Introduction
Hepatocellular carcinoma (HCC) is the third leading
cause of cancer-related death worldwide and a major cause of death
in patients with cirrhosis (1). By
the time HCC is detected, it has often reached an advanced stage
for which there is no curative treatment. In order to identify HCC
earlier, efforts have focused on patients with chronic hepatitis B
virus (HBV) infection, which affects 350 million persons worldwide
and is the most common risk factor for HCC (1). The ability to identify patients who are
at high risk for HBV-related HCC would benefit the prevention and
early detection of HCC.
Recent studies have indicated that measurement of
ferritin, the major cellular storage protein for iron (2), may allow for such early detection of
HCC. In vitro studies showed that iron induced increased
ferritin synthesis by hepatoma cell lines and enhanced tumor cell
growth (3,4). Elevated serum ferritin was also
associated with inflammation and liver diseases (2,5,6). Moreover, ferritin that is synthesized by
tumor cells may exert adverse effects on host immune responses and
defense mechanisms (3). Population
studies have clearly documented that HCC patients have a higher
level of ferritin than healthy subjects or patients with other
liver diseases (7–9), but retrospective studies cannot clarify
whether the increased level is the cause or consequence of HCC
because ferritin is also produced by the tumor cells. Meanwhile,
very few studies have prospectively evaluated the relationship
between serum ferritin level and HCC risk in HBV patients. A nested
case-control study in Taiwan reported that 192 men who developed
primary HCC and/or died of cancer had higher mean serum ferritin at
the start of the study, compared to those who were alive and free
of HCC at the date the case failed (developed HCC or died of
cancer) (10). A study conducted in
Korean patients with chronic hepatitis B and cirrhosis reported
that males with sustained high serum ferritin levels had a high
chance of developing HCC (5);
however, in this study, only 162 patients (46 females) were
included, ferritin levels were merely measured within the first 8
months, and covariates were not considered. We designed a
prospective cohort study with more than 10-year follow-up and
longitudinally collected serial blood samples from HBV-infected
patients in order to further clarify the association between
baseline ferritin levels and HCC risk in HBV-infected patients, and
to explore the dynamic change of ferritin level and HCC risk over
time.
Subjects and methods
Subjects
The subjects were identified from an ongoing
clinic-based patient cohort that was established in 1988 (11). The patients were recruited when they
visited the Liver Disease Prevention Center at Thomas Jefferson
University Hospital for the treatment of chronic HBV or HCV
infection and liver diseases including cirrhosis, fibrosis, and/or
HCC. There was no restriction on age, gender, and disease etiology
for patient recruitment. Majority (>90%) of the patients in the
cohort were of Korean ancestry. The patients included in the
current study met the following criteria: i) To eliminate the
confounding effects from disease etiologies, patients had HBV
infection only, without concomitant infection with HCV, HIV, or
other viruses; ii) to minimize the confounding effects of
population stratification and ethnic origin, patients were Korean
Americans; iii) patients had developed primary rather than
secondary HCC during follow-up; iv) patients were followed for at
least 1 year, and during which time HCC did not develop; v)
patients had both ferritin and α-fetoprotein (AFP) values measured
simultaneously at study entry; and vi) major demographic and
clinical data were available, such as date of HCC diagnosis. This
study was approved by the Institutional Review Board of Thomas
Jefferson University. Written informed consent was obtained from
each patient.
Data collection
Demographic and clinical data were obtained by
reviewing medical charts and/or consulting the treating physicians.
Related variables included age, gender, ethnicity, smoking status,
drinking status, cirrhosis status, and family history of cancer.
Ever smokers and drinkers were defined as previously (12). Liver cirrhosis and HCC were diagnosed
by the combination of clinical diagnosis and imaging techniques
(e.g., ultrasound, computed tomography, magnetic resonance
imaging), complemented by blood markers (e.g., AFP) (11). Ferritin levels for each patient were
detected at the initial visit, as well as at follow-up visits, with
the intervals determined by the treating physicians.
Statistical analysis
SAS (version 9.4; SAS Institute, Inc., Cary, NC,
USA) and Stata (version 12.0; StataCorp LP, College Station, TX,
USA) software packages were used for data analyses. All statistical
tests were two-sided, and a P-value of less than 0.05 was
considered as the threshold of statistical significance. The
clinical endpoint analyzed in this study was HCC development. Time
to HCC development was defined as the date from the study entry to
the date of HCC diagnosis or last follow-up. Patients who were free
of HCC at the last follow-up date were censored for analysis. The
patients were then divided into two, three, or four risk groups,
according to the clinical cut-off of 200 ng/ml (2,13), or
median/tertile/quartile of ferritin level in cancer-free HBV
patients. The cumulative incidence of HCC in each risk group was
plotted using the Nelson-Aalen method (14) and compared using the log-rank test.
The association between ferritin level and HCC risk was evaluated
using univariate and multivariate Cox proportional hazards
regression model, adjusting for age, gender, smoking status,
drinking status, cirrhosis, and family history of cancer. Because
all patients received standard-of-care treatment based on the AASLD
guidelines and adjustment of treatment did not affect the results,
we did not include treatment in the multivariate adjustment in the
current study. The proportional hazards assumption was validated
using the test based on Schoenfeld residuals. The significance and
strength of association was presented as hazard ratio (HR) with 95%
confidence interval (CI). Discrimination accuracy for predicting
the development of HCC was evaluated by constructing receiver
operating characteristic (ROC) curves and calculating the area
under the curve (AUC). Interactions between ferritin levels and
other variables on HCC risk were assessed by adding an interaction
term into the Cox regression model. Time-dependent effect of
ferritin levels on HCC risk was analyzed using the flexible
parametric survival model with a restricted cubic spline function
(15). To better understand the
dynamic change of ferritin during follow-up, we selected a
sub-cohort of 461 patients whose ferritin level was detected ≥3
times since the initial visit. The longitudinal trends of temporal
changes in average ferritin levels were plotted by fitting a
smoothing spline over time and compared between the patients who
developed HCC and those who remained cancer-free during follow-up
(16).
Results
Patient characteristics
A total of 1,152 HBV patients were included in this
study, and among them, 96 (8.3%) patients developed early-stage HCC
during follow-up. The patient characteristics are summarized in
Table I. The mean age of patients at
enrollment was 43.6 years old (age range: 18.7–77.8 years). The
majority of patients were males (66.1%, sex ratio: 1.95), never
smokers (59.7%), never drinkers (56.1%), without cirrhosis (71.8%)
or a family history of cancer (67.4%). As expected, HCC risk was
significantly increased among older patients (HR=2.23, 95% CI,
1.36–3.65), males (HR=1.88, 95% CI, 1.01–3.47), ever smokers
(HR=2.47, 95% CI, 1.39–4.38), cirrhotic patients (HR=7.62, 95% CI,
4.53–12.83), and patients with a high AFP value (HR=2.75, 95% CI,
1.78–4.25). Although increased HCC risk was observed in ever
drinkers and patients with a family history of cancer in the
univariate analyses, the associations were not statistically
significant after adjustment for covariates.
 | Table I.Characteristics of the study
population. |
Table I.
Characteristics of the study
population.
|
|
|
| Univariate
analysis | Multivariate
analysisa |
|---|
|
|
|
|
|
|
|---|
| Variables | Total HBV patients,
no. (%) | HBV patients who
developed HCC, no. (%) | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (years), mean
(SD) | 43.61 (11.75) | 50.97 (10.36) | 1.07
(1.05–1.08) |
<0.0001c | 1.05
(1.03–1.07) |
<0.0001c |
|
<43 | 550 (47.7) | 22 (22.9) | 1.00 |
| 1.00 |
|
|
≥43 | 602 (52.3) | 74 (77.1) | 3.48
(2.16–5.61) |
<0.0001c | 2.23
(1.36–3.65) | 0.0014c |
| Gender |
|
|
|
|
|
|
|
Female | 391 (33.9) | 15 (15.6) | 1.00 |
| 1.00 |
|
|
Male | 761 (66.1) | 81 (84.4) | 2.81
(1.62–4.88) | 0.0002c | 1.88
(1.01–3.47) | 0.0450c |
| Smoking status |
|
|
|
|
|
|
|
Never | 688 (59.7) | 35 (36.5) | 1.00 |
| 1.00 |
|
|
Ever | 382 (33.2) | 49 (51.0) | 2.61
(1.69–4.03) |
<0.0001c | 2.47
(1.39–4.38) | 0.0021c |
|
Unknown | 82 (7.1) | 12 (12.5) | 2.84
(1.47–5.47) | 0.0019 | 12.43
(2.92–52.97) | 0.0007 |
| Drinking
status |
|
|
|
|
|
|
|
Never | 646 (56.1) | 41 (42.7) | 1.00 |
| 1.00 |
|
|
Ever | 425 (36.9) | 45 (46.9) | 1.72
(1.12–2.62) | 0.0123c | 0.67
(0.38–1.16) | 0.1527 |
|
Unknown | 81 (7.0) | 10 (10.4) | 1.90
(0.95–3.79) | 0.0693 | 0.15
(0.03–0.71) | 0.0171 |
| Cirrhosis |
|
|
|
|
|
|
| No | 827 (71.8) | 19 (19.8) | 1.00 |
| 1.00 |
|
|
Yes | 325 (28.2) | 77 (80.2) | 12.25
(7.41–20.25) |
<0.0001c | 7.62
(4.53–12.83) |
<0.0001c |
| Family history of
cancer |
|
|
|
|
|
|
| No | 776 (67.4) | 57 (59.4) | 1.00 |
| 1.00 |
|
|
Yes | 376 (32.6) | 39 (40.6) | 1.50
(1.00–2.25) | 0.0523 | 1.21
(0.80–1.84) | 0.3657 |
| AFP (ng/ml), median
(IQR) | 3.10
(2.00–6.40) | 10.86
(5.15–47.05) | 1.62
(1.50–1.76)b |
<0.0001b,c | 1.51
(1.36–1.67)b |
<0.0001b,c |
|
<20 | 1015 (88.1) | 59 (61.5) | 1.00 |
| 1.00 |
|
|
≥20 | 137 (11.9) | 37 (38.5) | 5.39
(3.57–8.13) |
<0.0001c | 2.75
(1.78–4.25) |
<0.0001c |
Association between ferritin level and
HCC risk
HBV patients who developed HCC had a significantly
higher ferritin level [median 188.00 ng/ml, interquartile range
(IQR) 119.50–299.76 ng/ml] than those who remained cancer-free
(median 108.00 ng/ml, IQR 53.10–204.95 ng/ml, P<0.0001). We then
divided HBV patients into different risk groups according to the
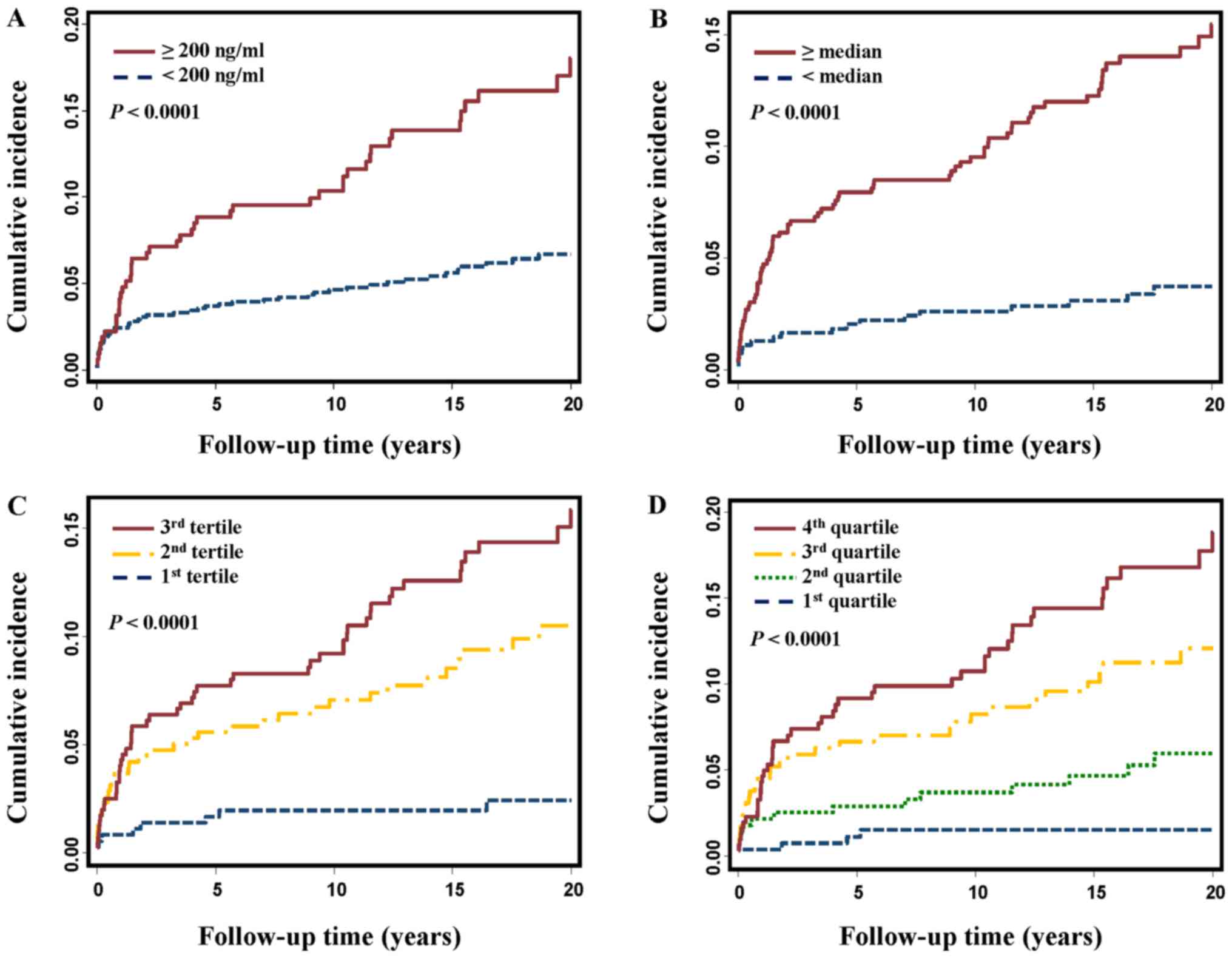
clinical cut-off or percentile cut-offs. As shown in Fig. 1, the cumulative HCC incidence was
significantly higher in patients with a high ferritin level than in
those with a low ferritin level, no matter which kind of cut-offs
were used (all log-rank P<0.0001). Compared to the patients with
a ferritin level less than 200 ng/ml, the patients with a high
ferritin level (≥200 ng/ml) had 2.43-fold increased risk of HCC
(HR=2.43, 95% CI, 1.63–3.63, Table
II). The association was still significant after adjusting for
age, gender, smoking status, drinking status, and family history of
cancer (HR=1.76, 95% CI, 1.16–2.65) and became borderline
significant when cirrhosis and AFP were further added to
multivariate adjustment (HR=1.45, 95% CI, 0.96–2.19). When patients
were categorized according to median/tertile/quartile cutoffs, the
association between ferritin and HCC risk was consistently observed
in univariate and multivariate analyses (Table II). Moreover, a trend of increasing
HRs along with elevated ferritin levels was noted (P for trend
<0.0001 in univariate analyses and <0.01 after multivariate
adjustment, Table II).
 | Table II.Associations between Ferritin level
and HCC risk. |
Table II.
Associations between Ferritin level
and HCC risk.
|
|
|
| Univariate
analysis | Multivariate
analysisa | Multivariate
analysisb | Multivariate
analysisc |
|---|
|
|
|
|
|
|
|
|
|---|
| Ferritin
values | HCC/total patients
(n) | Log-rank
P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| log
transformed | 96/1152 |
| 1.77
(1.47–2.14) |
<0.0001d | 1.55
(1.25–1.92) |
<0.0001d | 1.42
(1.16–1.74) | 0.0008d | 1.39
(1.14–1.70) | 0.0011d |
| By clinical
cut-off |
|
|
|
|
|
|
|
|
|
|
| <200
ng/ml | 51/832 |
<0.0001d | 1.00 |
| 1.00 |
| 1.00 |
| 1.00 |
|
| ≥200
ng/ml | 45/320 |
| 2.43
(1.63–3.63) |
<0.0001d | 1.76
(1.16–2.65) | 0.0074d | 1.44
(0.95–2.17) | 0.0848 | 1.45
(0.96–2.19) | 0.0748 |
| By median |
|
|
|
|
|
|
|
|
|
|
|
<Median | 19/548 |
<0.0001d | 1.00 |
| 1.00 |
| 1.00 |
| 1.00 |
|
|
≥Median | 77/604 |
| 3.92
(2.37–6.48) |
<0.0001d | 2.59
(1.54–4.35) | 0.0003d | 2.41
(1.43–4.06) | 0.0009d | 2.30
(1.37–3.88) | 0.0017d |
| By tertile |
|
|
|
|
|
|
|
|
|
|
| 1st
tertile | 9/361 |
<0.0001d | 1.00 |
| 1.00 |
| 1.00 |
| 1.00 |
|
| 2nd
tertile | 34/387 |
| 3.72
(1.78–7.76) | 0.0005d | 2.38
(1.12–5.03) | 0.0239d | 3.04
(1.44–6.42) | 0.0036d | 2.95
(1.39–6.28) | 0.0050d |
| 3rd
tertile | 53/404 |
| 5.66
(2.79–11.48) |
<0.0001d | 3.11
(1.50–6.47) | 0.0023d | 3.02
(1.47–6.20) | 0.0026d | 3.00
(1.46–6.16) | 0.0027d |
|
Ptrend |
|
|
|
<0.0001d |
| 0.0021d |
| 0.0070d |
| 0.0066d |
| By quartile |
|
|
|
|
|
|
|
|
|
|
| 1st
quartile | 5/269 |
<0.0001d | 1.00 |
| 1.00 |
| 1.00 |
| 1.00 |
|
| 2nd
quartile | 14/279 |
| 2.82
(1.02–7.83) | 0.0465d | 1.86
(0.66–5.20) | 0.2394 | 2.36
(0.84–6.67) | 0.1042 | 2.57
(0.91–7.26) | 0.0757 |
| 3rd
quartile | 32/295 |
| 6.26
(2.44–16.08) | 0.0001d | 3.40
(1.30–8.91) | 0.0127d | 4.23
(1.62–11.07) | 0.0033d | 4.13
(1.57–10.87) | 0.0040d |
| 4th
quartile | 45/309 |
| 8.70
(3.45–21.91) |
<0.0001d | 4.45
(1.73–11.48) | 0.0020d | 4.18
(1.63–10.71) | 0.0029d | 4.24
(1.66–10.84) | 0.0026d |
|
Ptrend |
|
|
|
<0.0001d |
|
<0.0001d |
| 0.0010d |
| 0.0012d |
Prediction performance of ferritin
combined with AFP
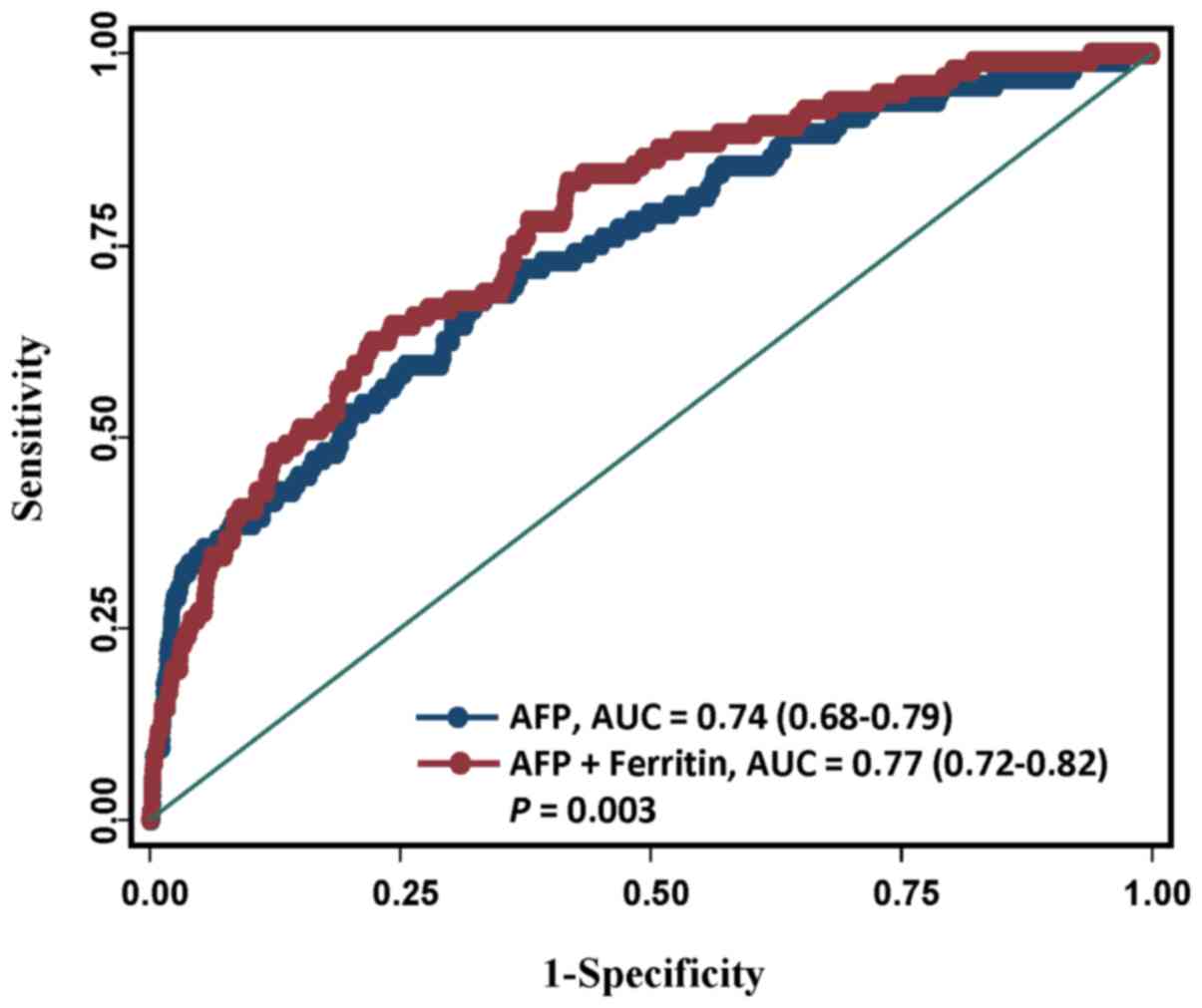
AFP is a commonly used tumor marker in HCC
diagnosis, although with a non-optimal discrimination accuracy
(17). We then investigated whether
incorporating ferritin into the AFP prediction model could improve
the performance of predicting HCC. The results showed that compared
to the performance in the model with AFP values, the prediction
performance significantly increased in the model in combination of
ferritin and AFP levels (AUC from 0.74 to 0.77, P=0.003; Fig. 2).
Time-dependent effect and dynamic
change of ferritin level
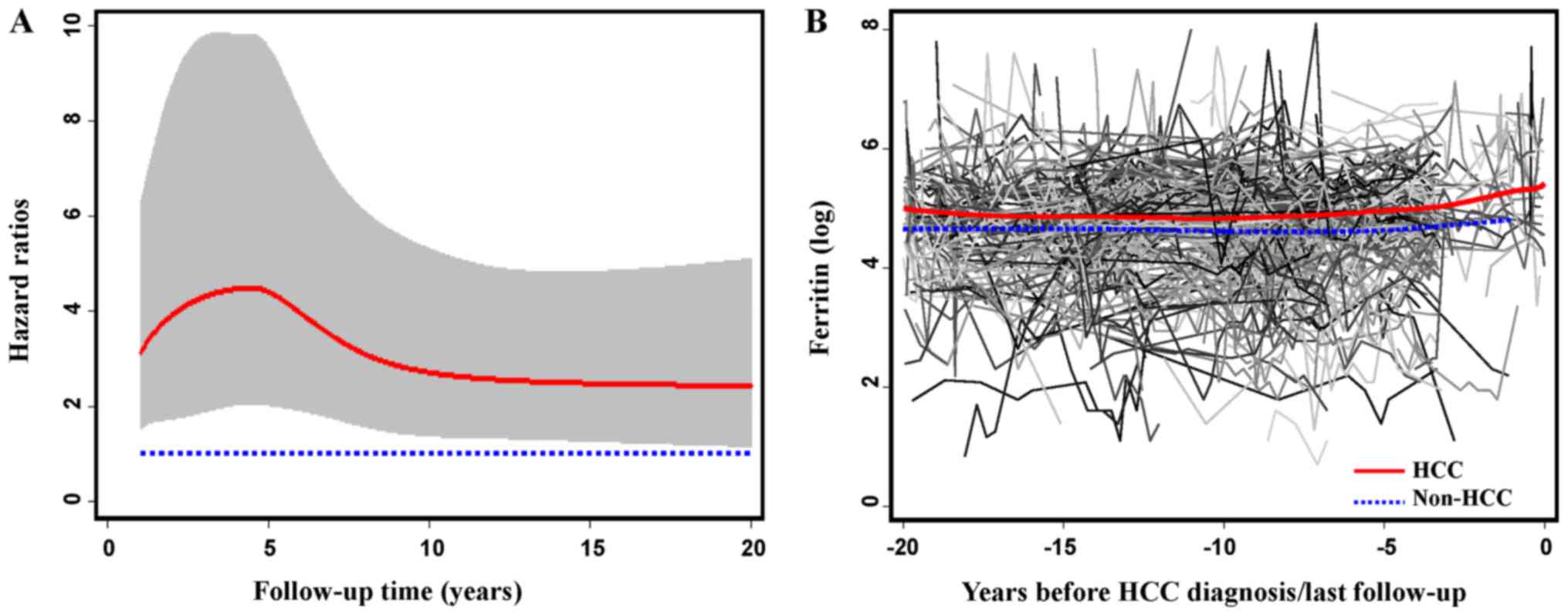
Time-dependent effect of ferritin level on HCC risk
was assessed using the flexible parametric model. As shown in
Fig. 3A, after the initial visit, HCC
risk significantly increased in the first 5 years, reached a peak
at approximately the fifth year, and kept stable after 10 years. To
evaluate dynamic change of ferritin, we also selected a sub-cohort
of 461 patients with 4309 detections of ferritin at both baseline
and follow-up visits, and depicted the longitudinal trend of
average ferritin values by fitting a smoothing spline over time
(Fig. 3B). The average ferritin level
in HBV patients who developed HCC (n=50) was persistently higher
than in those remaining cancer-free (n=411).
Stratified and joint effects of
ferritin level on HCC risk
We stratified the main effect analysis according to
patients' characteristics. In the univariate analyses, the
association of ferritin level with HCC risk was significant in both
strata divided by age, gender, drinking status, and family history
of cancer, and only evident in ever smokers (P=0.0233),
non-cirrhotic patients (P=0.0155), and patients with a AFP value
less than 20 ng/ml (P=0.0004; Table
III). After multivariate adjustment, we only observed a
borderline significant association in older patients (P=0.0940) and
patients with a low AFP value (P=0.0837).
 | Table III.Associations between Ferritin level
and HCC risk stratified by patients' characteristics. |
Table III.
Associations between Ferritin level
and HCC risk stratified by patients' characteristics.
|
|
|
| Univariate
analysis | Multivariate
analysisa |
|---|
|
|
|
|
|
|
|---|
| Variable | Ferritin values
(ng/ml) | HCC/total patients
(N) | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
<43 | <200 | 12/416 | 1 |
| 1 |
|
|
| ≥200 | 10/134 | 2.74
(1.18–6.33) | 0.0188b | 1.70
(0.70–4.17) | 0.2430 |
|
≥43 | <200 | 39/416 | 1 |
| 1 |
|
|
| ≥200 | 35/186 | 2.11
(1.34–3.33) | 0.0013b | 1.50
(0.93–2.40) | 0.0940 |
| P for
interaction |
|
|
| 0.6144 |
| 0.9970 |
| Gender |
|
|
|
|
|
|
|
Female | <200 | 9/353 | 1 |
| 1 |
|
|
| ≥200 | 6/38 | 7.29
(2.59–20.51) | 0.0002b | 1.25
(0.29–5.45) | 0.7695 |
|
Male | <200 | 42/479 | 1 |
| 1 |
|
|
| ≥200 | 39/282 | 1.64
(1.06–2.53) | 0.0269b | 1.45
(0.93–2.27) | 0.1040 |
| P for
interaction |
|
|
| 0.0087b |
| 0.9203 |
| Smoking status |
|
|
|
|
|
|
|
Never | <200 | 23/533 | 1 |
| 1 |
|
|
| ≥200 | 12/155 | 1.88
(0.94–3.78) | 0.0758 | 1.12
(0.54–2.33) | 0.7679 |
|
Ever | <200 | 23/236 | 1 |
| 1 |
|
|
| ≥200 | 26/146 | 1.92
(1.09–3.36) | 0.0233b | 1.48
(0.81–2.71) | 0.2064 |
| P for
interaction |
|
|
| 0.9982 |
| 0.5633 |
| Drinking
status |
|
|
|
|
|
|
|
Never | <200 | 26/505 | 1 |
| 1 |
|
|
| ≥200 | 15/141 | 2.23
(1.18–4.21) | 0.0136b | 1.14
(0.58–2.24) | 0.7121 |
|
Ever | <200 | 21/264 | 1 |
| 1 |
|
|
| ≥200 | 24/161 | 1.93
(1.08–3.47) | 0.0275b | 1.41
(0.76–2.64) | 0.2774 |
| P for
interaction |
|
|
| 0.7706 |
| 0.8010 |
| Cirrhosis |
|
|
|
|
|
|
| No | <200 | 10/639 | 1 |
| 1 |
|
|
| ≥200 | 9/196 | 3.04
(1.24–7.49) | 0.0155b | 1.27
(0.46–3.52) | 0.6515 |
|
Yes | <200 | 41/193 | 1 |
| 1 |
|
|
| ≥200 | 36/124 | 1.43
(0.91–2.24) | 0.1176 | 1.27
(0.80–2.03) | 0.3078 |
| P for
interaction |
|
|
| 0.1410 |
| 0.5747 |
| Family history of
cancer |
|
|
|
|
|
|
| No | <200 | 30/575 | 1 |
| 1 |
|
|
| ≥200 | 27/201 | 2.70
(1.61–4.54) | 0.0002b | 1.53
(0.89–2.65) | 0.1263 |
|
Yes | <200 | 21/257 | 1 |
| 1 |
|
|
| ≥200 | 18/119 | 1.95
(1.04–3.67) | 0.0375b | 1.25
(0.63–2.49) | 0.5233 |
| P for
interaction |
|
|
| 0.4255 |
| 0.8123 |
| AFP (ng/ml) |
|
|
|
|
|
|
|
<20 | <200 | 34/778 | 1 |
| 1 |
|
|
| ≥200 | 25/237 | 2.55
(1.52–4.28) | 0.0004b | 1.61
(0.94–2.77) | 0.0837 |
|
≥20 | <200 | 17/54 | 1 |
| 1 |
|
|
| ≥200 | 20/83 | 0.72
(0.38–1.38) | 0.3194 | 0.75
(0.38–1.46) | 0.3954 |
| P for
interaction |
|
|
| 0.0036b |
| 0.0583b |
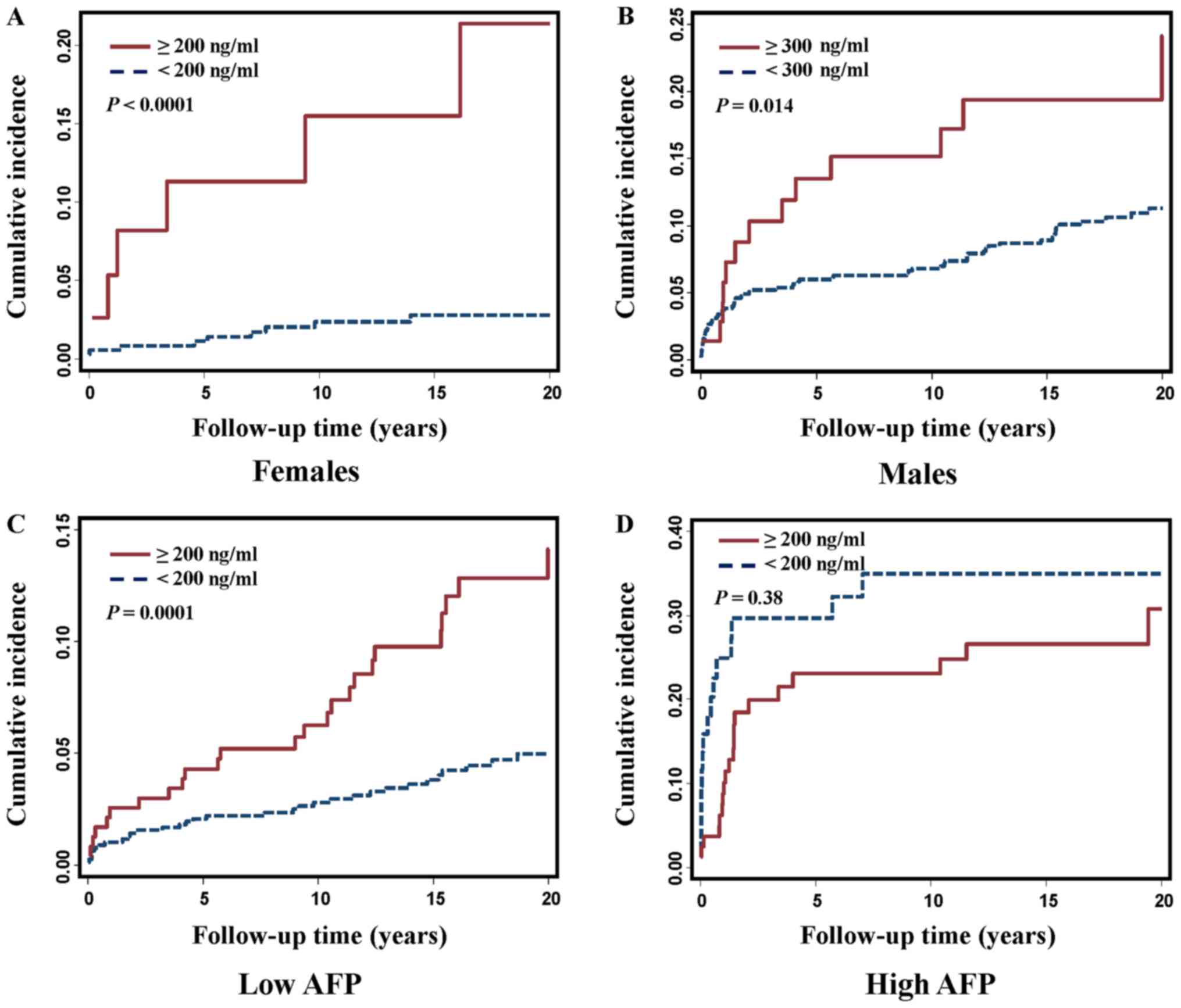
Because the clinical normal range differed between
females and males (13) and because
we detected a joint effect between ferritin level and gender on HCC
risk (P for interaction=0.0087), we further evaluated
gender-specific effect and compared the cumulative incidence in
females and males. As expected, ferritin levels were much higher in
males than in females (median 160 ng/ml vs. 50.40 ng/ml,
P<0.0001). Both female and male HBV patients who developed HCC
had significantly higher ferritin levels compared to those who were
still cancer-free (134 vs. 48.95 ng/ml, P=0.0003 in females; 188.90
vs. 154.80 ng/ml, P=0.0025 in males, Table IV). The significantly increased
cumulative incidence was observed in both female and male patients
with a high ferritin level when gender-specific clinical cut-offs
were used (Fig. 4A and B).
 | Table IV.Ferritin levels by gender. |
Table IV.
Ferritin levels by gender.
|
| Females
(n=391) | Males (n=761) |
|---|
|
|
|
|
|---|
| Variables | Non-HCC
(n=376) | HCC (n=15) | P-value | Non-HCC
(n=680) | HCC (n=81) | P-value |
|---|
| Ferritin
(ng/ml), | 48.95 | 134.00 | 0.0003a | 154.80 | 188.90 | 0.0025a |
| median (IQR) | (25.60–96.95) | (96.00–446.00) |
| (95.00–256.35) |
(124.00–296.52) |
|
We also detected a joint effect between ferritin and
AFP level on HCC risk (P for interaction=0.0036). We then compared
ferritin level and cumulative incidence in patients with a low or
high AFP value. We noticed that in patients with a low AFP value
(<20 ng/ml), the HBV patients who developed HCC had a
significantly higher level of ferritin than those who remained
cancer-free (178 vs. 103.25 ng/ml, P<0.0001; Table V); however, in patients with a high
AFP value (≥20 ng/ml), the ferritin level in the HCC patients was
slightly lower than that in the non-HCC patients (230 vs. 283
ng/ml), but the difference was not statistically significant.
Similarly, significantly increased cumulative incidence of HCC with
elevated ferritin level was observed in patients with a low AFP
value (Fig. 4C and D).
 | Table V.Ferritin levels by AFP level. |
Table V.
Ferritin levels by AFP level.
|
| AFP <20 ng/ml
(n=1,015) | AFP ≥20 ng/ml
(n=137) |
|---|
|
|
|
|
|---|
| Variables | Non-HCC
(n=956) | HCC (n=59) | P-value | Non-HCC
(n=100) | HCC (n=37) | P-value |
|---|
| Ferritin
(ng/ml), | 103.25 | 178.00 |
<0.0001a | 283.00 | 230.00 |
0.38750a |
| median (IQR) | (50.00–187.25) |
(119.00–253.30) |
|
(123.20–619.00) |
(120.00–495.00) |
|
Discussion
Serum ferritin is a widely available and easily
measured biochemical parameter in clinics. Previous studies
detected a higher level of ferritin in HCC patients; however, these
studies did not reveal whether there was a causal association
between serum ferritin level and hepatocarcinogenesis in HBV
infected patients. In the current study based on a well-established
prospective cohort, we demonstrated that baseline ferritin level
could independently predict the risk of HBV-related HCC, especially
in the first 10 years of follow-up. Moreover, the association
between ferritin level and HCC risk was noted in both males and
females, and was prominent in patients with a low AFP value.
The tumor enhancing effect of iron has been well
documented (18,19). Accumulating evidence has indicated
that an increased iron level predisposes a patient to infection,
induces oxidative stress, modifies the immune system, and
facilitates the growth of tumor cells (2,5,20,21).
Furthermore, antitumor effect of iron depletion by deferoxamine was
observed in nude mice bearing human HCC (22). As an iron storage protein, ferritin
makes iron available for critical cellular processes while
protecting lipids, DNA, and proteins from the potentially toxic
effects of iron (23). Serum ferritin
is an inexpensive biomarker in identifying clinically significant
iron overload (23). Elevated
ferritin concentrations could also be observed in inflammatory
processes, autoimmune diseases, neurodegenerative, metabolic
syndrome, and malignant diseases (23). In addition to the amount synthesized
by tumor cells, ferritin is also released into the circulation by
damaged hepatocytes in liver diseases. Cirrhosis is almost always
present when HCC is diagnosed and progressive hepatic iron loading
develops in one-third of patients with long-standing liver
cirrhosis (21); however, previous
studies failed to clarify whether ferritin is an independent
predictor of HCC or acts indirectly through coexisting cirrhosis. A
recent animal model of dietary iron overload reported iron-free
preneoplastic nodules and HCC developed in the absence of fibrosis
or cirrhosis, suggesting that ionic iron may also be directly
hepatocarcinogenic (24,25). In the current study, which was
conducted in chronic HBV infected patients, we demonstrated the
association between ferritin and HCC risk that was independent of
other liver diseases including cirrhosis (Table II).
It is well known that the average ferritin level in
males is much higher than in females, thus a gender-specific
cut-off is often suggested in clinics (2,13,26). A recent study reported an independent
predicting value of serum ferritin in the development of
HCV-related HCC, and this association was only observed in male
patients (27). Previous studies
conducted in HBV patients also identified increased HCC risk in
male patients with a high level of ferritin. But these studies were
either conducted in males alone (10)
or involved a small number of female patients (5). Our present study with 761 males and 391
females demonstrated that the association between ferritin level
and HCC risk was observed in both males and females, even when
males or females were stratified into different risk groups by a
gender-specific cut-off of ferritin (Fig.
3). It is unclear whether the inconsistent finding of ferritin
effect on HBV patients compared to HCV patients signifies that
hepatocarcinogenesis differs in patients with different disease
etiologies. It would be worthwhile to conduct additional studies to
validate our finding and clarify gender-specific effects.
AFP is a widely recognized tumor marker for the
diagnosis of HCC. However, there have been false-positive results
for HCC detection, as elevated AFP also occurs in chronic liver
disease and other malignancies than HCC; false-negative results
were reported as well, as not all HCCs secrete AFP (17,28,29). Our
results showed that the performance of HCC prediction significantly
improved after incorporating ferritin into the AFP prediction model
(Fig. 2), suggesting a complementary
role of ferritin in prediction of HCC development. Adult
hepatocytes re-express AFP mainly through mechanisms including
hepatocyte regeneration, and oxidative stress induced DNA damage
(30). Noritake et al
(31) reported that iron reduction by
therapeutic phlebotomy could reduce the serum AFP in HCV patients,
which probably mediated by the amelioration of enhanced hepatic
iron-mediated oxidative stress. Although the biological mechanisms
underlying the combined effect of AFP and Ferritin on HCC
development are still needed to be further investigated, the
present findings in our study demonstrated the potential of using
these two serum markers for the joint diagnosis of HCC.
Nevertheless, it should be noted that after adding ferritin to the
model, the diagnostic power was still moderate, albeit slightly
increased. Therefore, it would be worthwhile to study whether a
model including more risk factors, as well as novel molecular
markers could further improve the diagnostic performance. In
addition, in the stratification analyses, we found that the
association of ferritin level with HCC risk was more evident in
patients with a AFP value less than 20 ng/ml. Our result was
consistent with the finding of Zhou et al (32) who suggested that serum ferritin
estimation may be helpful in detection of HCC without elevated AFP,
and further supported the complementary information from ferritin
to AFP-based HCC diagnosis. Nevertheless, due to decreased sample
size in each stratum, especially in the patients with a high level
of AFP, it should be cautious when we explained the detected
interaction between ferritin and AFP, as well as, the failure in
identifying significant difference in ferritin levels between HCC
and non-HCC patients when the comparison was conducted in those
with elevated AFP.
Our study was based on a large-scale and homogenous
cohort of Korean patients with chronic HBV infection, so the
confounding effects of patient ethnicity and disease etiology have
been eliminated. We only included patients who were followed for at
least 1 year, and during which time HCC did not develop (1-year
exclusion window). This criterion ensured that all of the patients
were free of HCC at study entry. Therefore, the increased baseline
ferritin level was more likely linked to the development of HCC
rather than the release from existing tumor cells. In order to
minimize the confounding effect of any patient who had undiagnosed
HCC at baseline sample collection, we further restricted the
analyses to a sub-cohort of patients with a 2-year exclusion
window, that is, only included the patients who were followed for
at least 2 years and had not developed HCC within these 2 years.
The result from this sub-cohort analysis (HR=2.76, 95% CI,
1.60–4.78; adjusted HR=2.23, 95% CI, 1.25–3.97) was very similar to
that from the analysis based on the population with 1-year
exclusion window (HR=2.43, 95% CI, 1.63–3.63; adjusted HR=1.76, 95%
CI, 1.16–2.65, Table II), which
substantiates the robustness of our findings. Furthermore, ferritin
is a non-invasive, cost-effective, and easily obtained biomarker in
clinics so that it can be real-time detected and repetitively
monitored during follow-up.
Our study also has limitations. First, liver biopsy
is the gold standard for quantifying iron, while serum ferritin
levels do not always accurately reflect tissue iron (2,23,33). However, in the current study, very few
patients had baseline serum iron concentration or transferrin
saturation, and none of the patients had detection of tissue iron,
so we are unable to accurately assess the relationship between
ferritin, iron, and the development of HBV-related HCC. Second,
some important covariates, such as HBV DNA loading and body mass
index, which were reported to be associated with HCC risk and/or
correlate with serum ferritin level (12,34), were
not included in multivariate analyses due to a high percentage of
missing values. Future studies with more complete data could be
designed to evaluate the confounding effects from these covariates.
Third, this study was based on the data collected in a single
institute. Prospective studies from independent external cohorts
are required to validate our results. Moreover, the
generalizability of our findings to patients of other ethnicities
or disease etiologies is required to be assessed.
In short, our large scale cohort study indicated
that serum ferritin could prospectively predict
hepatocarcinogenesis in chronic HBV infected patients. Given
ferritin is a non-invasive blood-born maker which can be easily
obtained from routine laboratory test and be real-time monitored
during follow-up, the clinical significance of ferritin in early
diagnosis and effective management of HCC should not be
overlooked.
Acknowledgements
The authors would like to thank Jennifer Wilson
(Thomas Jefferson University, Philadelphia, PA, USA) for editorial
assistance.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81402328).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KT conceived the study and designed the project. ZB
and HWH designed the experiments. ZB, ZY, CY and YW performed the
experiments. ZB, WF, CW and SW analyzed the data. KT, CW, ZB and
HWH interpreted the data and wrote the article. All authors have
read and approved the article for publication.
Ethics approval and consent to
participate
The study was approved by the Institutional Review
Board of Thomas Jefferson University. Written informed consent was
obtained from all individual participants included in the
study.
Consent for publication
Written informed consent was obtained from all
individual participants included in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singal AG and El-Serag HB: Hepatocellular
carcinoma from epidemiology to prevention: Translating knowledge
into practice. Clin Gastroenterol Hepatol. 13:2140–2151. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fleming RE and Ponka P: Iron overload in
human disease. N Engl J Med. 366:348–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hann HW, Stahlhut MW and Hann CL: Effect
of iron and desferoxamine on cell growth and in vitro ferritin
synthesis in human hepatoma cell lines. Hepatology. 11:566–569.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwarzenbach H, Müller V, Milde-Langosch
K, Steinbach B and Pantel K: Evaluation of cell-free tumour DNA and
RNA in patients with breast cancer and benign breast disease. Mol
Biosyst. 7:2848–2854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hann HW, Kim CY, London WT and Blumberg
BS: Increased serum ferritin in chronic liver disease: A risk
factor for primary hepatocellular carcinoma. Int J Cancer.
43:376–379. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kowdley KV, Belt P, Wilson LA, Yeh MM,
Neuschwander-Tetri BA, Chalasani N, Sanyal AJ and Nelson JE: NASH
Clinical Research Network: Serum ferritin is an independent
predictor of histologic severity and advanced fibrosis in patients
with nonalcoholic fatty liver disease. Hepatology. 55:77–85. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakano S, Kumada T, Sugiyama K, Watahiki H
and Takeda I: Clinical significance of serum ferritin determination
for hepatocellular carcinoma. Am J Gastroenterol. 79:623–627.
1984.PubMed/NCBI
|
|
8
|
Simonetti RG, Craxi A, Dardanonì G,
Lanzarone F, Barbaria F, Cottone M and Pagliaro L: The clinical
value of serum ferritin in hepatocellular carcinoma.
Hepatogastroenterology. 32:276–278. 1985.PubMed/NCBI
|
|
9
|
Tatsuta M, Yamamura H, Iishi H, Kasugai H
and Okuda S: Value of serum alpha-fetoprotein and ferritin in the
diagnosis of hepatocellular carcinoma. Oncology. 43:306–310. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stevens RG, Beasley RP and Blumberg BS:
Iron-binding proteins and risk of cancer in Taiwan. J Natl Cancer
Inst. 76:605–610. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hann HW, Fu X, Myers RE, Hann RS, Wan S,
Kim SH, Au N, Xing J and Yang H: Predictive value of
alpha-fetoprotein in the long-term risk of developing
hepatocellular carcinoma in patients with hepatitis B virus
infection-results from a clinic-based longitudinal cohort. Eur J
Cancer. 48:2319–2327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu X, Wan S, Hann HW, Myers RE, Hann RS,
Au J, Chen B, Xing J and Yang H: Relative telomere length: A novel
non-invasive biomarker for the risk of non-cirrhotic hepatocellular
carcinoma in patients with chronic hepatitis B infection. Eur J
Cancer. 48:1014–1022. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adams P: Management of elevated serum
ferritin levels. Gastroenterol Hepatol (N Y). 4:333–334.
2008.PubMed/NCBI
|
|
14
|
Andersen PK, Geskus RB, de Witte T and
Putter H: Competing risks in epidemiology: Possibilities and
pitfalls. Int J Epidemiol. 41:861–870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Royston P and Parmar MK: Flexible
parametric proportional-hazards and proportional-odds models for
censored survival data, with application to prognostic modelling
and estimation of treatment effects. Stat Med. 21:2175–2197. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hann HW, Wan S, Lai Y, Hann RS, Myers RE,
Patel F, Zhang K, Ye Z, Wang C and Yang H: Aspartate
aminotransferase to platelet ratio index as a prospective predictor
of hepatocellular carcinoma risk in patients with chronic hepatitis
B virus infection. J Gastroenterol Hepatol. 30:131–138. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen CJ and Lee MH: Early diagnosis of
hepatocellular carcinoma by multiple microRNAs: Validity, efficacy,
and cost-effectiveness. J Clin Oncol. 29:4745–4747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hann HW, Stahlhut MW and Menduke H: Iron
enhances tumor growth. Observation on spontaneous mammary tumors in
mice. Cancer. 68:2407–2410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hann HW, Stahlhut MW and Blumberg BS: Iron
nutrition and tumor growth: Decreased tumor growth in
iron-deficient mice. Cancer Res. 48:4168–4170. 1988.PubMed/NCBI
|
|
20
|
Kew MC: Hepatic iron overload and
hepatocellular carcinoma. Liver Cancer. 3:31–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deugnier Y and Turlin B: Iron and
hepatocellular carcinoma. J Gastroenterol Hepatol. 16:491–494.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hann HW, Stahlhut MW, Rubin R and Maddrey
WC: Antitumor effect of deferoxamine on human hepatocellular
carcinoma growing in athymic nude mice. Cancer. 70:2051–2056. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Knovich MA, Storey JA, Coffman LG, Torti
SV and Torti FM: Ferritin for the clinician. Blood Rev. 23:95–104.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asare GA, Paterson AC, Kew MC, Khan S and
Mossanda KS: Iron-free neoplastic nodules and hepatocellular
carcinoma without cirrhosis in Wistar rats fed a diet high in iron.
J Pathol. 208:82–90. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Asare GA, Mossanda KS, Kew MC, Paterson
AC, Kahler-Venter CP and Siziba K: Hepatocellular carcinoma caused
by iron overload: A possible mechanism of direct
hepatocarcinogenicity. Toxicology. 219:41–52. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zacharski LR, Ornstein DL, Woloshin S and
Schwartz LM: Association of age, sex, and race with body iron
stores in adults: Analysis of NHANES III data. Am Heart J.
140:98–104. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Uchino K, Tateishi R, Fujiwara N, Minami
T, Sato M, Enooku K, Nakagawa H, Asaoka Y, Kondo Y, Yoshida H, et
al: Impact of serum ferritin level on hepatocarcinogenesis in
chronic hepatitis C patients. Hepatol Res. 46:259–268. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Giordano S and Columbano A: MicroRNAs: New
tools for diagnosis, prognosis, and therapy in hepatocellular
carcinoma? Hepatology. 57:840–847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang TS, Wu YC, Tung SY, Wei KL, Hsieh
YY, Huang HC, Chen WM, Shen CH, Lu CH, Wu CS, et al:
Alpha-fetoprotein measurement benefits hepatocellular carcinoma
surveillance in patients with cirrhosis. Am J Gastroenterol.
110:836–845. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Y, Zhao Y, Feng L, Zhang J, Zhang J
and Feng G: Association between alpha-fetoprotein and metabolic
syndrome in a Chinese asymptomatic population: A cross-sectional
study. Lipids Health Dis. 15:852016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Noritake H, Kobayashi Y, Ooba Y, Kitsugi
K, Shimoyama S, Yamazaki S, Chida T, Watanabe S, Kawata K and Suda
T: Improved serum alpha-fetoprotein levels after iron reduction
therapy in HCV patients. ISRN Hepatol. 2014:8751402014.PubMed/NCBI
|
|
32
|
Zhou XD, Stahlhut MW, Hann HL and London
WT: Serum ferritin in hepatocellular carcinoma.
Hepatogastroenterology. 35:1–4. 1988.PubMed/NCBI
|
|
33
|
Deugnier Y and Turlin B: Pathology of
hepatic iron overload. Semin Liver Dis. 31:260–271. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McKinnon EJ, Rossi E, Beilby JP, Trinder D
and Olynyk JK: Factors that affect serum levels of ferritin in
Australian adults and implications for follow-up. Clin
Gastroenterol Hepatol. 12(101–108): e42014.
|