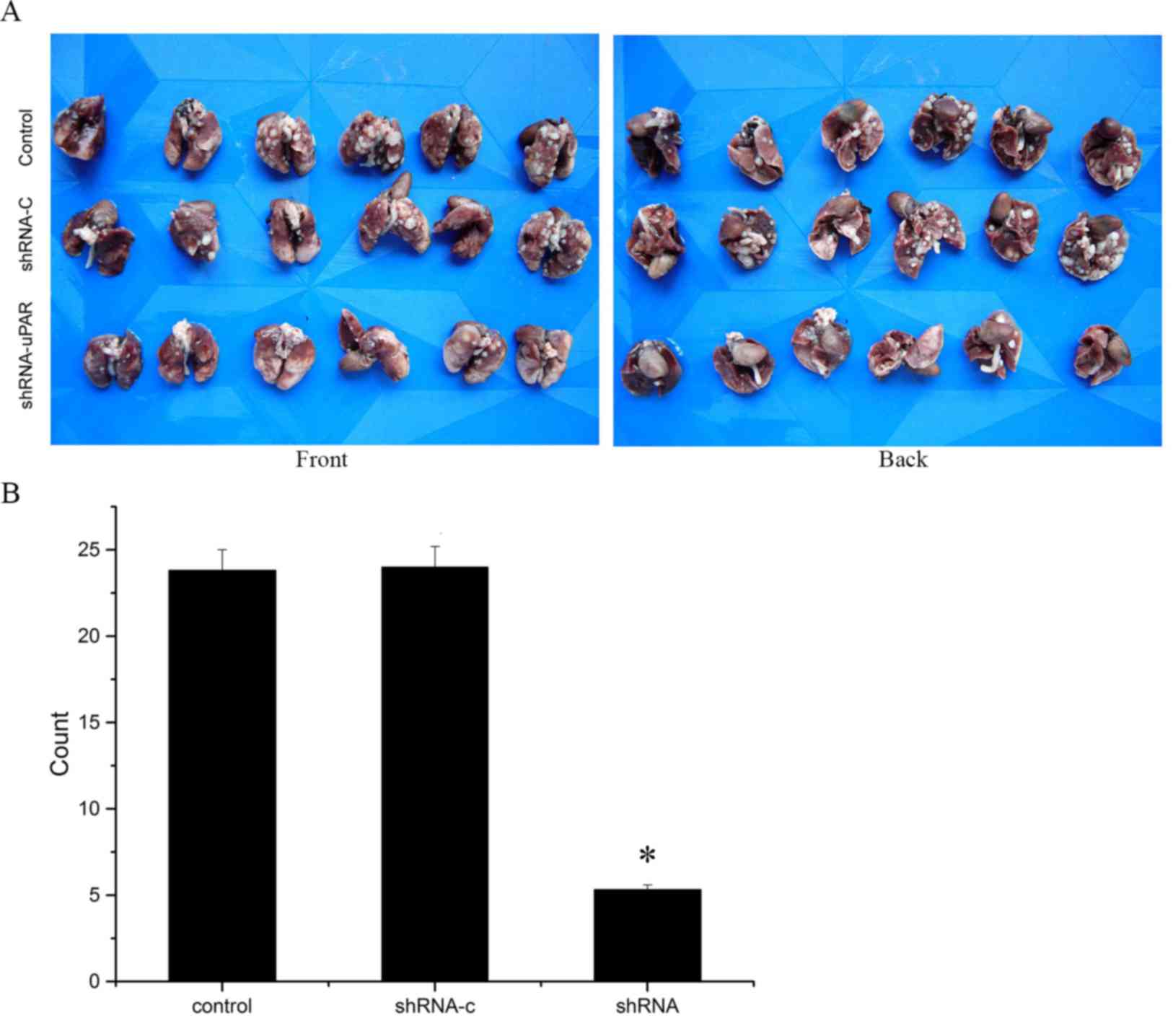
In vivo metastasis assay
A total of 18 female athymic nude mice (4–5 weeks
old; weight, 15–20 g) were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China) and were fed with food and water
ad libitum in a controlled atmosphere (temperature, 22±2°C;
humidity, 55±2%) on a 12/12 h light/dark cycle. The three groups of
1×108 cells in 200 µl culture medium (DMEM) were
injected into the tail vein of the nude mice (n=6). In accordance
with the principles of animal ethics and without affecting the
experimental results, we chose to sacrifice experimental animals by
cervical dislocation and their lungs were collected to determine
any metastases at 6 weeks after inoculation. Incidence of
metastasis was determined by counting the number of macroscopic
lesions on the surface of the lungs.
Statistical analysis
Data are presented as the mean ± standard deviation
of triplicate specimens per condition. The expression of uPAR in
OTSCC tumor specimens and adjacent non-cancerous specimens was
analyzed by the χ2 test. Differences between groups were
analyzed by one-way analysis of variance. Least Significant
Difference and Student-Newman-Keuls were used as the post hoc test
using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of uPAR in Ts cells
To investigate the protein expression level of uPAR
in OTSCC tumors and the association between tumor progression and
metastasis, immunohistochemical analysis for uPAR on formalin-fixed
OTSCC clinical tissue samples was conducted. Primary tissues
exhibited cytoplasmic staining for uPAR (Fig. 1). A total of 46 tumor samples (70.8%)
were uPAR positive, whilst adjacent tissue samples rarely exhibited
immunopositivity for uPAR (P<0.001; Table I). Further analysis of patient data
revealed that tumor metastasis and relapse were the
clinicopathological factors associated with uPAR positivity
(P<0.05). Other parameters, including age (P=0.68), sex
(P=0.653), pathological stage (P=0.839) and clinical stage
(P=0.388), did not differ significantly between uPAR-positive and
-negative groups (Table II).
 | Table I.Expression of uPAR in oral tongue
squamous cell carcinoma and adjacent tissues. |
Table I.
Expression of uPAR in oral tongue
squamous cell carcinoma and adjacent tissues.
|
|
| uPAR
expression |
|
|---|
|
|
|
|
|
|---|
| Tissue type | n | Positive | Negative | P-value |
|---|
| Cancer tissue | 65 | 46 | 19 | <0.001 |
| Adjacent
tissue | 28 | 6 | 22 |
|
 | Table II.Association between uPAR expression
and clinicopathological variables in 65 patients with oral tongue
squamous cell carcinoma. |
Table II.
Association between uPAR expression
and clinicopathological variables in 65 patients with oral tongue
squamous cell carcinoma.
|
| uPAR
expression |
|
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factor | Positive | Negative | χ2 | P-value |
|---|
| Sex |
|
| 0.17 | 0.68 |
|
Male | 29 | 13 |
|
|
|
Female | 17 | 6 |
|
|
| Age, years |
|
| 0.202 | 0.653 |
|
>60 | 27 | 10 |
|
|
|
≤60 | 19 | 9 |
|
|
| Pathological
stage |
|
| 0.351 | 0.839 |
| I | 13 | 6 |
|
|
| II | 22 | 7 |
|
|
|
III | 12 | 5 |
|
|
| Clinical stage |
|
| 3.023 | 0.388 |
| I | 5 | 3 |
|
|
| II | 11 | 8 |
|
|
|
III | 22 | 6 |
|
|
| IV | 8 | 2 |
|
|
|
Invasion/metastasis |
|
| 9.718 | 0.002 |
|
Yes | 24 | 2 |
|
|
| No | 22 | 17 |
|
|
| Relapse |
|
| 8.153 | 0.004 |
|
Yes | 25 | 3 |
|
|
| No | 21 | 16 |
|
|
Silencing of uPAR affects the
migratory potential of Ts cells in vitro
Following silencing uPAR in the Ts cell line, the
differences in migratory capacities were measured using a
wound-healing assay, in which the cells were scratched and then
migrated into the wound area. The control group and the shRNA-C
group demonstrated an ~90.0% wound closure by 24 h after the
initial wounding, the uPAR shRNA group demonstrated delayed
migration with ~58.7% wound closure in the same time period,
indicating a significantly delayed migratory potential (P<0.05;
Fig. 2).
Silencing of uPAR inhibited the
invasion of Ts cells
To assess the differences in invasive potentials
between the uPAR shRNA group and the parental Ts cells after 48 h,
Matrigel invasion assays were performed. Colorimetric analysis of
the crystal violet-stained cells indicated a ~60.0% decrease in the
number of cells in the uPAR siRNA group, compared with the control
group and the shRNA-C group, demonstrating a significantly lower
invasive potential following silencing of uPAR in Ts cells
(P<0.05; Fig. 3).
Silencing of uPAR decreases MMP-2,
MMP-9 and p-ERK protein expression levels
Subsequently, the effect of uPAR on the expression
of invasion-associated molecules in Ts cells was examined using
western blot analysis. Expression of matrix metalloproteinases is
important in the migration and invasion of cancer cells through the
basement membrane (16). It was
observed that MMP-2 and MMP-9 protein expression levels were
significantly decreased in the uPAR shRNA group, compared with the
shRNA-C and control groups (P<0.05). Furthermore, the effects on
the MEK/ERK and Akt signaling pathways were examined. The results,
depicted in Fig. 4, indicated no
significant change in Akt phosphorylation, but there was a 37.0%
decrease in ERK phosphorylation (P<0.05), indicating a possible
role of uPAR in the MEK/ERK signaling pathway involving mediation
of the invasion and migration effects.
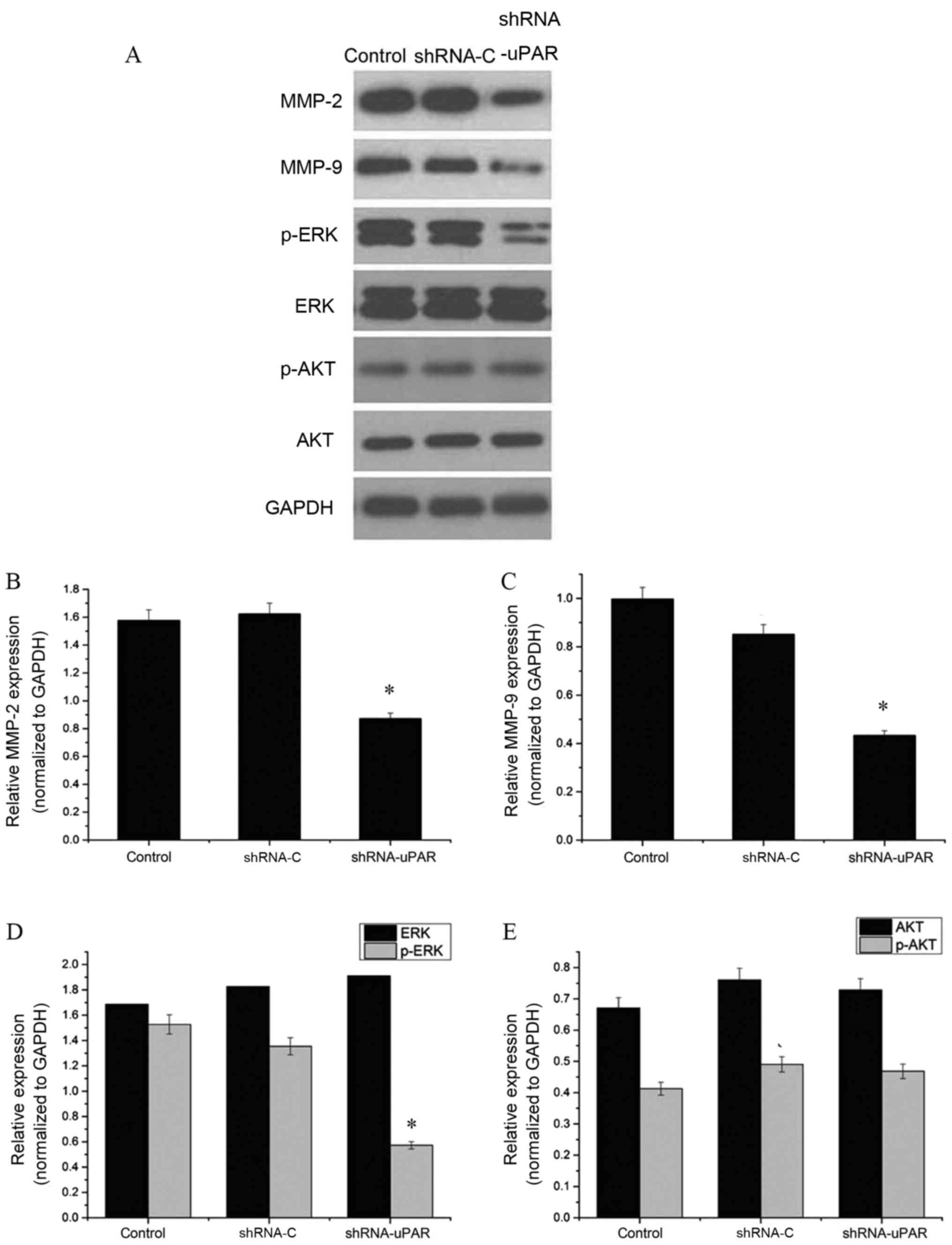 | Figure 4.Western blot analysis of
invasion-associated proteins. (A) The bands of western blot
analysis. GADPH was used as an internal control. (B) MMP-2 protein
expression levels were significantly decreased in the shRNA-uPAR
group, compared with the shRNA-C and control groups (*P<0.05).
(C) MMP-9 protein expression levels were significantly decreased in
the shRNA-uPAR group, compared with the shRNA-C and control groups
(*P<0.05). (D) ERK and p-ERK protein expression levels in the
shRNA-uPAR, shRNA-C and the control groups. P-ERK protein
expression levels were significantly decreased in the shRNA-uPAR
group (*P<0.05, compared with the control and shRNA-C groups).
(E) AKT and p-AKT protein expression levels in the shRNA-uPAR,
shRNA-C and the control groups. Silencing of uPAR had no effect on
the AKT protein expression levels (compared with the control and
shRNA-C groups). MMP, matrix metalloprotease; p-ERK,
phospho-extracellular signal-regulated kinase; p-Akt, phospho-Akt;
uPAR, urokinase-type plasminogen activator receptor; shRNA, short
hairpin RNA. |
Silencing of uPAR suppresses the
metastatic potential of Ts cells in vivo
To analyze the metastatic effect of uPAR in
vivo, Ts cells were injected into the tail vein of nude mice
and the presence of metastatic nodes in the lungs was evaluated
after 6 weeks. Results depicted in Fig.
5 indicated a significant decrease in the number of metastatic
nodes in the mice in the uPAR-shRNA group (P<0.05; Fig. 5).
Discussion
uPAR overexpression is associated with an increased
propensity for cancer progression and metastasis, and thus it has
emerged as a promising novel target for the treatment of cancer
(17). Previous studies indicated
that intact uPAR and its cleaved forms are associated with the
process of tumor initiation (18) and
metastasis (19). Results of the
study by Margheri et al (20)
indicated that silencing of uPAR altered the metastatic
characteristics of advanced cancer; however, to the best of our
knowledge, the mechanism underlying the role of uPAR in OTSCC
invasion and migration has not been previously studied. uPAR
activates cell-signaling pathways directed by proximal
transmembrane co-receptors, including the epidermal growth factor
receptor (21), and subsequently
functions as a broad-spectrum protease that has the ability to
degrade several extracellular matrix proteins (22) and activate latent growth factors and
MMPs (23). Therefore, the critical
role of uPAR along with the molecules involved in signaling
cascades are potential therapeutic targets for cancer
treatment.
In the present study, it was determined that uPAR
may be associated with the progression of OTSCC. From the 65
samples, statistical analysis also revealed that uPAR expression
was positively associated with tumor metastasis and relapse
(P=0.002 and P=0.004, respectively) and were not significantly
associated with sex (P=0.68), age (P=0.653), pathological stage
(P=0.839) and clinical stage (P=0.388), indicating that uPAR may
serve as a clinical factor for predicting a poor outcome. It was
previously reported that the positive uPAR expression observed in
breast cancer was correlated with tumor differentiation, clinical
stage and lymphatic metastasis, which is consistent with the
results of the present study (24).
Invasion and metastasis are not random, but are
controlled by concerted action of multiple genes, which is a
complex process and an important cause of cancer-associated
mortality (25). The present study
effectively demonstrated that silencing of uPAR inhibited the
migratory and invasive potential of Ts cells, indicating that uPAR
may contribute toward OTSCC metastasis, which is in accordance with
the results of previous studies that demonstrate that silencing of
uPAR upregulates the progression and invasiveness (26) of OTSCC. These data are in accordance
with the hypothesis that uPAR may serve a notable role in OTSCC
progression.
The MEK/ERK (27) and
phosphoinositide 3-kinase/Akt (28)
signaling pathways have been thoroughly characterized previously.
Previous studies have demonstrated that the ERK signaling pathway
serves a notable role in tumorigenesis (29). ERK was determined to be overexpressed
in various tumor types, including oral cancer types (30), malignant melanoma (31) and breast cancer (32). Activated Akt is required for a number
of events of the metastatic pathway, including the escape of cells
from the tumor environment (into and then out of the circulation),
activation of proliferation, blockage of apoptosis and activation
of angiogenesis (33). The results of
the present study indicated that the decrease in ERK may be
associated with the reduced invasion and migration, which was
determined in the uPAR shRNA group, compared with the shRNA-C and
control groups in vitro. Furthermore, western blot analysis
of Akt activation demonstrated no significant difference in Akt
phosphorylation; however, a previous study (34) indicated that downregulation of uPAR
and uPA caused the dephosphorylation of p-Akt. The results of the
present study indicated that there may be other methods to regulate
Akt phosphorylation. A previous study (35) determined that various oncoproteins and
tumor suppressors are implicated in cell signaling/metabolic
regulation convergence within the Akt signal transduction pathway;
however, further investigations are required. Furthermore, a
significant decrease in MMP-2 and MMP-9 expression was observed in
treated cells, indicating a causal role of uPAR in the invasion of
Ts cells. In agreement with the results of the present study,
Randle (36) demonstrated that
uPAR-induced invasion of prostate cells is mediated by MMP-2 and
MMP-9; therefore, we hypothesized that uPAR signaling may be
responsible for Ts cell signaling, which promotes tumor
progression.
Furthermore, the results of the present in
vivo experiments indicated a significant decrease in the number
of metastatic nodes; however, the tumor microenvironment also
contains other signals, which control tumor metastasis (37). Additionally, lung metastasis may also
be involved in multiple signaling pathways such as MAPK and SMAD1
signaling pathways (38). Further
research is required to confirm the present results; however, it is
notable that uPAR silencing influenced tumor metastasis in
vivo.
In conclusion, the present study demonstrated that
the inhibition of uPAR signaling modulates the invasion and
metastasis of Ts cells. Future studies to detect uPAR signaling in
various stages of tumor progression and metastasis may result in
the further development of a number of tumor-targeted therapies;
therefore, targeted silencing of uPAR-induced signaling would
provide novel treatment approaches for the management of OTSCC.
Acknowledgements
Not applicable.
Funding
The authors received a grant from the National
Natural Science Foundation of China (grant nos. 81372893 and
81773942), the International Scientific and Technological
Cooperation Project of Gansu Province (grant no. 17YF1WA165) and
Lanzhou University Research Funds of Stomatology (grant no.
201501-1).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG and SW wrote the manuscript and made
contributions to cell culture. QG and CG performed and analyzed the
wound-healing and tumor cell invasion assays. JC assisted with
immunohistochemistry. LZ and YZ contributed to western blot
analysis. JW conducted the in vivo metastasis assay. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All applicable international, national and/or
institutional guidelines for the care and use of animals were
followed. All procedures in studies involving human participants
were performed in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Declaration of Helsinki and its later amendments or comparable
ethical standards. The School of Stomatology ethics committee of
Lanzhou University (Lanzhou, China) approved the patient and animal
studies. Written informed consent was obtained from all individual
participants included in the study.
Patient consent for publication
Patients provided consent for the publication of the
present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sano D and Myers JN: Metastasis of
squamous cell carcinoma of the oral tongue. Cancer Metast Rev.
26:645–662. 2007. View Article : Google Scholar
|
|
3
|
Guerrero-Preston R, Soudry E, Acero J,
Orera M, Moreno-López L, Macía-Colón G, Jaffe A, Berdasco M,
Ili-Gangas C, Brebi-Mieville P, et al: NID2 and HOXA9 promoter
hypermethylation as biomarkers for prevention and early detection
in oral cavity squamous cell carcinoma tissues and saliva. Cancer
Prev Res (Phila). 4:1061–1072. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rasch MG, Lund IK, Almasi CE and
Hoyer-Hansen G: Intact and cleaved uPAR forms: Diagnostic and
prognostic value in cancer. Front Biosci. 13:6752–6762. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee EJ, Whang JH, Jeon NK and Kim J: The
epidermal growth factor receptor tyrosine kinase inhibitor ZD1839
(Iressa) suppresses proliferation and invasion of human oral
squamous carcinoma cells via p53 independent and MMP, uPAR
dependent mechanism. Ann N Y Acad Sci. 1095:113–128. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jing Y, Bejarano MT, Kovacs K and Merchan
J: Abstract 3545: Stromal selective targeting by uPAR retargeted
oncolytic measles virus inhibits breast cancer progression. Cancer
Res. 75 15 Suppl:Abstract nr 3545. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao N, Bozeman E, Qian W, Staley C, Wang
A, Mao H and Yang L: Abstract 4593: Targeted therapy of pancreatic
cancer by intraperitoneal delivery of uPAR-targeted theranostic
nanoparticles. Cancer Res. 74 19 Suppl:Abstract nr 4593. 2014.
View Article : Google Scholar
|
|
8
|
Gorantla B, Asuthkar S, Rao JS, Patel J
and Gondi CS: Suppression of the uPAR-uPA system retards
angiogenesis, invasion, and in vivo tumor development in pancreatic
cancer cells. Mol Cancer Res. 9:377–389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dass K, Ahmad A, Azmi AS, Sarkar SH and
Sarkar FH: Evolving role of uPA/uPAR system in human cancers.
Cancer Treat Rev. 34:122–136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zh XY, Wu JZ, Liu B, et al: Establishment
and characterization of a metastatic cell line from spinal cord
metastasis induced by injection of tongue cancer Tb cells in nude
mouse. J Chin Stomatol. 18:412–415. 2002.(In Chinese).
|
|
11
|
Wang J, Chen J, Ma M, et al: Construction
and identification of specific shRNA interference plasmid vector
targeted to uPAR gene. Chin Oncol. 19:904–909. 2009.(In
Chinese).
|
|
12
|
Wu YM, Zhang XN, Han ZY, Li CY and Chen
NC: Construction of shRNA expression vector pWH1 and its function
in silencing the HIF1 gene. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
24:115–118. 2008.(In Chinese). PubMed/NCBI
|
|
13
|
Lærum OD, Ovrebo K, Skarstein A,
Christensen IJ, Alpízar-Alpízar W, Helgeland L, Danø K, Nielsen BS
and Illemann M: Prognosis in adenocarcinomas of lower oesophagus,
gastro-oesophageal junction and cardia evaluated by
uPAR-immunohistochemistry. Int J Cancer. 131:558–569. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boonstra MC, Verspaget HW, Ganesh S,
Kubben FJ, Vahrmeijer AL, van de Velde CJ, Kuppen PJ, Quax PH and
Sier CF: Clinical applications of the urokinase receptor (uPAR) for
cancer patients. Curr Pharm Des. 17:1890–1910. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mahmood T and Yang PC: Western blot:
Technique, theory, and trouble shooting. N Am J Med Sci. 4:429–434.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brown GT and Murray GI: Current
mechanistic insights into the roles of matrix metalloproteinases in
tumour invasion and metastasis. J Pathol. 237:273–281. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pavón MA, Arroyo-Solera I, Céspedes MV,
Casanova I, León X and Mangues R: uPA/uPAR and SERPINE1 in head and
neck cancer: Role in tumor resistance, metastasis, prognosis and
therapy. Oncotarget. 7:57351–57366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Noh H, Hong S and Huang S: Role of
urokinase receptor in tumor progression and development.
Theranostics. 3:487–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Almholt K, Lærum OD, Nielsen BS, Lund IK,
Lund LR, Rømer J and Jögi A: Spontaneous lung and lymph node
metastasis in transgenic breast cancer is independent of the
urokinase receptor uPAR. Clin Exp Metastasis. 32:543–554. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Margheri F, Luciani C, Taddei ML, Giannoni
E, Laurenzana A, Biagioni A, Chillà A, Chiarugi P, Fibbi G and Del
Rosso M: The receptor for urokinase-plasminogen activator (uPAR)
controls plasticity of cancer cell movement in mesenchymal and
amoeboid migration style. Oncotarget. 5:1538–1553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu J, Muller KA, Furnari FB, Cavenee WK,
VandenBerg SR and Gonias SL: Neutralizing the EGF receptor in
glioblastoma cells stimulates cell migration by activating
uPAR-initiated cell signaling. Oncogene. 34:4078–4088. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Randle DD, Clarke S, Henderson V and
Odero-Marah VA: Snail mediates invasion through uPA/uPAR and the
MAPK signaling pathway in prostate cancer cells. Oncol Lett.
6:1767–1773. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kotipatruni RR, Nalla AK, Asuthkar S,
Gondi CS, Dinh DH and Rao JS: Apoptosis induced by knockdown of
uPAR and MMP-9 is mediated by inactivation of EGFR/STAT3 signaling
in medulloblastoma. PLoS One. 7:e447982012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang L and Han X: The urokinase
plasminogen activator system in breast cancer invasion and
metastasis. Biomed Pharmacother. 67:179–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang WG, Sanders AJ, Katoh M, Ungefroren
H, Gieseler F, Prince M, Thompson SK, Zollo M, Spano D, Dhawan P,
et al: Tissue invasion and metastasis: Molecular, biological and
clinical perspectives. Semin Cancer Biol. 35 Suppl:S244–S275. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Illemann M, Laerum OD, Hasselby JP,
Thurison T, Høyer-Hansen G and Nielsen HJ: Danish Study Group on
Early Detection of Colorectal Cancer, Christensen IJ:
Urokinase-type plasminogen activator receptor (uPAR) on
tumor-associated macrophages is a marker of poor prognosis in
colorectal cancer. Cancer Med. 3:855–864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marampon F, Gravina GL, Di Rocco A,
Bonfili P, Di Staso M, Fardella C, Polidoro L, Ciccarelli C,
Festuccia C, Popov VM, et al: MEK/ERK inhibitor U0126 increases the
radiosensitivity of rhabdomyosarcoma cells in vitro and in vivo by
downregulating growth and DNA repair signals. Mol Cancer Ther.
10:159–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vara Fresno JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang JY, Zong CS, Xia W, Yamaguchi H, Ding
Q, Xie X, Lang JY, Lai CC, Chang CJ, Huang WC, et al: ERK promotes
tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation.
Nat Cell Biol. 10:138–148. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hayashido Y, Kitano H, Sakaue T, Fujii T,
Suematsu M, Sakurai S and Okamoto T: Overexpression of integrin αv
facilitates proliferation and invasion of oral squamous cell
carcinoma cells via MEK/ERK signaling pathway that is activated by
interaction of integrin αvβ8 with type I collagen. Int J Oncol.
45:1875–1882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yajima I, Kumasaka MY, Thang ND, Goto Y,
Takeda K, Yamanoshita O, Iida M, Ohgami N, Tamura H, Kawamoto Y and
Kato M: RAS/RAF/MEK/ERK and PI3K/PTEN/AKT signaling in malignant
melanoma progression and therapy. Dermatol Res Pract.
2012:3541912012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ebi H, Costa C, Faber AC, Nishtala M,
Kotani H, Juric D, Della Pelle P, Song Y, Yano S, Mino-Kenudson M,
et al: PI3K regulates MEK/ERK signaling in breast cancer via the
Rac-GEF, P-Rex1. Proc Natl Acad Sci USA. 110:21124–21129. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hong SW, Jung KH, Lee HS, Choi MJ, Son MK,
Zheng HM and Hong SS: SB365 inhibits angiogenesis and induces
apoptosis of hepatocellular carcinoma through modulation of
PI3K/Akt/mTOR signaling pathway. Cancer Sci. 103:1929–1937. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gondi CS, Kandhukuri N, Dinh DH, Gujrati M
and Rao JS: Down-regulation of uPAR and uPA activates
caspase-mediated apoptosis and inhibits the PI3K/AKT pathway. Int J
Oncol. 31:19–27. 2007.PubMed/NCBI
|
|
35
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Randle DD, Clarke S and Odero-Marah V:
Abstract 2407: Snail 1 regulates invasion through uPA-uPAR and
MMP-9 signaling in prostate cancer cells. Cancer Res. 72 8
Suppl:Abstract nr 2407. 2012. View Article : Google Scholar
|
|
37
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oskarsson T, Batlle E and Massagué J:
Metastatic stem cells: Sources, niches, and vital pathways. Cell
Stem Cell. 14:306–321. 2014. View Article : Google Scholar : PubMed/NCBI
|